Abstract
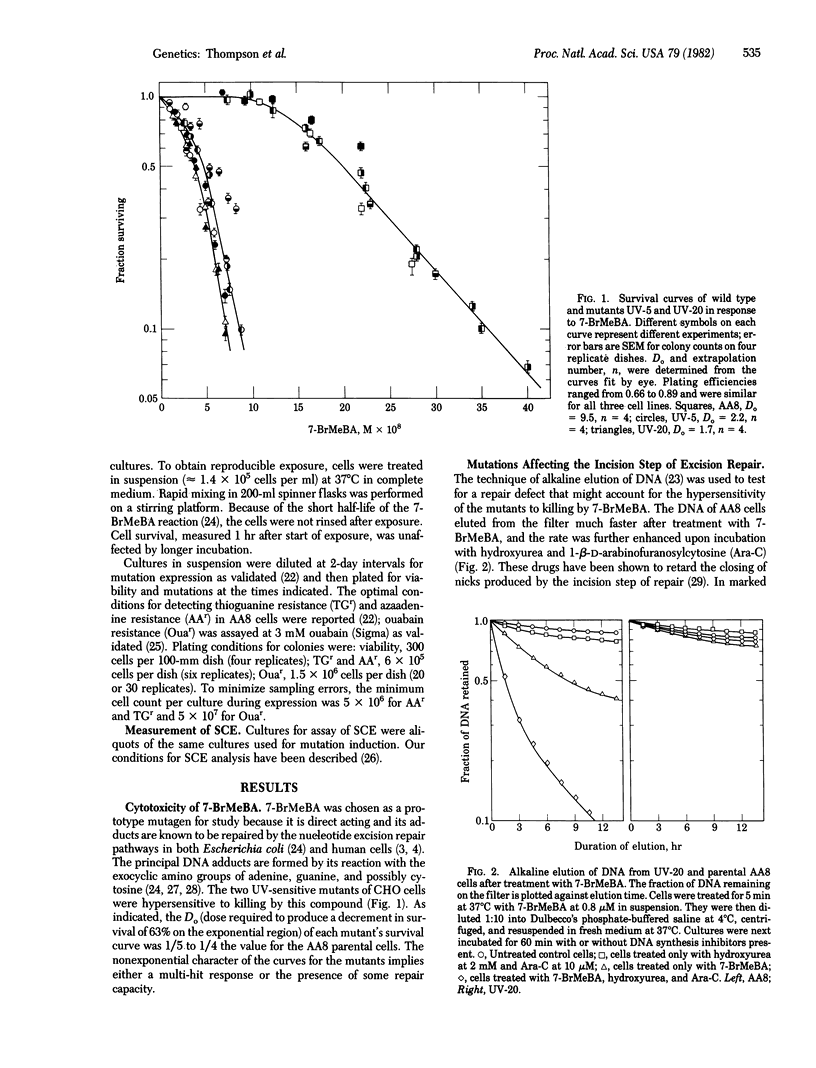
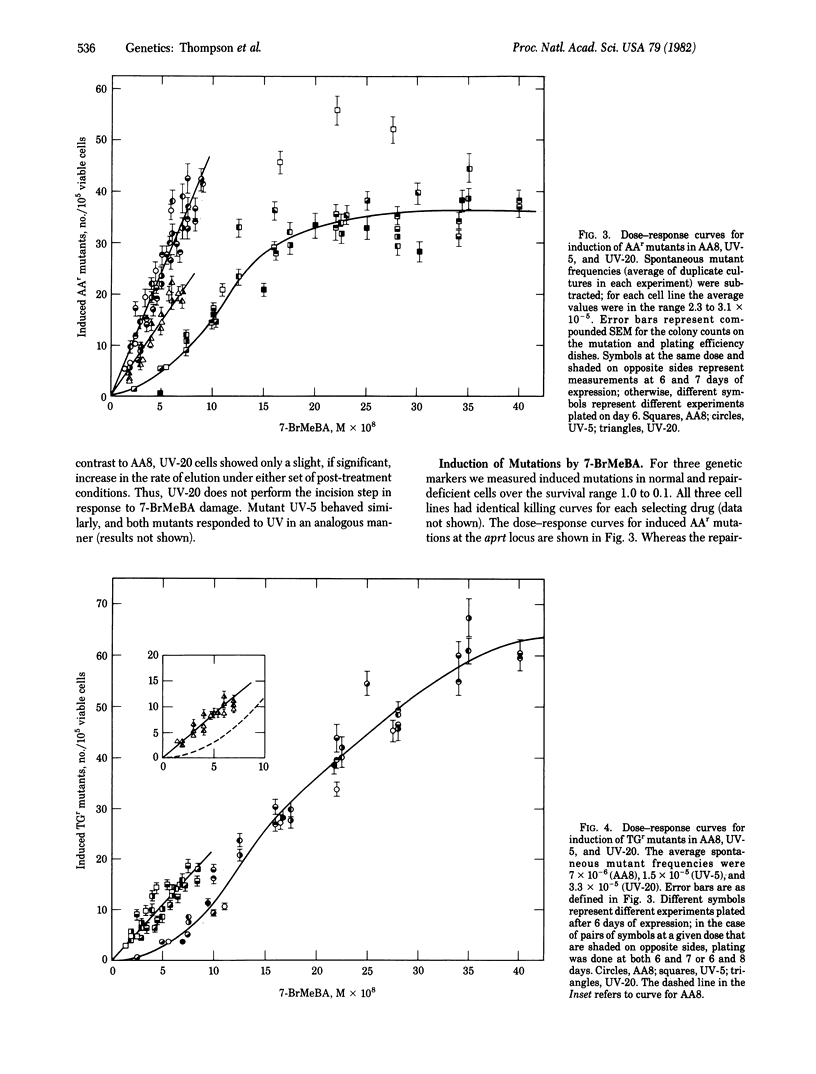
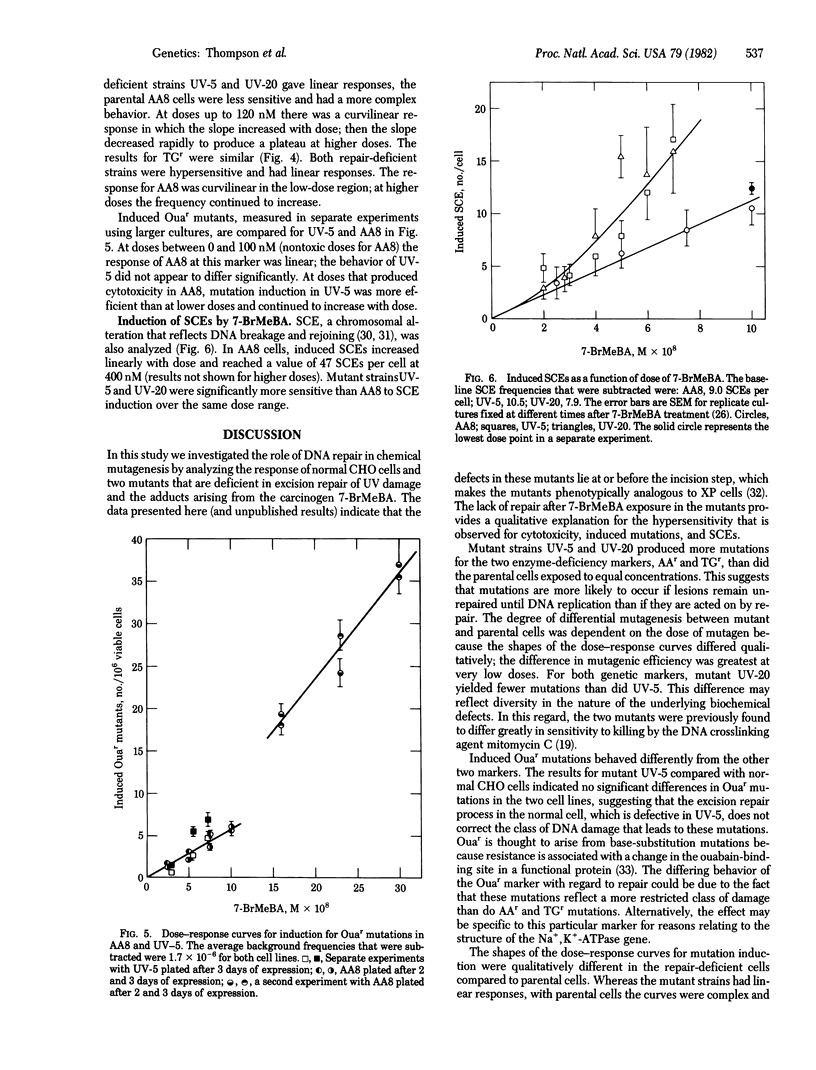
The role of DNA repair in mutagenesis was studied in normal, repair-proficient Chinese hamster ovary cells and in two mutant strains that are deficient in excision repair. By using the mutagen 7-bromomethylbenz[a]anthracene (7-BrMeBA) and the technique of alkaline elution of DNA, the mutants were found to be defective at or before the incision step of excision repair. Dose--responses were determined for cell killing, mutation induction at three loci, and sister chromatid exchanges over a survival range of 1.0--0.1 after 7-BrMeBA treatment. The mutants were 5-fold more sensitive to killing than were the normal cells, but the degree of hypersensitivity to mutation induction varied depending on the mutant strain, the genetic marker, and the dose of mutagen. In each instance, the dose--response curve for mutations was essentially linear in the repair-deficient cells. In the normal cells, however, the curves for induced resistance to thioguanine and azaadenine were complex and were curvilinear with increasing slope at low doses. This behavior may be attributable to saturation of the excision repair system. No difference was seen in the efficiency of inducing ouabain-resistant mutations in the repair-deficient cells compared to the normal cells, indicating a qualitatively different behavior of this marker. These results are consistent with excision repair of 7-BrMeBA damage being error-free in Chinese hamster ovary cells. Sister chromatid exchange, another manifestation of DNA damage, also was induced with greater efficiency in the repair-deficient cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch D. B., Cleaver J. E., Glaser D. A. Large-scale isolation of UV-sensitive clones of CHO cells. Somatic Cell Genet. 1980 May;6(3):407–418. doi: 10.1007/BF01542792. [DOI] [PubMed] [Google Scholar]
- Carrano A. V., Thompson L. H., Lindl P. A., Minkler J. L. Sister chromatid exchange as an indicator of mutagenesis. Nature. 1978 Feb 9;271(5645):551–553. doi: 10.1038/271551a0. [DOI] [PubMed] [Google Scholar]
- Carrano A. V., Thompson L. H., Stetka D. G., Minkler J. L., Mazrimas J. A., Fong S. DNA crosslinking, sister-chromatid exchange and specific-locus mutations. Mutat Res. 1979 Nov;63(1):175–188. doi: 10.1016/0027-5107(79)90114-3. [DOI] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipple A., Brookes P., Mackintosh D. S., Rayman M. P. Reaction of 7-bromomethylbenz(a)anthracene with nucleic acids, polynucleotides, and nucleosides. Biochemistry. 1971 Nov;10(23):4323–4330. doi: 10.1021/bi00799a026. [DOI] [PubMed] [Google Scholar]
- Dipple A., Roberts J. J. Excision of 7-bromomethylbenz[a]anthracene--DNA adducts in replicating mammalian cells. Biochemistry. 1977 Apr 5;16(7):1499–1503. doi: 10.1021/bi00626a039. [DOI] [PubMed] [Google Scholar]
- Heflich R. H., Hazard R. M., Lommel L., Scribner J. D., Maher V. M., McCormick J. J. A comparison of the DNA binding, cytotoxicity and repair synthesis induced in human fibroblasts by reactive derivatives of aromatic amide carcinogens. Chem Biol Interact. 1980 Jan;29(1):43–56. doi: 10.1016/0009-2797(80)90085-x. [DOI] [PubMed] [Google Scholar]
- Ikenaga M., Takebe H., Ishii Y. Excision repair of DNA base damage in human cells treated with the chemical carcinogen 4-nitroquinoline 1-oxide. Mutat Res. 1977 Jun;43(3):415–427. doi: 10.1016/0027-5107(77)90062-8. [DOI] [PubMed] [Google Scholar]
- Jenssen D., Ramel C. Relationship between chemical damage of DNA and mutations in mammalian cells. I. Dose-response curves for the induction of 6-thioguanine-resistant mutants by low doses of monofunctional alkylating agents, X-rays and UV radiation in V79 Chinese hamster cells. Mutat Res. 1980 Dec;73(2):339–347. doi: 10.1016/0027-5107(80)90199-2. [DOI] [PubMed] [Google Scholar]
- Kimball R. F. The relation of repair phenomena to mutation induction in bacteria. Mutat Res. 1978;55(2):85–120. doi: 10.1016/0165-1110(78)90018-0. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Erickson L. C., Ewig R. A., Friedman C. A. Fractionation of DNA from mammalian cells by alkaline elution. Biochemistry. 1976 Oct 19;15(21):4629–4637. doi: 10.1021/bi00666a013. [DOI] [PubMed] [Google Scholar]
- Laval F. Effect of uncouplers on radiosensitivity and mutagenicity in x-irradiated mammalian cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2702–2705. doi: 10.1073/pnas.77.5.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. UV mutagenesis in radiation-sensitive strains of yeast. Genetics. 1976 Feb;82(2):207–232. doi: 10.1093/genetics/82.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher V. M., Birch N., Otto J. R., MacCormick J. J. Cytotoxicity of carcinogenic aromatic amides in normal and xeroderma pigmentosum fibroblasts with different DNA repair capabilities. J Natl Cancer Inst. 1975 Jun;54(6):1287–1294. doi: 10.1093/jnci/54.6.1287. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Dorney D. J., Mendrala A. L., Konze-Thomas B., McCormick J. J. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res. 1979 Sep;62(2):311–323. doi: 10.1016/0027-5107(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Maher V. M., McCormick J. J., Grover P. L., Sims P. Effect of DNA repair on the cytotoxicity and mutagenicity of polycyclic hydrocarbon derivatives in normal and xeroderma pigmentosum human fibroblasts. Mutat Res. 1977 Apr;43(1):117–138. doi: 10.1016/0027-5107(77)90137-3. [DOI] [PubMed] [Google Scholar]
- McCaw B. A., Dipple A., Young S., Roberts J. J. Excision of hydrocarbon-DNA adducts and consequent cell survival in normal and repair defective human cells. Chem Biol Interact. 1978 Sep;22(2-3):139–151. doi: 10.1016/0009-2797(78)90121-7. [DOI] [PubMed] [Google Scholar]
- Prakash L. Lack of chemically induced mutation in repair-deficient mutants of yeast. Genetics. 1974 Dec;78(4):1101–1118. doi: 10.1093/genetics/78.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A., Benoit A. Induction of an error-prone mode of DNA repair in UV-irradiated monkey kidney cells. Mutat Res. 1980 Mar;70(1):71–81. doi: 10.1016/0027-5107(80)90059-7. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Repair deficient human disorders and cancer. Nature. 1978 Feb 23;271(5647):713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Simons J. W. Development of a liquid-holding technique for the study of DNA-repair in human diploid fibroblasts. Mutat Res. 1979 Feb;59(2):273–283. doi: 10.1016/0027-5107(79)90165-9. [DOI] [PubMed] [Google Scholar]
- Slor H. Induction of unscheduled DNA synthesis by the carcinogen 7-bromomethylbenz(a)anthracene and its removal from the DNA of normal and xeroderma pigmentosum lymphocytes. Mutat Res. 1973 Aug;19(2):231–235. doi: 10.1016/0027-5107(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sekiguchi M., Okada Y. Restoration of ultraviolet-induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus). Proc Natl Acad Sci U S A. 1975 Oct;72(10):4071–4075. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Rubin J. S., Cleaver J. E., Whitmore G. F., Brookman K. A screening method for isolating DNA repair-deficient mutants of CHO cells. Somatic Cell Genet. 1980 May;6(3):391–405. doi: 10.1007/BF01542791. [DOI] [PubMed] [Google Scholar]
- Venitt S., Tarmy E. M. The selective excision of arylalkylated products from the DNA of Escherichia coli treated with the carcinogen 7-bromomethylbenz(a)anthracene. Biochim Biophys Acta. 1972 Nov 16;287(1):38–51. doi: 10.1016/0005-2787(72)90328-0. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. L., Maher V. M., McCormick J. J. Error-free excision of the cytotoxic,mutagenic N2-deoxyguanosine DNA adduct formed in human fibroblasts by (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5933–5937. doi: 10.1073/pnas.77.10.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]



