Abstract
A number of cancers predominantly metastasize to bone, due to its complex microenvironment and multiple types of constitutive cells. Prostate cancer especially has been shown to localize preferentially to bones with higher marrow cellularity. Using an experimental prostate cancer metastasis model, we investigated the effects of cyclophosphamide, a bone marrow-suppressive chemotherapeutic drug, on the development and growth of metastatic tumors in bone. Priming the murine host with cyclophosphamide prior to intra-cardiac tumor cell inoculation was found to significantly promote tumor localization and subsequent growth in bone. Shortly after cyclophosphamide treatment, there was an abrupt expansion of myeloid lineage cells in the bone marrow and the peripheral blood, associated with increases in cytokines with myelogenic potential such as C-C chemokine ligand (CCL)-2, interleukin (IL)-6, and vascular endothelial growth factor (VEGF)-A. More importantly, neutralizing host-derived murine CCL2, but not IL-6, in the pre-metastatic murine host significantly reduced the pro-metastatic effects of cyclophosphamide. Together, our findings suggest that bone marrow perturbation by cytotoxic chemotherapy can contribute to bone metastasis via a transient increase in bone marrow myeloid cells and myelogenic cytokines. These changes can be reversed by inhibition of CCL2.
Keywords: Cyclophosphamide, prostate cancer, bone metastasis, myeloid cells, CCL2
INTRODUCTION
Bone is the predominant site of prostate cancer metastasis, and advanced-stage prostate cancer patients commonly develop metastatic bone lesions (1). Unfortunately, the pathophysiology of skeletal metastasis is not yet completely understood (2). One major obstacle to better understanding skeletal metastasis is the unusual complexity of the tumor microenvironment in bone (3), due to multiple constituent cell types. Emerging evidence supports that cells in the bone marrow microenvironment are actively involved in prostate cancer metastasis (4).
Bone marrow-derived myeloid lineage cells are critical regulators of tumor progression and metastasis (5–10). Yang et al. demonstrated that expansion of Gr-1+CD11b+ myeloid cells directly promotes tumor angiogenesis (6) via increased production of matrix metalloproteinase (MMP)-9 (7). Myeloid cells (expressing surface markers CD11b and/or Gr-1) are a major component of undifferentiated bone marrow cells, and ultimately differentiate into monocytes, macrophages and granulocytes (10). Parallel to the tumorigenic roles of myeloid cells, monocyte-macrophages also have been shown to participate in tumor metastasis (11–13). All of these data collectively support the critical roles of myeloid lineage cells in prostate cancer progression and bone metastasis. However, it is not clearly understood how the alterations in the bone marrow occur, which could provide clues for therapeutic approaches.
In clinical settings, chemotherapeutic drugs and/or irradiation perturb the bone marrow microenvironment, leading to alterations in marrow cellular composition. Although chemotherapy and irradiation are both bone marrow-suppressive, the subsequent recovery process may lead to temporary spikes of certain cell types, including monocytes and neutrophils (14, 15). Therefore, net effects of bone marrow-suppressive agents could have pro- or anti-tumorigenic effects. Interestingly, priming the murine host with cyclophosphamide (CY), a bone marrow-suppressive chemotherapeutic drug, promoted subcutaneous tumor growth and metastasis in several mouse models (16–19). CY is a DNA-alkylating drug commonly included in chemotherapeutic regimens against breast and lung cancers, and non-Hodgkin’s lymphoma. In addition, CY is used in the conditioning regimen for recipients of myeloablative bone marrow transplantation, to enhance engraftment and suppress the host immune reaction. Intriguing data showing opposite pro-metastatic effects of chemotherapeutic drugs remain poorly investigated. To our best knowledge, the effects of CY on skeletal metastasis have never been reported. Given that prostate cancer has been shown to utilize similar strategies as hematopoietic stem/progenitor cell homing, and that prostate cancer has long been known to home typically to bones enriched with red marrow (20), we hypothesized that alterations induced by CY in the bone marrow microenvironment would contribute to prostate cancer colonization in the bone and/or subsequent tumor growth. In the current study, we investigated pro-metastatic effects of bone marrow suppression in a prostate cancer skeletal metastasis model, and explored the underlying mechanisms that could be used to design methods of therapeutic intervention.
MATERIALS AND METHODS
Cells
Luciferase-labeled PC-3 cells (PC-3Luc) were established from the PC-3 cell line (ATCC), as previously described (20). PC-3Luc cells were regularly authenticated and matched short tandem repeat DNA profiles of the original PC-3 cell line (last tested on May 9, 2009).
Mouse models of prostate cancer
All experimental protocols were approved by the University of Michigan Institutional Animal Care and Use Committee. For a skeletal metastasis model, the procedure described by Park et al. was followed (21). Briefly, 2×105 PC-3Luc cells were injected into the left heart ventricle of male athymic mice (Harlan Laboratories). For an orthotopic bone tumor model, 1×103 PC-3Luc cells were injected in the proximal tibiae as described (21).
Ex vivo murine bone marrow microvascular angiography
Murine bone marrow vasculature was visualized by a modified method of Guldberg et al.(22) Mice were anesthetized and perfused sequentially with heparin supplemented-Ringer’s lactate (9mins), formalin (9mins), and Microfil® (FlowTech, 7mins) via the intra-cardiac route. Following polymerization, femurs were dissected, decalcified and scanned by micro-computed tomography (µCT).
Neutralizing antibodies
Anti-mouse CCL2 antibody (C1142, Janssen) and anti-mouse IL-6 antibody (R&D Systems) were provided by Janssen, LLC. C1142 is a rat/mouse chimeric antibody specific for mouse CCL2/MCP-1 and does not cross-react with human CCL2 or mouse MCP-5 (23–25). Non-specific IgG from mouse serum (Sigma-Aldrich) was used as a control antibody.
Flow cytometry
Bone marrow cells were collected by flushing femurs and tibiae. Lungs, liver and kidney were digested in complete DMEM supplemented with 0.5mg/ml collagenase (Sigma-Aldrich). One million cells were used for flow cytometry (BD Bioscience).
Complete blood counting (CBC) with white blood cell (WBC) differentials
Blood cell counting was performed in the University of Michigan Unit for Laboratory Animal Medicine, using a Forcyte™ automatic hematology analyzer (Oxford Science).
Quantitative PCR
mRNA samples were prepared from the flushed bone marrow cells, followed by RT-PCR for CD31 and mouse GAPDH (Applied Biosystems).
Statistical analyses
Experimental skeletal metastasis experiments were analyzed using linear mixed models. The primary outcome was the natural log transformed bioluminescence measurement. Fixed covariates in the model included the groups in the experiment and time (weeks) and the interaction between group and time. The repeated measures aspect of the model, due to multiple measurements over time within each mouse, was adjusted for using a single order autoregressive correlation structure. Contrasts were used to test the pair-wise comparisons of interest. Analyses were completed using SAS (SAS Institute) with a type I error of 5%.
All other statistical analyses, including Kaplan-Meier analyses of metastasis-free mice, Student’s t-tests comparing two groups, and Mann-Whitney U tests of samples failing to distribute normally, were performed with GraphPad Prism.
RESULTS
Cyclophosphamide enhanced experimental prostate cancer skeletal metastasis in vivo
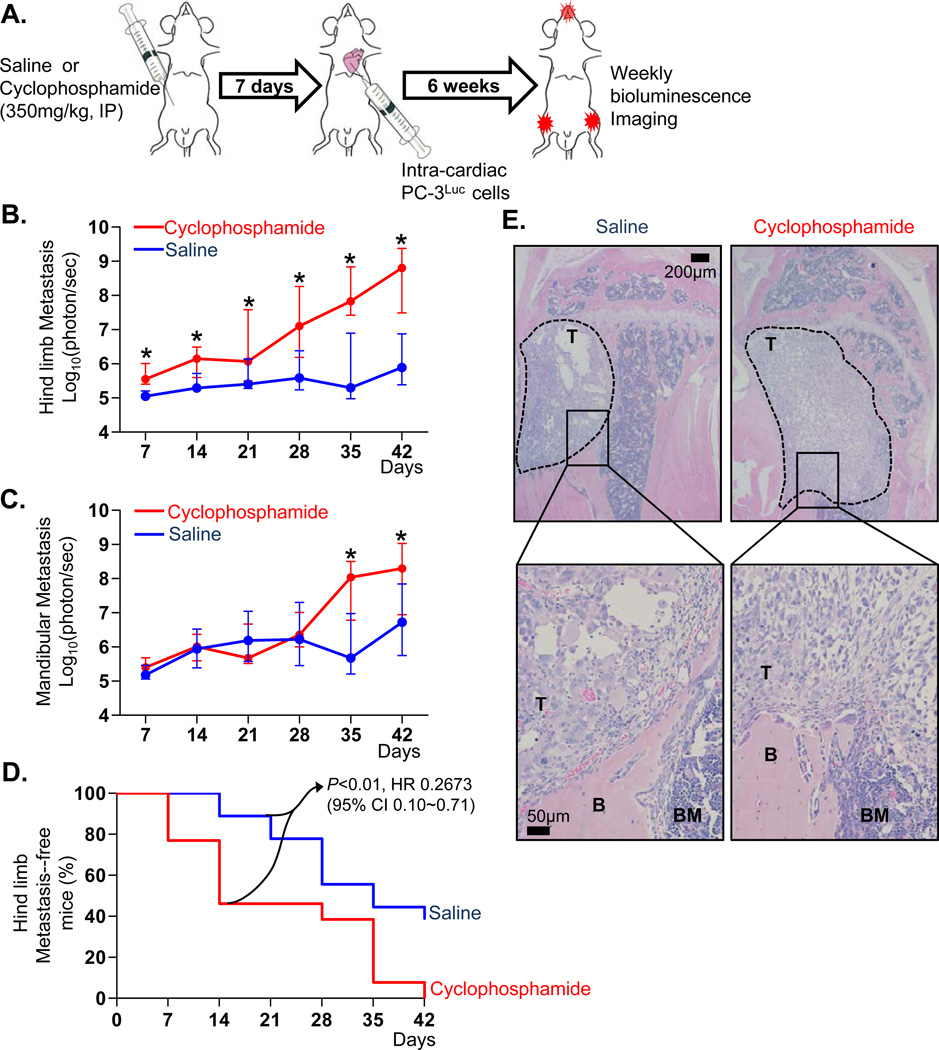
CY has been shown to promote subcutaneous tumor growth and experimental metastasis in various animal models (16–19). Initially, the effects of CY on prostate cancer skeletal metastasis were investigated. The experimental design is schematically shown in Figure 1A. The serum half-life of CY is less than 17minutes (in mice) and 6.5hours (in human), and mice were allowed 7 days of recovery to insure that the drug was completely cleared, in order to avoid any direct anti-tumor effects of CY (26, 27). Interestingly, mice primed with CY developed significantly larger tumors in the hindlimb bones after 7 days (Figure 1B). CY-treated mice exhibited increased tumor bioluminescence in the mandible also, but the effects were variable and not statistically significant until day-35 (Figure 1C). Because hindlimb skeletal metastases are more clinically relevant, and also murine mandibles are significantly different from human (e.g. continuous eruption of incisors), the hindlimb skeletal metastases were the focus of subsequent investigation. CY-primed mice developed hindlimb metastases at an earlier time point (i.e. increased incidence of metastases on day-7, 14 and 21; Figure 1D), compared to the saline-treated group that developed detectable hindlimb metastatic lesions only after 14 days. These data suggest that the larger tumor size on day-42 in the hindlimbs of CY-treated mice (Figure 1E) is attributable to the early events following tumor cell inoculation.
Figure 1. Priming mice with a single administration of CY (CY) enhanced experimental prostate cancer skeletal metastasis.
- Schematic representation of the experimental design. Male athymic mice were divided into two groups and treated with saline or CY. Following 7 days of recovery, PC-3Luc cells were injected into the left heart ventricle (n=18 for saline control and n=13 for CY group). Metastatic tumor growth was monitored by weekly in vivo bioluminescence imaging for 6 weeks.
- Hindlimb metastatic tumor size was measured by weekly in vivo bioluminescence imaging. Data are medians with interquartile range. Asterisks represent statistical significance (linear contrasts P<0.01).
- Mandibular metastatic tumor size was measured. Data are median ± interquartile range. Asterisks represent statistical significance (linear contrasts P<0.01).
- Percentage of hindlimb metastasis-free mice plotted in a Kaplan-Meier curve. Lesions emitting more than 1×105 photon/second were considered as metastases, and statistical significance determined by log-rank test (P<0.01). HR and CI stand for hazard ratio and confidence interval, respectively.
- Representative histological images of metastatic bone tumors. Tumor-bearing hindlimb tibiae were dissected, followed by H&E staining. The presence of metastatic tumor cells was confirmed microscopically. Tumor perimeter is indicated by dotted lines in lower magnification images (×4; upper panel). Higher magnification images (× 20; lower panel) show tumor, bone and bone marrow (denoted T, B and BM, respectively).
A single dose of CY significantly disrupted bone marrow vascular integrity
CY has been found to cause damage to endothelial cells, potentially promoting tumor cell seeding in the metastatic target organs (28). These data are consistent with the observation in Figure 1 showing outgrowth of metastatic tumors at earlier time points in the CY-treated hosts. Consequently, an experiment was designed to test whether a single dose of CY could perturb endothelial integrity in the bone marrow, which might in turn lead to increased extravasation of tumor cells immediately after inoculation. Because immunohistochemistry can only provide 2-dimensional images of selected cross-sections, a technique to reconstruct 3-dimensional vascular structures enclosed in calcified tissues was utilized (22) (Figure 2A). In Figure 2B, this technique clearly demonstrated 3-dimensional structures of microvessels in the epiphyses and the central sinusoidal vessels in the diaphyses of saline controls. In sharp contrast, a single dose of CY very obviously disrupted vascular integrity and continuity (Figure 2C). Quantification of the images in Figures 2B and C demonstrated that bone marrow vascular volume was significantly reduced by CY (Figure 2D). CD31 (an endothelial-specific marker) gene expression in bone was significantly suppressed with CY administration (Figure 2E), but not in lungs, liver and kidney (Supplemental Figure 1). In addition, bone marrow endothelial cells treated with 4-hydroperoxycyclophosphamide (a metabolite of CY with in vitro biological activity) had significantly increased apoptosis (Figure 2F). Taken together, CY-induced vascular disruption led to altered endothelial cells in the bone marrow.
Figure 2. A single administration of CY significantly disrupted bone marrow vascular integrity.
- Schematic representation of ex vivo murine bone marrow microvascular angiography. Male C57BL6/J mice were divided into two groups (n=13/group) and treated with saline or CY. Following 7 days of recovery, mice were perfused with liquid-phase radiopaque monomeric compound via the intra-cardiac route. After polymerization, femurs were dissected, decalcified and scanned with µCT.
- Five representative µCT images of ex vivo femoral angiography from the saline-treated group (n=13) are shown.
- Five representative µCT images of ex vivo femoral angiography from the CY-treated group (n=13) are shown.
- µCT data were analyzed to quantify the total vascular volume (per bone). Data are mean±SEM (P<0.05 by Mann Whitney U test).
- Femurs of saline- or CY-treated C57B6/J mice (n=10/group; the same dosage and schedule as described in A-C) were dissected, and bone marrow was harvested. The mRNA expression of CD31/PECAM endothelial cell marker was determined by quantitative RT-PCR. Data are mean±SD (P<0.01 by Student’s t-test).
- Human bone marrow endothelial cells were cultured and treated with low (3.5µM) and high (35µM) concentrations of 4-hydroperoxyCY, metabolite of CY with in vitro activity, for 24hours. The percentage of apoptotic cells was determined by flow cytometry (Annexin V+ and propidium iodide [PI]−). Data are mean±SEM (n=6/group; P<0.01 by Student’s t-test).
CY treatment did not cause systemic inflammation
We next ruled out the possibility that CY promoted metastasis by systemic inflammation secondary to the bone marrow suppression. CY-treated mice had significantly reduced body weight, compared to the saline control groups, and the effects lasted more than 2 weeks (Supplemental Figure 2A). However, CY-treated mice regained body weight with a similar trend to the saline-treated controls. In addition, CY-treated mice did not show any significant lethargy or signs of systemic inflammation, the latter often signaled by increased circulating levels of C-reactive protein (Supplemental Figure 2B).
Cyclophosphamide pre-treatment promoted orthotopic prostate tumor growth in bone
The potential role of disrupted bone marrow vascular integrity secondary to CY treatment in the increased metastatic tumor growth in the bone was further tested using an orthotopic approach (Figure 3A). This approach was designed to circumvent the effects of vascular disruption that could contribute to initial tumor cell seeding. PC-3 tumors grew larger after 6 weeks in the CY-treated bone marrow, compared to control (Figures 3B and C), suggesting that alterations in the CY-treated murine bone marrow, not a specific compromise of vascular integrity, were responsible for promoting tumor growth and/or metastasis.
Figure 3. CY pre-treatment directly promoted orthotopic PC-3 tumor growth in bone.
- Schematic representation of the experiment. Male athymic mice were divided into two groups (n=8/group) and treated with saline or CY. Following 7 days of recovery, PC-3Luc cells were injected into the bone marrow space of the right proximal tibiae. Tumor growth in bone was monitored by weekly in vivo bioluminescence imaging for 6 weeks.
- Intra-tibial tumor size was measured by weekly in vivo bioluminescence imaging (P<0.05 by Student’s t-test). Data are mean±SEM.
- Representative images of in vivo bioluminescence on day-42.
Cyclophosphamide transiently expanded myeloid lineage cells
Based on the observation in Figures 2 and 3, alterations induced by CY potentially contributing to tumor growth and/or metastasis were investigated. The changes of WBC differential counts were further investigated serially after CY administration. Total WBC counts were significantly reduced 3 to 15 days after CY, indicating that CY suppressed bone marrow, and that the effects lasted more than 2 weeks (Figure 4A). However, the WBC count was increased on day 7 compared to the day 3 CY group (Figure 4A). Furthermore, neutrophil number was below detection on day 3 but significantly spiked on day 7, immediately followed by suppression (Figure 4B). Additionally, monocyte counts showed a similar pattern to neutrophils (Figure 4C). Collectively, these data revealed that differentiated myeloid cells in the peripheral blood (i.e. monocytes and neutrophils) transiently increased during recovery from CY.
Figure 4. CY transiently expanded monocytes and neutrophils in the peripheral blood, and myeloid lineage cells in the bone marrow during the recovery phase after bone marrow suppression.
-
A. – C.Male C57BL6/J mice (n=8/group at each time point) were treated with saline (control) or CY, followed by total WBC (A), neutrophil (B), and monocyte (C) counting on 3, 7, 10 and 15 days after treatment. Data are mean ± SD. Asterisks indicate statistical significance (* P<0.01 and ** P<0.05 by Student’s t-test). Shade indicates standard range.
-
D.Male C57BL/6J mice (n=8 per group at each time point) were treated with saline (control) or CY followed by flushing bone marrow cells for flow cytometric analysis of myeloid lineage cell populations (expressing CD11b) on 3, 7, 10 and 15 days after treatment. Data are mean ± SD, and asterisks represent statistical significance (* P<0.01 and ** P<0.05 by Student’s t-test).
-
E. – G.CY treatment did not increase CD11b+ myeloid cells in solid organs. Male C57BL/6J mice (n=6/group) were treated with saline (control) or CY. After 7 days, kidney (E), lung (F) and liver (G) were surgically removed and digested for flow cytometric analyses of CD11b+ myeloid cells. Data are mean ± SD. N.S. indicates ‘not- significant’ by Student’s t-test.
-
H. – J.CY treatment increased myeloid-associated cytokines in serum. Male C57BL/6J mice (n=12 each) were treated with saline (control) or CY. After 7 days, serum cytokines including VEGF-A (H), IL-6 (I) and CCL2 (J) were measured by ELISA. Each dot represents an individual serum cytokine level, and bars represent median (P<0.01 by Mann Whitney U test).
Because both monocytes and neutrophils are differentiated from myeloid lineage cells in the bone marrow, the nature of the changes of myeloid lineage cells in the bone marrow was determined. Flow cytometric analyses of bone marrow cells from mice treated with CY after 3, 7, 10 and 15 days revealed that myeloid lineage cells (expressing CD11b) were significantly expanded 7 and 10 days after CY administration with suppression on days 3 and 15 (Figure 4D). In contrast, there was no change in the numbers of CD11b+ cells in other organs such as kidney, lung and liver (Figures 4E, F and G). We next determined the serum protein levels of VEGF-A, IL-6 and CCL2. All three molecules have angiogenic properties and also promote myeloid cell proliferation and differentiation (29–31). All three serum cytokines were significantly increased by CY treatment (Figures 4H, I and J).
CY-induced skeletal metastases overlap temporally with bone marrow myeloid cell expansion
In order to assess the temporal impact of CY on myeloid cell populations, the effects of tumor inoculation at various times after CY treatment were evaluated. PC-3Luc tumor cells were inoculated into the systemic circulation 3, 7 and 15 days after CY treatment (Figure 5A). The 7-day group had significantly more metastases, compared to the saline-treated control group, as observed previously. When tumor cells were injected at a later time point (i.e. 15 days after CY treatment), significantly fewer mice developed hindlimb metastatic lesions, suggesting that levels of bone marrow myeloid cell populations correlate with hindlimb metastases (Figure 5B and C). The 3-day group had a similar metastatic pattern as the 7-day group (Figure 5B) and increased tumor size compared to the 7-day group (Figure 5C), potentially because of prolonged survival of tumor cells in the systemic circulation overriding the expansion of bone marrow myeloid cells.
Figure 5. Recovery time after CY administration affected development of skeletal metastases.
- Schematic representation of the experiment. Male athymic mice were treated with saline or CY. Following 3, 7 or 15 days of recovery respectively, PC-3Luc tumor cells were inoculated via intra-cardiac injection. Development and subsequent growth of metastatic tumors were monitored by weekly in vivo bioluminescence imaging for 5 weeks.
- Percentage of metastasis-free mice was plotted in a Kaplan-Meier survival curve. The 7-day group had significantly increased hindlimb metastases compared to the saline-treated control group (P<0.01 by log-rank test, HR=0.07 with 95% CI of ratio=0.02~0.25). The 3-day group was similar to the 7-day group (P=0.67 by log-rank test, HR=0.78 with 95% CI=0.25~2.43), while the 15-day group had significantly slower development of metastases compared to the 7-day group (P<0.05 by log-rank test, HR=3.116 with 95% CI=1.1~8.9).
- Hindlimb metastatic tumor size was measured by weekly in vivo bioluminescence imaging. All CY-treated groups had significantly increased photon emission from the hindlimbs compared to the saline-treated mice (pair-wise linear contrasts P<0.01 at all time points). The 3-day group had significantly increased tumor burden compared to the 7-day group on week-5 imaging (pair-wise linear contrasts P<0.01), while the 15-day group had significantly reduced tumor burden compared to the 7-day group (pair-wise linear contrasts P<0.01). Data are median ± interquartile range.
Neutralizing host-derived murine CCL2, but not IL-6, inhibited CY-induced prostate cancer bone metastasis
The data described above collectively demonstrated that CY provided an environment conducive to experimental prostate cancer skeletal metastasis, potentially mediated by increase of serum cytokines and/or expansion of myeloid cells. The causal relationship of alterations induced by CY and tumor metastasis was determined using the intra-cardiac metastasis model in combination with neutralizing antibodies. Mice were treated with neutralizing antibodies targeting mouse IL-6 or mouse CCL2 during the 7 day recovery phase after CY treatment (Figure 6A). Consistent with the observation in Figure 1B, CY treatment significantly enhanced the development and subsequent growth of experimental bone metastasis (Figure 6B;). Neutralizing IL-6 did not prevent development of metastasis in CY-treated mice. However, neutralizing CCL2 significantly inhibited the CY-induced prostate cancer metastasis in vivo (statistical comparison shown in Figures 6C and D), indicating that the upregulation of CCL2 in response to CY contributed to the development and progression of metastasis. Moreover, administration of both antibodies against IL-6 and CCL2 had similar effects to the anti-CCL2 antibody alone group (Figures 6B–D). Importantly, neutralizing antibodies were administered before the tumor cell inoculation, in order to exclude the possibility of direct effects of the drug on the tumor cells. Therefore, the effects of neutralizing antibody were mainly due to the changes exerted on the host microenvironment. However, pre-clinical pharmacokinetic studies demonstrated that anti-CCL2 antibody can remain detectable in serum up to 10 days after administration, thus the possibility of direct effects may not be completely excluded (personal communication).
Figure 6. Neutralizing CCL2, but not IL-6, reverted CY-induced prostate cancer bone metastasis.
-
A.Schematic representation of the experiment. Male athymic mice were treated with saline (n=10) or CY in combination with control IgG (n=14; 10 mg/kg, i.p.), anti-mouse IL-6 (n=11; 20 mg/kg, i.p.), anti-mouse CCL2 (n=12; 10 mg/kg, i.p.), or a combination of anti-IL-6 and CCL2 antibodies (n=12). Three doses were given one day before CY treatment, and 3 and 6 days after CY treatment. On day-7 post-CY injection, PC-3Luc cells were injected into the left heart ventricle. Hindlimb metastatic tumors were monitored by weekly in vivo bioluminescence imaging for 6 weeks.
-
B.Serial images from five representative mice from each group are shown.
-
C. –D.Week 4 (C) and Week 6 (D) bioluminescence data were quantified and plotted. Tumor size was measured by photon/second from the hindlimb lesions in each group. Data are median ± interquartile range, and statistical significance was determined by Mann-Whitney U test.
An alternative chemotherapeutic drug, docetaxel, did not promote skeletal metastases
To further determine the causal role of CY-induced myeloid cell expansion to the development of skeletal metastasis, the effects of docetaxel, a chemotherapeutic agent commonly included in prostate cancer treatment regimens, were tested. In contrast to CY-mediated pro-metastatic effects, pre-treatment of mice with docetaxel decreased hindlimb skeletal metastasis (Figure 7B). In addition, CD11b+ cell enumeration in the docetaxel-treated bone marrow revealed similar but significantly blunted alterations in CD11b+ cells in comparison with the effects of CY (Figure 7C). Docetaxel-induced myeloid cell expansion (59.1±12.1%) at day-7 was not sufficient enough to increase myeloid cells (neutrophils and monocytes) in the peripheral blood (Figures 7D–F).
Figure 7. Docetaxel pre-treatment did not promote the development of hindlimb skeletal metastasis.
-
A.Schematic representation of the experiment. Male athymic mice were treated with saline or docetaxel. Following 7 days of recovery, PC-3Luc cells were injected into the left heart ventricle (n=10 for saline control and n=12 for docetaxel group). Hindlimb metastatic tumors were monitored by weekly in vivo bioluminescence imaging for 6 weeks.
-
B.Hindlimb metastatic tumor size was measured by weekly in vivo bioluminescence imaging. Data are medians with interquartile range. Asterisks represent statistical significance (linear contrasts P<0.01).
-
C.Docetaxel induced myeloid cell expansion similarly, but to a lesser extent than CY. Data are mean ± SD, and asterisks represent statistical significance (* indicates P<0.01 and ** indicates P<0.05 by Student’s t-test).
-
D. –F.Male C57BL6/J mice (n=10/group) were treated with saline or docetaxel (40 mg/kg, i.p.) followed by CBC with WBC differential counting 7 days after treatment. The numbers of WBC (D), neutrophils (E) and monocytes (F) are plotted. Data are mean ± SD. NS stands for ‘not-significant’ (P>0.05 by Student’s t-test).
DISCUSSION
Multiple mechanisms have been proposed to explain why bone provides a congenial metastatic microenvironment. For example, bone is enriched with cytokines and growth factors that promote tumor cell proliferation, migration, and survival (32). In addition, bone houses the primary hematopoietic organ (i.e. bone marrow), containing multiple types of progenitor cells and hematopoietic cells of various tumorigenic potential. Previously, Schneider et al. demonstrated that expansion of bone marrow cellularity before inoculation of prostate tumor cells significantly promoted skeletal metastasis (20), suggesting bones with increased cellularity constitute a more congenial microenvironment for metastasis. In this context, it is reasonable to expect that cytotoxic chemotherapy and/or irradiation may impact skeletal metastasis.
This study demonstrated for the first time that alterations induced by CY, a common chemotherapeutic drug, enhanced prostate cancer skeletal metastasis. Furthermore, we showed that the pro-metastatic effects of CY were significantly reversed by suppression of CCL2, which suggests the causal role of bone marrow myeloid lineage cell expansion. We demonstrated that a single dose of CY administration increased myelogenic cytokines, and correspondingly expanded the myeloid cell population in the bone marrow, as well as the numbers of monocytes and neutrophils transiently in the peripheral blood.
The unexpected “opposite” pro-tumorigenic effect of such a chemotherapeutic drug is not a novel observation in other non-skeletal sites. There have been several reports of chemotherapy-induced metastasis and/or tumor growth (18, 19, 33, 34). Most notably, Carmel and Brown demonstrated that pre-treatment of the host with CY, among many other chemotherapeutic drugs including actinomycin D, vinblastine, bleomycin, methotrexate and 5-fluorouracil, resulted in the most prominent pro-metastatic effects in a syngeneic sarcoma lung metastasis model (17). While most of the previous studies focused on an experimental pulmonary metastasis model, our data expanded the earlier observations by demonstrating the pro-metastatic effects of chemotherapy in a skeletal metastasis model (Figure 1 and Supplemental Figure 3). Data in the present study suggest that chemotherapeutic drugs with strong bone marrow suppression may have the adverse effect of promoting bone metastasis, a finding which has not been extensively investigated. CY is not a standard chemotherapeutic drug for prostate cancer patients, but recently low-dose metronomic administration of CY is in clinical trials as an anti-angiogenic therapy in prostate cancer (35, 36). In addition, CY is widely used for treatment of breast cancer, which also has a strong propensity for skeletal metastasis. Consequently, the effects of varying dosages and administration scheduling of CY on bone metastasis warrant extensive further studies.
The findings concerning the mechanisms involved in chemotherapy-enhanced metastasis have clinically therapeutic implications. We demonstrated that the numbers of bone marrow myeloid cells and myelomonocytic cells in the peripheral blood are significantly increased after CY administration, but not after docetaxel administration, potentially mediated by the increase of myelogenic cytokines. During the recovery phase after bone marrow suppression, spikes of monocytes and neutrophils are frequently observed in patients, and clinically considered a favorable prognostic sign (37). Data in the present study confirmed an abrupt increase of neutrophils and monocytes shortly after CY administration. Moreover, significant increases in CCL2, IL-6 and VEGF-A, all of which are potent myelogenic factors, were observed simultaneously or before the expansion of myelomonocytic cells, supporting the roles of these factors in the expansion of CD11b+ myeloid cells in the bone marrow. Results of this work confirmed that neutralizing CCL2, but not IL-6, significantly inhibited the pro-metastatic effects of CY . It should be noted that anti-CCL2 antibody is specific to the murine host-derived CCL2, and does not cross-react with prostate cancer-derived human CCL2, and that the neutralizing antibody was administered in only three dosages before tumor cell inoculation. Collectively, these data suggest that neutralizing CCL2 reconditions the pre-metastatic host microenvironment induced by chemotherapy.
Although the present data demonstrates the efficacy of anti-CCL2 antibody in the CY-induced prostate cancer bone metastasis model, increased expression of CCL2 (and subsequent expansion of myeloid cells) may not be the only mechanism of promoting metastasis after CY treatment. The first alternative explanation for the pro-metastatic effects of CY is that it could be mediated by the effects on bone cells. Given that inhibition of osteoclasts reversed the effects of GM-CSF on metastasis in a mouse model (38), it is possible that the effects of CCL2 neutralizing antibody in these results were, in part, mediated by inhibition of osteoclastogenesis. Secondly, while our results failed to confirm the causal role of CY-induced endothelial damage in metastasis, the possibility still remains for further investigation. CY is currently being tested for efficacy as anti-angiogenic therapy, and disruption of endothelial barrier function can promote extravasation of tumor cells in the metastatic microenvironment. Previously, Shirota and Tavassoli demonstrated that CY induces endothelial damage detectable by electron microscopy, and destroys the integrity of bone marrow sinus endothelium (indicated by red blood cells in the extra-vascular space), leading to enhanced engraftment of bone marrow transplantation (28). Therefore, CY effects on metastasis may be varied in different dosing schedules (i.e. metronomic low-dose) or different tumor models.
In conclusion, this study demonstrated that priming the murine host with CY altered the bone microenvironment, leading to promotion of prostate cancer bone metastasis. In addition, suppression of host CCL2 by antibody treatment significantly reduced the adverse effects of CY.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Rashesh Kapadia for µCT scanning, Evan Keller and Russell Taichman for discussions, and Chris Strayhorn for histology.
Financial support: This work was financially supported by the Department of Defense W81XWH-10-1-0546 (S.I.P.) and W81XWH-08-1-0037 (L.K.M.); the National Cancer Institute P01CA093900 (K.J.P. and L.K.M.) and P50CA69568 (K.J.P.); American Cancer Society Clinical Research Professorship (K.J.P.); and Janssen (L.K.M.)
Footnotes
Conflicts of interest: Linda Snyder and Jeffrey Nemeth are employed by Janssen, LLC. All other authors declare no financial conflict of interest.
REFERENCES
- 1.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 2.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 4.Park SI, Soki FN, McCauley LK. Roles of bone marrow cells in skeletal metastases: no longer bystanders. Cancer Microenviron. 2011;4:237–246. doi: 10.1007/s12307-011-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seandel M, Butler J, Lyden D, Rafii S. A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization. Cancer Cell. 2008;13:181–183. doi: 10.1016/j.ccr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A. 107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of Myeloid but not Endothelial Precursor Cells Facilitates Tumor Regrowth after Local Irradiation. Cancer Res. 2010;70:5679–5685. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn GO, Brown JM. Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives. Cell Cycle. 2009;8:970–976. doi: 10.4161/cc.8.7.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizutani K, Sud S, Pienta KJ. Prostate cancer promotes CD11b positive cells to differentiate into osteoclasts. J Cell Biochem. 2009;106:563–569. doi: 10.1002/jcb.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102:522–528. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47:1031–1039. [PubMed] [Google Scholar]
- 15.Abrams RA, Johnston-Early A, Kramer C, Minna JD, Cohen MH, Deisseroth AB. Amplification of circulating granulocyte-monocyte stem cell numbers following chemotherapy in patients with extensive small cell carcinoma of the lung. Cancer Res. 1981;41:35–41. [PubMed] [Google Scholar]
- 16.Vollmer TL, Conley FK. Effect of cyclophosphamide on survival of mice and incidence of metastatic tumor following intravenous and intracardial inoculation of tumor cells. Cancer Res. 1984;44:3902–3906. [PubMed] [Google Scholar]
- 17.Carmel RJ, Brown JM. The effect of cyclophosphamide and other drugs on the incidence of pulmonary metastases in mice. Cancer Res. 1977;37:145–151. [PubMed] [Google Scholar]
- 18.Wu YJ, Muldoon LL, Dickey DT, Lewin SJ, Varallyay CG, Neuwelt EA. Cyclophosphamide enhances human tumor growth in nude rat xenografted tumor models. Neoplasia. 2009;11:187–195. doi: 10.1593/neo.81352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi K, Yang M, Hayashi K, Jiang P, Yamamoto N, Tsuchiya H, et al. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 2008;68:516–520. doi: 10.1158/0008-5472.CAN-07-3063. [DOI] [PubMed] [Google Scholar]
- 20.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146:1727–1736. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 21.Park SI, Kim SJ, McCauley LK, Gallick GE. Pre-Clinical Mouse Models of Human Prostate Cancer and their Utility in Drug Discovery. Curr Protoc Pharmacol. 2011;51:145–527. doi: 10.1002/0471141755.ph1415s51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guldberg RE, Duvall CL, Peister A, Oest ME, Lin AS, Palmer AW, et al. 3D imaging of tissue integration with porous biomaterials. Biomaterials. 2008;29:3757–3761. doi: 10.1016/j.biomaterials.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, et al. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69:1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsui P, Das A, Whitaker B, Tornetta M, Stowell N, Kesavan P, et al. Generation, characterization and biological activity of CCL2 (MCP-1/JE) and CCL12 (MCP-5) specific antibodies. Hum Antibodies. 2007;16:117–125. [PubMed] [Google Scholar]
- 26.Bagley CM, Jr, Bostick FW, DeVita VT., Jr Clinical pharmacology of cyclophosphamide. Cancer Res. 1973;33:226–233. [PubMed] [Google Scholar]
- 27.Kline I, Gang M, Tyrer DD, Mantel N, Venditti JM, Goldin A. Duration of drug levels in mice as indicated by residual antileukemic efficacy. Chemotherapy. 1968;13:28–41. doi: 10.1159/000220528. [DOI] [PubMed] [Google Scholar]
- 28.Shirota T, Tavassoli M. Cyclophosphamide-induced alterations of bone marrow endothelium: implications in homing of marrow cells after transplantation. Exp Hematol. 1991;19:369–373. [PubMed] [Google Scholar]
- 29.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chantrain CF, Feron O, Marbaix E, Declerck YA. Bone marrow microenvironment and tumor progression. Cancer Microenviron. 2008;1:23–35. doi: 10.1007/s12307-008-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Man S, Zhang Y, Gao W, Yan L, Ma C. Cyclophosphamide promotes pulmonary metastasis on mouse lung adenocarcinoma. Clin Exp Metastasis. 2008;25:855–864. doi: 10.1007/s10585-008-9201-3. [DOI] [PubMed] [Google Scholar]
- 34.van Putten LM, Kram LK, van Dierendonck HH, Smink T, Fuzy M. Enhancement by drugs of metastatic lung nodule formation after intravenous tumour cell injection. Int J Cancer. 1975;15:588–595. doi: 10.1002/ijc.2910150408. [DOI] [PubMed] [Google Scholar]
- 35.Emmenegger U, Morton GC, Francia G, Shaked Y, Franco M, Weinerman A, et al. Low-dose metronomic daily cyclophosphamide and weekly tirapazamine: a well-tolerated combination regimen with enhanced efficacy that exploits tumor hypoxia. Cancer Res. 2006;66:1664–1674. doi: 10.1158/0008-5472.CAN-05-2598. [DOI] [PubMed] [Google Scholar]
- 36.Lord R, Nair S, Schache A, Spicer J, Somaihah N, Khoo V, et al. Low dose metronomic oral cyclophosphamide for hormone resistant prostate cancer: a phase II study. J Urol. 2007;177:2136–2140. doi: 10.1016/j.juro.2007.01.143. [DOI] [PubMed] [Google Scholar]
- 37.McClatchey KD, editor. Clinical Laboratory Medicine. Philadelphia, Pennsylvania, USA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 38.Dai J, Lu Y, Yu C, Keller JM, Mizokami A, Zhang J, et al. Reversal of Chemotherapy-Induced Leukopenia Using Granulocyte Macrophage Colony-Stimulating Factor Promotes Bone Metastasis That Can Be Blocked with Osteoclast Inhibitors. Cancer Res. 2010;70:5014–5023. doi: 10.1158/0008-5472.CAN-10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.