Abstract
Correlative data suggest that thyroid hormone receptor-β (TRβ) mutations could increase the risk of mammary tumor development, but unequivocal evidence is still lacking. To explore the role of TRβ mutants in vivo in breast tumor development and progression, we took advantage of a knock-in mouse model harboring a mutation in the Thrb gene encoding TRβ (ThrbPV mouse). Although in adult nulliparous females, a single ThrbPV allele did not contribute to mammary gland abnormalities, the presence of two ThrbPV alleles led to mammary hyperplasia in ~36% ThrbPV/PV mice. The ThrbPV mutation further markedly augmented the risk of mammary hyperplasia in a mouse model with high susceptibility to mammary tumors (Pten+/− mouse), as demonstrated by the occurrence of mammary hyperplasia in ~60% of Thrbpv/+Pten+/− and ~77% of ThrbPV/PV Pten+/− mice versus ~33% of Thrb+/+Pten+/− mice. The ThrbPV mutation increased the activity of signal transducer and activator of transcription (STAT5) to increase cell proliferation and the expression of the STAT5 target gene encoding β-casein in the mammary gland. We next sought to understand the molecular mechanism underlying STAT5 overactivation by TRβPV. Cell-based studies with a breast cancer cell line (T47D cells) showed that thyroid hormone (T3) repressed STAT5 signaling in TRβ-expressing cells through decreasing STAT5-mediated transcription activity and target gene expression, whereas sustained STAT5 signaling was observed in TRβPV-expressing cells. Collectively, these findings show for the first time that a TRβ mutation promotes the development of mammary hyperplasia via aberrant activation of STAT5, thereby conferring a fertile genetic ground for tumorigenesis.
Keywords: thyroid hormone receptor, breast cancer, mammary tumors, Pten, mouse models
Introduction
Thyroid hormone receptors (TRs) belong to the superfamily of nuclear receptors that are ligand-dependent transcription factors. Two TR genes, THRA and THRB, located on chromosomes 17 and 3, respectively, encode four thyroid hormone (T3)-binding receptors: TRα1, TRβ1, TRβ2 and TRβ3. They bind T3 that has critical roles in differentiation, growth and metabolism (Yen, 2001).
Several findings support the notion that mutations of TRs can be associated with cancer. Early evidence suggested that mutated TRs could be involved in carcinogenesis came from the discovery that v-erbA, a highly mutated chicken THRA1 that has lost the ability to activate gene transcription, leads to neoplastic transformation in erythroleukemia and sarcomas (Sap et al., 1989; Wallin et al., 1992; Thormeyer and Baniahmad, 1999). That male transgenic mice overexpressing v-erbA develop hepatocellular carcinomas, provided the evidence that v-erbA oncoprotein can promote neoplasia in mammals through its dominant-negative activity (Barlow et al., 1994). Knock-in mice harboring a germline mutation in Thrb (ThrbPV), leading to the loss of T3 binding and dominant-negative activity, develop thyroid cancer (Suzuki et al., 2002; Furumoto et al., 2005). Abnormal expression and somatic mutations of TRs have been observed in an array of human tumors, including those of the thyroid (Wallin et al., 1992; Bronnegard et al., 1994; Puzianowska-Kuznicka et al., 2002), liver (Lin et al., 1999) and breast (Silva et al., 2002; Li et al., 2002b).
Although the functions of most members of the nuclear receptor superfamily in breast tumor biology have been documented (Conzen, 2008), much less is known about TRs. Low circulating thyroid hormone levels (hypothyroidism) have been proposed to favor mammary hyperplasia in rodents and the development of breast tumors (Mittra, 1974). Loss of TRβ expression by gene deletion or promoter hypermethylation or production of abnormal TRβ proteins following THRB mutations has been reported in breast tumors. These correlative observations suggest that TRβ may well act as a tumor suppressor (Ali et al., 1989; Silva et al., 2002; Li et al., 2002b). However, a direct demonstration that TRβ mutations participate in the development and/or progression of breast tumors is still lacking.
The ThrbPV mouse (Kaneshige et al., 2000) offers us the unique opportunity to explore in vivo the role of a TRβ mutant (hereafter named TRβPV) in breast tumor development and progression. The ThrbPV mouse was created by a targeted mutation of the thyroid hormone receptor-β (TRβPV) through homologous recombination and Cre-LoxP system. TRβPV has a C insertion at codon 448 that produces a frameshift in the 14 C-terminal amino acids of TRβ1 (Parrilla et al., 1991). TRβPV has completely lost T3 binding and exhibits potent dominant-negative activity (Meier et al., 1992). In this study, we took advantage of the ThrbPV mouse model to analyze the effect of a single or double mutation of Thrb in nulliparous female mice. We also determined whether the TRβPV mutation can enhance the development and the progression of mammary tumors in a mouse model prone to mammary hyperplasia and tumors (Pten+/− mice) (Stambolic et al., 2000). The tumor suppressor Pten (phosphatase and tensin homolog deleted from chromosome 10) encodes a dual protein/lipid phosphatase, which counteracts the phosphatidylinositol 3-kinase signaling pathway (Eng, 2002).
Importantly, we show here that the homozygous ThrbPV mutation alone induces mammary gland hyperplasia, with extensive lobulo-alveolar development. In Pten+/− mice, the heterozygous or homozygous ThrbPV mutation further increased the occurrence of mammary hyperplasia. In addition, we show that the mutation of a single copy of Thrb doubled the percentage of Pten+/− females to develop mammary tumors. By characterizing mammary gland abnormalities in females with the ThrbPV mutation, we have demonstrated for the first time that mutations of TRβ increase the risks in mammary preneoplasia and tumor. We have also uncovered that TRβPV leads to sustained signal transducer and activator of transcription (STAT5) signaling to promote mammary hyperplasia and tumorigenesis.
Results
The TRβPV mutation increases the occurrence of mammary hyperplasia
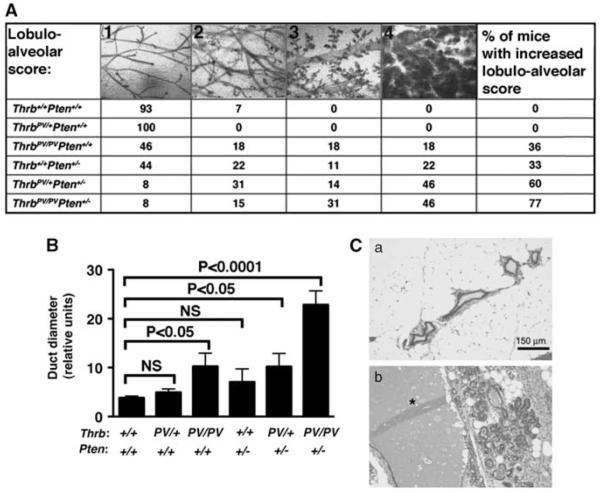
The effect of a dominant-negative TRβ mutation on the development of breast cancers was studied by using mice, heterozygous or homozygous for the TRβPV mutation (Thrbpv/+ and ThrbPV/PV mice). Mammary gland morphology was assessed on whole-mount preparations by analyzing the lobulo-alveolar development and duct diameter in 3-to 9-months-old nulliparous mice (Figure 1A; n = 9–14 mice per genotype). All mammary glands analyzed could be assigned the following stages: (1) normal structure similar to the one of young virgin mice, (2) increased number of ramifications and slightly increased alveolar size, (3) strongly increased number of ramifications with strongly increased alveolar size associated or not with moderate ductal dilatation and (4) extreme alveolar size covering the space between the ducts accompanied by ductal dilation, similar as what is observed in a midterm pregnant wild-type mouse (Figure 1A). Most wild-type mammary glands were at stage 1 and did not develop beyond stage 2. Whereas mammary gland development in Thrbpv/+ mice was similar to that of wild types, 36% of ThrbPV/PV mice displayed extensive development, which was classified into stages 3 (18%) and 4 (18%). The increased lobulo-alveolar development was accompanied by an approximately twofold increase in duct diameter as compared with wild-type mice. These results indicate that the homozygous ThrbPV mutation leads to mammary gland hyperplasia.
Figure 1.
Increased lobulo-alveolar development in the mammary glands of knock-in mice expressing TRβPV. (A) Mammary gland phenotypes of mice of the six genotypes. Whole mammary glands were stained with carmine red to visualize the structure of the ducts and alveolae. Four different lobulo-alveolar stages, scored from 1 to 4, could be distinguished as described in Results. The values in the table represent the percentage of mice of each genotype that could be related to one of the stages. The last column shows the percentage of animals with lobulo-alveolar development at stages 3 and 4. (B) Duct diameter in the six different genotypes. Whole mounts were photographed, and duct diameter was measured using NIH IMAGE software (ImageJ 1.34s; Wayne Rashband, NIH) (http:// rsb.info.nih.gov/ij). Shown is the average of the diameter sizes taken at four different locations on the largest duct. (C) Aberrant lobulo-alveolar development is associated with lactational changes. Hematoxylin and eosin mammary tissue sections of a wild-type (a) and a Thrbpv/+Pten+/− (b) mice. Note the dramatic dilatation of the duct (*) in the Thrbpv/+Pten+/− mouse.
To determine whether ThrbPV could further function as a modifier to enhance the occurrence of mammary hyperplasia in a mouse model predisposed to mammary hyperplasia and tumors, ThrbPV mutant mice were crossed with Pten+/− mice to obtain Thrbpv/+Pten+/− and ThrbPV/PVPten+/− mice. The loss of one Pten allele in female mice was previously reported to increase the predisposition of mammary glands to hyperplasia and tumors. In line with the previous observations that the loss of one Pten allele in female mice increases the risk of mammary hyperplasia (Stambolic et al., 2000), 33% of nulliparous Thrb+/+Pten+/− mice had extensive mammary development to stages 3 (11%) and 4 (22%) (Figure 1A). Strikingly, the ThrbPV mutation dramatically increased the occurrence of such abnormalities in Pten+/− females. Indeed, 60% of Pten+/− females heterozygous for ThrbPV(Thrbpv/+Pten+/− mice) had aberrant lobulo-alveolar development to stages 3 (14%) and 4 (46%) (Figure 1A), and increased ductal dilatation (Figure 1B). In Pten+/− females homozygous for ThrbPV (ThrbPV/PVPten+/− mice), this percentage was further increased to 77% (31% at stage 3 and 46% at stage 4) (Figure 1A), and was accompanied by a dramatically increased ductal dilation (approximately threefold; Figure 1B).
The TRβPV mutation increases the occurrence of mammary tumors in Pten+/− mice
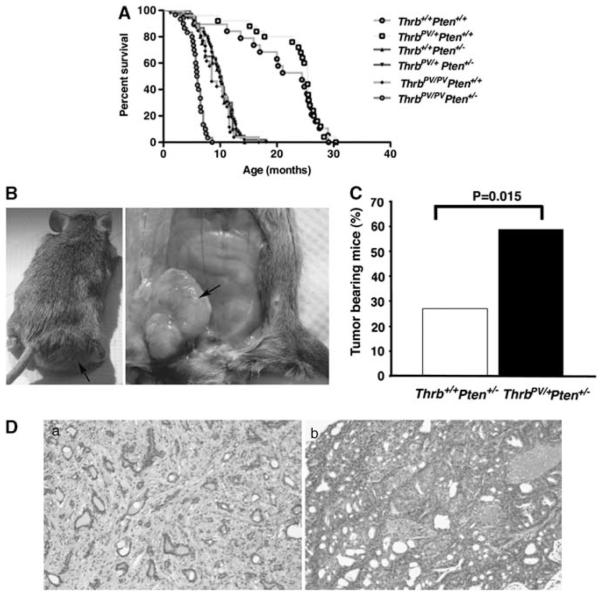
Mammary hyperplasia can proceed to tumorigenesis. To determine whether the ThrbPV mutation leads to mammary tumors, and/or enhances the occurrence of mammary tumors in Pten+/− mice, pathological analyses of the mammary glands were performed after the death or humane end point sacrifice of the animals. A low percentage (~5%) of wild-type females and Thrbpv/+Pten+/+ mice, which displayed similar life expectancy (~24.4 months and 25.4 months, respectively; Figure 2A) developed mammary tumors. As previously reported (Guigon et al., 2009), both ThrbPV/PVPten+/+ and ThrbPV/PVPten+/− died prematurely (~8.5 and ~6 months, respectively; Figure 2A) as a result of thyroid cancer. Owing to their early death, only a few of them displayed mammary tumors (6.6% (n = 1 out of 15 mice) and 7.7% (n = 2 out of 26 mice) of ThrbPV/PVPten+/+ and ThrbPV/PVPten+/− mice, respectively). In contrast, moribund Thrb+/+Pten+/− and Thrbpv/+Pten+/− females, both of which had similar life expectancies (~10 months, Figure 2A), did not present with thyroid cancer, but displayed mammary tumors (Figure 2B, arrow), as previously reported for the Pten+/− mouse(Stambolic et al., 2000). Notwithstanding, there was an increase by more than twofold in the percentage of Thrbpv/+Pten+/− females with mammary tumors (59%) as compared with Thrb+/+Pten+/− females (27.3%) (Figure 2C). Pathological analyses showed that both the genotypes displayed macroscopic fibroadenomas (Figure 2Da), which are benign lesions, and adenocarcinomas (Figure 2Db). Tumor latency was the same in both groups and reached ~11 months. These results demonstrate for the first time that a dominant-negative mutation of Thrb on a single allele enhances the predisposition of mammary glands to neoplastic transformation.
Figure 2.
TRβPV increases the occurrence of mammary tumors. (A) Survival curves of mice from the six genotypes. Wild-type Thrb+/+Pten+/+ mice (yellow curve) and Thrbpv/+Pten+/+ mice (orange curve) of our colony live about 24 months. ThrbPV/PVPten+/+ (green curve) and ThrbPV/PVPten+/− mice (red curve) die prematurely because of thyroid cancer compressing the trachea. Thrb+/+Pten+/− (purple curve) and Thrbpv/+Pten+/− mice (blue curve) die at around 10 months because of lymphoma and mammary tumors. (B) Presence of a large mass (a) in the inguinal region of a Thrbpv/+Pten+/− mouse (b) shown to be a mammary tumor at dissection. (C) The expression of a single copy of ThrbPV doubles the percentage of tumor bearing Pten+/− mice. (D) Hematoxylin- and eosinstained mammary tumors from Thrbpv/+Pten+/− mice displaying mammary fibroadenoma (a) or adenocarcinoma (b). Bar: 150 μm.
The TRβPV mutation leads to sustained activation of the STAT5 signaling pathway and increased cell proliferation in the mammary gland
The overactivation of the prolactin (PRL)-STAT5 path-way can lead to the development of mammary tumors (Wennbo et al., 1997; Vonderhaar, 1999; Iavnilovitch et al., 2004; Manhes et al., 2006; Eilon et al., 2007). Physiologically, PRL influences the mammary gland during development and growth, and stimulates milk gene transcription (Ormandy et al., 1997). Binding of PRL to its receptor induces the phosphorylation and activation of STAT5. Phosphorylated STAT5 dimerizes and translocates into the nucleus to activate genes involved in cell proliferation during pregnancy, and in production of milk, such as Csn2 encoding β-casein.
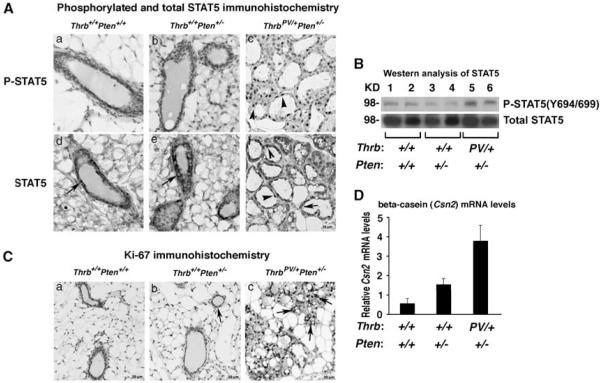
The fact that the ThrbPV mutation was associated with a higher occurrence of mammary hyperplasia and mammary tumors in Thrbpv/+Pten+/− females as compared with Thrb+/+Pten+/− females (Figures 1 and 2C) led us to hypothesize that the PRL–STAT5 signaling pathway could be aberrantly activated in Thrbpv/+Pten+/− mammary glands to contribute to mammary gland abnormalities. To test this hypothesis, we performed immunohistochemistry studies of phosphorylated STAT5 (P-STAT5) and total STAT5 in the mammary glands of nulliparous Thrb+/+Pten+/+, Thrb+/+Pten+/− and Thrbpv/+Pten+/− littermates. As shown in Figures 3Aa and b, P-STAT5 was undetectable in the mammary glands of Thrb+/+Pten+/+ and Thrb+/+Pten+/− females, although numerous epithelial cells showed a prominent total STAT5 staining in the cytoplasm (arrows, Figures 3Ad and e). In contrast, most of the epithelial cells were positively stained for both P-STAT5 and total STAT5 in the mammary glands of Thrbpv/+Pten+/− females (Figures 3Ac and f). While P-STAT5 was observed in the nucleus (arrowhead, Figure 3Ac), total STAT5 was detected in both the nuclear (arrowheads, Figure 3Af) and cytoplasmic compartments (arrow, Figure 3Af). The findings from immunohistochemical analysis were further confirmed by western blot analysis. Consistently, a marked increased phosphorylated STAT5 in the breast tumors of Thrbpv/+Pten+/− mice was evident (lanes 5 and 6, upper panel, Figure 3B) as compared with that in the mammary glands of nulliparous Thrb+/+Pten+/+ mice (lanes 1 and 2, upper panel, Figure 3B) and Thrb+/+Pten+/− mice (lanes 3 and 4, upper panel). In contrast, no apparent changes in total STAT5 were detected in the mammary glands of nulliparous Thrb+/+Pten+/+, Thrb+/+Pten+/− and Thrbpv/+Pten+/− littermates (lower panel, lanes 1–6, Figure 3B).
Figure 3.
The TRβPV mutation aberrantly activates STAT5 signaling to increase cell proliferation. (A) Immunohistochemistry of P-STAT5 on mammary tissue sections in Thrb+/+Pten+/+, Thrb+/+Pten+/− and Thrbpv/+Pten+/− mice. Tissue sections were counterstained with hematoxylin (blue). Thrb+/+Pten+/+and Thrb+/+Pten+/− mammary glands are devoid of P-STAT5 positively stained cells, whereas numerous P-STAT5 positively stained cells (brown nuclei) are present in the hyperplastic Thrbpv/+Pten+/−mammary gland. (B) Western analysis of P-STAT5 (upper panel) and total STAT5 (lower panel) on mammary tissue lysates from Thrb+/+Pten+/+ (lanes 1 and 2), Thrb+/+Pten+/− (lanes 3 and 4) and Thrbpv/+Pten+/− (lanes 5 and 6) mice. Total lysates (50 μg) were analyzed as described in Materials and methods. The lanes are marked (n 1/4 2 per genotype). (C) Assessment of cell proliferation byKi-67 immunohistochemistry on mammary tissue sections in Thrb+/+Pten+/+, Thrb+/+Pten+/− and Thrbpv/+Pten+/− mice. Tissue sections were counterstained with hematoxylin (blue). (D) Levels of Csn2 encoding β-casein in the mammary glands of Thrb+/+Pten+/+,Thrb+/+Pten+/− and Thrbpv/+Pten+/− mice. Shown are Csn2 mRNA levels determined by real-time reverse transcriptase–PCR and normalized to Gapdh (glyceraldehyde-3-phosphate dehydrogenase) mRNA levels.
We then evaluated the extent of cell proliferation in the mammary glands, by performing immunohistochemistry with the cell proliferation marker Ki-67. There were virtually no Ki-67-positive cells in wild-type Thrb+/+Pten+/+ mammary glands (Figure 3Ca), and very few Ki-67-positive cells in Thrb+/+Pten+/− glands (arrow, Figure 3Cb), indicative of no or minimal cell proliferation. In contrast, most of the mammary epithelial cells displayed Ki-67 staining in Thrbpv/+Pten+/− mammary glands, a result that is consistent with the occurrence of mammary hyperplasia (Figure 1) and the increased activation of STAT5 (Figures 3A and B). In addition, there was an increased expression of the STAT5 target gene Csn2 mRNA levels in Thrbpv/+Pten+/− mammary glands when compared with Thrb+/+Pten+/+ and Thrb+/+Pten+/− mammary glands (Figure 3D).
Liganded TRβ but not TRβPV represses STAT5 signaling to prevent cell proliferation
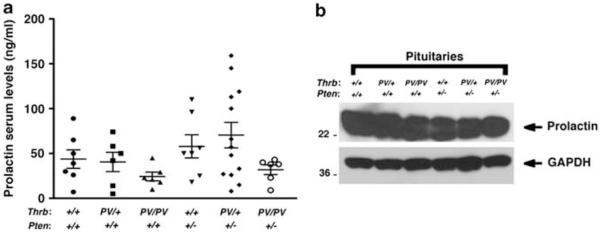
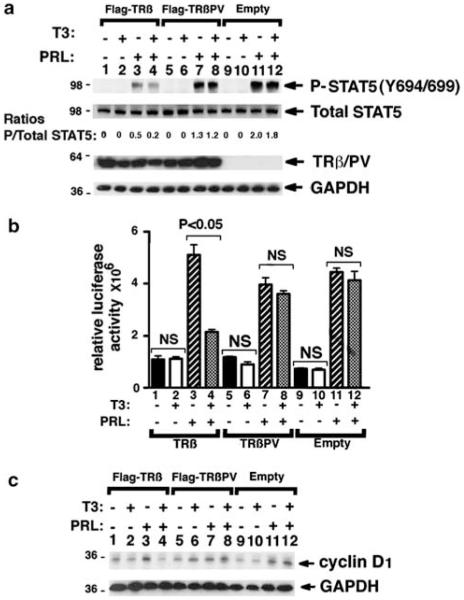
We then evaluated whether the increased STAT5 activation observed in the mammary glands of Thrbpv/+Pten+/− mice could result from increased secretion of PRL by the pituitary. However, in females with the TRβPV mutation, there was no significant change in the levels of PRL in the serum (Figure 4a) or in the pituitary (Figure 4b), suggesting that the abnormalities detected in Thrbpv/+Pten+/− mice were not due to the changes of serum PRL levels. As it was reported that PRL–STAT5 signaling pathway could be modulated by cross talk with members of the nuclear receptor family, such as glucocorticoid receptors (Zhang et al., 1997; Wyszo-mierski et al., 1999), we considered the possibility that TRβ could modulate the STAT5 signaling pathway. To test this hypothesis, we performed cell-based studies with a breast cancer cell line (T47D) that is responsive to PRL by expressing Flag-TRβ or Flag-TRβPV via adenoviral infection. Cells harboring vector only were used as negative controls. In the absence of PRL, total STAT5 was detected by western blot in the three groups, but P-STAT5 was barely detectable (Figure 5a, lanes 1,5 and 9). The treatment with T3 did not induce STAT5 phosphorylation (Figure 5a lanes 2, 6 and 10). In contrast, PRL treatment resulted in a robust increase in STAT5 phosphorylation in Flag-TRβ- and Flag-TRβPV-expressing cells or in control cells with the empty vector (Figure 5a, lanes 3, 7 and 11). Strikingly, the ratio of P-STAT5 to total STAT5 was decreased by approximately twofold after T3 treatment in Flag-TRβ cells, but neither in Flag-TRβPV cells nor in control cells (Figure 5a, compare lane 3 with 4, lane 7 with 8 and lane 11 with 12).
Figure 4.
Prolactin production is not significantly altered in mice with the TRβPV mutation. (a) Prolactin serum levels in nulliparous mice of the six genotypes. The estrous cycle was monitored daily with a vaginal impedance checker (Muromachi Kikai, Tokyo, Japan)and females were bled when not at the proestrus stage to avoid the serum prolactin peak. Serum prolactin levels were measured by radioimmunoassays at the National Hormone & Peptide Program, Harbor-UCLA Medical Center (Dr AF Parlow). (b) Prolactin content in the pituitaries of nulliparous mice of the six genotypes. Pituitary lysates preparation and western blots were carried out following a procedure previously described (Guigon et al., 2008). The anti-prolactin antibody was purchased from the National Hormone & Peptide Program, Harbor-UCLA Medical Center. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a loading control.
Figure 5.
Liganded TRβ but not TRβPV represses STAT5 signaling and target gene expression in T47D cells. (a) Western blot on whole-cell lysates from T47D cells for P-STAT5 (Y694/ 699), total STAT5, Flag, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a loading control. T47D cells expressing Flag-TRβ (lanes 1–4), Flag-TRβPV (lanes 5–8) or with the empty vector (lanes 9–12) were incubated with or without T3 and PRL for 5 h, as described in Materials and methods. (b) STAT5 activity in T47D cells determined by reporter assay. T47D cells were co-transfected with pcDNA3.1-TRβ (bars 1–4), pcDNA3.1-TRβPV (bars 5–8) or with pcDNA3.1 (bars 9–12) and the pGL4-CISH reporter gene containing four STAT5 binding sites. Cells were incubated with or without T3 and PRL for 5 h, as described in Materials and methods. (c) Western blot on whole-cell lysates from T47D cells for cyclin D1, and GAPDH as a loading control. T47D cells infected with Flag-TRβ (lanes 1–4), Flag-TRβPV (lanes 5–8)adenovirus constructs or with the vector only adenovirus (lanes 9–12) were incubated with or without T3 and PRL for 24 h, as described in Materials and methods.
We further determined the effect of T3 treatment on STAT5 activity by reporter gene luciferase assays with the pGL4-CISH reporter gene containing four STAT5 binding sites (Fang et al., 2008). T47D cells were transfected with pcDNA3.1-TRβ, pcDNA3.1-TRβPV or pcDNA3.1. Minimal reporter activity was detected in the three groups in the absence of PRL (Figure 5b). In control cells, as in TRβ- and TRβPV-expressing cells, PRL treatment strongly increased luciferase activity (Figure 5b). The co-treatment with T3 induced an ~2.5-fold decrease in luciferase activity in Flag-TRβ cells (Figure 5b, compare lanes 3 and 4). In contrast, there was no effect of T3 in TRβPV-expressing cells (Figure 5b compare lanes 7 and 8) or in control cells (Figure 5b, compare lanes 11 and 12), which displayed sustained luciferase activity. These results indicate that STAT5 activity is normally repressed by TRβ in the presence of T3, but that it is not repressed by TRβPV which has lost T3 binding capacity, thus resulting in the persistent activation of STAT5 signaling pathway in TRβPV-expressing cells.
Ccnd1, encoding cyclin D1, is a known STAT5 downstream target gene (Turkson, 2004) that has a critical role in early checkpoint regulation at the G1 phase of the cell cycle. We therefore evaluated whether PRL–STAT5-induced activation of cyclin D1 could be affected by TRβ in the presence of T3. Indeed, in Flag-TRβ-expressing cells, cyclin D1 protein abundance was decreased in the presence of T3 (compare lanes 3 and 4, Figure 5c). In contrast, in Flag-TRβPV-expressing cells, no apparent decreases were detected in the presence of T3 (compare lanes 7 and 8, Figure 5c), or in control cells (lanes 11 and 12, Figure 5c). These findings indicate that in the presence of T3, TRβ acts to suppress cell proliferation via decreasing cyclin D1 protein levels. In contrast, persistent activation of STAT5-cyclin D1 was sustained in Flag-TRβPV-expressing cells.
Discussion
Breast cancer is the most common neoplasia and the second cause of cancer deaths in women in western countries (Jemal et al., 2010). Genetic mutations, either inherited or sporadic, as well as dysregulation of ovarian hormone signaling are known to contribute to the development and progression of breast cancers. A potential role of T3 was first postulated by Beatson’s (1896) study, in which the use of thyroid extracts to treat a patient with metastatic breast cancer was described. Since then, many studies have investigated the potential association of hypo- or hyperthyroid disorders with breast cancer in the past decades, but without clear conclusions (Zumoff et al., 1981; Kalache et al., 1982; Brinton et al., 1984; Lemaire and Baugnet-Mahieu, 1986; Takatani et al., 1989; Franceschi et al., 1990; Goldman et al., 1992; Smyth, 1993; Giani et al., 1996; Simon et al., 2002; Turken et al., 2003; Kuijpens et al., 2005; Saraiva et al., 2005; Agarwal et al., 2007; Ditsch et al., 2010; Tosovic et al., 2010). The reasons for controversial findings among these studies are not immediately clear. Several reports have shown the presence of TRs in normal mammary gland (Hayden and Forsyth, 1997) and breast tumors (Alvarado-Pisani et al., 1986; Alberg and Helzlsouer, 1997; Conde et al., 2006). Moreover, silencing of the THRB gene by promoter hypermethylation, or the expression of truncated TRβ has been reported in human breast cancers (Silva et al., 2002; Li et al., 2002b). However, the relation between thyroid status or TRβ mutations and the pathogenesis of human breast cancer is not understood (Hedley et al., 1981; Smyth, 1997; Strain et al., 1997).
The study of a unique model with a mutation in Thrb offers the opportunity to evaluate for the first time the role of TRβ mutants in mammary tumorigenesis. Here, we report that the mutation of Thrb on two alleles led to mammary gland hyperplasia in more than one-third of the animals. In addition, we found that ThrbPV dramatically enhanced the occurrence of mammary hyperplasia in Pten+/− mice that are prone to develop mammary hyperplasia and tumors, as shown by the observation that ~60% of nulliparous Thrbpv/+Pten+/− mice and ~77% of ThrbPV/PVPten+/− mice displayed extensive lobulo-alveolar development as compared with ~33% in Thrb+/+Pten+/− female counterparts. That the appearance of mammary hyperplasia can proceed to mammary gland tumorigenesis was illustrated by the development of mammary tumors in both Thrb+/+Pten+/− and Thrbpv/+Pten+/− mice as mice aged. Consistent with the significant increase in the occurrence of mammary hyperplasia in Thrbpv/+Pten+/− mice, these mice displayed an approximately twofold increase in the occurrence of mammary tumors as compared with Thrb+/+Pten+/− mice. As homozygous ThrbPV mice with or without Pten deficiency died of thyroid cancer prematurely, only a low percentage of spontaneously developed mammary tumors could be observed at their deaths. Owing to their deaths at relatively young age from thyroid cancer, it was not possible to ascertain extensively the impact of mutations of two Thrb alleles on the development of mammary tumors in ThrbPV/PVPten+/− mice.
PRL is mainly synthesized in the pituitary (for review by Bachelot A, Binart N, 2007). Consistent with the well-established role of PRL in normal mammary development and function, multiple studies using various experimental approaches implicate PRL in both the pathogenesis and the progression of mammary neoplasia in rodents (Vonderhaar, 1999; Barash, 2006). Binding of PRL to its receptor induces the phosphorylation and activation of the transcription factor STAT5. Phosphorylated STAT5 translocates to the nucleus, where it regulates the expression of milk genes, such as β-casein, and genes involved in cell proliferation and survival. In line with the observation that excessive PRL stimulation of the mammary gland leads to the development of tumors, overexpression of constitutively active STAT5 in the mammary gland leads to the development of mammary tumors in multiparous mice (Iavnilovitch et al., 2004). High levels of STAT5 phosphorylation have been observed in human breast tumors (Cotarla et al., 2004). These observations prompted us to determine whether overactivated PRL–STAT5 pathway could contribute to the increased occurrence of mammary tumors in Thrbpv/+Pten+/− mice as compared with Thrb+/+Pten+/− mice. Our in vivo studies showed a strong activation of STAT5 in the mammary glands of Thrbpv/+Pten+/− mice, whereas, as previously reported (Li et al., 2002a), minimal STAT5 activity was detected in the mammary glands of wild-type and Thrb+/+Pten+/− littermates. Consistent with the stimulatory effect of STAT5 signaling on cell proliferation, we observed a strong increase in epithelial cell proliferation in the mammary glands of Thrbpv/+Pten+/− mice together with a dramatic augmentation in the expression level of the STAT5 target gene, Csn2.
Determination of PRL concentrations in the serum and in the pituitary showed no significant increase that could account for STAT5 overactivation in Thrbpv/+Pten+/− mammary glands. We postulated that endogenous TRβ and TRβPV could regulate STAT5 activity. Cell-based studies with a PRL-responsive breast cancer cell line, T47D, showed that STAT5 activity was significantly repressed by T3 in TRβ-expressing cells. However, the T3-induced repression of STAT5 activity did not occur in TRβPV-expressing cells, consistent with the fact that TRβPV has lost T3 binding capacity. STAT5 signaling was repressed by T3 in TRβ-expressing cells through decreasing STAT5-mediated transcription activity and target gene expression, whereas sustained STAT5 activity was observed in TRβPV-expressing cells. Thus, these results suggest that TRβPV leads to persistent activation of the STAT5 signaling to stimulate cell proliferation, thereby contributing to mammary hyperplasia.
In summary, this report indicates that the ThrbPV mutation increases the occurrence of mammary epithelial hyperplasia and mammary tumors in nulliparous females through aberrant activation of the STAT5 pathway. The increased susceptibility of mammary gland abnormalities is not found in heterozygous ThrbPV mice, but in homozygous ThrbPV mice, suggesting that gene dosage is important. The observation that mice heterozygous for ThrbPV but deficient in Pten exhibit a high occurrence of mammary gland hyperplasia and tumors suggests the existence of a genetic interaction between ThrbPV signaling and Pten to contribute to mammary gland abnormalities. It was reported that Pten deficiency leads to phosphatidylinositol 3-kinase/Akt signaling overactivation in the mammary glands (Li et al., 2002a; Alimonti et al., 2010). Consistent with these findings, we observed an increased activation of the phosphatidylinositol 3-kinase/Akt pathway in the mammary glands of Thrb+/+Pten+/− and Thrbpv/+Pten+/− mice as assessed by western blot analysis of phosphorylated and total Akt (Supplementary Figure A). Therefore, it may be hypothesized that the overactivation of the phosphatidylinositol 3-kinase/ Akt signaling pathway due to Pten deficiency together with the overactivation of STAT5 pathway resulting from the ThrbPV mutation are required for mammary gland hyperplasia and tumors. Collectively, our work shows for the first time that a TRβ mutation predisposes to the development of mammary preneoplastic lesions. Moreover, it would be of interest to assess the impact ofdifferent THRB mutations or of THRB loss on the mammary gland in the Pten+/− mouse model. Besides, because the relation between thyroid status and the pathogenesis of breast cancer is still being debated, further studies are required to analyze the effect of hypo- and hyper-thyroidism on the occurrence of mammary preneoplasia in these mice.
The novel findings from this study have important clinical implications. Germ-line inactivating mutations of Pten are commonly associated with breast tumors in Cowden’s syndrome patients. Moreover, several studies revealed the occurrence of somatic Pten inactivation in sporadic human breast tumors (Lynch et al., 1997; Rhei et al., 1997; Banneau et al, 2010). Similarly, THRB mutations have been reported in breast tumors (Silva et al., 2002). Given the dual involvement of Thrb mutation and Pten deficiency in mouse mammary tumorigenesis shown in this study, it would be of importance to search for genetic alterations in both genes in human breast tumors. Thus, the identification of a TRβ mutation as a potential oncogene has provided new insights into tumorigenesis of the breast, and thereby provides new therapeutic strategies.
Materials and methods
Mice and T47D cell line
The care and handling of the animals used in this study were approved by the National Cancer Institute Animal Care and Use Committee. Mice harboring the ThrbPV gene (Thrbpv/+ and ThrbPV/PV mice) were prepared and genotyped as described earlier (Kaneshige et al., 2000). Pten+/− mice were kindly provided by Dr Ramon Parsons (Columbia University, New York, NY, USA; (Podsypanina et al., 1999)). Thrbpv/+Pten+/− mice were obtained by crossing Pten+/− mice with Thrbpv/+ mice, and then Thrbpv/+Pten+/− males were crossed with Pten+/− females to obtain the different genotypes for our study: Thrb+/+Pten+/+, Thrbpv/+Pten+/+, ThrbPV/PVPten+/+, Thrb+/+Pten+/−, Thrbpv/+Pten+/− and ThrbPV/PVPten+/− mice. Littermates with a similar genetic background were used in all experiments. The human breast cancer cell line T47D was generously provided by Erika Ginsburg (NCI, Bethesda, MD, USA).
Whole-mount mammary gland staining and histology
For whole-mount staining, the fourth mammary glands were spread on microscope slides, and fixed in a mix of glacial acetic acid (1 volume) and ethanol 100% (3 volumes) overnight or longer. They were then hydrated in ethanol 70% and tap-distilled water, and stained with carmine alum stain (1% carmine, 2.5% aluminum potassium sulfate, Sigma, St Louis, MO, USA) overnight or longer, at room temperature. After staining, the slides were dehydrated through increasing ethanol concentrations, cleared in xylene (Sigma) and mounted with Permount (Fisher Scientific, Hudson, NH, USA). Mammary glands, lungs and lymph nodes were dissected and embedded in paraffin. Sections of 5-μm thickness were prepared and stained with hematoxylin and eosin (HistoServ, Germantown, MD, USA). For each animal, single random sections through the mammary gland and the lung were examined by our pathologist (Mark C Willingham). For mammary gland, morphological evidence of a single section of fibroadenoma and adenocarcinoma was routinely counted. Evidence of any of these changes in any section was counted as positive for that change.
RNA extraction and real-time reverse transcriptase–PCR
Total RNA from mammary glands was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) as indicated by the manufacturer’s protocol. Real-time reverse transcriptase–PCR was carried out using a QuantiTect SYBR green reverse transcriptase–PCR kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. Primers were as followed: β-casein, Csn2 forward: 5′-CCTTGCCAGTCTTGCTAATC-3′; Csn2 reverse: 5′-GAATGTGGAGTGGCAG-3′. Gapdh (glyceraldehyde-3-phosphate dehydrogenase), forward: 5′-ACATCA TCCCTGCATCCACT-3′; Gapdh, reverse, 5′-GTCCTCAGTGT AGCCCAA-3′.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed paraffin mammary sections, as previously described (Guigon et al., 2009). Primary antibodies used were Ki-67 antibody (dilution 1:300; Thermo Scientific, Fremont, CA, USA; #RB-9043-P0), phosphorylated STAT5 primary antibody (dilution 1:200; Cell Signaling Technologies, Beverly, MA, USA, #9359) and total STAT5A/B (#9363 dilution 1:100; Cell Signaling Technologies, #9359). Staining was developed with 3,3′ diaminobenzidine (DAB) using the DAB substrate kit for peroxidase (Vector laboratories, Burlingame, CA, USA, SK-4100).
Western blot analysis
Mammary gland tissues were homogenized on ice in lysis buffer containing 50 mm Tris, 100 mm HCl, 0.1% Triton X-100, 0.2 μm okadaic acid, 100 mm NaF and 2 mm Na3VO4, and a proteinase inhibitor tablet (Complete Mini EDTA-free; Roche, Mannheim, Germany), followed by incubation on ice for 10 min with occasional vortexing. The lysate was centrifuged for 5 min at 20 000g at 4 °C, and the supernatant was collected. The protein concentration for each lysate was determined by the Bradford assay (Pierce Chemical, Rockford, IL, USA) using bovine serum albumin (Pierce Chemical) as the standard. The protein sample (40 μg) was loaded and separated by SDS–polyacrylamide gel electrophoresis. After electrophoresis, the protein was electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA, USA). Antibodies used: phospho-STAT5A/B (Tyr694/699) (dilution 1:1000; Millipore, #05-495), total STAT5A/B (dilution 1:1000), phosphorylated Akt (Ser473, #9363), total Akt (#9272) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (dilution 1:1000; #2118) from Cell Signaling Technologies, cyclin D1 (dilution 1:200; Santa Cruz Biotechnologies, Santa Cruz, CA, USA, sc-450) and anti-Flag antibody (dilution 0.5 ug/ml; Sigma, #F7425). Band intensities were quantified by using NIH IMAGE software (ImageJ 1.34s; Wayne Rashband, NIH) (http://rsb.info.nih.gov/ij).
Expression of Flag-TRβ and Flag-TRβPV in the breast cancer T47D cell line
Adenoviral constructs encoding Flag-tagged TRβ and TRβPV or the empty vector were generated by Dom Esposito’s cloning Optimization Group, and adenovirus production was performed by Viral Technology Laboratory in NCI-Fredrick/ NIH. 8 × 105 T47D cells per 60-mm dishes (for protein expression studies) or 2.5 × 106 T47D cells per 10-cm dishes (for immunoprecipitation studies) were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum, insulin (1 μg/ml) and 1 mm of glutamine for 24 h. Adenovirus infectionwas performed in OptiMEM (Invitrogen) at a multiplicity of infection of 10. After 2 h, the medium was changed with RPMI1640 containing 10% T3-depleted (Td) serum. After 18 h of culture, cells were treated with or without human prolactin (200 ng/ml, Sigma #L4021) and T3 (100 nm) for 5 or 24 h. Cells were then lysed and protein extracted as previously described (Guigon et al., 2008).
Luciferase assays
T47D cells were plated at a density of 1 × 105 cells per well in 12-well plates. After 24 h, cells were cultured in serum-free OptiMEM1 and transfected with pcDNA3.1-TRβ, pcDNA3.1-TRβPV or the pcDNA3.1 plasmid, and the reporter plasmid pGL4-CISH (generously provided by Dr Clevenger, (Fang et al., 2008)) using Lipofectamine2000 (Invitrogen), according to the manufacturer’s protocol. After a 7-h incubation, transfection medium was replaced by fresh Dulbecco’s modified Eagle’s medium containing 10% T32-depleted calf serum. Eighteen hours later, cells were cultured with or without T3 (100 nm) and PRL (200 ng/ml) for an additional 5 h. Cells were lysed in reporter lysis buffer (3 × cell lysis buffer; BD Bioscience Pharmingen, Franklin Lakes, NJ, USA). Lysates were assayed for luciferase activity and normalized to total protein concentration.
Statistical analysis
Data are expressed as mean±s.e.m. Statistical analysis was performed with the use of analysis of variance, and P<0.05 was considered significant unless otherwise specified. GraphPad Prism 5.0a (San Diego, CA, USA) was used to perform Kaplan-Meier cumulative survival analysis, and unpaired Student’s t-test, with Welch’s correction when variances were unequal.
Supplementary Material
Acknowledgements
We thank Dr L Fozzatti for her help in mouse dissection, Dr C Lu for taking mouse body pictures, and Drs B Vonderhaar and E Ginsburg for providing T47D cells and helpful technical advice. This research was supported by the Intramural Research Program of Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Agarwal DP, Soni TP, Sharma OP, Sharma S. Synchronous malignancies of breast and thyroid gland: a case report and review of literature. J Cancer Res Ther. 2007;3:172–173. doi: 10.4103/0973-1482.37413. [DOI] [PubMed] [Google Scholar]
- Alberg AJ, Helzlsouer KJ. Epidemiology, prevention, and early detection of breast cancer. Curr Opin Oncol. 1997;9:505–511. doi: 10.1097/00001622-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Ali IU, Lidereau R, Callahan R. Presence of two members of c-erbA receptor gene family (c-erbA beta and c-erbA2) in smallest region of somatic homozygosity on chromosome 3p21-p25 in human breast carcinoma. J Natl Cancer Inst. 1989;81:1815–1820. doi: 10.1093/jnci/81.23.1815. [DOI] [PubMed] [Google Scholar]
- Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, et al. Subtle variations in Pten dose determine cancer susceptibility. Nat Genet. 2010;42:454–458. doi: 10.1038/ng.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Pisani AR, Chacon RS, Betancourt LJ, Lopez-Herrera L. Thyroid hormone receptors in human breast cancer: effect of thyroxine administration. Anticancer Res. 1986;6:1347–1351. [PubMed] [Google Scholar]
- Banneau G, Guedj M, MacGrogan G, de Mascarel I, Velasco V, Schiappa R, et al. Molecular apocrine differentiation is a common feature of breast cancer in patients with germline PTEN mutations. Breast Cancer Res. 2010;12:R63. doi: 10.1186/bcr2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash I. Stat5 in the mammary gland: controlling normal development and cancer. J Cell Physiol. 2006;209:305–313. doi: 10.1002/jcp.20771. [DOI] [PubMed] [Google Scholar]
- Barlow C, Meister B, Lardelli M, Lendahl U, Vennstrom B. Thyroid abnormalities and hepatocellular carcinoma in mice transgenic for v-erbA. EMBO J. 1994;13:4241–4250. doi: 10.1002/j.1460-2075.1994.tb06744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. The Lancet. 1896;2:162–165. [PMC free article] [PubMed] [Google Scholar]
- Brinton LA, Hoffman DA, Hoover R, Fraumeni JF., Jr Relationship of thyroid disease and use of thyroid supplements to breast cancer risk. J Chronic Dis. 1984;37:877–893. doi: 10.1016/0021-9681(84)90062-6. [DOI] [PubMed] [Google Scholar]
- Bronnegard M, Torring O, Boos J, Sylven C, Marcus C, Wallin G. Expression of thyrotropin receptor and thyroid hormone receptor messenger ribonucleic acid in normal, hyperplastic, and neoplastic human thyroid tissue. J Clin Endocrinol Metab. 1994;79:384–389. doi: 10.1210/jcem.79.2.8045952. [DOI] [PubMed] [Google Scholar]
- Conde I, Paniagua R, Zamora J, Blanquez MJ, Fraile B, Ruiz A, et al. Influence of thyroid hormone receptors on breast cancer cell proliferation. Ann Oncol. 2006;17:60–64. doi: 10.1093/annonc/mdj040. [DOI] [PubMed] [Google Scholar]
- Conzen SD. Minireview: nuclear receptors and breast cancer. Mol Endocrinol. 2008;22:2215–2228. doi: 10.1210/me.2007-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotarla I, Ren S, Zhang Y, Gehan E, Singh B, Furth PA. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer. 2004;108:665–671. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- Ditsch N, Liebhardt S, Von Koch F, Lenhard M, Vogeser M, Spitzweg C, et al. Thyroid function in breast cancer patients. Anticancer Res. 2010;30:1713–1717. [PubMed] [Google Scholar]
- Eilon T, Groner B, Barash I. Tumors caused by overexpression and forced activation of Stat5 in mammary epithelial cells of transgenic mice are parity-dependent and developed in aged, postestropausal females. Int J Cancer. 2007;121:1892–1902. doi: 10.1002/ijc.22954. [DOI] [PubMed] [Google Scholar]
- Eng C. Role of PTEN, a lipid phosphatase upstream effector of protein kinase B, in epithelial thyroid carcinogenesis. Ann N Y Acad Sci. 2002;968:213–221. doi: 10.1111/j.1749-6632.2002.tb04337.x. [DOI] [PubMed] [Google Scholar]
- Fang F, Antico G, Zheng J, Clevenger CV. Quantification of PRL/Stat5 signaling with a novel pGL4-CISH reporter. BMC Biotechnol. 2008;8:11. doi: 10.1186/1472-6750-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, la Vecchia C, Negri E, Parazzini F, Boyle P. Breast cancer risk and history of selected medical conditions linked with female hormones. Eur J Cancer. 1990;26:781–785. doi: 10.1016/0277-5379(90)90151-i. [DOI] [PubMed] [Google Scholar]
- Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, et al. An unliganded thyroid hormone beta receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol. 2005;25:124–135. doi: 10.1128/MCB.25.1.124-135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani C, Fierabracci P, Bonacci R, Gigliotti A, Campani D, De Negri F, et al. Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metab. 1996;81:990–994. doi: 10.1210/jcem.81.3.8772562. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Monson RR, Maloof F. Benign thyroid diseases and the risk of death from breast cancer. Oncology. 1992;49:461–466. doi: 10.1159/000227093. [DOI] [PubMed] [Google Scholar]
- Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng SY. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol Cell Biol. 2008;28:4598–4608. doi: 10.1128/MCB.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigon CJ, Zhao L, Willingham MC, Cheng SY. PTEN deficiency accelerates tumour progression in a mouse model of thyroid cancer. Oncogene. 2009;28:509–517. doi: 10.1038/onc.2008.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden TJ, Forsyth IA. Thyroid hormone binding in rat mammary gland. J Endocrinol. 1997;75:38P–39P. [PubMed] [Google Scholar]
- Hedley AJ, Jones SJ, Spiegelhalter DJ, Clements P, Bewsher PD, Simpson JG, et al. Breast cancer in thyroid disease: fact or fallacy? Lancet. 1981;1:131–133. doi: 10.1016/s0140-6736(81)90712-1. [DOI] [PubMed] [Google Scholar]
- Iavnilovitch E, Cardiff RD, Groner B, Barash I. Deregulation of Stat5 expression and activation causes mammary tumors in transgenic mice. Int J Cancer. 2004;112:607–619. doi: 10.1002/ijc.20484. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Kalache A, Vessey MP, McPherson K. Thyroid disease and breast cancer: findings in a large case-control study. Br J Surg. 1982;69:434–435. doi: 10.1002/bjs.1800690731. [DOI] [PubMed] [Google Scholar]
- Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, et al. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA. 2000;97:13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpens JL, Nyklictek I, Louwman MW, Weetman TA, Pop VJ, Coebergh JW. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid. 2005;15:1253–1259. doi: 10.1089/thy.2005.15.1253. [DOI] [PubMed] [Google Scholar]
- Lemaire M, Baugnet-Mahieu L. Thyroid function in women with breast cancer. Eur J Cancer Clin Oncol. 1986;22:301–307. doi: 10.1016/0277-5379(86)90395-0. [DOI] [PubMed] [Google Scholar]
- Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, et al. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002a;129:4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- Li Z, Meng ZH, Chandrasekaran R, Kuo WL, Collins CC, Gray JW, et al. Biallelic inactivation of the thyroid hormone receptor beta1 gene in early stage breast cancer. Cancer Res. 2002b;62:1939–1943. [PubMed] [Google Scholar]
- Lin KH, Shieh HY, Chen SL, Hsu HC. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol Carcinog. 1999;26:53–61. doi: 10.1002/(sici)1098-2744(199909)26:1<53::aid-mc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lynch ED, Ostermeyer EA, Lee MK, Arena JF, Ji H, Dann J, et al. Inherited mutations in PTEN that are associated with breast cancer, cowden disease, and juvenile polyposis. Am J Hum Genet. 1997;61:1254–1260. doi: 10.1086/301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhes C, Kayser C, Bertheau P, Kelder B, Kopchick JJ, Kelly PA, et al. Local over-expression of prolactin in differentiating mouse mammary gland induces functional defects and benign lesions, but no carcinoma. J Endocrinol. 2006;190:271–285. doi: 10.1677/joe.1.06829. [DOI] [PubMed] [Google Scholar]
- Meier CA, Dickstein BM, Ashizawa K, McClaskey JH, Muchmore P, Ransom SC, et al. Variable transcriptional activity and ligand binding of mutant beta 1 3,5,3′-triiodothyronine receptors from four families with generalized resistance to thyroid hormone. Mol Endocrinol. 1992;6:248–258. doi: 10.1210/mend.6.2.1569968. [DOI] [PubMed] [Google Scholar]
- Mittra I. Mammotropic effect of prolactin enhanced by thyroidectomy. Nature. 1974;248:525–526. doi: 10.1038/248525a0. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two ‘hot spot’ regions of the ligand binding domain. J Clin Invest. 1991;88:2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M, Krystyniak A, Madej A, Cheng SY, Nauman J. Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab. 2002;87:1120–1128. doi: 10.1210/jcem.87.3.8296. [DOI] [PubMed] [Google Scholar]
- Rhei E, Kang L, Bogomolniy F, Federici MG, Borgen PI, Boyd J. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in primary breast carcinomas. Cancer Res. 1997;57:3657–3659. [PubMed] [Google Scholar]
- Sap J, Munoz A, Schmitt J, Stunnenberg H, Vennstrom B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989;340:242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Saraiva PP, Figueiredo NB, Padovani CR, Brentani MM, Nogueira CR. Profile of thyroid hormones in breast cancer patients. Braz J Med Biol Res. 2005;38:761–765. doi: 10.1590/s0100-879x2005000500014. [DOI] [PubMed] [Google Scholar]
- Silva JM, Dominguez G, Gonzalez-Sancho JM, Garcia JM, Silva J, Garcia-Andrade C, et al. Expression of thyroid hormone receptor/erbA genes is altered in human breast cancer. Oncogene. 2002;21:4307–4316. doi: 10.1038/sj.onc.1205534. [DOI] [PubMed] [Google Scholar]
- Simon MS, Tang MT, Bernstein L, Norman SA, Weiss L, Burkman RT, et al. Do thyroid disorders increase the risk of breast cancer? Cancer Epidemiol Biomarkers Prev. 2002;11:1574–1578. [PubMed] [Google Scholar]
- Smyth PP. Thyroid disease and breast cancer. J Endocrinol Invest. 1993;16:396–401. doi: 10.1007/BF03348865. [DOI] [PubMed] [Google Scholar]
- Smyth PP. The thyroid and breast cancer: a significant association? Ann Med. 1997;29:189–191. doi: 10.3109/07853899708999335. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/- mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- Strain JJ, Bokje E, van’t Veer P, Coulter J, Stewart C, Logan H, et al. Thyroid hormones and selenium status in breast cancer. Nutr Cancer. 1997;27:48–52. doi: 10.1080/01635589709514500. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- Takatani O, Okumoto T, Kosano H, Nishida M, Hiraide H, Tamakuma S. Relationship between the levels of serum thyroid hormones or estrogen status and the risk of breast cancer genesis in Japanese women. Cancer Res. 1989;49:3109–3112. [PubMed] [Google Scholar]
- Thormeyer D, Baniahmad A. The v-erbA oncogene (review) Int J Mol Med. 1999;4:351–358. [PubMed] [Google Scholar]
- Tosovic A, Bondeson AG, Bondeson L, Ericsson UB, Malm J, Manjer J. Prospectively measured triiodothyronine levels are positively associated with breast cancer risk in postmenopausal women. Breast Cancer Res. 2010;12:R33. doi: 10.1186/bcr2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken O, NarIn Y, DemIrbas S, Onde ME, Sayan O, KandemIr EG, et al. Breast cancer in association with thyroid disorders. Breast Cancer Res. 2003;5:R110–R113. doi: 10.1186/bcr609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–422. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK. Prolactin involvement in breast cancer. Endocr Relat Cancer. 1999;6:389–404. doi: 10.1677/erc.0.0060389. [DOI] [PubMed] [Google Scholar]
- Wallin G, Bronnegard M, Grimelius L, McGuire J, Torring O. Expression of the thyroid hormone receptor, the oncogenes c-myc and H-ras, and the 90 kD heat shock protein in normal, hyperplastic, and neoplastic human thyroid tissue. Thyroid. 1992;2:307–313. doi: 10.1089/thy.1992.2.307. [DOI] [PubMed] [Google Scholar]
- Wennbo H, Kindblom J, Isaksson OG, Tornell J. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology. 1997;138:4410–4415. doi: 10.1210/endo.138.10.5461. [DOI] [PubMed] [Google Scholar]
- Wyszomierski SL, Yeh J, Rosen JM. Glucocorticoid receptor/ signal transducer and activator of transcription 5 (STAT5) interactions enhance STAT5 activation by prolonging STAT5 DNA binding and tyrosine phosphorylation. Mol Endocrinol. 1999;13:330–343. doi: 10.1210/mend.13.2.0232. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jones S, Hagood JS, Fuentes NL, Fuller GM. STAT3 acts as a co-activator of glucocorticoid receptor signaling. J Biol Chem. 1997;272:30607–30610. doi: 10.1074/jbc.272.49.30607. [DOI] [PubMed] [Google Scholar]
- Zumoff B, O’Connor J, Levin J, Markham M, Strain GW, Fukushima DK. Plasma levels of thyroxine and triiodothyronine in women with breast cancer. Anticancer Res. 1981;1:287–291. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.