Abstract
Background
Previous evidence indicated that incidence rates of non-Hodgkin's lymphoma (NHL) are high in Egypt although little is known about risk factors.
Materials and methods
Using data from the population-based cancer registry of Gharbiah governorate in Egypt, we assessed the 1999–2005 incidence of hematopoietic cancers (HCs) based on the ICD-O3 by age- and sex-specific urban–rural distribution.
Results
NHL showed the highest incidence among all HCs (11.7 per 100 000). Urban incidence of HCs was higher than rural incidence. Incidence rates of Hodgkin's lymphoma (HL) and NHL were high especially among urban males up to the 64-year age category. Rural incidence of HL and NHL was high below age 20. Among the districts of the governorate, we observed NHL incidence pattern similar to that observed for hepatocellular carcinoma because of the possible link to hepatitis C virus for both cancers. Comparison to the published HCs data from Algeria, Cyprus, and Jordan showed the highest NHL rate in Egypt than the other countries in the region.
Conclusions
Future studies should define the role of environmental exposures in hematopoietic carcinogenesis in this population. In-depth studies should also investigate the role of access to health care in the urban–rural variation of HC distribution in this population.
Keywords: Egypt, hematopoietic cancer, Hodgkin's lymphoma, incidence, non-Hodgkin's lymphoma
introduction
Hematopoietic cancers (HCs) are diverse groups of diseases including leukemias, lymphomas, plasma cell tumors, myelodysplastic syndromes, and mastocytosis. They arise primarily from two categories of immunological cell types—myeloid and lymphoid cells [1]. Tumors involving the lymphoid group are more common and the classification between lymphomas and leukemias has become blurred over the last few years [1]. Among lymphomas, Hodgkin's lymphoma (HL) has low incidence rates across the world with small variations. In the United States, the incidence of HL is ∼3 per 100 000, while it is <1 per 100 000 in parts of Asia and sub-Saharan Africa [2]. Non-Hodgkin's lymphoma (NHL) though has much higher global incidence than HL with wider variations across the world [3]. Most developed countries as well as West Asia report rates of NHL >10 per 100 000, while parts of South and East Asia and Africa report rates of <5 per 100 000 [2]. Incidence rates of leukemia are intermediate in the East Mediterranean region between lowest rates in Africa, e.g. Uganda's incidence rate of 1.3 per 100 000 in males and 1.4 per 100 000 in females and highest incidence rates in New Zealand (14.8 per 100 000 in males and 8.7 per 100 000 in females) [4, 5].
Among developing countries, the incidence of HC is low with Egypt being one of the exceptions. Egypt has one of the highest incidence rates of lymphoma in the world, mainly NHL, which is higher than even the United States [4, 5] as well as other developed nations where HCs are more common. In Egypt, NHL is the second most common cancer in adults and lymphoma is the most common cancer in children [6]. Previous studies of HC have been carried out in Egypt, but they mostly contain data from pathology-based laboratories [7] or localized registries from predominantly urban areas with limited variations in risk factors and exposures [8]. As a part of this study, we explored patterns of incidence of HCs in the Gharbiah governorate, situated in the center of the Nile Delta Region (NDR) of Egypt, from the country's only population-based cancer registry. This study capitalized on the unique opportunities to investigate HC in this region since our previous studies from NDR have depicted distinct variations in environmental and occupational exposures [9–12], variations based on urban and rural residence [13–15], and distribution of possible risk factors for HC [15–19].
materials and methods
The Gharbiah population-based cancer registry (GPCR) was created in 1998 as a part of the Middle East Cancer Consortium (MECC) with funding from the National Cancer Institute in Bethesda and is located in Tanta, the capital of Gharbiah Governorate [6]. The active registry also collects information on cancer patients who reside in Gharbiah but are diagnosed in neighboring governorates. Methods for ensuring data quality, eliminating duplication, and information on registry staff training and experience have been documented previously [15, 16].
study population
The case definition for the study population consisted of all patients in the GPCR diagnosed with any hematopoietic malignancy (ICD-O3 morphology codes 9590-9989) between the years 1999 and 2005. The following variables were obtained for each case: registry number, sex, birth date, age at diagnosis, address, region, marital status, smoking status, occupation, family history, date, place and basis of diagnosis, topography, morphology, laterality, grade, stage, follow-up and cause of death information, medical record number, treatment, and place of reference. Missing values and inconsistencies in these variables were addressed by examining medical records and not considered in further analysis, if unresolved. While not all cases were deceased, it was not within the scope of this study to independently acquire additional risk factor information or supplement missing values through interviews. Use of human subject data was approved by the University of Michigan Institutional Review Board and the Gharbiah Cancer Society Research Ethics Committee.
The Gharbiah governorate consists of eight districts, each with its own main city of the same name while the rest of the districts are rural with a total of 316 villages in the governorate. According to the recent 2006 Central Agency for Public Mobilization and Statistics (CAPMAS) census [20], Gharbiah has a population of slightly > 4 million (49% female, 51% male). Approximately 30% of the population resides in urban areas with ∼52% of the population below the age of 24 and 3.8% above the age of 65.
Census data for Gharbiah governorate were obtained from the 1996, 2006, and 2011 census reports of CAPMAS [20]. A constant linear growth of the population was assumed to project populations in the intervening years. At the district level, the census data consisted of 16 age categories (5-year intervals). Pediatric cases were defined as <15 years of age, whereas adults were defined as 15 years of age and above. These population figures formed the denominators to calculate the overall, age-specific, district-specific, and urban–rural incidence rates for hematopoietic malignancies.
The urban–rural classification followed the CAPMAS coding of urban and rural areas. Urban areas consisted of the capital cities of the eight districts of the Gharbiah governorate, while the remaining areas in the governorate were considered rural. Each case in the registry is assigned a residence code based on their city or village of residence and this code was used to classify patients as urban or rural.
data management and statistical analysis
Nodal lymphomas included those tumors arising in lymph nodes, Waldeyer's ring, the tonsils, and the spleen. All other topologies were considered extranodal lymphomas [21]. Aggressiveness of NHL tumors was assessed similar to the REAL classification system [22].
For comparability to other data, the subtypes were grouped using the World Health Organization (WHO) classification according to the HAEMACARE project classification [23].
Annual and average age- and sex-specific incidence rates for the period 1999–2005 were calculated using the number of hematopoietic malignancy cases as the numerator and the corresponding age- and sex-specific population data from CAPMAS as the denominator. Univariate analyses were carried out with demographic indicators and other registry variables to better understand the characteristics of the study population. Interannual variation in the number of cases was examined using χ2 testing. Incidence rate ratios (IRRs) were calculated and used when comparing the eight districts to each other. Additionally rates were calculated to compare urban and rural regions in Gharbiah. Direct age-adjusted incidence rates were calculated by direct age standardization for the districts and Gharbiah using WHO's world standard population [2]. These rates were then compared with data of the registries of Algeria, Cyprus, and Jordan published in the MECC monograph, Cancer Incidence in Five Continents version IX and GlobalCan [4, 5, 24]. All statistical operations were carried out in SAS 9.2 (SAS Institute, Cary, NC).
results
There were 4288 cases (58.6% male, 41.4% female) of HCs in GPCR from the years 1999–2005 (Table 1). Most of the cases were rural (58.2%) and belonged to the district of Tanta (29.6%) followed by the district of El-Mehalla (22.2%). Approximately half the lymphoma cases had presented in later stages of the disease (54%) and had been diagnosed by the histology of the primary tumor (85.9%). The majority of the lymphomas were nodal (73.5%) and were moderately aggressive (49.9%) (Table 1).
Table 1.
Characteristics of the study population and patterns of hematopoietic cancers in Gharbiah, Egypt (1999–2005)
| Variable | Descriptive category | Total No. | Cases (%) |
|---|---|---|---|
| Total cases | 4288 | 100.0 | |
| Sex | Male | 2511 | 58.6 |
| Female | 1777 | 41.4 | |
| Residence | Urban | 1793 | 41.8 |
| Rural | 2495 | 58.2 | |
| Year of diagnosis | 1999 | 577 | 13.5 |
| 2000 | 585 | 13.6 | |
| 2001 | 601 | 14.0 | |
| 2002 | 588 | 13.7 | |
| 2003 | 643 | 15.0 | |
| 2004 | 645 | 15.0 | |
| 2005 | 649 | 15.1 | |
| Districts | Tanta | 1269 | 29.6 |
| El-Mehalla | 952 | 22.2 | |
| Kafr El Zayat | 437 | 10.2 | |
| Zefta | 365 | 8.5 | |
| Samanood | 202 | 4.7 | |
| Santa | 377 | 8.8 | |
| Kotour | 381 | 8.9 | |
| Basyoon | 305 | 7.1 | |
| Stage of cancera | Localized (I) | 536 | 12.5 |
| Regional (Dir. Ext; Ext. LN; and NOS, II) | 824 | 19.2 | |
| Distant (III, IV) | 2315 | 54.0 | |
| Unknown | 613 | 14.3 | |
| Basis of diagnosis | Histology of primary | 3683 | 85.9 |
| Cytology/hematology | 261 | 6.1 | |
| Clinical/Ult./X-ray | 110 | 2.6 | |
| Death certificate only | 204 | 4.8 | |
| Otherb | 30 | 0.7 | |
| Tumor site(3081)c | Nodal | 1759 | 73.5 |
| Extranodal | 635 | 26.5 | |
| Aggressiveness of tumor(2462)c | Indolent | 745 | 30.3 |
| Aggressive | 1229 | 49.9 | |
| Highly aggressive | 488 | 19.8 |
aStage of cancer was defined according to the SEER Summary Staging Manual, 2000. Stages of cancer were categorized as: localized: stage I lymphoma and solitary plasmacytoma; regional, NOS: stage II lymphoma; distant: stage III and IV lymphoma as well as all leukemia, multiple myeloma, and myelodysplastic syndrome.
bOther includes: clinical only, exploratory surgery/autopsy, specific biochemical/immunological test, and histology of metastases.
cLymph node involvement and aggressive nature were assessed for lymphomas only, with sample sizes of 2394 and 2462, respectively. Dir.ext., direct extension; Ext. LN, extranodal lymph node; NOS, not otherwise specified; Ult., ultrasound.
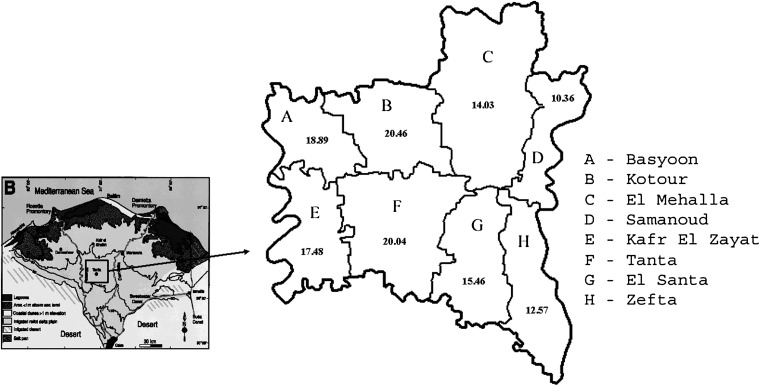
Table 2 depicts the crude age-specific as well as age-specific incidence rates of the major groups of HCs compared by urban–rural status residence of cases. Crude rates showed higher incidence for all HL, NHL, and leukemia in males than females, except for HL in the age group of 65–74, and leukemia in the age-group of 50–64, where females had higher incidence rates than males. In addition, incidence rates of all three malignancies showed steady increase with aging (Table 2). Overall age-standardized incidence rates of lymphomas and leukemias were higher among males than females in both urban and rural populations. Also, urban incidence was higher than rural incidence when comparing overall incidence rates with most age-specific incidence rates. The maximum urban–rural differences were observed for urban incidence of leukemia (IRR = 4.89; 95% CI = 1.53–15.59) and HL (IRR = 3.91; 95% CI = 0.35–43.15) in the 75+ age category. Distribution of incidence of HCs by districts of Gharbiah (Figure 1) depicted highest incidence rates in the central districts of Kotour and Tanta.
Table 2.
Crude and age-standardizeda incidence rates (100 000) by sex, overall, and by urban–rural status for Hodgkin's lymphoma, non-Hodgkin's lymphoma, and leukemia in Gharbiah, Egypt
| Cancer site | Gharbiah overall IRb |
Gharbiah overall IRa |
Gharbiah urban IRa |
Gharbiah rural IRa |
Urban–rural IRR (95% CI)b |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| Hodgkin's lymphoma (C81) | ||||||||||
| <20 | 1.96 | 0.91 | 0.79 | 0.36 | 1.13 | 0.58 | 0.65 | 0.28 | 1.72 (1.19–2.50) | 2.09 (1.20–3.62) |
| 20–49 | 2.46 | 1.11 | 0.99 | 0.45 | 1.55 | 0.58 | 0.74 | 0.38 | 2.10 (1.51–2.93) | 1.51 (0.92–2.50) |
| 50–64 | 2.84 | 2.05 | 0.37 | 0.27 | 0.48 | 0.26 | 0.31 | 0.27 | 1.55 (0.82–2.92) | 0.96 (0.43–2.16) |
| 65–74 | 2.19 | 2.40 | 0.11 | 0.12 | 0.12 | 0.13 | 0.11 | 0.11 | 1.11 (0.27–4.66) | 1.18 (0.29–4.71) |
| 75+ | 2.58 | 0.74 | 0.05 | 0.01 | 0.10 | 0.00 | 0.03 | 0.02 | 3.91 (0.35–43.15) | 0.00 |
| Total | 2.28 | 1.15 | 2.30 | 1.21 | 3.37 | 1.56 | 1.83 | 1.07 | 1.84 (1.47–2.31) | 1.46 (1.05–2.02) |
| Non-Hodgkin's lymphoma (C82–C85, C96) | ||||||||||
| <20 | 2.58 | 1.31 | 1.03 | 0.52 | 1.37 | 0.53 | 0.90 | 0.52 | 1.52 (1.10–2.12) | 1.03 (0.62–1.70) |
| 20–49 | 10.12 | 6.35 | 4.05 | 2.54 | 5.37 | 3.30 | 3.47 | 2.19 | 1.55 (1.31–1.83) | 1.51 (1.23–1.87) |
| 50–64 | 38.11 | 32.55 | 4.95 | 4.23 | 6.06 | 5.11 | 4.31 | 3.75 | 1.40 (1.18–1.67) | 1.36 (1.12–1.65) |
| 65–74 | 61.57 | 48.20 | 3.08 | 2.41 | 3.67 | 3.93 | 2.76 | 1.76 | 1.33 (1.02–1.73) | 2.23 (1.67–2.98) |
| 75+ | 60.25 | 52.57 | 1.21 | 1.05 | 1.63 | 1.44 | 0.99 | 0.90 | 1.65 (1.03–2.64) | 1.59 (0.99–2.57) |
| Total | 11.45 | 8.42 | 14.32 | 10.75 | 18.10 | 14.31 | 12.43 | 9.12 | 1.46 (1.32–1.61) | 1.57 (1.39–1.77) |
| Leukemia (C91–95) | ||||||||||
| <20 | 3.53 | 2.27 | 1.41 | 0.91 | 1.79 | 1.27 | 1.27 | 0.77 | 1.41 (1.06–1.87) | 1.65 (1.16–2.35) |
| 20–49 | 3.10 | 2.90 | 1.24 | 1.16 | 1.34 | 1.47 | 1.19 | 1.02 | 1.12 (0.82–1.54) | 1.45 (1.06–1.98) |
| 50–64 | 7.71 | 7.80 | 1.00 | 1.01 | 1.19 | 1.27 | 0.89 | 0.87 | 1.33 (0.90–1.97) | 1.45 (0.98–2.16) |
| 65–74 | 9.30 | 8.79 | 0.47 | 0.44 | 0.70 | 0.71 | 0.34 | 0.32 | 2.09 (1.06–4.09) | 2.22 (1.12–4.39) |
| 75+ | 12.05 | 11.11 | 0.24 | 0.22 | 0.51 | 0.21 | 0.10 | 0.23 | 4.89 (1.53–15.59) | 0.94 (0.30–2.96) |
| Total | 4.00 | 3.36 | 4.36 | 3.74 | 5.53 | 4.94 | 3.80 | 3.20 | 1.46 (1.22–1.73) | 1.54 (1.27–1.86) |
aAge-standardized to the world population.
bCrude rate.
CI, confidence interval; IR, incidence; IRR, incidence rate ratio.
Figure 1.
Crude incidence rates for hematopoietic cancers in the eight districts of Gharbiah, 1999-2005 (Map from Lehman et al., 2008 [16]).
Supplemental Table S1 (available at Annals of Oncology online) and Table 3 present the crude incidence rates for lymphoid and myeloid malignancies in the Gharbiah registry by age and morphologic types based on the WHO and HEMACARE classification [23]. ‘Diffuse B-cell lymphoma’, ‘malignant lymphoma, large B-cell diffuse NOS’, ‘malignant lymphoma, small B-cell lymphocytic’, and ‘B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma’ were the most common types among the mature B-cell neoplasms. Among the HL, mixed cellularity was the most common type in this group (supplemental Table S1, available at Annals of Oncology online). Among the myeloproliferative neoplasms, chronic myelocytic leukemia was the most common type (Table 3).
Table 3.
Number of cases and crude incidence rate (IR) per 100 000 for myeloid malignancies diagnosed in Gharbiah 1999–2005 by sex and morphologic type
| HAEMACARE groupings | ICD-O-3 code | ICD-O-3 description | Total |
Males |
Females |
|||
|---|---|---|---|---|---|---|---|---|
| No. of cases | IR | No. of cases | IR | No. of cases | IR | |||
| Acute myeloid leukemia | 266 | 1.03 | 140 | 1.07 | 126 | 0.99 | ||
| Subgroup 1 | 9840 | Acute erythroid leukemia | 0 | 0 | 0 | 0 | 0 | 0 |
| 9861 | AML, NOS | 71 | 0.27 | 37 | 0.28 | 34 | 0.27 | |
| 9867 | Acute myelomonocytic leukemia | 23 | 0.09 | 9 | 0.07 | 14 | 0.11 | |
| 9870 | Acute basophilic leukemia | 1 | 0.00 | 1 | 0.01 | 0 | 0 | |
| 9872 | AML, minimal differentiation | 8 | 0.03 | 6 | 0.05 | 2 | 0.02 | |
| 9873 | AML without maturation | 36 | 0.14 | 20 | 0.15 | 16 | 0.13 | |
| 9874 | AML with maturation | 65 | 0.25 | 38 | 0.29 | 27 | 0.21 | |
| 9891 | Acute monocytic leukemia | 22 | 0.09 | 10 | 0.08 | 12 | 0.09 | |
| 9910 | Acute megakaryoblastic leukemia | 3 | 0.01 | 1 | 0.01 | 2 | 0.02 | |
| 9930 | Myeloid sarcoma | 1 | 0.00 | 0 | 0.00 | 1 | 0.01 | |
| Subgroup 2 | 9866 | Acute promyelocytic leukemia t(15; 17) (q22; q11-12) | 30 | 0.12 | 16 | 0.12 | 14 | 0.11 |
| 9871 | AML with abnormal marrow eosinophils | 1 | 0.00 | 0 | 0 | 1 | 0.01 | |
| 9896 | AML, t(8,21) (q22,q22) | 1 | 0.00 | 0 | 0.00 | 1 | 0.01 | |
| 9897 | AML, 11q23 abnormalities | 0 | 0 | 0 | 0 | 0 | 0 | |
| Subgroup 3 | 9895 | AML, with multilineage dysplasia | 2 | 0.01 | 1 | 0.01 | 1 | 0.01 |
| 9984 | Refractory anemia with excess blasts in transformation (obsolete) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Subgroup 4 | 9931 | Acute panmyelosis with myelofibrosis | 2 | 0.01 | 1 | 0.01 | 1 | 0.01 |
| Myeloproliferative neoplasms | 220 | 0.85 | 103 | 0.79 | 117 | 0.92 | ||
| CML | 9863 | CML, NOS | 201 | 0.78 | 95 | 0.72 | 106 | 0.83 |
| 9875 | Chronic myelogenous leukemia, BCR/ABL positive | 6 | 0.02 | 1 | 0.01 | 5 | 0.04 | |
| Other myeloproliferative neoplasms | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Subgroup 1 | 9950 | Polycythemia vera | 3 | 0.01 | 2 | 0.02 | 1 | 0.01 |
| 9961 | Myelosclerosis with myeloid metaplasia | 7 | 0.03 | 2 | 0.02 | 5 | 0.04 | |
| 9962 | Essential thrombocythemia | 1 | 0 | 1 | 0.01 | 0 | 0 | |
| 9963 | Chronic neutrophilic leukemia | 2 | 0.01 | 2 | 0.02 | 0 | 0 | |
| 9964 | Hypereosinophilic syndrome | 0 | 0 | 0 | 0 | 0 | 0 | |
| Subgroup 2 | 9960 | Chronic myeloproliferative disease, NOS | 0 | 0 | 0 | 0 | 0 | 0 |
| Myelodysplastic syndrome | 36 | 0.14 | 16 | 0.12 | 20 | 0.16 | ||
| 9980 | Refractory anemia | 19 | 0.07 | 8 | 0.06 | 11 | 0.09 | |
| 9982 | Refractory anemia with sideroblasts | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9983 | Refractory anemia with excess blasts | 9 | 0.03 | 3 | 0.02 | 6 | 0.05 | |
| 9985 | Refractory cytopenia with multilineage dysplasia | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9986 | Myelodysplastic syndrome 5q deletion | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9989 | Myelodysplastic syndrome, NOS | 8 | 0.03 | 5 | 0.04 | 3 | 0.02 | |
| Myelodysplastic/myeloproliferative neoplasms | 5 | 0.02 | 2 | 0.02 | 3 | 0.02 | ||
| 9945 | Chronic myelomonocytic leukemia | 4 | 0.02 | 2 | 0.02 | 2 | 0.02 | |
| 9876 | Atypical CML, BCR/ABL-1 negative | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9946 | Juvenile myelomonocytic leukemia | 1 | 0 | 0 | 0 | 1 | 0.01 | |
| 9975 | Myelodysplastic/myeloproliferative | 0 | 0 | 0 | 0 | 0 | 0 | |
| Unknown myeloid neoplasms | 198 | 0.77 | 119 | 0.91 | 79 | 0.62 | ||
| Leukemia, NOS | 9800 | Leukemia, NOS | 172 | 0.66 | 99 | 0.75 | 73 | 0.57 |
| 9801 | Acute leukemia, NOS | 26 | 0.10 | 20 | 0.15 | 6 | 0.05 | |
| 9805 | Acute leukemia, ambiguous lineage | 0 | 0 | 0 | 0 | 0 | 0 | |
| Myeloid leukemia, NOS | 9806 | Myeloid leukemia, NOS | 0 | 0 | 0 | 0 | 0 | 0 |
| All myeloid malignancies | 725 | 2.80 | 380 | 2.90 | 345 | 2.70 | ||
AML, acute myeloid leukemia; NOS, not otherwise specified; CML, chronic myeloid leukemia.
When categorized by clinical types, the incidence of NHL was the highest (11.7 per 100 000) followed by leukemia (5.30 per 100 000) and HL (1.72 per 100 000) (supplemental Table S2, available at Annals of Oncology online). When the rates of HL, NHL, and leukemia of Egypt were compared with the rates of countries from the Middle Eastern region (Algeria, Cyprus, and Jordan) [4, 5, 24], Egypt showed the highest incidence of NHL than other Middle Eastern countries (supplemental Table S2, available at Annals of Oncology online).
On analyzing the extent of involvement of nodal sites with regards to lymphoma, apart from lymph nodes of multiple regions (C77.8) (43.9%), the maximum involvement was of lymph nodes of head and neck (C77.0) (14.0%) followed by abdominal lymph nodes (C77.2) (12.4%). Among the extranodal sites, maximum involvement was seen for gastrointestinal tract (GIT) (29.7%) followed by head and neck (20.0%). The top five primary sites for extranodal lymphoma were stomach (C16) (15.4%), bone (C40-41) (12.9%), soft tissue (C49) (11.0%), skin (C44) (9.4%), and nasopharynx (C11) (8.7%).
discussion
This study is the largest and only population-based study on HCs from Egypt. The study has five interesting observations. First, we observed a higher urban than rural incidence of HCs with a maximum difference for leukemia and HL in the 75+ age category. Second, we identified a regional variation of HCs among the districts with highest rates seen for central districts followed by the western districts and the lowest rates in the most eastern districts. Third, NHL was the most common malignancy with diffuse B-cell neoplasms being the most common type of NHL. Chronic lymphocytic leukemia was the most common subtype of leukemia. Fourth, the most common extranodal sites were GIT, head and neck, and bone. Fifth, there were international variations of HCs when the Egyptian rates were compared with the rates of neighboring countries. Incidence of NHL in Egyptians was higher than the incidence in Algeria, Cyprus, and Jordan, but the incidences of HL and leukemia in Egypt were intermediate compared with the incidences of these two HC types in Algeria, Jordan, and Cyprus [4, 5, 25].
In this study, we observed a higher urban incidence for all HCs albeit the differences in urban and rural incidence were <100%; we did see an approximately four times higher incidence of leukemia and HL among the 75+ year urban males. We do not believe that there is a significant difference in access to primary health care between rural and urban regions in our study population. Egypt has one of the best coverage of primary health care systems in the world through rural health units and urban health centers [25]. However, complete unbiased ascertainment and accurate diagnosis of different subtypes of HC are challenging even in developed countries [26]. In addition, socioeconomic factors did not show to be a factor in HC subtype differences in developed countries [27]. However, variation of health care access and detailed case ascertainment are worthy of future thorough investigations in developing countries like Egypt.
NHL has a similar risk profile with higher occurrence seen in immune-suppressed individuals or individuals infected with hepatitis C virus (HCV), Epstein–Barr virus, or HIV [28, 29]. There is also limited evidence suggesting association of NHL with higher exposure to ultraviolet light [30] and pesticides [31]. Ionizing radiation and alkylating agents such as those used for chemotherapy have been investigated in relation to HC [32–37]. However, none of the studies that investigated these exposures in relation to HC were conducted in Egypt or other African countries.
Prevalence of HCV infection is ∼13.9% of the healthy population with higher rates of up to 15.8% in Northern Egypt, including Gharbiah [38]. Such a high rate of HCV infection is related to the increasing incidence of hepatocellular cancer (HCC) in Egypt [16] while at the same time is also probably responsible for the high rates of NHL, especially B-cell type NHL, observed in our present study. The common role of HCV infections for HCC and NHL is also supported by the pattern of incidence of HCs in the districts of Gharbiah (Figure 1) which is mainly driven by NHL and is quite similar to that seen for HCC [16]. HCV infection has also been linked to increased risk of B-cell NHL [39] and to diffuse large B-cell marginal zone and follicular lymphoma in studies from the National Cancer Institute of Cairo University in Egypt [40]. The role of HCV infection in NHL is further strengthened by immunohistochemical studies that detected HCV RNA in malignant NHL tissues from Egypt [41]. A recent report of cancer incidence in Aswan in South Egypt showed a significantly lower rate of NHL (crude rate of 1.9 per 100 000 and age-standardized rate of 1.9 per 100 000) than the rate reported from Gharbiah in this study [42]. However, the rates of HL and leukemia in Aswan were not significantly different from the rates in Gharbiah (1.2 per 100 000 and 1.3 per 100 000, crude and adjusted rates, respectively, for HL and 5.3 per 100 000 and 6.6 per 100 000, crude and adjusted rates, respectively, for leukemia in Aswan). Higher rates of NHL in Gharbiah than South Egypt and other neighboring countries are likely due to the higher infection rate of HCV in Gharbiah than in South Egypt and the Middle East [43].
HCV has been attributed as a causal role in NHL in the past and there are putative reasons to support this from a large number of epidemiological studies with estimates suggesting a two to four times higher risk of NHL occurrence among HCV-positive individuals [43, 44]. HCV infection mainly affects B cells resulting in a benign lymphoproliferation and mixed cryoglobulinemia [45, 46]. The association of HCV infection and NHL is clear in Egypt since the pattern of incidence of lymphomas is quite similar to the pattern of incidence of HCC (which can be considered a proxy for HCV infection) in the districts of Gharbiah [16]. Unfortunately, there are no available results on the exact infection rate of HCV among the population of the Gharbiah governorate.
Smoking has been linked to HC but the associations have been either weak or nonexistent. For example, weak-positive association was observed for cigarette smoking and HL [47] and for follicular but not other types of NHL [48]. Other studies showed no excess risk of NHL with tobacco smoking [49–52] with other exceptions [53–55]. Rates of smoking are high and increasing in Egypt [56, 57] but smoking has not been studied in relation to HC.
The majority of lymphomas in our study were nodal and the proportion was consistent with the values seen in the United States where two-thirds of lymphoid malignancies arise in the lymph nodes, while only one-third arise in extranodal sites [1]. However, among the extranodal sites, it was interesting to note that gastrointestinal and head and neck sites were the most commonly involved sites. It is important to note that the rate of extranodal lymphoma in our study (26.5%) is significantly higher than the extranodal rates reported in recent studies from Iran (11.5%) and Korea (12.4%) [58, 59].
Our study had a number of strengths, chief among which was the fact that this was the first study on HCs from Egypt from a population-based registry. The population in this region of Egypt is quite stable with low migration rates [20] and as such our estimates on the geographical variations of incidence rates were quite valid. However, our study also had inherent shortcomings of registry data, in general, with respect to lack of detailed information on the distribution of risk factors among individual cases.
In conclusion, this study showed urban–rural as well as district-level geographical differences of the types of hematopoietic malignancies in the NDR of Egypt. The study highlights the geographical distribution for both NHL and HCC in the study region and the possible parallel link between HCV and both cancers. Future detailed studies at the individual level in this population should consider the possible, unique infections, lifestyle factors and environmental risk factors, especially for NHL.
funding
CMH was supported by the Cancer Epidemiology Education in Special PopulationsProgram of the University of Michigan through funding from the National Institutes of Health (R25 CA112383).
disclosure
The authors do not have conflict of interest or funding sources that might generate a conflict of interest.
Supplementary Material
references
- 1.Robbins SL, Kumar V, Cotran RS. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2010. [Google Scholar]
- 2.Parkin DM, Whelan SL, Ferlay J, et al. Cancer Incidence in Five Continents. Vol. 8. Lyon, France: International Agency for Research on Cancer; 2002. [Google Scholar]
- 3.Boyle P, Levin B, editors. World Cancer Report 2008. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 4.Freedman LS National Cancer Institute, Middle East Cancer Consortium. Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared with US SEER. 2006. NIH Pub. No. 06-5873. Bethesda, MD: 150. [Google Scholar]
- 5.Curado MP, Edwards B, Shin HR, et al., editors. Cancer Incidence in Five Continents. Vol. 9. Lyon, France: International Agency for Research on Cancer; 2007. 101. [Google Scholar]
- 6.Soliman AS, Boffetta P. Lymphoma and leukemia. In: Freedman LS, Edwards BK, Ries LAG, Young JL, editors. Cancer Incidence in the Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Bethesda, MD: National Cancer Institute; 2006. pp. 131–140. [Google Scholar]
- 7.Mokhtar N, Gouda I, Adel I. Cancer Pathology Registry 2003–2004 and Time Trend Analysis. Cairo, Egypt: National Cancer Institute, Cairo University; 2007. [PubMed] [Google Scholar]
- 8.Abdel-Fattah MM, Yassine OG. Non-Hodgkin's lymphomas in Alexandria, Egypt; incidence rates and trend study (1995–2004) Eur J Cancer Prev. 2007;16:479–485. doi: 10.1097/01.cej.0000243858.91642.c9. doi:10.1097/01.cej.0000243858.91642.c9. [DOI] [PubMed] [Google Scholar]
- 9.Soliman AS, Smith MA, Cooper SP, et al. Serum organochlorine pesticide levels in patients with colorectal cancer in Egypt. Arch Environ Health. 1997;52:409–415. doi: 10.1080/00039899709602219. doi:10.1080/00039899709602219. [DOI] [PubMed] [Google Scholar]
- 10.Soliman AS, Wang X, DiGiovanni J, et al. Serum organochlorine levels and history of lactation in Egypt. Environ Res. 2003;92:110–117. doi: 10.1016/s0013-9351(02)00056-7. [DOI] [PubMed] [Google Scholar]
- 11.Kriegel AM, Soliman AS, Zhang Q, et al. Serum cadmium levels in pancreatic cancer patients from the East Nile Delta region of Egypt. Environ Health Perspect. 2006;114:113–119. doi: 10.1289/ehp.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soliman AS, Wang X, Stanley JD, et al. Geographical clustering of pancreatic cancers in the Northeast Nile Delta region of Egypt. Arch Environ Contam Toxicol. 2006;51:142–148. doi: 10.1007/s00244-005-0154-0. Published on 20060201. doi:10.1007/s00244-005-0154-0. [DOI] [PubMed] [Google Scholar]
- 13.Dey S, Soliman AS, Hablas A, et al. Urban-rural differences in breast cancer incidence by hormone receptor status across 6 years in Egypt. Breast Cancer Res Treat. 2010;120:149–160. doi: 10.1007/s10549-009-0427-9. Published on 20090623. doi:10.1007/s10549-009-0427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey S, Hablas A, Seifeldin IA, et al. Urban-rural differences of gynecological malignancies in Egypt (1999–2002) BJOG. 2010;117:348–355. doi: 10.1111/j.1471-0528.2009.02447.x. Published on 20091216. doi:10.1111/j.1471-0528.2009.02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey S, Soliman AS, Hablas A, et al. Urban-rural differences in breast cancer incidence in Egypt (1999–2006) Breast. 2010;19:417–423. doi: 10.1016/j.breast.2010.04.005. Published on 20100508. doi:10.1016/j.breast.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehman EM, Soliman AS, Ismail K, et al. Patterns of hepatocellular carcinoma incidence in Egypt from a population-based cancer registry. Hepatol Res. 2008;38:465–473. doi: 10.1111/j.1872-034X.2007.00299.x. Published on 20071127. doi:10.1111/j.1872-034X.2007.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felix AS, Soliman AS, Khaled H, et al. The changing patterns of bladder cancer in Egypt over the past 26 years. Cancer Causes Control. 2008;19:421–429. doi: 10.1007/s10552-007-9104-7. Published on 20080110. doi:10.1007/s10552-007-9104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedewa SA, Soliman AS, Ismail K, et al. Incidence analyses of bladder cancer in the Nile delta region of Egypt. Cancer Epidemiol. 2009;33:176–181. doi: 10.1016/j.canep.2009.08.008. Published on 20090916. doi:10.1016/j.canep.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soliman AS, Hung CW, Tsodikov A, et al. Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. Hepatol Int. 2010;4:681–690. doi: 10.1007/s12072-010-9187-1. Published on 20100819. doi:10.1007/s12072-010-9187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Census of Egypt and Statistical Year Book. 3rd edition. Cairo, Egypt: Central Agency for Public Mobilization and Statistics (CAPMAS); 2011. http://www.capmas.gov.eg/faq.aspx?lang=2. [Google Scholar]
- 21.Fritz A, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology. Geneva, Switzerland: World Health Organization; [Google Scholar]
- 22.Shipp MA, Harrington DP, Anderson JR, et al. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. doi:10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 23.Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–3734. doi: 10.1182/blood-2010-05-282632. Published on 20100727. doi:10.1182/blood-2010-05-282632. [DOI] [PubMed] [Google Scholar]
- 24.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. http://globocan.iarc.fr (1 February 2012, date last accessed) [Google Scholar]
- 25.United Nations, Economic Commission for Africa, Subregional Office for North Africa. Economic and Social Conditions in North Africa, Part III: The Economic Participation of Women in North Africa. http://www.uneca.org/na/Women.pdf (29 March 2012, date last accessed). 2005. [Google Scholar]
- 26.Smith A, Roman E, Howell D, et al. The Haematological Malignancy Research Network (HMRN): a new information strategy for population based epidemiology and health service research. Br J Haematol. 2010;148:739–753. doi: 10.1111/j.1365-2141.2009.08010.x. Published on 20091201. doi:10.1111/j.1365-2141.2009.08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–1692. doi: 10.1038/bjc.2011.450. Published on 20111101. doi:10.1038/bjc.2011.450; 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartge P, Wang SS, Bracci PM, et al. Non-Hodgkin's Lymphoma. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2009. pp. 898–918. [Google Scholar]
- 29.International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans, Vol. 67, Human Immunodeficiency Viruses and Human t-cell lymphotropic viruses. Geneva, Switzerland: IARCPress; 1996. [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong BK, Kricker A. Sun exposure and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:396–400. doi: 10.1158/1055-9965.EPI-06-1068. Published on 20070302. doi:10.1158/1055-9965.EPI-06-1068. [DOI] [PubMed] [Google Scholar]
- 31.Boffetta P, de Vocht F. Occupation and the risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:369–372. doi: 10.1158/1055-9965.EPI-06-1055. doi:10.1158/1055-9965.EPI-06-1055. [DOI] [PubMed] [Google Scholar]
- 32.Linet MS, Schubauer-Berigan MK, Weisenburger DD, et al. Chronic lymphocytic leukemia: an overview of aetiology in light of recent developments in classification and pathogenesis. Br J Haematol. 2007;139:672–686. doi: 10.1111/j.1365-2141.2007.06847.x. doi:10.1111/j.1365-2141.2007.06847.x. [DOI] [PubMed] [Google Scholar]
- 33.Larson RA, Le Beau MM. Prognosis and therapy when acute promyelocytic leukemia and other “good risk” acute myeloid leukemias occur as a therapy-related myeloid neoplasm. Mediterr J Hematol Infect Dis. 2011;3:e2011032. doi: 10.4084/MJHID.2011.032. Published on 20110708. doi:10.4084/MJHID.2011.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leone G, Fianchi L, Voso MT. Therapy-related myeloid neoplasms. Curr Opin Oncol. 2011;23:672–680. doi: 10.1097/CCO.0b013e32834bcc2a. doi:10.1097/CCO.0b013e32834bcc2a. [DOI] [PubMed] [Google Scholar]
- 35.Bari A, Marcheselli L, Marcheselli R, et al. Therapy-related myeloid neoplasm in non-hodgkin lymphoma survivors. Mediterr J Hematol Infect Dis. 2011;3:e2011065. doi: 10.4084/MJHID.2011.065. Published on 20111220. doi:10.4084/MJHID.2011.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eichenauer DA, Engert A. Therapy-related myeloid neoplasms in patients treated for hodgkin lymphoma. Mediterr J Hematol Infect Dis. 2011;3:e2011046. doi: 10.4084/MJHID.2011.046. Published on 20111024. doi:10.4084/MJHID.2011.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Tang G, Medeiros LJ, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2011;25:237–245. doi: 10.1038/modpathol.2011.158. doi:10.1038/modpathol.2011.158; 10.1038/modpathol.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehman EM, Wilson ML. Epidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy people in Egypt: a systematic review and meta-analysis. Int J Cancer. 2009;124:690–697. doi: 10.1002/ijc.23937. doi:10.1002/ijc.23937. [DOI] [PubMed] [Google Scholar]
- 39.Cowgill KD, Loffredo CA, Eissa SA, et al. Case-control study of non-Hodgkin's lymphoma and hepatitis C virus infection in Egypt. Int J Epidemiol. 2004;33:1034–1039. doi: 10.1093/ije/dyh183. Published on 20040520. doi:10.1093/ije/dyh183. [DOI] [PubMed] [Google Scholar]
- 40.Goldman L, Ezzat S, Mokhtar N, et al. Viral and non-viral risk factors for non-Hodgkin's lymphoma in Egypt: heterogeneity by histological and immunological subtypes. Cancer Causes Control. 2009;20:981–987. doi: 10.1007/s10552-009-9316-0. Published on 20090305. doi:10.1007/s10552-009-9316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gouda I, Nada O, Ezzat S, et al. Immunohistochemical detection of hepatitis C virus (genotype 4) in B-cell NHL in an Egyptian population: correlation with serum HCV-RNA. Appl Immunohistochem Mol Morphol. 2010;18:29–34. doi: 10.1097/PAI.0b013e3181ae9e82. doi:10.1097/PAI.0b013e3181ae9e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egypt National Cancer Registry. Aswan Profile-2008. Cairo, Egypt: Egypt National Cancer Registry; 2010. RR1. [Google Scholar]
- 43.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100, Part B: Biological Agents. Geneva, Switzerland: WHO Press; 2009. [Google Scholar]
- 44.Mazzaro C, Tirelli U, Pozzato G. Hepatitis C virus and non-Hodgkin's lymphoma 10 years later. Dig Liver Dis. 2005;37:219–226. doi: 10.1016/j.dld.2005.01.003. doi:10.1016/j.dld.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Viswanatha DS, Dogan A. Hepatitis C virus and lymphoma. J Clin Pathol. 2007;60:1378–1383. doi: 10.1136/jcp.2007.051870. doi:10.1136/jcp.2007.051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charles ED, Dustin LB. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009;76:818–824. doi: 10.1038/ki.2009.247. Published on 20090715. doi:10.1038/ki.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hjalgrim H, Ekstrom-Smedby K, Rostgaard K, et al. Cigarette smoking and risk of Hodgkin lymphoma: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:1561–1566. doi: 10.1158/1055-9965.EPI-07-0094. doi:10.1158/1055-9965.EPI-07-0094. [DOI] [PubMed] [Google Scholar]
- 48.Morton LM, Hartge P, Holford TR, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph) Cancer Epidemiol Biomarkers Prev. 2005;14:925–933. doi: 10.1158/1055-9965.EPI-04-0693. doi:10.1158/1055-9965.EPI-04-0693. [DOI] [PubMed] [Google Scholar]
- 49.Zahm SH, Weisenburger DD, Holmes FF, et al. Tobacco and non-Hodgkin's lymphoma: combined analysis of three case-control studies (United States) Cancer Causes Control. 1997;8:159–166. doi: 10.1023/a:1018412027985. [DOI] [PubMed] [Google Scholar]
- 50.Adami J, Nyren O, Bergstrom R, et al. Smoking and the risk of leukemia, lymphoma, and multiple myeloma (Sweden) Cancer Causes Control. 1998;9:49–56. doi: 10.1023/a:1008897203337. [DOI] [PubMed] [Google Scholar]
- 51.Miligi L, Seniori Costantini A, Crosignani P, et al. Occupational, environmental, and life-style factors associated with the risk of hematolymphopoietic malignancies in women. Am J Ind Med. 1999;36:60–69. doi: 10.1002/(sici)1097-0274(199907)36:1<60::aid-ajim9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 52.Willett EV, Smith AG, Dovey GJ, et al. Tobacco and alcohol consumption and the risk of non-Hodgkin lymphoma. Cancer Causes Control. 2004;15:771–780. doi: 10.1023/B:CACO.0000043427.77739.60. doi:10.1023/B:CACO.0000043427.77739.60. [DOI] [PubMed] [Google Scholar]
- 53.Freedman DS, Tolbert PE, Coates R, et al. Relation of cigarette smoking to non-Hodgkin's lymphoma among middle-aged men. Am J Epidemiol. 1998;148:833–841. doi: 10.1093/oxfordjournals.aje.a009708. [DOI] [PubMed] [Google Scholar]
- 54.Stagnaro E, Tumino R, Parodi S, et al. Non-Hodgkin's lymphoma and type of tobacco smoke. Cancer Epidemiol Biomarkers Prev. 2004;13:431–437. [PubMed] [Google Scholar]
- 55.Talamini R, Polesel J, Montella M, et al. Smoking and non-Hodgkin lymphoma: case-control study in Italy. Int J Cancer. 2005;115:606–610. doi: 10.1002/ijc.20891. doi:10.1002/ijc.20891. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2009: Implementing Smoke-Free Environments. Geneva, Switzerland: WHO Press; 2009. [Google Scholar]
- 57.Khan AAM, Dey S, Taha AH, et al. Attitudes of Cairo University medical students toward smoking: the need for tobacco control programs in medical education. J Egypt Public Health Assoc. 2012;87(1-2):1–7. doi: 10.1097/01.EPX.0000411467.14763.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mozaheb Z, Aledavood A, Farzad F. Distributions of major sub-types of lymphoid malignancies among adults in Mashhad, Iran. Cancer Epidemiol. 2011;35:26–29. doi: 10.1016/j.canep.2010.09.009. Published on 20101030. doi:10.1016/j.canep.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Yoon SO, Suh C, Lee DH, et al. Distribution of lymphoid neoplasms in the Republic of Korea: analysis of 5318 cases according to the World Health Organization classification. Am J Hematol. 2010;85:760–764. doi: 10.1002/ajh.21824. doi:10.1002/ajh.21824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.