Abstract
Myocardial ischemia reperfusion injury contributes to adverse cardiovascular outcomes after myocardial ischemia, cardiac surgery or circulatory arrest. Primarily, no blood flow to the heart causes an imbalance between oxygen demand and supply, named ischemia (from the greek isch-, restriction and -haema, blood), resulting in damage or dysfunction of the cardiac tissue. Instinctively, early and fast restoration of blood flow has been established to be the treatment of choice to prevent further tissue injury. Indeed, the use of thrombolytic therapy or primary percutaneous coronary intervention is the most effective strategy for reducing the size of a myocardial infarct and improving the clinical outcome. Unfortunately, restoring blood flow to the ischemic myocardium, named reperfusion, can also induce injury. This phenomenon was therefore termed myocardial ischemia reperfusion injury. Subsequent studies in animal models of acute myocardial infarction suggest that myocardial ischemia reperfusion injury accounts for up to 50% of the final size of a myocardial infarct. Consequently many researchers aim to understand the underlying molecular mechanism of myocardial ischemia reperfusion injury to find therapeutic strategies ultimately reducing the final infarct size. Despite of the identification of numerous therapeutic strategies at the bench, many of them are just in the process of being translated to bedside. In the current review, we will discuss the most striking basic science findings made during the last decades that are currently under clinical evaluation, with the ultimate goal to treat patients who are suffering from myocardial ischemia and reperfusion associated tissue injury.
Introduction
In the United States alone, approximately one million people per year suffer from a myocardial infarction. Additionally 700 patients undergoing cardioplegic arrest for various cardiac surgeries. Despite the different etiologies that lead to the partial or complete arrest of cardiac circulation both patient groups share myocardial ischemia and reperfusion injury as a common pathophysiological feature.1
Myocardial ischemia and reperfusion injury (I/R) was first described by Jennings et al. 1960 utilizing a canine heart as a coronary ligation model.2 The authors of the study observed that reperfusion appeared to accelerate the development of necrosis. The histological changes found after only 30–60 minutes of I/R were comparable to the degree of necrosis normally seen after 24 hours of permanent coronary occlusion.
This initial observation kindled a discussion that lasted for decades whether reperfusion itself is responsible for tissue injury. It was not until the discovery of ischemic preconditioning that the independent effects of ischemia and reperfusion began to be unraveled from one another. Murry and Reimer reported in 1986 that repetitive short episodes of ischemia followed by a prolonged period of ischemia with reperfusion resulted in a significantly decreased infarct size.3 The term “Ischemic preconditioning” was coined and subsequently this phenomenon was confirmed in a number of animal models as well as in humans.4 It is now well accepted that ischemic preconditioning is an evolutionarily highly conserved mechanism. Subsequent experiments revealed that the reperfusion event is key to the initiation of molecular cascades that have the potential to mediate cardioprotection. Despite the substantial progress in understanding mechanisms of I/R based on models of acute MI, and the associated enthusiasm for translating these findings into patient care, results of clinical studies have been largely disappointing.1 However, many mechanisms identified at the bench, have not been extensively investigated yet and some larger clinical trials evaluating promising interventions from bench to bedside have just started.
Pathophysiologic Mechanisms of Myocardial I/R Injury
Fifteen-20 seconds after the occlusion of coronary vessels, anaerobic glycolysis supervenes as the only significant source of new high-energy phosphate. This is sufficient to meet at least the most basic energy demand of cardiomyocytes, however within 60 to 90 minutes of ischemia the affected area of the heart develops contracture-rigor.5 The critical role of anaerobic glycolysis in providing ATP in severe ischemia is dramatically illustrated in experiments in which anaerobic glycolysis was inhibited. As a consequence no glycolytic ATP is formed. In less than five minutes, the reserve supplies of energy phosphates are depleted totally and the heart undergoes contracture-rigor.5
On reperfusion, mitochondrial oxidative phosphorylation returns to pre-ischemic levels within seconds, however contractile power drags behind and only gradually reaches pre-ischemic values. This phenomenon is termed myocardial stunning.6,7 Stunned myocardium has relative excess oxygen consumption for a given rate of contractile work, and thus has a decreased mechanical efficiency. This may be in part due to a rapid recovery of the intracellular pH during reperfusion: During ischemia H+ accumulates intracellular as a consequence of anaerobic glycolysis. Once perfusion is restored, H+ is transported into the extracellular space in order to normalize the pH in exchange for Na+. The resultant increase in intracellular Na+ in turn activates the sarcolemmal 2Na+/Ca2+ exchanger, resulting in exchange of intracellular Na+ with extracellular Ca2+. A high rate of 2Na+/Ca2+ exchange can finally lead to Ca2+ overload and cell death (Figure 1 Ischemia Reperfusion Injury).8
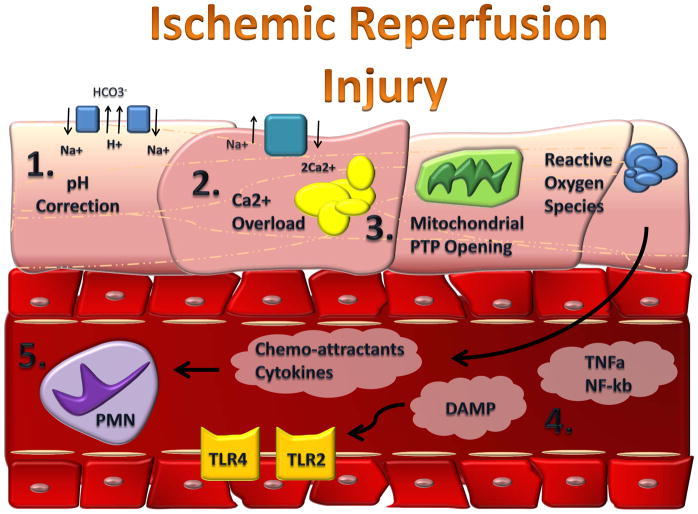
Figure 1. Mediators in Myocardial Reperfusion Injury.
During myocardial reperfusion the rapid restoration of physiological pH (1), Ca2+ overload (2) and further ATP-depletion through opening of the mitochondrial permeability transition pore, mPTP (3) leads to the generation of reactive oxygen species (ROS, 4). ROS is responsible for the release of pro inflammatory factors (TNF-α, NF-κb, TLRs and DAMPs, 5). The release of these chemoattractants leads to the invasion of neutrophils (6).
Studies in animals have demonstrated that during myocardial reperfusion there is an overshoot in the rate of fatty acid oxidation, impaired pyruvate oxidation and accelerated anaerobic glycolysis. High rates of fatty acid beta-oxidation dramatically inhibit glucose oxidation. This results in a marked imbalance between glycolysis and glucose oxidation. Pyruvate oxidation is likely further inhibited in the clinical situation by the high plasma free fatty acid concentration observed with acute myocardial infarction. This ‘uncoupling’ is a major source of the net H+ production in the heart. If glycolysis is coupled to glucose oxidation, H+ production from glycolysis is zero. However, if glycolysis is uncoupled from glucose oxidation, and pyruvate derived from glycolysis is converted to lactate, there is a net production of 2 H+ from each glucose molecule, which originates from the hydrolysis of glycolytically derived ATP.9–11
Based on these metabolic studies, the important role of the mitochondrial Permeability Transition Pore (PTP), a nonselective channel of the inner mitochondrial membrane became a critical determinant of lethal reperfusion injury.12 Mitochondrial permeability is characterized by permeabilization of an otherwise relatively impermeable mitochondrial inner membrane. During reperfusion, the fate of the cell is determined by the extent of mitochondrial permeabilization. If minimal, the cell may recover; if moderate, the cell may undergo programmed cell death; if severe, the cell may die from necrosis due to inadequate energy production. Opening the channel collapses the mitochondrial membrane potential and uncouples oxidative phosphorylation, resulting in ATP depletion and cell death. During myocardial ischemia, the mitochondrial PTP remains closed, only to open within the first few minutes after myocardial reperfusion in response to mitochondrial Ca2+ overload, oxidative stress, restoration of a physiologic pH, and ATP depletion. Therefore, the mitochondrial PTP represent an important new target for cardioprotection during reperfusion.13
The intracellular changes during ischemia and reperfusion, such as accumulation of H+ and Ca2+ as well as the disruption of mitochondrial membrane potential, lead to the formation of free radical or reactive oxygen species (ROS). ROS accumulation and the subsequent activation of pro-inflammatory pathways play an important role in ischemia reperfusion injury. Therefore a crucial mediator of I/R injury are oxygen derived free radicals, leading to different forms of oxygen species.14 Reactive oxygen intermediates cause direct damage to cellular DNA, protein, and lipids in addition to activating pathways of stress response. This nonspecific injury initiates a cytokine-mediated cascade, which results in the production of tumor necrosis factor alpha (TNFa).15 Excessive TNFa expression and subsequent cardiomyocyte TNF receptor type 1 stimulation induce contractile dysfunction, hypertrophy, fibrosis and cell death.16 Additionally, the increase in the intracellular Ca2+ concentration with generation of calcium pyrophosphate complexes and the formation of uric acid is a potent stimulator of inflammation. Calcium phosphate complexes and uric acid belong to a group of so called danger signals, and can bind to intracellular protein complexes called inflammasomes.17 The inflammasomes include different adaptor molecules that mediate an increase of the production and secretion of interleukin-1 (IL-1)β. Furthermore, Toll-like receptors are stimulated through danger signals eventually stimulating the secretion of further pro-inflammatory cytokines and chemokines through an activation of NF-κB.18
Finally, the release of chemoattractants draw neutrophils into the infarct zone during the first 6 hours of myocardial reperfusion, and during the next 24 hours they migrate into the myocardial tissue. This process is facilitated by cell-adhesion molecules. These neutrophils cause vascular plugging and release degradative enzymes and more reactive oxygen species.8
As outlined above, a key feature of hypoxia on tissue is the development of metabolic acidosis. As such, these metabolic changes have a direct impact on tissue inflammation, tissue integrity and therefore cell survival. Therefore it is not surprising that e.g trimetazidine, which acts by inhibiting a mitochondrial enzyme and shifts the preference for energy substrate away from fatty acid metabolism and toward glucose metabolism, reduces ischemia-reperfusion damage as well as tissue inflammation.19,20 This effect appears to be predominantly caused by a selective block of long-chain 3-ketoacyl-CoA thiolase activity, the last enzyme involved in β-oxidation. In fact, a meta-analysis of randomized controlled trials in heart failure revealed that trimetazidine had a significant protective effect for all-cause mortality and cardiovascular events and hospitalization.21 These recent data once again alerts us to the increasingly un-ignorable evidence for the benefit of metabolic modulation in myocardial disease and the urgency for a definitive clinical trial.22
On the other hand, one may question if anti-inflammatory drugs could also have a beneficial effect on heart metabolism during ischemia and reperfusion injury. In fact, it has been shown that IL-6 suppresses myocardial glucose metabolism via inhibition of AMP-activated protein kinase (AMPK).23 Since AMPK activation and glucose metabolism provide an important source of energy for the heart during ischemia and reperfusion,10 anti-inflammatory agents therefore bear the potential to positively influence cardiac metabolism (Figure 2 Ischemia-Metabolism-Inflammation). However, there is a need for future studies at the bench and the bedside to fully understand the interrelationship between inflammation and metabolism during cardiac ischemia and reperfusion injury.
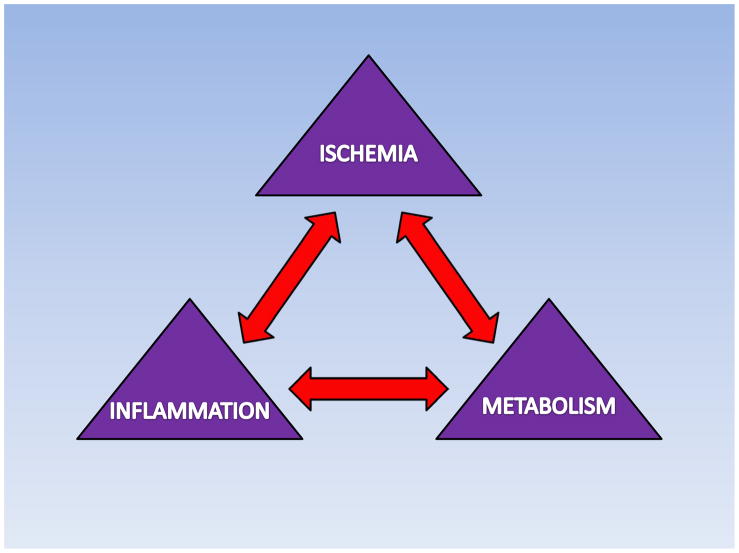
Figure 2. Relationship between ischemia, metabolism and inflammation.
Ischemia and Reperfusion leads to significant metabolic changes followed by inflammatory processes. Therapeutic interventions have demonstrated that metabolic modulators have a positive influence on metabolism and heart function with concomitant inhibition of inflammation.
Ischemic Preconditioning
Physicians managing patients with acute coronary syndrome, including severe unstable angina and acute myocardial infarction, occasionally used to report “the cardiac warm-up phenomenon”.24 This means that patients with at least one episode of prodromal angina showed less severe ischemic damage after subsequent exposure to a longer period of ischemia. In 1986, Murry et al. first documented this phenomenon experimentally through brief intermittent periods of myocardial ischemia, which significantly reduced the size of the infarct from a subsequent total occlusion.3 This observation was termed “ischemic preconditioning” and has been confirmed repeatedly in various species. For the first time it was shown that infarct size limitation was possible. The observed protective effect was indeed so powerful that this phenomenon has been described by several investigators as “the strongest form of in vivo protection against myocardial ischemic injury other than early reperfusion”.4
Although direct ischemic preconditioning does reduce reperfusion injury as well as its systemic consequences, its main disadvantage is direct stress to the target organ and mechanical trauma to major vascular structures, which have limited its clinical application. Remote ischemic preconditioning (RIPC) is a novel method where ischemia followed by reperfusion of one organ is believed to protect remote organs either due to release of biochemical messengers in the circulation or activation of nerve pathways, resulting in release of messengers that have a protective effect.25 RIPC was first demonstrated in the myocardium by McClanahan in 1993.25 He found that ischemia in the kidney followed by reperfusion protected myocardium from ischemia and reduced infarct size. In animal models brief ischemia reperfusion of the limb, gut, mesenteric, or kidney reduced myocardial infarct size. In humans skeletal preconditioning has been used for myocardial protection with the beneficial effect being attributed to regulation of endothelial protection.
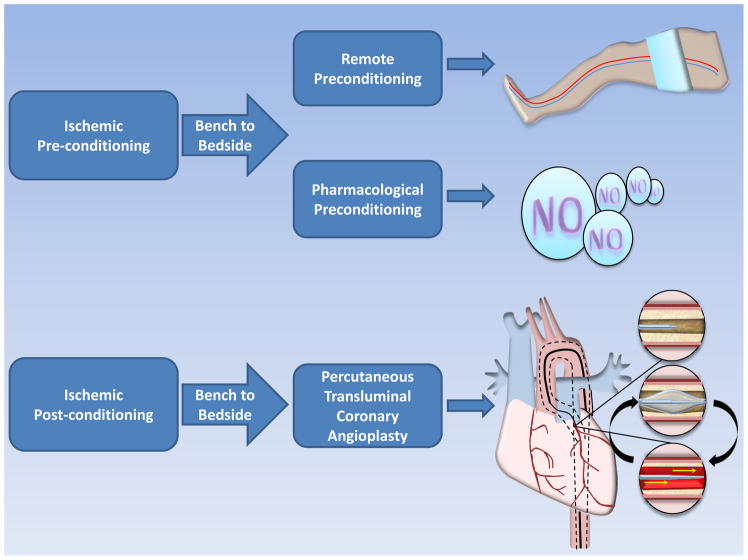
Since RIPC may be superior over IP it is not surprising that RIPC, one of the most striking mechanisms identified at the bench, is currently under intense clinical evaluation (Table 1, Clinical Trials). As such there are several ongoing studies on RIPC in different clinical settings.26 Although the underlying mechanisms and pathways need further clarification, the close relationship to classical preconditioning makes it a promising approach in a clinical setting (Figure 2, From Bench to Bedside).
Table 1.
Current clinical trials on myocardial reperfusion injury (ClinicalTrials.gov)
| Mechanism | Current clinical trials studies (Number) | Intervention | Example (largest ongoing clinical trials) |
|---|---|---|---|
| Preconditioning | 5 | Remote ischemic preconditioning (RIPC), Levosimendan, Nitrate | Remote Ischaemic Preconditioning for Heart Surgery (RIPHeart- Study) Number of enrolled patients: 2070 |
| Postconditioning | 9 | Remote ischemic postconditioning, Erythropoietin, Epoetin alpha | DANish Study of Optimal Acute Treatment of Patients With ST- elevation Myocardial Infarction (DANAMI-3) Number of enrolled patients: 2000 |
| Inflammation | 4 | Acetaminophen, Etanercept, Adenosine | Myocardial Protection With Adenosine During Primary Percutaneous Coronary Intervention in Pts With STEMI (PROMISE) Number of enrolled patients: 200 |
| Metabolism | 14 | Melatonin, Metformin, Dipyridamol, Atorvastatin, Aminoimidazol- Carboxamid-Ribonukleotid (AICAR), Glucose-insulin-potassium (GIK), 5- Seco-4-nor-cholestan-5-one oxime-3- ol (TRO40303), Exenatide, Vitamin C, Perhexellin | Melatonins Effect on Ischemia-reperfusion Injury Following Acute Myocardial Infarction Number of enrolled patients: 60 |
Nitric oxide (NO), a soluble gas continuously synthesized in endothelial cells by the eNOS regulates basal vascular tone and endothelial function, and maintains blood oxygenation via hypoxic pulmonary vasoconstriction. Many studies have implicated the endogenous production of NO, or its therapeutic application in ischemia and reperfusion. Administration of NO or NO donors prior to ischemia attenuates the consequences of myocardial ischemia/reperfusion injury and thereby reduces infarct size and endothelial dysfunction.27 These beneficial effects of NO are related to a pharmacological type of preconditioning.28 Thus, it is not surprising there are currently many clinical trials investigating the infusion of nitrates, which is non-enzymatically converted to nitric oxide in the setting of ischemia (Table 1, Clinical Trials).
Postconditioning
Even though classical preconditioning may work in a clinical setting such as heart surgery, it is not feasible in patients with acute MI because the coronary artery is already occluded at the time of hospital admission of the patient.
Zhao et al. was the first who described a phenomenon called “postconditioning” in a canine model.29 Where preconditioning is triggered by brief episodes of ischemia-reperfusion performed just before a prolonged coronary artery occlusion, postconditioning is induced by a comparable sequence of reversible ischemia-reperfusion but is applied just after the prolonged ischemic insult. Protection by postconditioning was shown to be as potent as that provided by preconditioning, whatever the species and experimental preparation. Unlike preconditioning, the experimental design of postconditioning theoretically allows direct application to the clinical settings, especially during percutaneous transluminal coronary angioplasty (PTCA). In this case, inflation and deflation of the angioplasty balloon after reopening of the coronary artery can mimic repetitive coronary artery clamping performed in postconditioning animal models (Figure 2, From Bench to Bedside). Several clinical studies already suggested that postconditioning by coronary angioplasty protects the human heart during acute myocardial infarction.30–34 Based on these promising findings with lower patient numbers, postconditioning is currently investigated in nine ongoing clinical studies. If the effect is real, the magnitude of the benefit will be remarkably large (35% relative reduction in infarct size) in an era in which it is increasingly difficult to demonstrate additional benefits of new therapies beyond current management due to relatively small infarct size and low mortality of patients with STEMI enrolled in clinical trials.35 However, to move forward, large multicenter clinical trials of cardiac and remote post-conditioning that have adequate statistical power to detect a reduction in clinical end points are needed.36 Hopefully, studies like the DANish Study of Optimal Acute Treatment of Patients With ST-elevation Myocardial Infarction (DANAMI-3) with 2000 anticipated patient enrollments, will fulfill such criteria (Table 1, Clinical Trials).
Inflammation
Myocardial ischemia and reperfusion leads to an inflammatory response that causes further damage to viable tissue around the infarct, likely through accelerated apoptosis. Acute and chronic immune responses elicited by myocardial ischemia have an important role in the functional deterioration of the heart.37 Initially, research on modulating the inflammatory response was focused on effector mediators such as leukocytes. However, increasing evidence indicates that various endogenous ligands that act as ‘danger signals’, also called danger-associated molecular patterns (DAMPs), are released upon injury and modulate inflammation. Originally described as part of the first-line defense against invading microorganisms, several Toll-like receptors (TLRs) on leukocytes and parenchymal cells have now been shown to respond to such signals and to have a pivotal role in non-infectious pathological cardiovascular conditions, such as ischemia-reperfusion injury and heart failure. From a therapeutic perspective, DAMPs are attractive targets owing to their specific induction after injury. 37
TLRs are implicated in myocardial I/R injury38, suggested by the use of the TLR4 antagonist, Eritoran in mice where it protects against this injurious process (Figure 4 Inflammation).39 This protective mechanism may be attributed to attenuated inflammation, such as decreased myeloperoxidase activity, leading to smaller infarcts compared to controls. Further studies have suggested that extracellular heat shock cognate protein 70 (HSC70)39, released by the myocardium during MI/R injury, plays a key role in the post-ischemic inflammatory response via TLR-4 signaling.40 TLR2 also plays a role in MI/R injury, likely related to its modulation of leukocyte activity, which mediates coronary endothelial dysfunction.41 However, TLR antagonists are still under clinical development and have to be tested in a clinical setting of myocardial ischemia and reperfusion injury.42
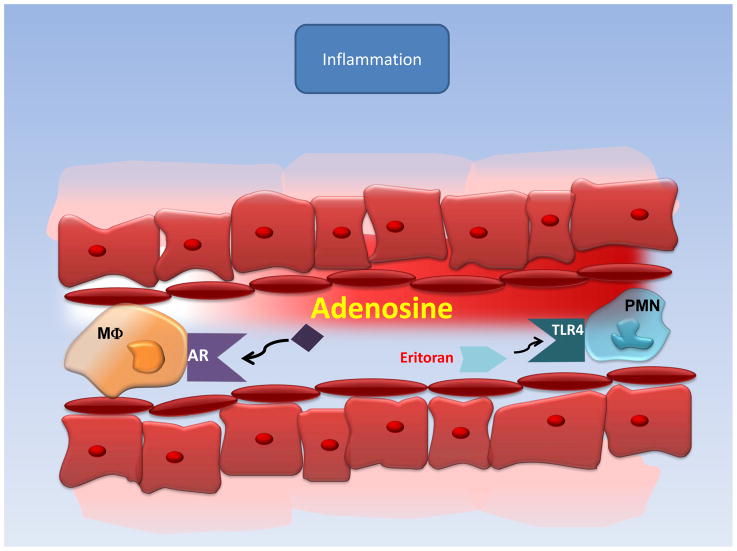
Figure 4. Anti-Inflammatory effects of Adenosine.
Adenosin receptor (ARs) or Toll-like receptor (TLR) activation protects the heart from ischemia-reperfusion injury by inactivating the ischemia-reperfusion-induced inflammatory response.
Even though substantial research efforts have been dedicated to identify agents modulating the inflammatory response after MI no promising data from clinical trials have been observed so far. As such a multicenter, double-blind, placebo-controlled, randomized clinical trial demonstrated that an antibody to CD11/CD18 leukocyte integrin receptor did not reduce infarct size in patients who underwent primary angioplasty.43 However, multiple studies have suggested that the very old drug ‘adenosine’ is critical for protection against inflammation mediated ischemia-reperfusion injury.44–47 This mechanism of protection by adenosine includes direct effects on organ parenchymal cells, vasodilatation of coronary arteries but also inhibition of leukocyte-mediated inflammatory response (Figure 4 Inflammation). The protective effect of extracellular adenosine is mediated through four adenosine receptors (ARs; Adora1, Adora2a, Adora2b and Adora3).48 All ARs have been associated with cardiac tissue protection in different settings. In particular the Adora2b receptor has been implicated in ischemic preconditioning49 and postconditioning.50 Although systemic adenosine infusion did not reduce overall mortality in the ‘famous’ AMISTAD-II trial, some effect on infarct size was noted.50 Based on these findings a multicenter, randomized, placebo-controlled double-blind study on the safety and efficacy of a brief intracoronary infusion of adenosine applied at the time of reperfusion to limit infarct size is currently being performed (Table 1, Clinical Trials). In addition, future studies using more specific adenosine receptor agonists during reperfusion may be necessary to fully elucidate the benefits of adenosine signaling for ischemia reperfusion injury.49
Metabolism
Cardiac fatty acid and glucose metabolism, specifically fatty acid β-oxidation and glucose oxidation, are highly regulated processes that meet the majority of myocardial energetic requirements. Cardiac ischemia and reperfusion is characterized by complex alterations in fatty acid and glucose oxidation that ultimately have a negative impact on cardiac efficiency and function. Pharmacologically shifting the balance between fatty acid β-oxidation and glucose oxidation by enhancing glucose oxidation at the expense of fatty acid oxidation can improve the efficiency of ATP generation and hydrolysis during reperfusion. Such alterations in energy substrate metabolism can limit the deficits in cardiac efficiency and function that occur during cardiac ischemia and reperfusion (Figure 5 Metabolism).10,11,51
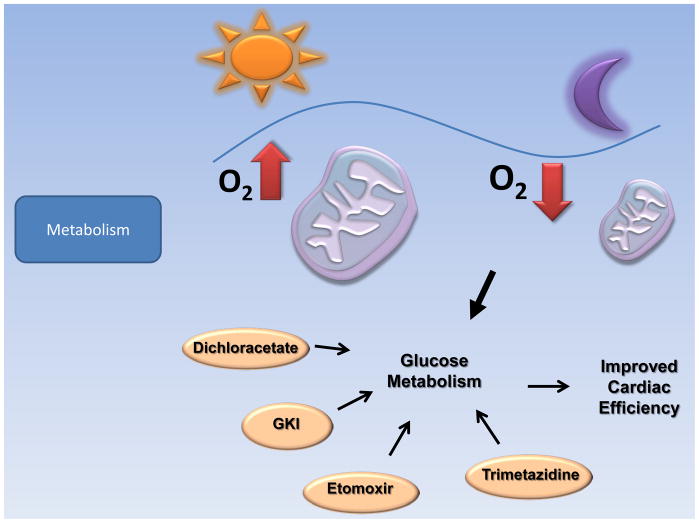
Figure 5. Metabolism as therapeutic target in I/R.
Light as a central regulator of circadian rhythmicities may be a novel therapeutic option in the treatment of patients suffering from myocardial ischemia by improving cardiomyocyte cell metabolism after myocardial ischemia and reperfusion. Modulators of metabolism like Dichloracetate, Glucose-Potassium-Insulin (GKI), Etoxomir and Trimetazidine are already used in a clinical setting and can improve cardiac function through shifting cell metabolism from fatty acid β-oxidation to preferred glucose oxidation.
As the rate-limiting enzyme mediating mitochondrial fatty acid uptake, CPTI (Carnitine palmitoyltransferase I) is an attractive target for inhibiting myocardial fatty acid β-oxidation. Etomoxir, an irreversible inhibitor of CPTI, has been shown to improve myocardial function, concomitant with an increase in glucose oxidation after global ischemia in rat hearts.52
Trimetazidine, a partial fatty acid β-oxidation inhibitor that competitively inhibits long chain 3-ketoacyl-CoA thiolase, is also able to increase glucose oxidation. Results from clinical studies have confirmed the effectiveness of trimetazidine as an anti-ischemic agent.53 Treatment of angina with trimetazidine has been shown to increase the time to 1-mm ST segment depression.54
Ranolazine, another partial fatty acid β-oxidation inhibitor, reciprocally increases glucose oxidation. Clinically, ranolazine has been approved in the United States for the treatment of chronic stable angina. Ranolazine monotherapy has been shown to increase exercise capacity and time to 1-mm ST segment depression, and to reduce the number of weekly angina attacks.55
Glucose–insulin–potassium (GIK) therapy has been shown to increase the rates of glycolysis and also decrease circulating concentrations of FFA.9 The shift toward glucose utilization decreases infarct size and improves post-ischemic cardiac function. A number of studies also demonstrate that GIK therapy is beneficial when administered at reperfusion.56 The Glucose–Insulin–Potassium Study demonstrates a reduction in mortality, although the benefit is limited to patients without heart failure. A more recent Dutch GIK study showed a potentially higher mortality in the GIK group.57,58 Based on these uncertainties about GIK, currently a clinical trial on the effect of Glucose-insulin-potassium Solution on Myocardial Protection During Off-pump Coronary Bypass Surgery is underway that will provide more answers to the grade of beneficial effects of this therapeutic approach.
Directly increasing myocardial glucose oxidation represents another approach to improve cardiac function. Dichloroacetate (DCA) stimulates the mitochondrial PDH complex via the inhibition of the activity of PDK. Improved coupling between glycolysis and glucose oxidation contributes to the mechanism(s) by which DCA exerts its cardioprotective effects.59 In a small clinical study, where DCA was given to patients with coronary artery disease via intravenous infusion, improvements in LV stroke volume was observed in the absence of changes in heart rate, left ventricular end diastolic pressure or myocardial oxygen consumption.60
As outlined earlier, with the onset of reperfusion after ischemia, the first pathophysiological event is represented by metabolic changes that can lead to cell death and a pro-inflammatory phenotype that further enhances tissue injury. Targeting metabolic events seems to be a promising strategyto reduce ischemia reperfusion injury. In fact, counting the number of ongoing clinical trials with the purpose to target metabolism reveals a total number of 14 studies (Table 1, Clinical Trials).
Another emerging approach to target metabolism is based on the observation that multiple extracardiac stimuli, such as workload and circulating nutrients (e.g., fatty acids), are known to influence myocardial metabolism and contractile function and exhibit marked circadian rhythms.61–67 Thus, it was found that there are circadian rhythmicities in myocardial oxidative and non-oxidative metabolism as well as responsiveness of the rat heart to changes in workload and fatty acid availability.67 Almost all living organisms have developed biological rhythms linked to the day/night or light/dark cycles of the sun. The impact that such rhythms exert on a variety of physiological functions in humans has been recognized for a long time. The internal oscillator, or control station regulating the body’s circadian clock, is the suprachiasmatic nucleus, located in the hypothalamus, above the optic chiasm. The suprachiasmatic nucleus processes external signals such as ambient light and inputs from the brain to regulate a variety of cyclic functions, including body temperature, sleep/wake cycles, and secretion of hormones such as cortisol, melatonin, thyroxin, and vasopressin. 68–72
What is clear is that mutations in the Clock gene affect metabolism in each genetic background tested, but in different ways.73 Disruption of another circadian protein, Bmal1, also shows metabolic abnormalities with impaired insulin responsiveness and reduced gluconeogenesis.74
Since external signals such as ambient light are central regulators of the circadian rhythm, the question arises if regulated daylight exposure of patients could alter metabolism. The use of daylight and the maintenance of a physiological day/night rhythm in a clinical setting could therefore represent a strategy which may improve cardiac metabolism after myocardial ischemia and reperfusion. Indeed a recent study using red light found acceleration of myocardial contractility recovery and reduction in the amount of molecular products of lipid peroxidation after ischemia.75 Future studies directed towards the understanding of the circadian rhythm in cardioprotection therefore bears the potential to uncover novel, powerful and easy applicable treatment options to prevent or ameliorate the consequences of myocardial ischemia and reperfusion injury in a perioperative setting or in the context of acute myocardial infarction.
Conclusion
Although translating findings from bench to bedside has been largely disappointing, many striking findings at the bench such as Ischemic Preconditioning, Postconditioning or Remote Preconditioning are just under clinical evaluation.
In addition, based on the new understanding of inflammation or metabolism during ischemia and reperfusion, the development of new drugs targeting structures like Toll like receptors or heart metabolism give hope to implement these strategies into a clinical setting.
Figure 3. From Bench to Bedside: Ischemic Preconditioning, Pharmacological Preconditioning and Ischemic Postconditioning in the Clinical Setting.
Remote preconditioning (RIPC) can be achieved by intermittent cuff inflation before heart surgery mimicking ischemic preconditioning, one of the most powerful cardioprotective mechanism found at the bench. Pharmacolgical preconditioning can be achieved by the administration of nitrates prior to surgery, where they are metabolized to NO under ischemic conditions. Ischemic postconditioning during percutaneous transluminal coronary angioplasty is induced by repetitive inflating and deflating of the catheter balloon with the onset of successful reperfusion.
Acknowledgments
The present research work is supported by National Heart, Lung, and Blood Institute Grant 1K08HL102267 to TE, the American Heart Association (AHA) Scientist Development (SD) Grant to T. E and a Deutsche Forschungsgemeinschaft (DFG) research fellowship to M. K.
References
- 1.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–8. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- 3.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation. 1998;97:1848–67. doi: 10.1161/01.cir.97.18.1848. [DOI] [PubMed] [Google Scholar]
- 5.Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu Rev Med. 1991;42:225–46. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- 6.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–67. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- 7.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–9. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 8.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 9.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–50. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Lopaschuk GD. AMP-activated protein kinase control of energy metabolism in the ischemic heart. Int J Obes (Lond) 2008;32 (Suppl 4):S29–35. doi: 10.1038/ijo.2008.120. [DOI] [PubMed] [Google Scholar]
- 11.Lopaschuk GD, Stanley WC. Malonyl-CoA decarboxylase inhibition as a novel approach to treat ischemic heart disease. Cardiovasc Drugs Ther. 2006;20:433–9. doi: 10.1007/s10557-006-0634-0. [DOI] [PubMed] [Google Scholar]
- 12.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151–4. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 13.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009;1787:1402–15. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Cadenas S, Aragones J, Landazuri MO. Mitochondrial reprogramming through cardiac oxygen sensors in ischaemic heart disease. Cardiovasc Res. 2010;88:219–28. doi: 10.1093/cvr/cvq256. [DOI] [PubMed] [Google Scholar]
- 15.Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, Harken AH. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–18. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Kleinbongard P, Schulz R, Heusch G. TNFalpha in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev. 2011;16:49–69. doi: 10.1007/s10741-010-9180-8. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 18.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8:292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 19.Fragasso G, Salerno A, Lattuada G, Cuko A, Calori G, Scollo A, Ragogna F, Arioli F, Bassanelli G, Spoladore R, Luzi L, Margonato A, Perseghin G. Effect of partial inhibition of fatty acid oxidation by trimetazidine on whole body energy metabolism in patients with chronic heart failure. Heart. 2011;97:1495–500. doi: 10.1136/hrt.2011.226332. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz JD, Chirkov YY, Kennedy JA, Sverdlov AL. Modulation of myocardial metabolism: an emerging therapeutic principle. Curr Opin Cardiol. 2010;25:329–34. doi: 10.1097/HCO.0b013e328339f191. [DOI] [PubMed] [Google Scholar]
- 21.Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97:278–86. doi: 10.1136/hrt.2010.208751. [DOI] [PubMed] [Google Scholar]
- 22.Ashrafian H, Neubauer S. Metabolic modulation in heart failure: high time for a definitive clinical trial. Heart. 2011;97:267–8. doi: 10.1136/hrt.2010.214932. [DOI] [PubMed] [Google Scholar]
- 23.Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, Kim JK. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–46. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomai F. Warm up phenomenon and preconditioning in clinical practice. Heart. 2002;87:99–100. doi: 10.1136/heart.87.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res. 2008;150:304–30. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 26.Bein B, Meybohm P. Organ protection by conditioning. Anasthesiol Intensivmed Notfallmed Schmerzther. 2010;45:254–61. doi: 10.1055/s-0030-1253094. quiz 262. [DOI] [PubMed] [Google Scholar]
- 27.Tocchetti CG, Stanley BA, Murray CI, Sivakumaran V, Donzelli S, Mancardi D, Pagliaro P, Gao WD, van Eyk J, Kass DA, Wink DA, Paolocci N. Playing with cardiac “redox switches”: the “HNO way” to modulate cardiac function. Antioxid Redox Signal. 2011;14:1687–98. doi: 10.1089/ars.2010.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha SS, Shiva S, Gladwin MT. Myocardial protection by nitrite: evidence that this reperfusion therapeutic will not be lost in translation. Trends Cardiovasc Med. 2008;18:163–72. doi: 10.1016/j.tcm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 30.Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF, Finet G, Andre-Fouet X, Macia JC, Raczka F, Rossi R, Itti R, Kirkorian G, Derumeaux G, Ovize M. Long-term benefit of postconditioning. Circulation. 2008;117:1037–44. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 31.Xue F, Yang X, Zhang B, Zhao C, Song J, Jiang T, Jiang W. Postconditioning the human heart in percutaneous coronary intervention. Clin Cardiol. 2010;33:439–44. doi: 10.1002/clc.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin ZX, Zhou JJ, Xin M, Peng DR, Wang XM, Bi SH, Wei XF, Yi DH. Postconditioning the human heart with adenosine in heart valve replacement surgery. Ann Thorac Surg. 2007;83:2066–72. doi: 10.1016/j.athoracsur.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Darling CE, Solari PB, Smith CS, Furman MI, Przyklenk K. ‘Postconditioning’ the human heart: multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic Res Cardiol. 2007;102:274–8. doi: 10.1007/s00395-007-0643-6. [DOI] [PubMed] [Google Scholar]
- 34.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L’Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–8. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 35.Prasad A. Post-conditioning for cardioprotection during reperfusion therapy: too good to be true? JACC Cardiovasc Interv. 2010;3:56–7. doi: 10.1016/j.jcin.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Lonborg J, Holmvang L, Kelbaek H, Vejlstrup N, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Treiman M, Jensen JS, Engstrom T. ST-Segment resolution and clinical outcome with ischemic postconditioning and comparison to magnetic resonance. Am Heart J. 2010;160:1085–91. doi: 10.1016/j.ahj.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Eltzschig HK, Eckle T. Ischemia and Reperfusion - From Mechanism to Translation. Nat Med. 2011 doi: 10.1038/nm.2507. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckle T, Eltzschig HK. Toll-like receptor signaling during myocardial ischemia. Anesthesiology. 2011;114:490–2. doi: 10.1097/ALN.0b013e31820a4d78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang RP, Yao Q, Xiao YB, Zhu SB, Yang L, Feng JM, Li DZ, Li XL, Wu JJ, Chen J. Toll-like receptor 4/nuclear factor-kappa B pathway is involved in myocardial injury in a rat chronic stress model. Stress. 2011;14:567–75. doi: 10.3109/10253890.2011.571729. [DOI] [PubMed] [Google Scholar]
- 40.Ao L, Zou N, Cleveland JC, Jr, Fullerton DA, Meng X. Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: mediating KC and MCP-1 expression induced by extracellular HSC70. Am J Physiol Heart Circ Physiol. 2009;297:H21–8. doi: 10.1152/ajpheart.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296:H1–12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kats S, Schonberger JP, Brands R, Seinen W, van Oeveren W. Endotoxin release in cardiac surgery with cardiopulmonary bypass: pathophysiology and possible therapeutic strategies. An update. Eur J Cardiothorac Surg. 2011;39:451–8. doi: 10.1016/j.ejcts.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Faxon DP, Gibbons RJ, Chronos NA, Gurbel PA, Sheehan F. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199–204. doi: 10.1016/s0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 44.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–15. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eltzschig HK. Targeting Hypoxia-induced Inflammation. Anesthesiology. 2011;114:239–42. doi: 10.1097/ALN.0b013e3182070c66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 48.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 50.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–14. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–5. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- 52.Lopaschuk GD, McNeil GF, McVeigh JJ. Glucose oxidation is stimulated in reperfused ischemic hearts with the carnitine palmitoyltransferase 1 inhibitor, Etomoxir. Mol Cell Biochem. 1989;88:175–9. doi: 10.1007/BF00223440. [DOI] [PubMed] [Google Scholar]
- 53.Rosano GM, Vitale C, Sposato B, Mercuro G, Fini M. Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: a double-blind placebo-controlled study. Cardiovasc Diabetol. 2003;2:16. doi: 10.1186/1475-2840-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grabczewska Z, Bialoszynski T, Szymanski P, Sukiennik A, Swiatkiewicz I, Kozinski M, Kochman W, Grzesk G, Kubica J. The effect of trimetazidine added to maximal anti-ischemic therapy in patients with advanced coronary artery disease. Cardiol J. 2008;15:344–50. [PubMed] [Google Scholar]
- 55.Chaitman BR. Efficacy and safety of a metabolic modulator drug in chronic stable angina: review of evidence from clinical trials. J Cardiovasc Pharmacol Ther. 2004;9 (Suppl 1):S47–64. doi: 10.1177/107424840400900105. [DOI] [PubMed] [Google Scholar]
- 56.Turel B, Gemici K, Baran I, Yesilbursa D, Gullulu S, Aydinlar A, Serdar A, Kazazoglu AR, Kumbay E, Cordan J. Effects of glucose-insulin-potassium solution added to reperfusion treatment in acute myocardial infarction. Anadolu Kardiyol Derg. 2005;5:90–4. [PubMed] [Google Scholar]
- 57.Kloner RA, Nesto RW. Glucose-insulin-potassium for acute myocardial infarction: continuing controversy over cardioprotection. Circulation. 2008;117:2523–33. doi: 10.1161/CIRCULATIONAHA.107.697979. [DOI] [PubMed] [Google Scholar]
- 58.Apstein CS, Opie LH. A challenge to the metabolic approach to myocardial ischaemia. Eur Heart J. 2005;26:956–9. doi: 10.1093/eurheartj/ehi200. [DOI] [PubMed] [Google Scholar]
- 59.Wang P, Lloyd SG, Chatham JC. Impact of high glucose/high insulin and dichloroacetate treatment on carbohydrate oxidation and functional recovery after low-flow ischemia and reperfusion in the isolated perfused rat heart. Circulation. 2005;111:2066–72. doi: 10.1161/01.CIR.0000162466.06150.D4. [DOI] [PubMed] [Google Scholar]
- 60.Cesar LA, Gowdak LH, Mansur AP. The metabolic treatment of patients with coronary artery disease: effects on quality of life and effort angina. Curr Pharm Des. 2009;15:841–9. doi: 10.2174/138161209787582075. [DOI] [PubMed] [Google Scholar]
- 61.Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res. 2010;106:647–58. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durgan DJ, Young ME. Linking the cardiomyocyte circadian clock to myocardial metabolism. Cardiovasc Drugs Ther. 2008;22:115–24. doi: 10.1007/s10557-008-6086-y. [DOI] [PubMed] [Google Scholar]
- 63.Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int. 2011;28:187–203. doi: 10.3109/07420528.2010.550406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–69. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 65.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–50. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, Shaw CA, Bray MS, Hardin PE, Young ME. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530–41. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- 67.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–47. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 68.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–56. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardin PE, Krishnan B, Houl JH, Zheng H, Ng FS, Dryer SE, Glossop NR. Central and peripheral circadian oscillators in Drosophila. Novartis Found Symp. 2003;253:140–50. discussion 150–60. [PubMed] [Google Scholar]
- 70.Hardin PE. Essential and expendable features of the circadian timekeeping mechanism. Curr Opin Neurobiol. 2006;16:686–92. doi: 10.1016/j.conb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Hardin PE. The circadian timekeeping system of Drosophila. Curr Biol. 2005;15:R714–22. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 72.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dufour CR, Levasseur MP, Pham NH, Eichner LJ, Wilson BJ, Charest-Marcotte A, Duguay D, Poirier-Heon JF, Cermakian N, Giguere V. Genomic convergence among ERRalpha, PROX1, and BMAL1 in the control of metabolic clock outputs. PLoS Genet. 2011;7:e1002143. doi: 10.1371/journal.pgen.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monich V, Drugova O, Lazukin V, Bavrina A. Low-power light and isolated rat hearts after ischemia of myocardium. J Photochem Photobiol B. 2011;105:21–4. doi: 10.1016/j.jphotobiol.2011.06.006. [DOI] [PubMed] [Google Scholar]