Abstract
Prostate carcinoma frequently metastasizes to bone where the microenvironment facilitates its growth. Inhibition of bone resorption is effective in reducing tumor burden and bone destruction in prostate cancer. However, whether drugs that inhibit osteoclast function inhibit tumor growth independent of inhibition of bone resorption is unclear. Calcium is released during bone resorption and the calcium sensing receptor is an important regulator of cancer cell proliferation. The goal of this investigation was to elucidate the role of calcium released during bone resorption and to determine the impact of drugs which suppress bone resorption on tumor growth in bone. To compare tumor growth in a skeletal versus non-skeletal site, equal numbers of canine prostate cancer cells expressing luciferase (ACE-1luc) prostate cancer cells were inoculated into a simple collagen matrix, neonatal mouse vertebrae (vossicles), human de-proteinized bone, or a mineralized collagen matrix. Implants were placed subcutaneously into athymic mice. Luciferase activity was used to track tumor growth weekly and at one month tumors were dissected for histologic analysis. Luciferase activity and tumor size were greater in vossicles, de-proteinized bone and mineralized collagen matrix versus non-mineralized collagen implants. The human osteoblastic prostate carcinoma cell line C4-2b also grew better in a mineral rich environment with a greater proliferation of C4-2b cells reflected by Ki-67 staining. Zoledronic acid (ZA), a bisphosphonate, and recombinant OPG-Fc, a RANKL inhibitor, were administered to mice bearing vertebral implants (vossicles) containing ACE-1 osteoblastic prostate cancer cells. Vossicles or collagen matrices were seeded with ACE-1luc cells subcutaneously in athymic mice (2 vossicles, 2 collagen implants/mouse). Mice received ZA (5μg/mouse, twice/week), (OPG-Fc at 10mg/kg, 3 times/week) or vehicle, and luciferase activity was measured weekly. Histologic analysis of the tumors, vossicles and endogenous bones and serum biochemistry were performed. Antiresorptive administration was associated with decreased serum TRAP5b and reduced osteoclast numbers, increased tibia and vossicle bone areas. ZA significantly decreased bone marrow calcium concentrations without affecting serum calcium. ZA and OPG-Fc significantly inhibited tumor growth in bone but not in collagen implants. In conclusion, the inhibitory effects of ZA or OPG-Fc on prostate tumor growth in bone are mediated via blocking bone resorption and calcium release from bone.
Keywords: Zoledronic acid (ZA), osteoprotegrin (OPG), anti-resorptive, prostate tumor, calcium, bone resorption
Introduction
Skeletal metastasis is a major cause of morbidity and mortality associated with prostate and breast cancer. Approximately 400,000 new patients per year in the United States develop skeletal metastases and more than 70% of patients with carcinoma of the breast, prostate, and kidneys will eventually develop bone metastases (1). Prostate skeletal metastasis results in characteristic osteoblastic or mixed osteoblastic/osteolytic lesions in skeletal sites. Prostate cancer often metastasizes to the axial skeleton and long bone metaphyses, which are sites that have active bone remodeling and increased marrow cellularity (2). Previous studies from our laboratory showed that reduced levels of the calcium sensing receptor in prostate carcinoma cells significantly reduced tumor growth in vitro and in vivo, which implicates the calcium-mediated signaling pathway as promoting prostate cancer cell growth (3). Similarly, calcium was suggested to enhance breast carcinoma growth in vivo (4). Low dietary calcium was associated with higher bone turnover and promoted tumor growth in bone, independent of the action of parathyroid hormone (4). Osteoclastogenesis and bone resorption are independent steps leading to the development of skeletal metastases and are mutually essential for prostate cancer establishment in the bone microenvironment (5). Increased bone resorption or bone turnover enhanced growth of skeletal metastases has been reported by multiple groups (2, 6-11). However, most studies have focused on growth factors and cytokines released during bone resorption and their contribution to tumor growth and neo-angiogenesis. Since the skeleton has an abundant apatite store which releases calcium during resorption, it is important to understand the role of calcium in skeletal metastasis which has the potential to improve therapy and prevention of skeletal metastases.
Bisphosphonates are potent anti-resorptive drugs that are used in cancer patients with skeletal diseases caused by multiple myeloma, prostate or breast cancer. It is still controversial whether bisphosphonates are able to directly inhibit tumor cell growth independent of their effect on bone in vivo (12-18). In addition, how bisphosphonates suppress prostate tumor growth using experimental models that evoke an osteoblastic response has not been well studied. The ACE-1 cell line was developed from a canine prostate carcinoma, and similar to the human condition, it forms typical osteoblastic lesions (19). In the present study, a novel xenograft vossicle co-implant tumor model using ACE-1 cells (20, 21), was applied to determine the roles of calcium and antiresorptives in prostate tumor growth in bone.
Materials and methods
Cell Culture
The ACE-1 canine prostate cancer cell line with luciferase (ACE-1luc) (19) and the human prostate cancer cell line LNCaP and C4-2b were maintained at 37°C and 5% CO2 in RPMI 1640 media containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen Corp., Carlsbad, CA). The LNCaP cell line was originally isolated and expanded from a needle aspiration biopsy of the left supraclavicular lymph node of a 50-year-old Caucasian male with confirmed diagnosis of metastatic prostate carcinoma (www.ATCC.org). The C4-2b cell line was originally derived from LNCaP tumors maintained in castrated and intact athymic male mice (22). The ACE-1 cell line was generated from a canine prostate cancer metastasis to bone (19). Both ACE-1 and C4-2b cells are osteotropic prostate cancer cell lines which form typical osteoblastic lesions in vivo.
In vivo prostate cancer models
All experimental animal procedures were performed in compliance with institutional ethical requirements approved by the University of Michigan Committee for the Use and Care of Animals. Male athymic mice (4-5 weeks old) were purchased from Harlan (Indianapolis, IN). For subcutaneous implantation of the scaffolds loaded with tumor cells, two 1cm incisions were made along the backs of mice, pouches were made on both sides of the incision with blunt dissection, and the tumor scaffolds were implanted. Collagen matrices (Gelfoam®, a gelatin-based sponge prepared from purified type A gelatin) (12-7mm, Pfizer, NY) were pre-cut into 5×5×7mm cubes, soaked with 100,000 or 500,000 tumor cells in 8 μl Hank’s buffered saline solution (Invitrogen). Two collagen sponges were subcutaneously implanted into one flank of each mouse. In the same mouse, the other side was implanted with two scaffolds with equal number of tumor cells in a fresh bone tissue implant (vossicle), de-proteinized bone implants (DP-bone), or collagen pre-loaded with mineral (mineralized collagen).
The bone implants were performed as previously described, with minor modification (20, 21). Briefly, vertebrae were isolated under sterile conditions from 9-day old mice. Soft tissue was dissected, and vertebrae were sectioned into vertebral bodies (vossicles) with a scalpel blade. For de-proteinized bone implants, one Puros™ (an allograft bone grafting material with lipids and protein removed) block allograft (15mm, Zimmer Dental Inc.), was evenly cut to 40 pieces. Mineralized collagen matrix with or without mineral (hydroxyapatite) composite, was prepared as described in the literature (23). In brief, mineralized collagen sponges were prepared by immersing the sponges in 200 mM CaCl2 for 3 min followed by immersion in 120 mM Na2HPO4 for another 3 min. Then after mineral deposition at 45 °C, the sponges were rinsed in double-distilled water to remove all of the calcification solution and dried at 37 °C. Over-drying was prevented to minimize sponge shrinkage (23). Each implant was soaked with 100,000 ACE-1luc cells or 500,000 C4-2b cells in 8 μl Hank’s buffered saline solution.
Treatment with zoledronic acid (ZA) or OPG-Fc
One week after tumor implantation, mice with collagen and vossicle implants were randomly divided to two groups, one group was treated with vehicle, the other group with ZA or OPG-Fc (provided by Amgen, Seattle, WA) for up to 8 weeks. The dose used was 200μg/kg, twice/week for ZA and 10mg/kg, 3 times/week for OPG-Fc. The dose of ZA was selected based on our previous work which demonstrated significant inhibition of bone resorption (2). The dose of OPG-Fc was selected based on the manufacturer’s suggestion. Saline was used as vehicle control for both ZA and OPG-Fc.
Bioluminescent imaging (BLI)
In vivo bioluminescent imaging was carried out at the University of Michigan Small Animal Imaging Resource facility weekly after tumor implantation. Mice were injected i.p. with 100 μL of 40 mg/mL luciferin dissolved in PBS. Imaging was performed under 1.75% isoflurane/air anesthesia on a cryogenically cooled IVIS system equipped with a 50-mm lens and coupled to a data acquisition PC running Living Image software 2.6 (Xenogen Corp., Alameda, CA). Ventral images were acquired 12 minutes after injection. Pseudocolor images of photon emissions were overlaid on grayscale images of mice to aid in determining signal spatial distribution. Photon quantifications were calculated within regions of interest (ROI).
Histology and immunohistochemistry
After 4-8 weeks, mice were sacrificed under anesthesia (i.p. injection of a mixture of ketamine [90 mg/kg] and xylazine [5 mg/kg]) and xenograft tumors were harvested and fixed in fresh 10% formalin. Tibiae were decalcified in 10% EDTA for 14 days prior to paraffin embedding. Paraffin-embedded specimens were sectioned (5 μm) and stained with either hematoxylin and eosin (H&E), Trichrome (bone), tartate-resistant acid phosphatase (TRAP) (osteoclasts) (Acid Phosphatase, Leukocyte Kit, Sigma, St. Louis, MO) or Ki-67 (antibody from Neomarker, Fremont, CA). Mayer’s hematoxylin (Sigma) was used for counterstaining. ImageJ software (the U. S. National Institutes of Health; http://rsb.info.nih.gov/ij/) was used for quantification of Ki-67 immunohistochemical staining. Briefly, images of the positively-stained nuclei were extracted using the color deconvolution macro (24), followed by nuclei counting using the ‘Analyze Particles’ function in ImageJ. Total cell numbers per image were determined by counting hematoxylin-stained nuclei in the same manner.
TRAP5b and Calcium measurement
Serum TRAP5b activity was measured by ELISA (Immunodiagnostic Systems, Inc.) following the manufacturer’s instructions. Total calcium levels in serum and bone marrow aspirates (supernatant) were measured by a quantitative colorimetric assay with Calcium Reagent Set (Pointe Scientific, Inc., Canton, MI).
Statistical analysis
One-way ANOVA or Student’s t-test for independent analysis was used to evaluate differences using the GraphPad Instat 3 software program (GraphPad Software, San Diego, CA). Fisher’s exact test was used to compare incidence and the value p<0.05 was considered statistically significant. All assays were repeated at least twice with similar results.
Results
Mineral rich sites enhanced growth of prostate tumor Xenografts
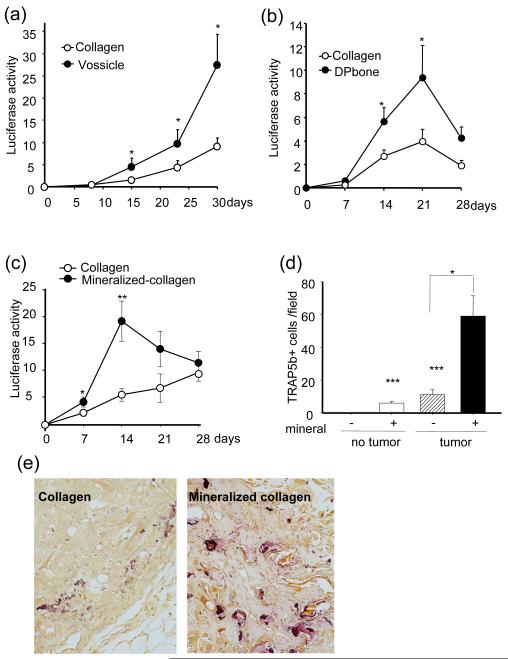
Prostate carcinoma frequently metastasizes to the skeleton suggesting that the bone microenvironment facilitates tumor cell growth. This was confirmed with the xenograft tumor models using vossicles (neonatal mouse vertebrae) as a scaffold. As demonstrated in Fig. 1a, ACE-1luc cells developed tumors more aggressively in vossicle implants compared to collagen as demonstrated by significantly greater luciferase activity in vossicle implants after 2 weeks. To determine the influence of the bone mineral composite in prostate carcinoma cell growth in vivo, two strategies were applied. First, tumor development was compared in a bone scaffold where the protein component was removed (de-proteinized bone, DPbone) versus collagen matrix (Fig. 1b); secondly, tumor development was compared in mineral-loaded collagen matrix (mineralized collagen) versus a simple collagen matrix (Fig. 1c-e). Both scaffolds (de-proteinized bone and mineralized collagen matrix) enhanced ACE-1luc cell xenograft tumor growth compared to non-mineralized collagen as reflected by higher luciferase activity in deproteinized-bone (DPbone) implants and mineralized collagen compared to regular collagen implants at week 2 and/or 3 after implantation (Fig. 1 b-c). Unlike vossicle implants, both DPbone and mineralized collagen matrix lack viable bone cells. In order for mineral degradation and calcium release, osteoclasts need to be recruited to the subcutaneous implants from the host peripheral blood. As speculated, the mineralized collagen matrix/tumor sections had strong TRAP-positive staining and the number of TRAP-positive cells was significantly higher than in collagen implants lacking mineral (Fig. 1d-e).
Fig. 1. ACE-1 tumors grew larger in mineralized bone compared to collagen matrices.
Vertebral bodies (vossicles), human de-proteinized bone (DPbone), mineralized collagen or simple collagen matrices were implanted with ACE-1 cells (100,000 cells per implant). BLI imaging was recorded weekly. Luciferase activity (109p/sec/cm2/Sr) of collagen tumor implants was less than tumor cells in; (a) vossicles, (b) de-proteinized bone, or (c) mineralized collagen. Tumors in mineralized collagen matrices had more TRAP5b- positive cells than tumors in collagen matrices (d-e). (n=8, *p<0.05, **p<0.001 versus collagen tumor implant, ***p<0.05 versus plain collagen implant).
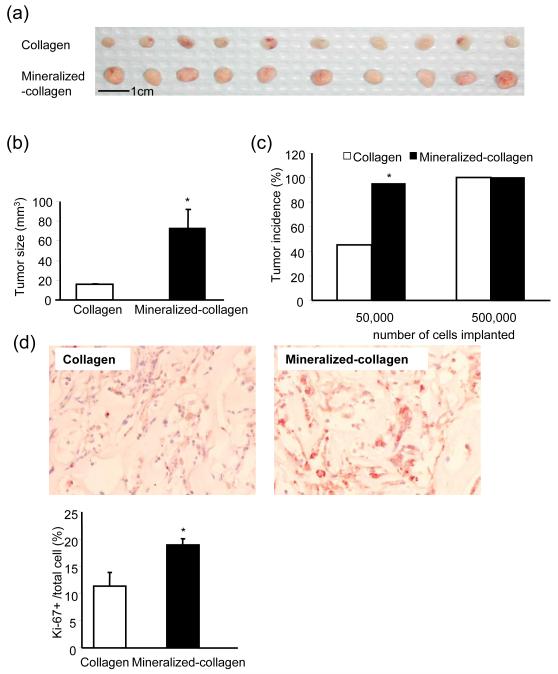
A second osteoblastic prostate carcinoma cell line was used to validate the beneficial effects of a mineralized matrix on tumor cell growth. Equal numbers of human C4-2b prostate carcinoma cells, were inoculated into simple collagen matrices and mineralized collagen matrices and implanted in athymic mice for 2-3 weeks. The size of tumors grown in mineralized collagen was significantly larger than those in non-mineralized collagen (Fig. 2a-b). The incidence of tumor formation was also higher in mineralized versus non-mineralized collagen (Fig. 2c). Immunostaining for human Ki-67 protein on tumor sections revealed higher positive staining in mineralized collagen implants, which indicates increased cell proliferation (Fig. 2d). It is known that, unlike C4-2b cells, LNCaP cells do not have calcium sensing receptor [3] thus, as expected, the presence or absence of calcium did not significantly impact LNCaP cell tumor growth (Supplementary data Table 1). Similar tumor incidences were observed among implants with LNCaP cells in vossicles, mineralized-collagen or simple collagen matrices. .
Fig. 2. C4-2b tumors were larger with greater cell proliferation in mineralized collagen matrices versus simple collagen matrices.
Mineralized collagen and simple collagen matrices were implanted with 50,000 or 500,000 C4-2b cells and implanted subcutaneously into 4-week-old athymic mice. The tumor sizes from mice implanted with 500,000 C4-2b cells were compared at 2-weeks (a-b) (n=10, *p<0.01, versus collagen/tumor implant). The incidence of tumor formation was greater in mineralized compared to simple collagen implants in mice implanted with 50,000 C4-2b cells (c) (n=10, *p<0.01, versus collagen/tumor implant). (d) Representative images and quantitative results of of Ki-67 staining in regular and mineralized collagen/tumor sections. (n=8, * p<0.02).
Anti-resorptives selectively inhibited prostate tumor growth in vossicles but not collagen matrices
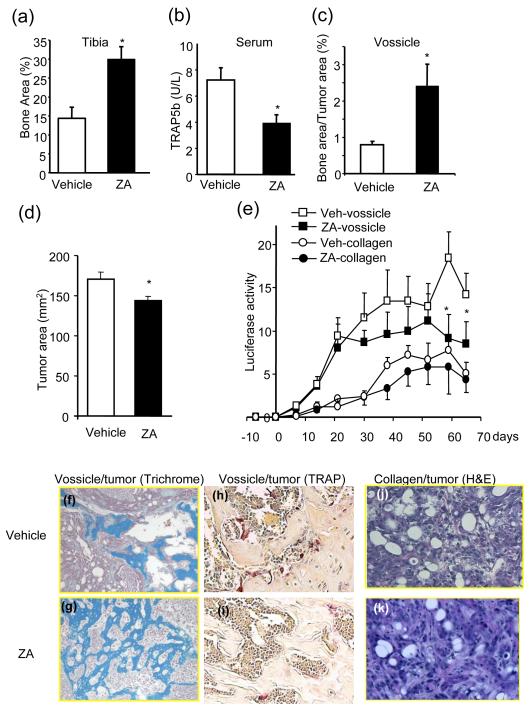
The inhibitory effects of ZA or OPG-Fc were investigated in a tumor implantation model. First, the effects of ZA in bone were confirmed, as demonstrated in Fig. 3 a-c. Both endogenous (tibia) and exogenous (vossicle) bones were protected from bone resorption in the ZA-treated mice. Also, serum TRAP5b concentrations, which reflect bone resorption activity, were significantly decreased with ZA-treatment (Fig. 3b). Consistently, TRAP staining was weaker in sections of tibia isolated from ZA-treated mice (Fig. 3h-i). Tumor size was reduced in vossicles implanted in ZA treated mice (Fig. 3d). Tumor implants in bone (vossicles) or collagen matrix from the same mice responded to ZA treatment differentially (Fig. 3e). The vossicle implants in mice treated with ZA progressed slower versus vossicle implants in mice treated with vehicle. Interestingly, tumor implants in the simple collagen matrix grew similarly whether they were from ZA- or vehicle-treated mice. This result suggests that ZA suppressed tumor growth in a bone but not in a collagen microenvironment. The histology of tumor sections from vossicles (Fig. 3f-g) and collagen tumors (Fig. 3j-k) showed greater residual bone remaining in vossicle tumors from ZA-treated mice while the morphology of tumors remained the same in collagen tumors.
Fig. 3. ZA protected bone area and suppressed tumor growth in vossicles.
Vossicle and collagen matrices were implanted with luciferase-positive ACE-1 prostate cancer cells (100,000 cells per implant) and placed subcutaneously into athymic mice. ZA or vehicle was administered 7-days later and BLI imaging recorded weekly. (a) Trabecular bone area in tibia per tissue area was significantly greater in ZA-treated mice (n=8, *p<0.05). (b) Serum TRAP5b (osteoclast marker) was significantly decreased with ZA treatment (n=8, *p<0.05). (c) Vossicle bone area per tumor area was higher in the ZA group (n=8, *p<0.05). (d) Tumor area was reduced in vossicles from the ZA group. (e) ZA-treated vossicles had lower luciferase activity (n=8, 109p/sec/cm2/Sr, *p<0.05 versus veh-vossicle, **p<0.05 versus veh-collagen) than vehicle-treated vossicles. (f-g) Vossicles in ZA-treated groups had more bone (Mason’s Trichrome mineralized tissue blue). (h-i) TRAP5b staining showed reduced osteoclast numbers in ZA-treated mice (j-k) H&E staining revealed no morphological differences in collagen implanted tumor treated with ZA or vehicle.
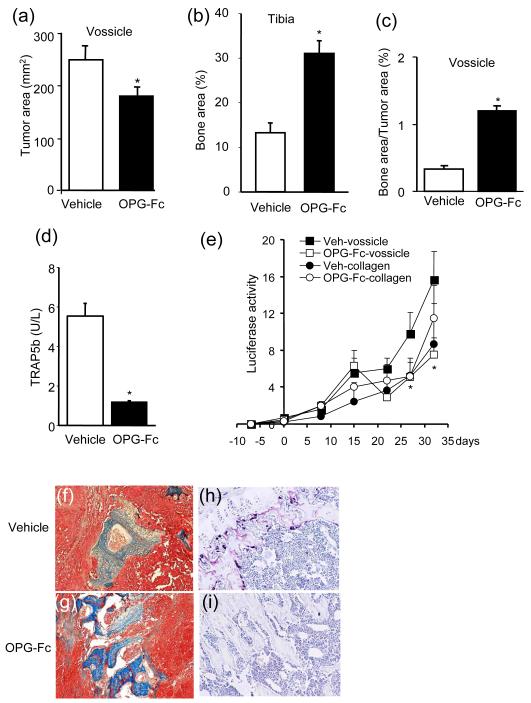
Additional experiments were performed using an anti-resorptive agent that acts by a different mechanism. OPG-Fc was used in the same model system to inhibit osteoclastic bone resorption and evaluate its impact on tumor growth (Fig. 4). OPG is a decoy receptor of RANKL, and a potent inhibitor of bone resorption. However, the circulating half-life of native OPG is brief partially due to its heparin-binding domain (25). OPG-Fc is a modified form of OPG with deletion of the heparin-binding domain and fusion to the Fc portion of human IgG1 (26). OPG-Fc has a long circulating half life and causes sustained suppression of bone turnover (26, 27). The protective effects of OPG-Fc on bone area were demonstrated in both vossicle implants and tibiae (Fig. 4a-c, f-g). Serum TRAP5b and TRAP-positive staining of osteoclasts in the tibiae were decreased in the OPG-Fc-treated group (Fig. 4d, h-i). The OPG-Fc-treated group also had significantly less luciferase activity (live tumor cell number) in vossicle implants but not in the collagen matrix (Fig. 4e). The reduction in BLI progression (tumor growth) in vossicles after OPG-Fc treatment was independently verified by measuring tumor size by histology (Fig. 4a).
Fig. 4. Inhibition of bone resportion by OPG-Fc (OPG) suppressed tumor growth in vossicles.
Vossicle and collagen matrices were implanted with luciferase-positive ACE-1 prostate cancer cells (100,000 cells) and placed subcutaneously into athymic mice. (a) Tumor area was reduced in vossicles from the OPG-Fc group. (b) Tibial bone area per tissue area (%) was significantly greater in the OPG-Fc-treated group. (c) The OPG-Fc-treated group had increased bone area in vossicle tumors, and (d) decreased serum TRAP5b (n=8, *p<0.05). (e) OPG decreased tumor luciferase activity in vossicles (n=8, 109p/sec/cm2/Sr, *p<0.05 versus veh-vossicle, **p<0.05 versus veh-collagen). Representative histology of vossicle tumors with Mason’s Trichrome staining (f-g) and TRAP staining of tibiae (h-i).
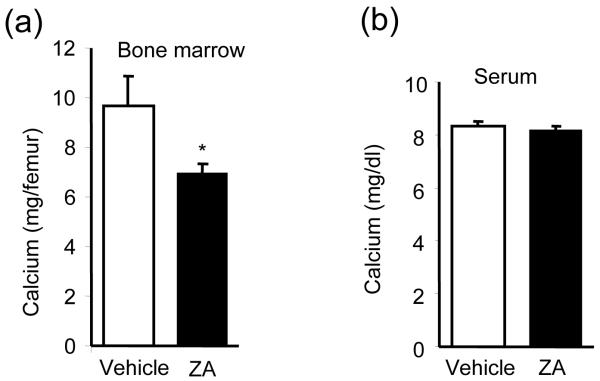
Zoledronic Acid decreased calcium levels locally but not systemically
The existence of bone or mineral facilitated ACE-1 prostate carcinoma cell growth in the s.c. xenograft models. Both ZA and OPG-Fc selectively suppressed tumor growth in xenografts with bone (vossicles) but failed to suppress tumor growth in non-bone (collagen) implants. Since bone scaffolds depleted of protein components or mineralized collagen enhanced tumor growth, this suggests that anti-resorptive treatment decreased mineral release. Calcium concentration was used as a marker of bone resorption since calcium is the major ion in hydroxyapatite. ZA significantly decreased calcium concentrations in whole bone marrow aspirates without changing serum calcium concentrations (Fig. 5).
Fig. 5. ZA decreased calcium concentrations in bone marrow but not in the serum.
C57/B6 mice were treated with ZA or Vehicle before bone marrow aspiration and serum calcium levels were measured (n=8). (a) ZA treatment decreased bone marrow calcium concentrations. (*p<0.05) (b) Serum calcium concentrations were not altered by ZA.
Discussion
The strategy of targeting bone resorption to limit tumor growth in skeletal lesions has proven to be effective although the mechanism is not fully understood. The role of bone minerals, the major component of bone matrix, has not been extensively studied. In the present investigation, which utilizes a novel xenograft model, it was found that bone mineral increased prostate carcinoma cell growth in vivo regardless of the presence of proteins. The significance of these experiments was the direct comparison between tumor growth with mineral (vossicles, de-proteinized bones and mineralized collagen matrix) and without mineral (simple collagen matrix) in the same host. Growth of prostate cancer xenografts decreased from week 3 in de-proteinized bones and week 2 in mineralized collagen matrices. It is likely that the bone minerals become depleted at that point with resultant reduced tumor growth. Interestingly, subcutaneous implantation of collagen matrices with minerals or tumor cells demonstrated positive TRAP staining while collagen implants alone had no TRAP-positive cells. The presence of both mineral and prostate cancer cells demonstrated the highest TRAP-positive staining in the implants. This suggests that both minerals and cancer cells are necessary to recruit osteoclast progenitor cells from peripheral blood. The detection of active TRAP activity in the tumor sections further supported that bone resorption was vital to growth of osteoblastic prostate carcinoma cells.
Recent clinical studies reported a correlation between serum calcium concentration in patients and prostate tumor progression (28, 29). It was demonstrated that high serum calcium or a factor associated with it (such as high serum parathyroid hormone) increased the risk for prostate cancer. These findings support the hypothesis that serum calcium is a prospective biomarker of prostate cancer outcomes. Moreover, it was reported that biochemical markers of bone turnover significantly decreased with ZA treatment in children with osteogenesis imperfecta or osteoporosis (30). Not surprisingly, this was associated with a trend in decreased serum calcium. However, there was no definitive hypocalcaemia since at the end of 12 months of treatment the serum calcium levels were within the normal reference range (30).
It has been speculated that blocking bone resorption leads to lower serum calcium concentrations, with reports of serum calcium reduction by treatment with bisphosphonates (31-33). Serum calcium concentrations are a vital physiological parameter with regulation by multiple feedback loops mediated by parathyroid hormone, calcitonin and vitamin D through organs such as the kidney, intestine and bone (34). Thus, the effects of bisphosphonates on calcium balance may not be easily detected. In most cases, bisphosphonates were found to decrease serum calcium during hypercalcaemia (31-33, 35, 36), but may not alter serum calcium concentrations in a normocalcemic patient due to the tight regulation of calcium homeostasis (30, 37).
In the present study, there was no significant change in the serum calcium concentrations in the mice treated with ZA despite a significant decrease in TRAP5b, a bone resorption marker. In contrast, ZA significantly reduced the calcium concentration in the bone marrow. Similarly, there was a trend of reduced calcium levels in the OPG-Fc treated group versus vehicle group after 3-weeks. Bisphosphonates are very potent in blocking bone resorption and have been speculated to decrease calcium concentrations in bone, but there have been no reports that measured calcium in the bone marrow. To our knowledge, this is the first report of a decrease in bone marrow calcium concentration by ZA. These data suggest that bone minerals supported tumor growth and blocking bone resorption with ZA may reduce tumor growth in bone by inhibiting calcium release from the mineral matrix in addition to reducing growth factor release from osteoclasts.
The inhibition of tumor growth by ZA and OPG-Fc were most effective in the bone microenvironment (18, 38). Since ZA and OPG-Fc are antiresorptives with different mechanisms, this suggests that both agents inhibited tumor growth in bone indirectly versus directly. Our results are consistent with previous reports (16, 38-40) that bisphosphonates suppressed tumor growth in bone. In the present study, ZA and OPG-Fc were administered to the tumor-bearing mice in a therapeutic regimen versus a prevention protocol where the drugs would be administered prior to tumor implantation. In our study, there was a two-week lag before the mice treated with ZA or OPG-Fc had inhibition of tumor growth.
In summary, the inhibitory effects on tumor growth of bisphosphonates or OPG-Fc are dependent on their reduction in bone resorption. The data presented may not definitely establish that high calcium levels in bone solely lead to increased prostate cancer cell proliferation as other pathways involved in prostate cancer growth in bone have not been ruled out. The data do support engaging in further studies targeting calcium signaling as a potential therapeutic strategy to treat prostate cancer skeletal metastasis.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Rodrigo Neiva for preparation of de-proteinized bone scaffolds and Fabiana Soki for her technical assistance. This work was supported by the National Cancer Institute award P01-CA093900 (LKM and KJP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146:1727–36. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 3.Liao J, Schneider A, Datta NS, McCauley LK. Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 2006;66:9065–73. doi: 10.1158/0008-5472.CAN-06-0317. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Zhou H, Modzelewski JR, Kalak R, Blair JM, Seibel MJ, Dunstan CR. Accelerated bone resorption, due to dietary calcium deficiency, promotes breast cancer tumor growth in bone. Cancer Res. 2007;67:9542–8. doi: 10.1158/0008-5472.CAN-07-1046. [DOI] [PubMed] [Google Scholar]
- 5.Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- 6.Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, Peng XH, Chirgwin JM, Guise TA. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirbe AC, Rubin J, Uluckan O, Morgan EA, Eagleton MC, Prior JL, Piwnica-Worms D, Weilbaecher KN. Disruption of CXCR4 enhances osteoclastogenesis and tumor growth in bone. Proc Natl Acad Sci U S A. 2007;104:14062–7. doi: 10.1073/pnas.0705203104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69:1685–92. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–53. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 10.Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80:1546–56. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Russell MR, Jamieson WL, Dolloff NG, Fatatis A. The alpha-receptor for platelet-derived growth factor as a target for antibody-mediated inhibition of skeletal metastases from prostate cancer cells. Oncogene. 2009;28:412–21. doi: 10.1038/onc.2008.390. [DOI] [PubMed] [Google Scholar]
- 12.Brown HK, Holen I. Anti-tumour effects of bisphosphonates--what have we learned from in vivo models? Curr Cancer Drug Targets. 2009;9:807–23. doi: 10.2174/156800909789760339. [DOI] [PubMed] [Google Scholar]
- 13.Clezardin P, Fournier P, Boissier S, Peyruchaud O. In vitro and in vivo antitumor effects of bisphosphonates. Curr Med Chem. 2003;10:173–80. doi: 10.2174/0929867033368529. [DOI] [PubMed] [Google Scholar]
- 14.Fournier PG, Stresing V, Ebetino FH, Clezardin P. How do bisphosphonates inhibit bone metastasis in vivo? Neoplasia. 12:571–8. doi: 10.1593/neo.10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004;10:4559–67. doi: 10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- 16.Li YY, Chang JW, Chou WC, Liaw CC, Wang HM, Huang JS, Wang CH, Yeh KY. Zoledronic acid is unable to induce apoptosis, but slows tumor growth and prolongs survival for non-small-cell lung cancers. Lung Cancer. 2008;59:180–91. doi: 10.1016/j.lungcan.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Ory B, Heymann MF, Kamijo A, Gouin F, Heymann D, Redini F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer. 2005;104:2522–9. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Zhou H, Brennan K, Blair JM, Modzelewski JR, Seibel MJ, Dunstan CR. Inhibition of bone resorption, rather than direct cytotoxicity, mediates the anti-tumour actions of ibandronate and osteoprotegerin in a murine model of breast cancer bone metastasis. Bone. 2007;40:471–8. doi: 10.1016/j.bone.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 19.LeRoy BE, Thudi NK, Nadella MV, Toribio RE, Tannehill-Gregg SH, van Bokhoven A, Davis D, Corn S, Rosol TJ. New bone formation and osteolysis by a metastatic, highly invasive canine prostate carcinoma xenograft. Prostate. 2006;66:1213–22. doi: 10.1002/pros.20408. [DOI] [PubMed] [Google Scholar]
- 20.Koh AJ, Demiralp B, Neiva KG, Hooten J, Nohutcu RM, Shim H, Datta NS, Taichman RS, McCauley LK. Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone. Endocrinology. 2005;146:4584–96. doi: 10.1210/en.2005-0333. [DOI] [PubMed] [Google Scholar]
- 21.Pettway GJ, McCauley LK. Ossicle and vossicle implant model systems. Methods Mol Biol. 2008;455:101–10. doi: 10.1007/978-1-59745-104-8_7. [DOI] [PubMed] [Google Scholar]
- 22.Lin DL, Tarnowski CP, Zhang J, Dai J, Rohn E, Patel AH, Morris MD, Keller ET. Bone metastatic LNCaP-derivative C4-2B prostate cancer cell line mineralizes in vitro. Prostate. 2001;47:212–21. doi: 10.1002/pros.1065. [DOI] [PubMed] [Google Scholar]
- 23.Rohanizadeh R, Swain MV, Mason RS. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med. 2008;19:1173–82. doi: 10.1007/s10856-007-3154-y. [DOI] [PubMed] [Google Scholar]
- 24.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–9. [PubMed] [Google Scholar]
- 25.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 26.Capparelli C, Morony S, Warmington K, Adamu S, Lacey D, Dunstan CR, Stouch B, Martin S, Kostenuik PJ. Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): a pharmacodynamic and pharmacokinetic analysis in rats. J Bone Miner Res. 2003;18:852–8. doi: 10.1359/jbmr.2003.18.5.852. [DOI] [PubMed] [Google Scholar]
- 27.Kostenuik PJ, Bolon B, Morony S, Daris M, Geng Z, Carter C, Sheng J. Gene therapy with human recombinant osteoprotegerin reverses established osteopenia in ovariectomized mice. Bone. 2004;34:656–64. doi: 10.1016/j.bone.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Skinner HG, Schwartz GG. Serum calcium and incident and fatal prostate cancer in the National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:2302–5. doi: 10.1158/1055-9965.EPI-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner HG, Schwartz GG. A prospective study of total and ionized serum calcium and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:575–8. doi: 10.1158/1055-9965.EPI-08-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannesen J, Briody J, McQuade M, Little DG, Cowell CT, Munns CF. Systemic effects of zoledronic acid in children with traumatic femoral head avascular necrosis and Legg-Calve-Perthes disease. Bone. 2009;45:898–902. doi: 10.1016/j.bone.2009.04.255. [DOI] [PubMed] [Google Scholar]
- 31.Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88:4569–75. doi: 10.1210/jc.2003-030050. [DOI] [PubMed] [Google Scholar]
- 32.Siris ES, Lyles KW, Singer FR, Meunier PJ. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(Suppl 2):P94–8. doi: 10.1359/jbmr.06s218. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Burton DW, Geller J, Hillegonds DJ, Hastings RH, Deftos LJ, Hoffman RM. The bisphosphonate olpadronate inhibits skeletal prostate cancer progression in a green fluorescent protein nude mouse model. Clin Cancer Res. 2006;12:2602–6. doi: 10.1158/1078-0432.CCR-05-2050. [DOI] [PubMed] [Google Scholar]
- 34.Bronner F. Extracellular and intracellular regulation of calcium homeostasis. ScientificWorldJournal. 2001;1:919–25. doi: 10.1100/tsw.2001.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mastrandrea LD, Albini CH. Bisphosphonate treatment of tumor-induced hypercalcemia in a toddler: case report and review of related literature. Endocr Pract. 2006;12:670–5. doi: 10.4158/EP.12.6.670. [DOI] [PubMed] [Google Scholar]
- 36.Waked A, Geara A, El-Imad B. Hypercalcemia, metabolic alkalosis and renal failure secondary to calcium bicarbonate intake for osteoporosis prevention - ‘modern’ milk alkali syndrome: a case report. Cases J. 2009;2:6188. doi: 10.4076/1757-1626-2-6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravn P. Bisphosphonates for prevention of postmenopausal osteoporosis. Dan Med Bull. 2002;49:1–18. [PubMed] [Google Scholar]
- 38.Corey E, Brown LG, Quinn JE, Poot M, Roudier MP, Higano CS, Vessella RL. Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clin Cancer Res. 2003;9:295–306. [PubMed] [Google Scholar]
- 39.Brubaker KD, Brown LG, Vessella RL, Corey E. Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer. 2006;6:15. doi: 10.1186/1471-2407-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miwa S, Mizokami A, Keller ET, Taichman R, Zhang J, Namiki M. The bisphosphonate YM529 inhibits osteolytic and osteoblastic changes and CXCR-4-induced invasion in prostate cancer. Cancer Res. 2005;65:8818–25. doi: 10.1158/0008-5472.CAN-05-0540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.