Abstract
Background
Approximately 25% of patients with esophageal cancer (EC) who undergo preoperative chemoradiation, achieve a pathologic complete response (pathCR). We hypothesized that a model based on clinical parameters could predict pathCR with a high (≥60%) probability.
Patients and methods
We analyzed 322 patients with EC who underwent preoperative chemoradiation. All the patients had baseline and postchemoradiation positron emission tomography (PET) and pre- and postchemoradiation endoscopic biopsy. Logistic regression models were used for analysis, and cross-validation via the bootstrap method was carried out to test the model.
Results
The 70 (21.7%) patients who achieved a pathCR lived longer (median overall survival [OS], 79.76 months) than the 252 patients who did not achieve a pathCR (median OS, 39.73 months; OS, P = 0.004; disease-free survival, P = 0.003). In a logistic regression analysis, the following parameters contributed to the prediction model: postchemoradiation PET, postchemoradiation biopsy, sex, histologic tumor grade, and baseline EUST stage. The area under the receiver-operating characteristic curve was 0.72 (95% confidence interval [CI] 0.662–0.787); after the bootstrap validation with 200 repetitions, the bias-corrected AU-ROC was 0.70 (95% CI 0.643–0.728).
Conclusion
Our data suggest that the logistic regression model can predict pathCR with a high probability. This clinical model could complement others (biomarkers) to predict pathCR.
Keywords: chemoradiation, esophageal cancer, esophageal preservation, nomogram, prediction of response
introduction
Esophageal cancer (EC) poses a significant health burden around the world [1, 2]. The incidence of adenocarcinoma has been rising dramatically in the West for decades [3–5]. EC is often diagnosed in its late stages, but ∼50% of patients have potentially curable disease. In patients who are physiologically fit for surgery and have a technically resectable EC, surgery alone results in a cure rate of <20% (stage II or III disease) [6]. Therefore, surgery is not favored as primary therapy for patients with clinical disease stage greater than cT1bN0. Combined modality therapeutic strategies have been implemented over the last 20 years to improve the cure rate. Preoperative chemoradiation [7] is favored over preoperative chemotherapy as a component of multimodality therapy [8–10] due to higher efficacy.
Approximately 25% of patients with EC who undergo preoperative chemoradiation achieve a pathologic complete response (pathCR), defined as the absence of malignant cells in the resected specimen [11–15]. Many investigators have observed that a pathCR is associated with a longer overall survival (OS) duration [11–15]. Residual disease implies that the tumor is aggressive, chemoradiation resistant, and likely to have high metastatic potential [16]. Currently, no clinical parameters, including imaging studies, can be used to predict which patients will achieve a pathCR [17–21]. Similarly, biomarker studies have not established a validated signature that leads to the prediction of pathCR [22, 23]. Thus, because pathCR cannot be predicted, every patient who receives preoperative chemoradiation stands at a 25% probability of achieving a pathCR. However, the development of a model that predicts pathCR with a probability of ≥60% could allow for the investigation of novel treatment strategies.
Previous studies have shown that the rate of local recurrence after surgery is low in patients who achieve a pathCR [11, 16], but we cannot know the rate of local recurrence without surgery in patients who achieve a pathCR because one cannot determine who has had a pathCR without examining the surgical specimen. The benefit of surgery in patients with EC who achieve a pathCR is unclear and is an important avenue for research. If pathCR could be predicted with high probability, then surgery could be used as a salvage procedure rather than as a planned procedure.
We hypothesized that if the relevant clinical parameters were selected (via univariate and multivariate analyses) in a large number of patients with EC (thus accounting for the inherent heterogeneity among these patients) and combined using the logistic regression method, a model to predict pathCR with a high probability (≥60%) could emerge. Such model could spur esophageal preservation strategies.
patients and methods
patients
We searched the prospectively maintained EC database in the Department of Thoracic and Cardiovascular Surgery at The University of Texas MD Anderson Cancer Center, to find consecutive patients who met all the required criteria to be included in the analysis.
All the patients who had a technically resectable tumor and were medically fit for surgery were eligible for this analysis. All the patients had chemoradiation followed by surgery, and their surgical specimens were scored for pathologic response using a validated process [24]. The patients were included only if they had all of the following additional information: baseline histologic confirmation, baseline histologic grade, results of upper gastrointestinal endoscopy with baseline clinical staging via endoscopic ultrasonography (EUS), baseline positron emission tomography (PET) results, postchemoradiation PET (done 5 or 6 weeks after the completion of chemoradiation) results, and postchemoradiation endoscopic biopsy results. Patient survival data were also collected. No other selection criteria were implemented. Staging was determined using the American Joint Committee on Cancer staging system [25]. The Institutional Review Board at MD Anderson approved this analysis.
therapy
Chemotherapy consisting of a fluoropyrimidine (i.v. or oral) and either a platinum compound or a taxane was given concurrently with a total radiation dose of 45–50.4 Gy, delivered in daily fractions of 1.8 Gy. The details of radiation therapy were similar to those published recently [26–28].
Approximately 5 or 6 weeks after the completion of chemoradiation, all the patients underwent esophagectomy and lymph node dissection with curative intent. Either transthoracic (Ivor-Lewis), transhiatal, total (three-field technique), or minimally invasive esophagectomy was performed at the discretion of the treating surgeon.
follow-up and survival
The patients were monitored periodically until at least 5 years after surgery or until death. Additional follow-up data were obtained from the MD Anderson tumor registry, hospital records, and the Social Security Death Index. Follow-up time was calculated from the date of surgery to the event (death, recurrence, or to the date of last contact).
statistical analysis
Data were collected prospectively using a standardized protocol. The death and event rates were calculated according to the Kaplan–Meier method, and the differences were assessed using the log-rank test. A univariate logistic regression model was utilized to examine the association between each clinicopathologic parameter and pathCR. Odds ratios with 95% confidence intervals (CIs) were used to quantify the strength of the association between parameters and pathCR. The preoperative parameters with a P value of ≤0.25 in the univariate analysis were entered into a multivariate logistic regression model. Using the Wald stepwise selection method with P = 0.10 as the entry and removal probability, we obtained the final model for the dataset.
All parameters that were statistically significant in the multivariate analysis were then used to construct a nomogram for predicting pathCR. A concordance index was obtained for the nomogram. Internal validation using the bootstrap method was then carried out to calculate a bias-corrected concordance index. All statistical analyses were carried out using the S-Plus 8.0 (rpart library; Tibco Software Inc., Palo Alto, CA) and SPSS 17.0 (SPSS Inc., Chicago, IL). Statistical significance was defined as P < 0.05.
results
patients
Supplementary Table S1, available at Annals of Oncology online shows relevant baseline patient and disease characteristics. As anticipated, most patients were men, and most patients had adenocarcinoma. The median tumor length as determined by baseline endoscopy was 5 cm. Of 322 specimens, 201 had poorly differentiated histology. The median baseline maximum standardized uptake value (SUVmax) of the primary tumor was 10.1 (range: 1–60).
Supplementary Table S2, available at Annals of Oncology online outlines patient and disease characteristics after therapy (postchemoradiation data). The median postchemoradiation SUVmax was 4 (0–44.1). Intriguingly, postchemoradiation biopsy revealed no cancer cells in almost 79% of patients but only 21.74% of patients had a pathCR.
OS and DFS
The median OS of the entire population was 48.033 months (95% CI 41.893–53.334 months). As of this writing, 26.1% of pathCR patients and 40.9% of non-pathCR patients have died. The median OS was 79.767 months (95% CI 56.402–77.528 months) in pathCR patients and 39.733 months (95% CI 41.893–53.334 months) in non-pathCR patients. This difference was statistically significant (P = 0.004; Supplementary Figure S1A, available at Annals of Oncology online).
The median DFS of the entire cohort was 42.10 months (95% CI 43.981–55.179 months). As of this writing, 32.8% of pathCR patients and 47.5% of non-pathCR patients have had recurrence and/or disease-specific death. The median DFS was 79.767 months (95% CI 53.273–75.142 months) in pathCR patients and 30.40 months (95% CI 38.263–49.979 months) in non-pathCR patients. This difference was statistically significant (P = 0.003; Supplementary Figure S1B, available at Annals of Oncology online).
univariate analysis
Variables were selected for inclusion in multivariate analysis on the basis of their significance in the univariate analysis (Supplementary Table S3, available at Annals of Oncology online); in addition, we selected other variables that have prognostic relevance (e.g. tumor length [29], grade of differentiation [30], postchemoradiation SUVmax [31], and postchemoradiation endoscopic biopsy [32]). However, the inclusion of variables such as age, sex, and baseline T and N categories in the logistic regression analyses was generic. Our goal was to create a model that could be easily integrated in community practice and would be amenable to prospective validation.
Several parameters (e.g. age, tumor length, baseline, and postchemoradiation SUVmax) were tested as continuous variables in addition to their dichotomized values based on the median. Several variables [sex, histology, differentiation, baseline SUV, postchemoradiation SUV, primary tumor length, baseline T (but not N) category, and postchemoradiation biopsy results] were significantly associated with pathCR in the univariate analysis and were included in the multivariate analysis.
multivariate analysis
Table 1 shows the finalized multivariate analysis, in which five variables were associated with a higher chance of achieving pathCR (female sex, well or moderately differentiated histology, the absence of cancer cells on postchemoradiation biopsy specimens, lower postchemoradiation SUVmax, and baseline T category). It is not clear why the female gender was associated with pathCR, but the absence of cancer cells on postchemoradiation biopsy specimens and lower postchemoradiation SUV are consistent with a chemoradiation-sensitive EC. None of these variables can individually predict pathCR with a high (≥60%) probability; therefore, we constructed a nomogram by combining these significant variables.
Table 1.
Finalized multivariate logistic regression for outcome of pathCR
| Variable | Subcategories | Frequency | P | Odds ratio | 95% CI |
|
|---|---|---|---|---|---|---|
| Upper bound | Lower bound | |||||
| Sex | Male (reference) | 282 | 0.024 | 1.0 | ||
| Female | 40 | 2.438 | 1.122 | 5.297 | ||
| Differentiation | Poorly differentiated (reference) | 201 | 0.021 | 1.0 | ||
| Well or moderately differentiated | 121 | 1.966 | 1.107 | 3.491 | ||
| Postchemoradiation biopsy results | Cancer (reference) | 68 | 0.021 | 1.0 | ||
| No cancer | 254 | 4.647 | 1.706 | 12.658 | ||
| Baseline T category | T3 (reference) | 268 | 0.015 | 1.0 | ||
| T1+2 | 45 | 0.107 | 1.844 | 0.877 | 3.878 | |
| T4 | 9 | 0.011 | 7.035 | 1.575 | 31.419 | |
| Postchemoradiation SUV | Continuous | 322 | 0.030 | 0.869 | 0.766 | 0.987 |
SUV, standardized unit value; pathCR, pathologic complete response; CI, confidence interval.
nomogram
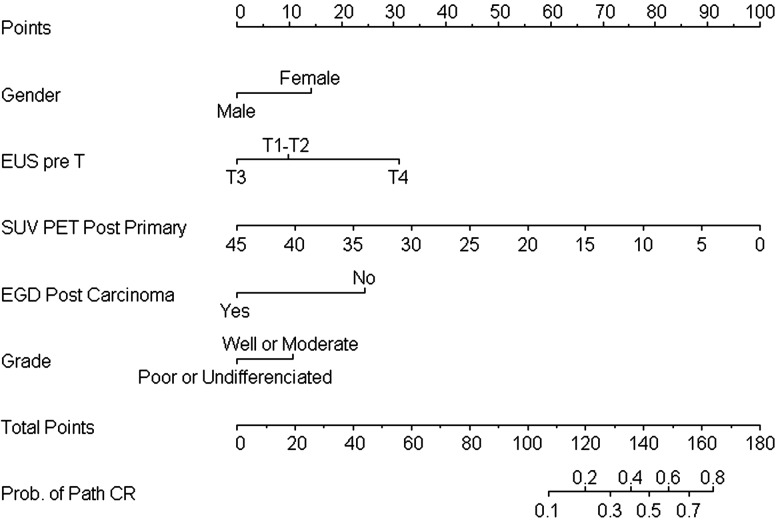
The nomogram (Figure 1) demonstrates that combining five variables can increase the probability of predicting pathCR to as high as 80% if a patient scores >160 points. Among the most influential factors for attaining the highest scores for predicting pathCR were lower postchemoradiation SUVmax and the absence of cancer cells on postchemoradiation biopsy specimens. Nine patients with resectable T4 disease skewed our data on the influence of baseline T category. Our results suggest that a patient with EUST4 disease at baseline would score more points than a patient with EUST1–3 disease at baseline, but this finding was likely due to the small number of patients with EUST4 disease. However, to avoid any selection bias, we elected not to remove patients with EUST4 disease from this analysis (all the patients who met the minimum eligibility requirements for this project were included from our entire database). Supplementary Table S4, available at Annals of Oncology online provides a three-patient scenario with total scores and predicted probability of pathCR.
Figure 1.
Nomogram for predicting pathCR based on clinical variables.
The area under the receiver operating characteristic curve (AU-ROC) was 0.72 (95% CI 0.662–0.787), and after bootstrap validation with 200 repetitions, the bias-corrected AU-ROC was 0.70 (95% CI 0.643–0.728).
discussion
The outcomes of patients treated primarily with surgery and their OS curves for pathologic stage demonstrate that EC is a heterogeneous disease [6]. However, similar degree of heterogeneity is obvious even after multimodality therapy [27]. The aggressive tumor biology and resistance to therapy are most likely driven by genotypic alterations in the tumor DNA and its ability to adapt to injury (e.g. from chemoradiation) and evade apoptosis. In that respect, localized EC is an excellent model to study therapy resistance because most patients with localized EC receive preoperative chemoradiation, an examination of the surgical specimen shows the therapeutic effect (i.e. pathCR or non-pathCR), and this information is prognostic [11–15]. If we could predict the extreme categories of therapeutic effects such as pathCR or high degrees of resistance (defined by gross residual disease in the surgical specimen) [12, 24], then perhaps we could develop individualized treatment approaches according to these predictions. The tools that provide such information with high accuracy are desirable.
Could we preserve the esophagus of a patient who is expected to achieve a pathCR? Could we avoid chemoradiation-associated morbidity in a patient who has chemoradiation-resistant EC? Can we correctly identify a patient with EC who will benefit from chemoradiation and surgery? These questions cannot be answered until we develop models that can predict outcomes in patients with EC and those models are easily replicated. In this analysis, we focused on the prediction of pathCR. By combining variables that were independently associated with pathCR, we created a predictive model. We found that postchemoradiation SUV had the highest contribution in the nomogram followed by postchemoradiation biopsy results. If a patient with EC scores >160 points (before surgery), then the likelihood of achieving a pathCR is ≥60%. Clearly, this model must be replicated and validated before it can be further tested in the clinical decision-making process. As developed, this nomogram cannot be implemented in the clinic but will need considerable refinement and the development of complementary models. A focused investigation of predictive biomarkers could be of value. If a biomarker signature could be developed for the prediction of pathCR, it too could be integrated in this nomogram. However, the development of such a biomarker would be quite challenging and would require considerable effort.
We acknowledge the weaknesses in our analysis; they include its retrospective nature, the limited number of patients analyzed, and the need for replication and validation. However, our analysis was strengthened by the inclusion of variables that are practical and transportable to community oncology, its cross-validation, and its novel findings.
Our practical model for predicting pathCR in patients with EC is a preliminary step toward the development of an esophagus preservation strategy. The presented model needs to be replicated and then prospectively validated before it can be implemented in clinical practice. The integration of relevant biomarkers in this model may further improve its usefulness.
funding
This work was supported in part by the Dallas, Park, Smith, and Cantu family funds, the Kevin Fund; the Sultan Fund; the River Creek Foundation; and the Aaron and Martha Schecter Private Foundation. This work was also supported by the Multidisciplinary Research Program at MD Anderson Cancer Center, Houston, USA, and by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672 (provided by the National Cancer Institute, Bethesda, USA).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 doi: 10.1002/ijc.25516. doi:10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Research UK. Oesophageal (gullet) cancer. [on-line] 2008 http://info.cancerresearchuk.org/cancerstats/types/oesophagus/incidence/ (14 October 2008, date last accessed) [Google Scholar]
- 4.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100(16):1184–1187. doi: 10.1093/jnci/djn211. doi:10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 6.Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22(1):1–8. doi: 10.1111/j.1442-2050.2008.00901.x. doi:10.1111/j.1442-2050.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaast AV, van Hagen P, Hulshof M, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer. Results from a multicenter randomized phase III study. Am Soc Clin Oncol. 2010:5s. [Google Scholar]
- 8.Ajani JA. Resectable esophageal cancer: surgery as primary therapy is not the answer, but then, what is and why? J Clin Oncol. 2010 doi: 10.1200/JCO.2009.26.7591. doi:10.1200/JCO.2009.26.7591. [DOI] [PubMed] [Google Scholar]
- 9.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067. doi: 10.1200/JCO.2009.22.2083. doi:10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 10.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719–3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 11.Rohatgi P, Swisher SG, Correa AM, et al. Characterization of pathologic complete response after preoperative chemoradiotherapy in carcinoma of the esophagus and outcome after pathologic complete response. Cancer. 2005;104(11):2365–2372. doi: 10.1002/cncr.21439. [DOI] [PubMed] [Google Scholar]
- 12.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 13.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25(5):507–512. doi: 10.1200/JCO.2006.08.0101. [DOI] [PubMed] [Google Scholar]
- 15.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87(2):392–398. doi: 10.1016/j.athoracsur.2008.11.001. discussion 398–399 doi:10.1016/j.athoracsur.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohatgi PR, Swisher SG, Correa AM, et al. Failure patterns correlate with the proportion of residual carcinoma after preoperative chemoradiotherapy for carcinoma of the esophagus. Cancer. 2005;104(7):1349–1355. doi: 10.1002/cncr.21346. [DOI] [PubMed] [Google Scholar]
- 17.Murthy SB, Patnana SV, Xiao L, et al. The standardized uptake value of 18-fluoro-deoxy glucose positron emission tomography after chemoradiation and clinical outcome in patients with localized gastroesophageal carcinoma. Oncology. 2010;78(5–6):316–322. doi: 10.1159/000319938. [DOI] [PubMed] [Google Scholar]
- 18.Flamen P, Van Cutsem E, Lerut A, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13(3):361–368. doi: 10.1093/annonc/mdf081. [DOI] [PubMed] [Google Scholar]
- 19.Swisher SG, Erasmus J, Maish M, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101(8):1776–1785. doi: 10.1002/cncr.20585. [DOI] [PubMed] [Google Scholar]
- 20.Wieder HA, Ott K, Lordick F, et al. Prediction of tumor response by FDG-PET: comparison of the accuracy of single and sequential studies in patients with adenocarcinomas of the esophagogastric junction. Eur J Nucl Med Mol Imaging. 2007;34(12):1925–1932. doi: 10.1007/s00259-007-0521-3. [DOI] [PubMed] [Google Scholar]
- 21.Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19(12):3058–3065. doi: 10.1200/JCO.2001.19.12.3058. [DOI] [PubMed] [Google Scholar]
- 22.Izzo JG, Malhotra U, Wu TT, et al. Association of activated transcription factor nuclear factor kappab with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24(5):748–754. doi: 10.1200/JCO.2005.03.8810. [DOI] [PubMed] [Google Scholar]
- 23.Luthra R, Wu TT, Luthra MG, et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol. 2006;24(2):259–267. doi: 10.1200/JCO.2005.03.3688. doi:10.1200/JCO.2005.03.3688. [DOI] [PubMed] [Google Scholar]
- 24.Wu TT, Chirieac LR, Abraham SC, et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. Am J Surg Pathol. 2007;31(1):58–64. doi: 10.1097/01.pas.0000213312.36306.cc. [DOI] [PubMed] [Google Scholar]
- 25.Greene FL. AJCC Cancer Staging Atlas/American Joint Committee on Cancer. New York, NY: Springer; 2006. [Google Scholar]
- 26.Ajani JA, Correa AM, Walsh GL, et al. Trimodality therapy without a platinum compound for localized carcinoma of the esophagus and gastroesophageal junction. Cancer. 2010;116(7):1656–1663. doi: 10.1002/cncr.24935. [DOI] [PubMed] [Google Scholar]
- 27.Javeri H, Arora R, Correa AM, et al. Influence of induction chemotherapy and class of cytotoxics on pathologic response and survival after preoperative chemoradiation in patients with carcinoma of the esophagus. Cancer. 2008;113(6):1302–1308. doi: 10.1002/cncr.23688. doi:10.1002/cncr.23688. [DOI] [PubMed] [Google Scholar]
- 28.Javeri H, Xiao L, Rohren E, et al. The higher the decrease in the standardized uptake value of positron emission tomography after chemoradiation, the better the survival of patients with gastroesophageal adenocarcinoma. Cancer. 2009;115(22):5184–5192. doi: 10.1002/cncr.24604. doi:10.1002/cncr.24604. [DOI] [PubMed] [Google Scholar]
- 29.Bolton WD, Hofstetter WL, Francis AM, et al. Impact of tumor length on long-term survival of pT1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2009;138(4):831–836. doi: 10.1016/j.jtcvs.2009.02.003. doi:10.1016/j.jtcvs.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Patel PR, Yao JC, Hess K, et al. Effect of timing of metastasis/disease recurrence and histologic differentiation on survival of patients with advanced gastric cancer. Cancer. 2007;110(10):2186–2190. doi: 10.1002/cncr.23046. [DOI] [PubMed] [Google Scholar]
- 31.Murthy SB, Patnana SV, Xiao L, et al. The standardized uptake value of 18-fluorodeoxyglucose positron emission tomography after chemoradiation and clinical outcome in patients with localized gastroesophageal carcinoma. Oncology. 78(5–6):316–322. doi: 10.1159/000319938. doi:10.1159/000319938. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Cleary KR, Yao JC, et al. Significance of post-chemoradiation biopsy in predicting residual esophageal carcinoma in the surgical specimen. Dis Esophagus. 2004;17(1):38–43. doi: 10.1111/j.1442-2050.2004.00355.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.