Abstract
Human autoimmune disease involves local activation of antigen-specific CD4+ T cells that produce inflammatory Th1 cytokines leading to the further recruitment and activation of lymphocytes and monocytes, resulting ultimately in the destruction of target tissue. Antigen presenting cells (APCs) initiate activation of CD4+ T cells in a multistep process that minimally involves co-ligation of the TCR and CD4 by the MHC class II/peptide complex and costimulation through additional T cell surface molecules such as CD28. Disruption of this highly orchestrated series of events can result in the direct modulation of CD4+ T cell behavior. The interaction between MHC and TCR holds unique promise as a focal point for therapeutic intervention in the pathology of CD4+ T cell-mediated diseases, and MHC class II-derived Recombinant TCR Ligands (“RTLs”) have emerged as a new class of therapeutics with potent clinical efficacy in a diverse set of animal models for multiple sclerosis. Here I review the systemic effect that RTL therapy has on the intact immune system and present an overview of a molecular mechanism by which RTL therapy could induce these systemic changes.
Keywords: Recombinant TCR ligand, EAE, T lymphocytes, Immunoregulation, MHC/peptides, multiple sclerosis, T cell receptor
BACKGROUND
CD4+ T cells and autoimmune disease
The definitive classification of a disease as “autoimmunity” has traditionally been based on the detection of autoantibodies reacting with an affected tissue or cell [1]. Brute-force molecular and biochemical methods coupled with computational algorithms based on structural considerations of peptide binding to MHC molecules provided an exponential increase in the number of self-antigens documented to be associated with specific autoimmune diseases [1], and inflammatory antigen-specific CD4+ T cells have taken center stage in the pathogenesis of a variety of human autoimmune diseases [2–13]. Disease progression appears to be mediated by CD4+ T cells homing to the target tissue where autoantigen is present and selectively producing T-helper type 1 lymphokines [14]. This cascade of events leads to the recruitment and activation of lymphocytes and monocytes that ultimately destroy the target tissue [15].
Multiple sclerosis (MS) for example, is a demyelinating disease characterized by chronic inflammation directed against myelin antigens in the central nervous system (CNS) [16]. Autoreactive CD4+ T cell responses to myelin basic protein (MBP), proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG), and 2’3’-cyclic nucleotide 3-phosphodiesterase (CNPase) have been clearly documented, and reactivity to some or all of these target antigens probably plays a major role in MS pathology [17–28]. Modulation or elimination of specific T cell responses, “tolerance,” forms the basis of a generalizable and rational approach toward therapeutic intervention in MS and other CD4+ T cell mediated diseases.
The idea of selective induction of Ag-specific T cell tolerance has deep roots. In the late 1960’s Mitchison documented that gross overstimulation of T cells with large doses of soluble Ag induced “immunological paralysis” [29]. This Ag-specific tolerance, also termed high dose suppression or high zone tolerance, involves extrathymic mechanisms [30], one in particular being a now clearly delineated program within mature T cells that results in activation induced cell death (AICD) [31]. The practical clinical utility of high dose suppression is limited because it carries with it the risk of exacerbating the ongoing disease process. Smaller doses of Ag have been employed successfully to control autoimmunity in animal models by coupling the Ag-peptide to a larger carrier protein such as a mAb [32–34]. This approach greatly reduced but did not completely ablate the inflammatory T cell response [32]. A different approach using inhibitory “altered peptide ligands” (APLs) appeared to hold promise, proving efficacious for therapeutic intervention in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), by blocking T cell activation after requisite processing and presentation by APCs. Multiple mechanisms of action have been proposed including alteration of the pathogenic T cell population at the level of the TCR via partial agonism or antagonism followed by T cell anergy [35–37], and the induction of immunoregulatory T cells specific for the APLs that produced anti-inflammatory cytokines, i.e. 'bystander' suppression [38]. The latter appeared to be the mechanism by which APLs worked on humans in vivo [39]. Unfortunately, the first human clinical trials using APLs documented that the administration of APLs activated the disease-mediating T cells rather than suppressing them [39, 40]. Despite the disappointing clinical results, the data documented the validity of myelin specific T cells (MBP-85–99-specific) as a target for antigen-specific immunomodulation in defined patient populations with MS [39]. The work also pointed out the hazards associated with trying to extrapolate animal model data toward human therapeutic intervention [39, 40].
As our understanding of the molecular processes involved in T cell activation expand, we are presented with another opportunity for antigen-specific clinical disease intervention. In simple terms, activation of CD4+ T cells is a multi-step process initiated by co-ligation of the TCR and CD4 by the MHC class II/peptide complex present on APC (signal 1), as well as co-stimulation through additional T cell surface molecules such as CD28 (signal 2). Stimulation through the TCR by MHC class II/peptide in the absence of co-stimulation, rather than being a neutral event, induces a state of unresponsiveness, "anergy", to subsequent optimal antigen presentation [41, 42]. As this field of study has matured it has grown far more complex, providing as many new questions as answers. Stimulation through the TCR by MHC class II/peptide in the absence of costimulation can generate a range of responses, from anergy to apoptosis [43–45]. These responses depend on a variety of influences, including the affinity of the MHC/peptide complex for the TCR [46–49]. Apparently the adaptive immune system has developed mechanisms for controlling the expansion as well as the behaviour of CD4+ T cells by regulating the context in which presentation of signal 1 (antigen plus MHC) occurs. Regulation could potentially occur on a number of different levels, including cellular control in the form of self MHC class II presentation [50].
Thus, all hope is not lost for Ag-specific immunotherapy, but refocused. A clinically useful approach toward antigen-driven immunosuppression would be to present the complete ligand, antigen plus MHC, in the absence of co-stimulatory signals that are normally provided by APCs, using the MHC/peptide interaction with the TCR as a starting point for controlling antigen-directed T cell activation and pathogenesis.
Structure of the MHC class II Molecule and Design of RTLs
Class II molecules are membrane-bound glycoproteins made up of non-covalently associated α- and β-polypeptide subunits, each of which consists of a short cytoplasmic tail, a single membrane-spanning sequence, and two extracellular domains. X-ray crystallographic studies have demonstrated that peptides from processed antigen bind to MHC molecules in the membrane distal pocket formed by the β1 and α1 domains [51, 52]. Class II molecules co-ligate CD4 through the β2 domain [53, 54] and perhaps through a secondary site on the β1 domain [55], with co-ligation of CD4 apparently serving the role of broadening the TCR repertoire by potentiating productive TCR signaling and clonal expansion in response to the engagement of low-affinity antigenic ligands [56].
The idea of developing soluble class II molecules has taken a variety of forms. MHC class II molecules shed by APCs as part of a vesicle termed the “exosome” are being exploited for potential clinical utility [57–61]. Soluble recombinant MHC class II molecules in various forms with and without covalently tethered Ag peptide have been developed, including detergent solubilized full-length MHC class II [43, 62], extracellular α1α2/β1β2 heterodimeric class II domains with and without covalently tethered Ag peptide [63–65], Fos/Jun leucine-zipper stabilized class II α1α2/β1β2 class II (α1α2/β1β2)2-IgG dimers [66, 67]. Work in my laboratory has taken a reductionist approach, focused on engineering the smallest possible molecule that retains the peptide binding/TCR recognition features of the class II molecule. It appears we have achieved that goal in the form of single chain class II-derived Recombinant TCR Ligands (RTLs) consisting of the α1 and β1 domains of MHC class II molecules genetically linked into a single polypeptide chain [68–71]. The design of the RTLs was based on extensive molecular modeling studies of MHC class II proteins including crystal structures of human DR [72–76] and murine I-Ak with covalently bound single peptides [77], and modeling studies predicted that the antigen binding domain would remain stable in the absence of the α2 and β2 Ig-fold domains [69, 71, 78].
To date, genes encoding MHC class II-derived RTLs have been constructed from Lewis (LEW) rat RT1.B, murine I-Au and I-As, and human HLA-DR, HLA-DP and HLA-DQ. In all three species the design involved re-engineering domains of MHC class II, genetically coupling the amino terminus of the alpha-1-domain to the carboxyl terminus of the beta-1-domain. Along with "empty" RTLs that can be loaded exogenously with synthetic peptides, we have produced a variety of genetically-encoded variants with antigenic peptide attached by a linker to the N-terminus of the beta-chain (Table I) [64, 69, 79, 80]. RTL molecules have been used for studying binding specificity in vitro [71, 81, 82], for exploring primary TCR signaling events independent of co-stimulatory input associated with the MHC II α2 and β2 domains or with other molecules expressed by antigen presenting cells [83], and for treating CD4+ T cell-mediated autoimmune disease in an MHC II/epitope-specific manner [68, 70, 80, 83, 84].
Table I.
RTLs (Recombinant TCR Ligands)
| RTL | Description | RTL | Description |
|---|---|---|---|
| RAT | HUMAN | ||
| 100 | RT1.B β1α1 (empty) | 300 | DR2 β1α1 (F150L) [DRA*0101,DRB1*1501] |
| 101 | RT1.B β1α1 (Q12R) (empty) | 301 | DR2 β1α1 w/covalent hu-MBP-85-99 (F120L) |
| 103 | 101-A488 | 302 | 300 (empty) (wild-type) |
| 200 | 100 w/ covalent rat-MBP-72-89 | 303 | 302 w/covalent hu-MBP-85-99 |
| 201 | 100 w/ covalent Gp-MBP-72-89 | 304 | 300 w/ carboxy-terminal biotinylation sequence |
| 305 | 301 w/ carboxy-terminal biotinylation sequence | ||
| 202 | 100 w/ covalent rat-MBP-55-69 | 306 | 302 w/ carboxy-terminal biotinylation sequence |
| 203 | 100 w/ covalent rat-CM-2 | 307 | 303 w/ carboxy-terminal biotinylation sequence |
| 204 | 200 w/carboxyl his-tag | 308 | 300 w/ covalent Gp-MBP-72-89 |
| 205 | 201 w/carboxyl his-tag | 309 | 300 w/ covalent Rt-MBP-72-89 |
| 206 | 202 w/carboxyl his-tag | 310 | 302 w/ covalent Gp-MBP-72-89 |
| 207 | 201 w/carboxyl his-tag | 311 | 302 w/ covalent BCR-ABL peptide |
| 209 | 200-A488 chromophore | 312 | 302 w/ covalent mu-MOG-35-55 |
| 210 | 201-A488 chromophore | 313 | 302 w/ covalent hu-MOG-35-55 |
| 211 | 202-A488 chromophore | 314 | 302 w/ covalent hu-αβ-crystallin peptide |
| 212 | 203-A488 chromophore | 320 | 303 w/ (5 β-sheet Ser mutations) monomer |
| 213 | 201 w/ C49A, C111A | 322 | 312 w/ (5 β-sheet Asp mutations) monomer |
| 214 | 201 w/ C49S, C111S | ||
| 215 | 201 w/ C49A, C111S | 340 | 303 w/ (5 β-sheet Asp mutations) monomer |
| 216 | 201 w/ C49S, C111A | 342 | 312 w/ (5 β-sheet Asp mutations) monomer |
| 217 | 101 w/ carboxy-terminal biotinylation sequence | 360 | 303 w/CD4-binding site mutations |
| 218 | 201 w/ carboxy-terminal biotinylation sequence | ||
| 600 | DP2 β1α1 [DPA1*0103,DPB1*0201] | ||
| MURINE | 601 | 600 w/ E79K | |
| 400 | Murine (SJL) β1α1 (I-AS) | ||
| 401 | 400 w/ PLP-139-151 peptide | 700 | DP4 β1α1 [DPA1*0103,DPB1*0401] |
| 402 | 400 w/ carboxy-terminal biotinylation sequence | ||
| 800 | DQ2 β1α1 [DQA1*05/DQB1*02] | ||
| 500 | Murine (B10.PL) β1α1 (I-AU) | ||
| 501 | 500 w/ MBP-1-11 peptide | 900 | DQ8 β1α1 [DQA1*03/DQB1*0302] |
| 502 | 500 w/ carboxy-terminal biotinylation sequence |
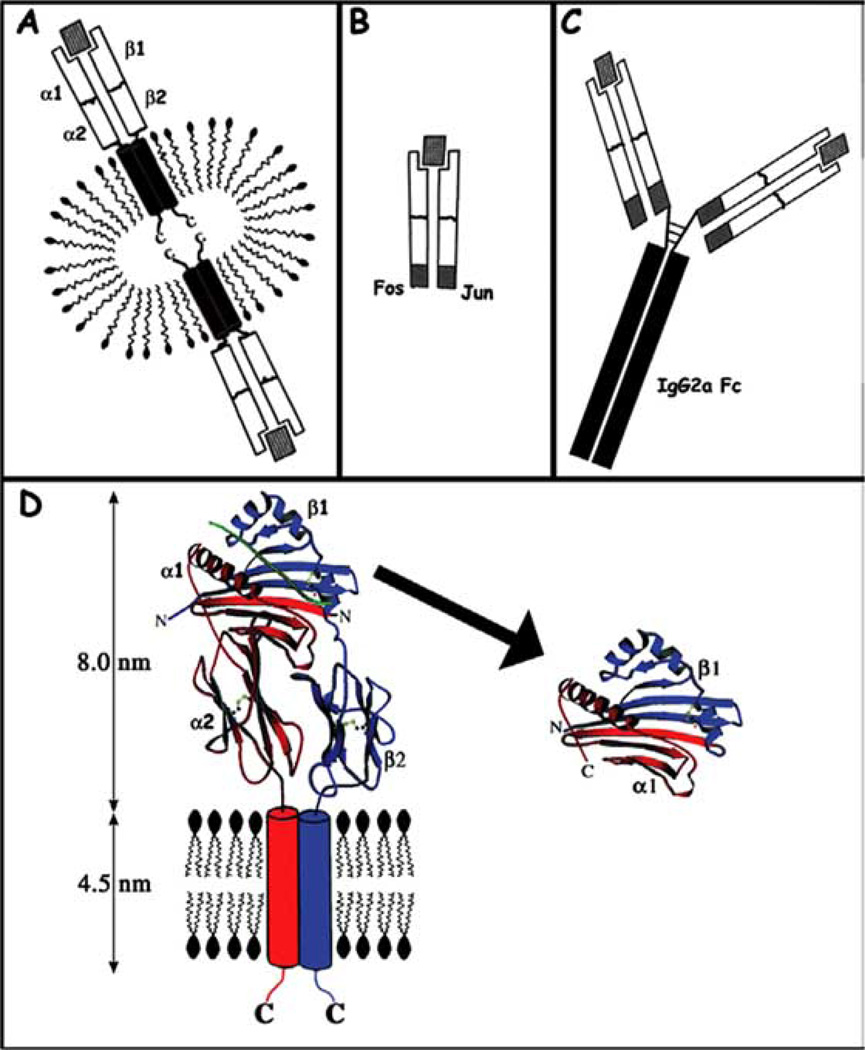
The mechanism by which autoreactive T cells can be specifically activated is fundamental to our understanding of how autoimmunity may be triggered. Strikingly different clinical effects are observed as a result of treatment with the various soluble class II constructs, compared schematically in (Fig. 1). The most obvious difference between the RTL constructs and other soluble class II constructs is the shear difference in size between the molecules. The effects that have been documented on T cells in vitro and in vivo and for these different constructs include anergy induction [67], antigen-specific apoptosis [43, 85], a combination of clonal anergy induction and upregulation of anti-inflammatory cytokines [86] and cytokine-switching [70, 84]. These various class II-derived therapeutics have yet to be tested head-to-head under identical clinical conditions in the same animal models. Thus, it is premature to predict why the clinical effects vary, as differences in the animal models may play a role. We have compared RTL therapy in a variety of animal models and different clinical mechanisms do appear to be involved, with some basic general mechanisms emerging as the key to the clinical efficacy of these compounds. In this review I present an overview of the clinical outcome of RTL therapy in two rodent models of EAE, including the systemic effect RTL treatment has on the target organ and periphery. The review is concluded with an overview of the signal transduction events that occur within T cells following RTL binding in the absence of co-stimulatory events between T cell and APC, deduced by mapping the information cascade from TCR to transcriptional regulation of the IL-2 gene.
Fig. (1). Comparison of soluble MHC class II constructs.
(A) Detergent solubilized class II. (B) Extracellular domains of class II stabilized using a Fos/Jun leucine zipper. (C) MHC class II dimers created by replacing Fv region of IgG2a with beta chain of class II. Please note that structures depicted in A, B, and C are not to scale. (D) Rational design of RTLs. Left: A ribbon diagram of the MHC class II molecule HLA-DR2 on the surface of an APC. Right: RTL302, the soluble single-chain molecule derived from the antigen-binding/T cell recognition domains. The structures are based on the crystallographic coordinates of HLA-DR2 (PDB accession code 1BX2), and the transmembrane domains are shown schematically as 0.5 nm cylinders, roughly the diameter of a poly-glycine alphahelix. Color scheme: alpha-chain, red; beta-chain, blue. Bound antigenic peptide is green. The amino and carboxyl termini of HLA-DR2 and RTL302 are labeled N, C, respectively. Disulfide bonds are displayed as ball and stick models.
SUPPRESSION AND TREATMENT OF A MONOPHASIC DISEASE
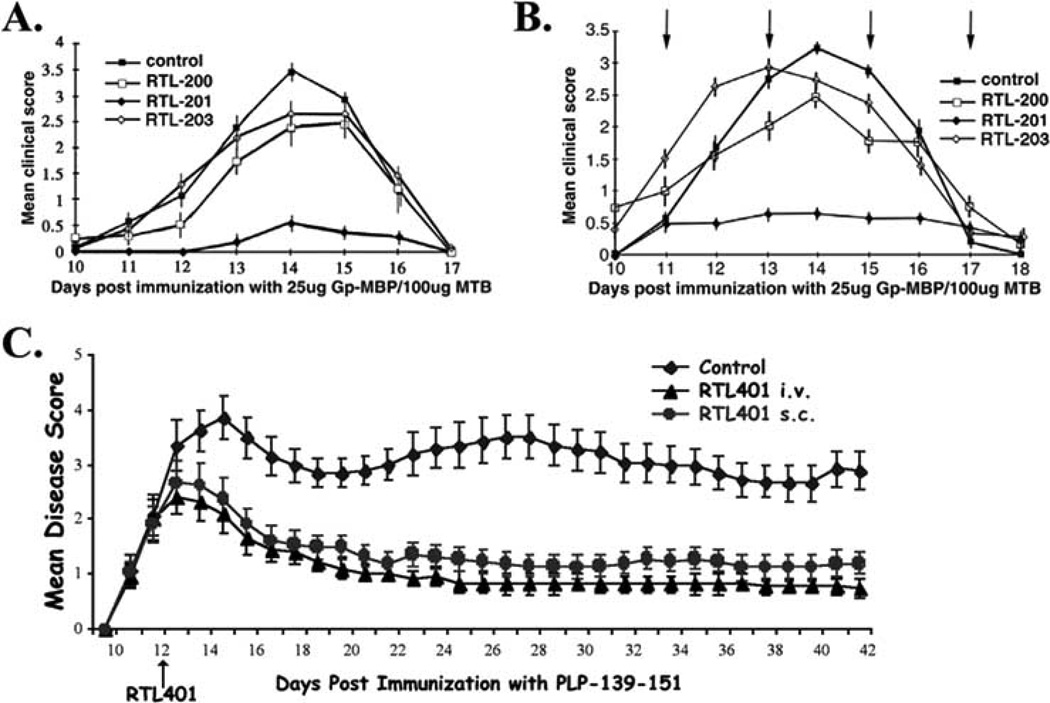
Our earliest studies evaluated RTLs for their ability to suppress and treat Gp-MBP-induced EAE in Lewis (LEW) rats [79, 80]. LEW rats are susceptible to experimental autoimmune encephalomyelitis (EAE) induced with guinea pig myelin basic protein (MBP), or synthetic peptides corresponding to MBP-73-86, MBP-69-89, and MBP-87-99 peptides. T cell responses of LEW rats to MBP-69-89 are I-A (RT1.B)-restricted, whereas responses to MBP-87-99 are I-E (RT1.D)-restricted [87–89]. Intravenous RTL therapy (RT1.B-derived RTL201 carrying the dominant encephalitogenic epitope MBP-72-89; See Table I) suppressed the induction of clinical (Fig. 2A) and histological signs of EAE in an antigen-specific manner. Animals that were left untreated or that received control RTLs containing Ag-peptides other than Gp-MBP-72-89 developed paralytic EAE. Animals that received peptide alone (Gp-MBP-69-89 at the dose of free peptide equivalent to complete cleavage of the peptide from RTL201 in vivo) developed paralytic EAE, effectively ruling out the possibility that free peptide cleaved from RTL201 accounted for protection. Rats with EAE showed a 15% loss in body weight whereas animals treated with RTL201 showed no significant loss of body weight throughout the course of the experiment.
Fig. (2). Clinical efficacy of RTLs.
(A) Suppression of EAE in LEW rats. Groups of LEW rats (n = 5) were injected with 25 µg of Gp-MBP/CFA/100µg MTB to induce active EAE. On days 3, 7, 9, 11, and 14 after disease induction, rats were given 300 µg RTL200 (encoding rat-MBP-72-89), 300 µg RTL201 (encoding Gp-MBP-72-89), 300 µg RTL203 (encoding rat-CM-2), or were left untreated, as indicated. A single representative experiment is shown; the experiment was done three times. Values indicate mean clinical score ± SEM on each day of clinical disease. (B) Treatment of established, actively-induced EAE in LEW rats. Groups of LEW rats (n = 6) were injected with 25 µg of Gp-MBP/CFA to induce active EAE. On the day of onset of clinical signs (day 11), day 13, and day 15, rats were given 300 µg RTL200 (encoding rat-MBP-72-89), 300 µg RTL201 (encoding Gp-MBP-72-89), 300 µg RTL203 (encoding rat-CM-2), as indicated by arrows, or were left untreated (control). A single representative experiment is shown; the experiment was done twice. Values indicate mean clinical score ± SEM on each day of clinical disease. (C) Intravenous or subcutaneous administration of RTL401 improves EAE in SJL/J mice. SJL females were immunized with PLP139-151. At disease onset (day 12), mice were treated daily with vehicle, 100 ug RTL401 i.v. or 100 ug RTL401 s.c. for 8 days. Data presented is the mean of 2 experiments for each group.
Experiments with RTL treatment on the first day of disease onset, with follow-up injections 48 and 96 hours later, defined a temporal window within which RTL therapy was effective (Fig. 2B). RTL201 essentially eliminated the infiltration of activated inflammatory cells into the CNS, with no inflammatory lesions in spinal cord histological sections. The number of mononuclear cells isolated after recovery from EAE was reduced 4–5-fold in RTL201 protected animals (0.24 × 106 cells/spinal cord) compared to control animals (1.10 × 106 cells/spinal cord), or animals receiving irrelevant-Ag control RTLs (1.17 × 106 cells/spinal cord). RTL201 protected animals also had 8–10-fold fewer activated (OX40+) T cells in the spinal cord than control animals. RTL201 also completely blocked the induction of clinical and histological signs of EAE after passive transfer of 10 × 106 blasting Gp-MBP-69-89 specific T cells [80]. RTL201 treatment was very specific and had no effect on EAE induced by passive transfer of I-E (RT1.D)-restricted MBP-87-99-specific T cells [80].
Consistent with inhibition of EAE in vivo, RTL201 specifically inhibited proliferation responses of T cells cultured ex vivo. Draining lymph node cells (DLN) were recovered from both treated and control animals at the peak of actively-induced EAE and stimulated in vitro with Gp-MBP-72-89 or whole Gp-MBP, and the proliferative response was measured 72 hrs later using a standard 3H-thymidine incorporation assay. T cells from RTL201 treated animals showed an approximately 3-fold decrease in their proliferative response to Gp-MBP-72-89 or whole Gp-MBP compared to T cells from untreated animals. T cells from RTL200 and RTL203 treated animals showed a mild (about 25%) decrease in proliferative response to Gp-MBP-72-89 or whole Gp-MBP, consistent with the effect of these molecules on suppression of EAE in vivo [80].
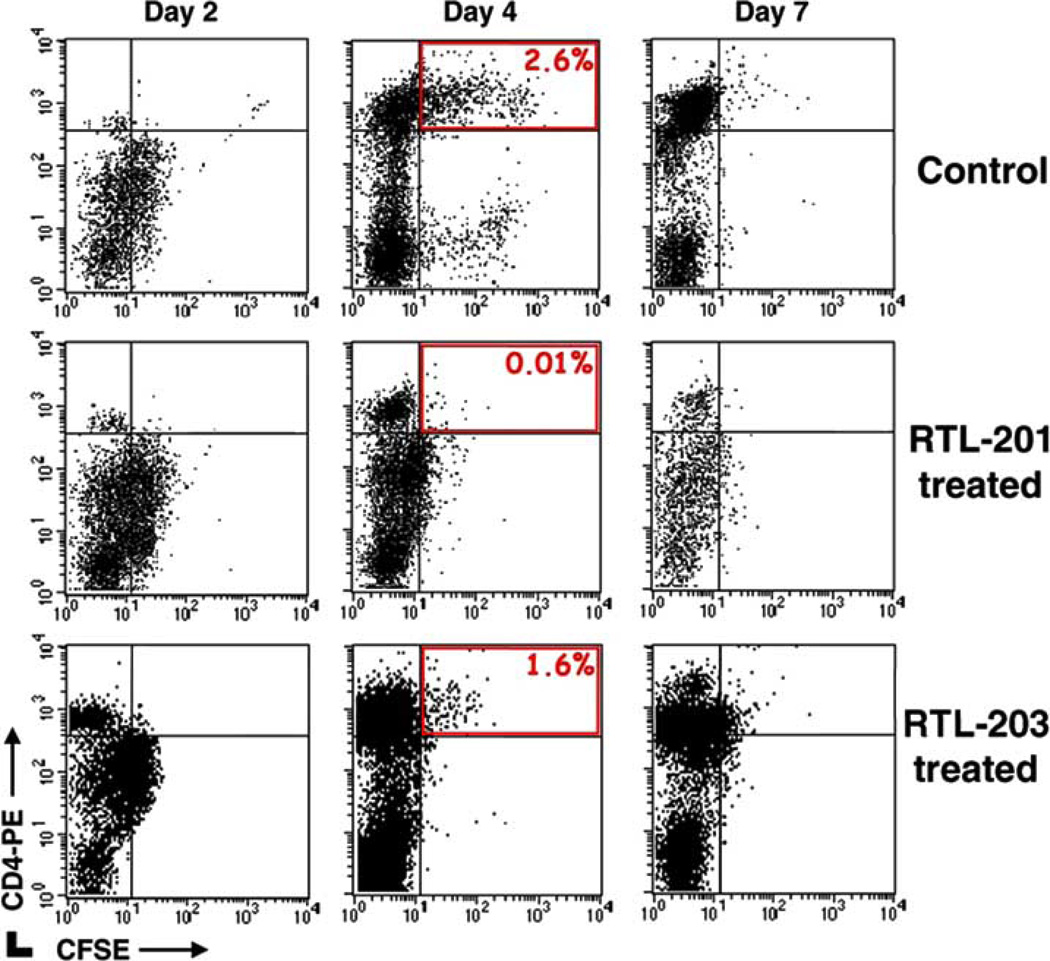
To more clearly define a potential mechanism of activity for the efficacy of RTL treatment in vivo, passive transfer experiments with activated Gp-MBP-72-89 specific T cells labeled with the fluorescent dye 5- (6-) carboxyfluorescein diacetate succinimidyl ester (CFSE) were performed. RTL201 inhibited infiltration of donor derived CD4+ T cells (CFSE+) into the CNS (Fig. 3, upper right quadrants). In passively transferred EAE, onset of disease typically occurred between days 3 and 5. Two days after passive transfer (prior to disease induction), there was a >10-fold decrease in the number of labeled CD4+ T cells in the CNS of RTL201 treated animals vs. RTL203 treated or untreated controls (40 vs. 450–500 cells/spinal cord), and at onset of disease (Day 4), there was a 300-fold difference (200 vs. 45–60,000 cells/spinal cord).
Fig. (3). Decreased encephalitogenic T cells in the CNS after RTL treatment.
Short-term T cell lines specific for Gp-MBP-72-89 were stained with CFSE and injected into animals (10 × 106 cells/300µl RPMI). On days 1, 3 and 5 after passive transfer, rats were given 300 µg RTL201 (encoding Gp-MBP-72-89) or RTL203 (encoding rat-CM-2) i.v. or were left untreated. On days 2, 4 and 7 animals were sacrificed and cells infiltrating the CNS were analyzed by FACS. RTL201 treatment inhibits trafficking of donor (CD4+/CFSE+) derived cells into the CNS. A single representative experiment is shown; the experiment was done twice.
Three key observations could be drawn from experiments in the LEW rat model regarding RTL therapy. First, treatment of EAE required the combination of both MHC and peptide components of the RTL. Second, RTL therapy significantly decreased the number of inflammatory cells that penetrated the blood-brain-barrier after treatment, and third, RTL therapy resulted in a decreased ability of T cells harvested from draining lymph nodes (DLN) to proliferate in response to Ag challenge ex vivo. Treatment appeared to directly modulate encephalitogenic T cell behavior.
SJL/J MOUSE STUDIES: RTL THERAPY INDUCES SYSTEMIC IMMUNOMODULATION
The murine SJL/J relapsing/remitting model presents a more complex clinical paradigm of EAE than the monophasic LEW rat model, and provided an opportunity to explore how well RTL therapy worked in a model that shares most of the clinical characteristics of human multiple sclerosis [90]. RTLs had a potent protective effect in the SJL/J relapsing/remitting mouse model of EAE [84], and of interest from a drug delivery point of view for future clinical applications, subcutaneous administration worked as well as i.v. treatment (Fig. 2C) [84].
The SJL model allowed us to more completely evaluate peptide specificity of RTL treatment in vivo. While RTL401 (I-As-derived, PLP-139-151-covalently tethered Ag; See Table I) had a significant effect on EAE induced with PLP-139-151, disease induced with I-As-restricted encephalitogenic peptides PLP-178-191 and MBP-84-104 was not significantly affected by RTL401 treatment [84]. Of note, day 42 LN responses in PLP-178-191 and MBP-84-104 peptide-immunized mice with EAE were specific only for the immunizing peptide, and no responses were observed to PLP-139-151 peptide, indicating a lack of epitope spreading [84].
Further evidence for the requirement of MHC and peptide specificity in RTL treatment was obtained by using RTL401 to treat EAE induced by either PLP-139-151 peptide or MOG-35-55 peptide in (C57BL/6×SJL)F1 mice. These mice express both I-As and I-Eb MHC class II molecules that restrict PLP-139-151 (I-As) and MOG-35-55 (I-Eb) peptides, in both cases producing an encephalitogenic response. While treatment with RTL401 significantly reduced the severity of EAE induced by PLP-139-151 peptide, it had no effect on EAE induced by MOG-35-55 peptide [84]. These data strongly supported our previous observation in LEW rats that RTL treatment of EAE is specific for the cognate combination of MHC and neuroantigen peptide.
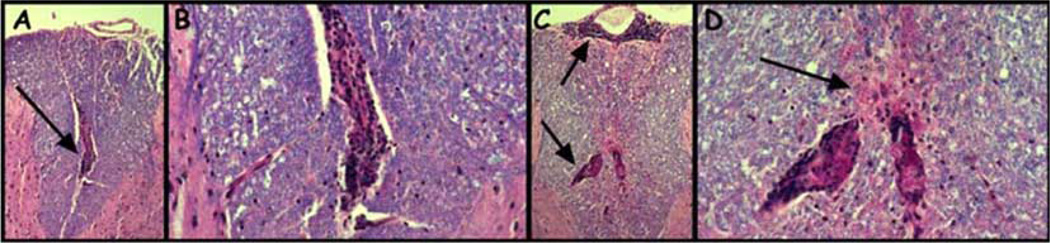
Lymph node and spleen cells from vehicle control and RTL401 treated SJL/J mice with EAE were analyzed during the course of treatment for proliferation and cytokine responses to the immunizing PLP-139-151 peptide. Immune cell responses were assessed just after disease onset but prior to treatment (day 11), 24h after initiation of treatment (day 13), at the peak of the initial clinical episode (day 15), at the first remission (day 18), at the beginning of the first relapse (day 22), at the peak of the first relapse (day 28), and at the end of the first relapse (day 42). RTL401 treatment nominally inhibited proliferation responses to PLP-139-151 peptide in LN cultures, and significantly enhanced proliferation of splenocyte cultures [84]. Additionally, RTL401 had mixed effects on cytokine secretion from PLP-139-151-stimulated splenocytes that appeared to be coupled to disease progression. One day after initiation of RTL401 treatment (day 13), there were no significant changes in cytokine responses. Surprisingly, at the peak of the first episode of EAE (day 15), there was enhanced secretion of inflammatory (TNF-α, IFN-γ, MCP-1, and IL-6) and anti-inflammatory (IL-10) factors in splenocyte cultures from RTL401-treated mice. During remission from the first episode of EAE (day 18), strongly reduced levels of IFN-γ were observed, levels of MCP-1 were still enhanced, but no significant differences in TNF-α, IL-6, or IL-10 were observed in RTL401 treated mice. At onset of the first relapse (day 22), there was again a significant decrease in secreted IFN-γ in RTL401-treated mice, but no significant differences in the other inflammatory factors. Of possible importance for systemic regulation, there was a significant increase in secreted IL-10 levels by PLP-139-151-specific splenocytes from RTL401-treated mice at both the onset and peak of the first relapse (days 22 and 28, respectively). Both IgG1 and IgG2a antibodies were detected in serum during the course of EAE, but levels showed only minor fluctuations as a result of RTL401 treatment [84]. Histological sections of spinal cords taken on day 28 showed reduced inflammatory and demyelinating lesions in RTL401 treated vs. control mice (Fig. 4). This reduction in inflammatory activity found in RTL401-treated mice was reflected by a reduction in the number of inflammatory mononuclear cells obtained from brain and spinal cord tissue over the course of treatment, consistent with the observations made in the LEW rat EAE following RTL therapy. Reduction of inflammatory cells was most pronounced at onset and peak of the first clinical episode (days 13 and 15), and at onset of the first relapse (day 22), was marked by an overall decrease of CD4+ T cells (from 43% to 23%) but an increase in CD11b+ monocytes/macrophages (from 38% to 60%) as determined by FACS analysis. Particularly intriguing, expression of adhesion/homing markers VLA-4 and LFA-1 [91, 92] on CD3+ mononuclear cells was consistently reduced in brains and spinal cords from RTL401-treated mice on days 22, 28, and 42 after EAE induction [84]. RT-PCR analysis of spinal cord tissue from RTL401-treated mice also showed moderate to strong reduction in expression of mRNA for inflammatory cytokines (IFN-γ, TNF-α, IL-6) and chemokines (RANTES, MIP-2, and IP-10), but enhanced expression of TGF-β3 [84]. Expression of IL-10 in spinal cord was very low throughout the EAE disease course in spinal cords from RTL-treated mice, with only a slight enhancement in RTL401-treated mice during the first relapse (Day 22). Interestingly, expression of most chemokine receptors (CCR1, CCR2, CCR5, CCR6, CCR7 & CCR8) was moderately to strongly reduced in spinal cord tissue from RTL401-treated mice beginning at the peak of the first episode (Day 15), consistent with earlier observations [93–98]. In contrast, expression of CCR3 (Th2 associated) appeared to be uniquely enhanced during the first relapse in spinal cord tissue collected from RTL401-treated mice [84, 99].
Fig. (4).
Fixed, paraffin-embedded spinal cord sections from vehicle-treated (A and B) or RTL401-treated (C and D) SJL/J mice 46 days after induction of EAE. Spinal cord from vehicle-treated mouse showed dense mononuclear infiltration (Panel A, arrow) with only very slight or no apparent loss of myelin stain (blue stain, luxol fast blue) in the surrounding myelinated tissue (Panel B). Spinal cord from RTL401-treated mouse showed multiple regions of dense mononuclear cell infiltration (Panel C, Arrows) with considerable diffuse loss of myelin stain in the regions adjacent to the mononuclear infiltrate (Panel C and Panel D, Arrow). Magnification: A and C, 50×; B and D, 150×.
Data from the SJL model reflect the temporal (here defined as the Time period during the course of disease at which the observation is made) and spatial (here defined as the location within the animal at which the observation is made) complexity of a relapsing disease state. In general, variations in expression of inflammatory cytokines mirrored periods of EAE relapses and remission in control SJL/J mice, with more expression noted on days 15 (peak of initial episode) and 22 (first relapse) than on day 18 (remission).
Our findings in the SJL/J model suggest that a single course of i.v. or s.c. RTL therapy prevented relapses and induced long-term clinical benefit that appeared to be mediated by a cytokine switch mechanism involving IL-10, TGF-β3, and altered CCR expression including CCR3, leading to a moderation of CNS inflammation and demyelination. The cytokine switch mechanism postulated here differs from an anergy mechanism reported previously in SJL/J mice by Sharma et al. using purified full-length I-As molecules loaded with PLP-139-151 peptide [100]. Furthermore, significant systemic changes followed RTL therapy, including a reduction in the number of inflammatory cells infiltrating the CNS, and, of the cells that still could cross the blood–brain barrier, significantly reduced expression of VLA-4 and LFA-1. A summary of the systemic changes induced following RTL treatment is listed in Table II.
Table II.
Systemic Changes Following RTL Treatment
| ↑/↓* | ||
|---|---|---|
| CNS | SPLEEN | |
| INFLAMMATORY FACTORS | ||
| Cytokines | ||
| INF-γ | ↓ | ↓ |
| TNF-α | ↓ | nc |
| IL-6 | ↓ | nc |
| Chemokines | ||
| RANTES | ↓ | nd |
| MIP-2 | ↓ | nd |
| MCP-1 | nd | ↑ |
| IP-10 | ↓ | |
| Chemokine Receptors | ||
| CCR1 | ↓ | nd |
| CCR2 | ↓ | nd |
| CCR5 | ↓ | nd |
| CCR6 | ↓ | nd |
| CCR7 | ↓ | nd |
| CCR8 | ↓ | nd |
| ANTI-INFLAMMATORY FACTORS | ||
| Cytokines | ||
| TGF-β3 | ↑ | nd |
| IL-10 | ↑ | ↑ |
| Chemokine Receptors | ||
| CCR3 | ↑ | |
| ADHESION MOLECULES | ||
| VLA-4 | ↓ | nd |
| LFA-1 | ↓ | nd |
*↑/↓ Effect of RTL treatment on inflammatory factors, anti-inflammatory factors and adhesion molecules involved in cell trafficking. ↑, increase, ↓, decrease, nc, no change, nd, not done. Cytokine production monitored in the spleen was quantified by cytokine bead array assay. Adhesion molecules on CD3+ T cells infiltrating the CNS were quantifed by FACS. Cytokine, chemokine and chemokine receptor gene expression was quantified from whole frozen spinal cords by real-time PCR. Expression of each gene was calculated relative to the expression of the housekeeping gene, L32.
RTL THERAPY INDUCED SYSTEMIC IMMUNOMODULATION IN AUTOIMMUNITY: DISSECTING THE MOLECULAR MECHANISM
The systemic effects of RTL therapy appear to block encephalitogenic T cell trafficking to the CNS (LEW rat, Fig. 3) and promote a cytokine switch in response to encephalitogenic peptides (SJL/J mouse, Table II). Most clearly observed in the SJL/J mouse model, RTL therapy appeared to allow a non-encephalitogenic Ag-specific T cell to persist that retains some ability to infiltrate CNS tissue. Significantly, the infiltrating cells clearly had reduced inflammatory capability, enhanced secretion of anti-inflammatory factors, and enhanced expression of a protective CCR. The potential therapeutic implication is that Ag-specific therapy could promote a cytokine switch from an encephalitogenic phenotype to a memory population that, at least modestly, could return to the target organ, down-modulate inflammatory events, and potentially induce a form of bystander regulation. What are the molecular mechanisms that control this cytokine switch at the cellular level? In order to comprehensively characterize the effect of RTL binding directly to the TCR, we began a detailed molecular characterization of the signal transduction pathways within T cells that are directly altered following RTL engagement with the TCR that we recently documented by surface plasmon resonance using soluble single chain TCR (SCTCR) [82].
Our studies to date have focused on mapping the information cascade from TCR to the nuclear events that control the autocrine cytokine IL-2 [101]. The earliest detectable change following RTL binding was in the CD3ζ phosphorylation pattern. Both immobilized RTL201 (containing Gp-MBP-72-89 peptide) and anti-CD3ε triggered a prominent emergence of p23 (the fully phosphorylated pattern of mouse CD3ζ) and enhanced p21 (the partially phosphorylated pattern of mouse CD3ζ). The p23/p21 ratio was increased 2-fold by RTL201 (from 0.25 to 0.5), and almost 4-fold by immobilized α-CD3ε (from 0.25 to 0.93). RTL200, which contains the rat MBP-72-89 antigen peptide (threonine instead of serine at position 80 of the myelin basic protein peptide), consistently showed an increased level of p21, but did not alter the p23/p21 ratio significantly from vehicle control. We also monitored CD3ζ chain-associated ZAP-70, which is required for TCR function [102, 103]. Immunoblot analysis for Tyr319-phosphorylated ZAP-70 was used to detect the activation of ZAP-70 from whole cell lysates. RTL201 treatment significantly increased the phosphorylation levels of ZAP-70 Tyr319 within 5 minutes of treatment, while RTL200 induced no significant increase in ZAP-70 phosphorylation. Immobilized α-CD3ε was consistently more effective than RTL201 in raising the level of ZAP-70 phosphorylation. Of note, while RTL201 increased the p23/p21 ratio of CD3ζ and increased ZAP-70 phosphorylation, these primary signal transduction events induced after RTL engagement with the TCR were weaker than those induced by anti-CD3ε. Importantly, the effect of RTL treatment was exquisitely antigen specific, with a single amino acid substitution in the RTL-coupled Ag, as demonstrated by RTL200 treatment, showing very little effect on the p23/p21 ratio of CD3ζ and virtually no increase in ZAP-70 phosphorylation.
RTL201 and anti-CD3ε induce differential calcium signaling. Using single cell imaging and recording, both immobilized RTL201 and α-CD3ε caused Fura-2/AM-loaded cells to turn bright green, indicating elevation of intracellular calcium levels. Quantitatively the peak level of intracellular calcium induced by anti-CD3ε treatment was almost two-fold higher than that induced by RTL201 treatment (2.61 vs 1.40). Calcium activation by RTL201 was Ag-specific, as no calcium elevation was observed following RTL200 treatment. The different percentage of cells activated by anti-CD3ε and RTL201 (83% and 39%, respectively) are also indicative of differential early downstream consequences following treatment with the two agents. The reagents had stimulatory effects only when immobilized on a surface. Treatment with soluble RTL201, anti-CD3ε, or anti-CD28 did not induce calcium elevation. EGTA (2mM), a cell impermeable Ca2+ chelator, dramatically decreased the percentage of cells activated by anti-CD3ε and the relative peak calcium concentration to a level similar to that induced by RTL201 treatment with or without EGTA present. Xestospongin C (XeC, a membrane-permeable blocker of IP3-mediated calcium release, 1 µM), drastically blocked calcium elevation in cells treated with either RTL201 or anti-CD3ε, and EGTA plus XeC, completely blocked calcium mobilization in cells stimulated with anti-CD3ε. The differential effects of signaling inhibitors and calcium chelators provided a clear picture of the sources of calcium mobilized by these different reagents, demonstrating that anti-CD3ε mobilized calcium from both internal and external sources, while RTL201 treatment mobilized calcium only from internal stores. PLCγ is a common mediator between receptor stimulation and calcium mobilization [104]. Pretreatment of A1 cells with the PLCγ inhibitor U73122 (10 µM) prevented calcium mobilization by both RTL201 and a-CD3ε. U73343 (an analogue of U73122 with minimal biological function, used as a negative control, 10 µM) did not block calcium mobilization (data not shown). The results showed that calcium mobilization by both RTL201 and α-CD3ε is strictly PLCγ-dependent.
RTL201 and α-CD3ε induced differential activation of transcription factors. Using an electrophoretic mobility shift assay (EMSA), we monitored transcriptional activation with specific probes for NFAT and NFκB. A 30 minute treatment with RTL201 or α-CD3ε resulted in a marked increase in NFAT activity, although α-CD3ε treatment showed a significantly stronger effect. This difference in potency was consistent with the differences in amplitude of calcium flux observed. Treatment with ionomycin (Iono, a calcium ionophore, 250 µg/ml) to pump in calcium from the extracellular milieu, also resulted in NFAT activation. RTL200 (containing the MBP-72-89 S80T mutant negative control) showed very little effect on NFAT activity. The increase in NFAT activity observed following RTL201 or α-CD3ε treatment was blocked by TMB8 (a cell-permeable calcium antagonist, 1 mM) and cyclosporine A (CsA, an inhibitor of the calcium/calmodulin-dependent phospholipase calcineurin, 2 µM) [105], indicating that NFAT was activated via a calcium/calcineurin-dependent pathway. A 30 minute treatment with α-CD3ε led to a dramatic increase in NFκB activity, while no significant increase in NFκB activity was detected after RTL201 or RTL200 treatment. These EMSA results were further confirmed by immunoblot assay of IκB phosphorylation, which releases active NFκB when it is phosphorylated or degraded [106]. IκB was phosphorylated after α-CD3ε treatment, but not after RTL201 or RTL200 treatment. No IκB degradation was observed at the 30 minute time point of our study.
Although both NFAT and NFκB are essential for T cell function and activation, other signaling factors are required for full T cell proliferation and IL-2 production. In T cells, phosphorylation of ERKs leads to the formation of the AP-1 complex [107], which is a partner of NFAT in the initialization of IL-2 mRNA transcription [108]. Treatment with α-CD3ε(10 µg/ml) induced strong ERK phosphorylation, which peaked at 30 minutes and declined to the basal level by 60 minutes. In contrast, RTL201 (10 µg/ml) showed no significant effect on ERK phosphorylation levels. The dose dependent induction of ERK phosphorylation following α-CD3ε treatment could be readily detected as low as 5 µg/ml, whereas no induction of ERK phosphorylation was observed following RTL201 treatment, even at 20 µg/ml.
RTL201 engagement with the TCR appeared to induce a subset of the signal transduction events that were initiated by binding of α-CD3ε to the TCR. What was the result at the effector cytokine level? Intracellular IL-2 levels were significantly increased after 4 hour treatment with immobilized RTL201, α-CD3ε, or Ionomycin/PMA, while RTL200 induced no significant change above the basal level. However, IL-2 production after RTL201 treatment was short-lived and returned to background levels by 16 hours. This was dramatically different from the observed IL-2 production following treatment with plate-bound α-CD3ε or ionomycin/PMA, in which case IL-2 was still accumulating at 16 hours. IL-2 production induced by RTL201 and α-CD3ε was calcium dependent, shown by using TMB8 (1 mM), an intracellular calcium antagonist. IL-2 production induced by RTL201 and α-CD3ε was also PLCγ-dependent, shown by using U73122 (10 µM), a PLCγ inhibitor, and its analogue U73343 (10 µM) as a negative control. Thus, α-CD3ε and ionomycin/PMA induced a sustained increased IL-2 production that was both calcium and PLCγ-dependent. RTL201 induced a significantly increased level of IL-2 production that was also calcium and PLCγ-dependent, but this increase was transient, returning to basal levels by 16 hrs.
Our results demonstrated that RTLs triggered specific downstream signaling events that deplete intracellular calcium stores without fully activating T cells, resulting in activation of the transcription factor NFAT uncoupled from the activation of NFκB or ERKs, limiting specific short term effector activities including limited production of IL-2. This data provided the first clues to how Ag-specific TCR engagement with RTLs could modify T cell phenotype and produce the profound systemic effects observed in our in vivo and ex vivo animal studies. Differential modulation of calcium signaling appeared to be responsible for the differences between RTL201 and α-CD3ε treatments observed at the transcriptional level, a result of mobilization of calcium from internal stores by RTL201 without triggering influx of extracellular calcium.
We have recently extended this work to human T cell clones, using DR2 and DR7 homozygous donor-derived Ag-specific T cell clones expressing a single TCR BV gene, to evaluate HLA-DR2-derived RTLs ability to directly modify the behavior of T cells [70]. RTL treatment induced a distinct change in CD3ζ phosphorylation that reached a minimum at 10 minutes. Only the cognate RTLs containing the peptide for which the clones were specific induced this altered CD3ζ phosphorylation, typically observed after T cell activation by antagonist ligands [104, 105].
RTL303 treatment induced a sustained high calcium signal in the DR2 homozygous T cell clone MR#3-1 specific for the MBP-85-99 peptide, whereas RTL301 (identical to RTL303 except a single point mutation that altered folding properties, F150L) showed no increase in calcium signal over the same time period. Within 15 min after treatment with RTLs, the level of ERK-P was shown to be drastically reduced in an Ag-specific fashion, with 20 µM RTL303 reducing ERK-P by 80% in clone MR#3-1 and 20 µM RTL311 reduced ERK-P by 90% in the C-ABL peptide specific clone MR#2-87. Upon activation with APC plus Ag, clone MR#3-1 (MBP-85-99 specific) and MR#2-87 (CABL specific) showed classic Th1 cytokine profiles that included IL-2 production, high IFN-g and little or no detectable IL-4 or IL-10. Activation with anti-CD3ε antibody induced an initial burst of strong proliferation and production of IL-2, IFN-γ, and surprisingly, IL-4, but no IL-10. In contrast, upon treatment with RTL303, clone MR#3-1 continued production of IFN-γ, but in addition dramatically increased its production of IL-10. IL-10 appeared within 24 hours after addition of RTL303 and its production continued for more than 72 hours, to three orders of magnitude above the untreated or RTL311 treated control. In contrast, IL-2 and IL-4 levels did not show RTL induced changes. Similarly, the C-ABL-specific MR#2-87 clone also showed a dramatic increase in production of IL-10 within 24 hours after RTL311 treatment that continued for greater than 72 hours above the untreated or RTL303 treated control. Again, IL-2 and IL-4 levels did not show detectable RTL induced changes, and IFN-γ production remained relatively constant. The switch to IL-10 production was exquisitely Ag-specific, with the clones responding only to the cognate RTL carrying peptide antigen for which the clones were specific. A DR7 homozygous T cell clone CP#1-15 specific for MBP-85-99 showed no response to DR2-derived RTLs indicating that RTL induction of IL-10 was also MHC restricted. To assess the effects of RTL pre-treatment on subsequent response to antigen, T cell clones pretreated with anti-CD3ε or RTLs were restimulated with APC/peptide, and cell surface markers, proliferation and cytokine production were monitored. RTL pre-treatment had no effect on the cell surface expression levels of CD25, CD69 or CD134 (OX40) induced by restimulation with APC/peptide compared to T cells stimulated with APC/peptide that had never seen RTLs, and there were no apoptotic changes observed over a 72 hour period using Annexin V staining. As anticipated, α-CD3ε pretreated T cells were strongly inhibited, exhibiting a 71% decrease in proliferation and >95% inhibition of cytokine production, with continued IL-2R (CD25) expression, a pattern consistent with classical anergy [109]. Clone MR#3-1 showed a 42% inhibition of proliferation when pretreated with 20 µM RTL303, and clone MR#2-87 showed a 57% inhibition of proliferation when pretreated with 20 µM RTL311. Inhibition of proliferation was also MHC class II-specific, as MBP85-99 specific clone CP#1-15 (HLA-DR7 homozygous donor) showed little change in proliferation after pretreatment with RTL303 or RTL311. Clone MR#3-1 pretreated with RTL303 followed by restimulation with APC/Ag showed a 25% reduction in IL-2, a 23% reduction in IFN-γ, and no significant changes in IL-4 production. Similarly, clone MR#2-87 showed a 33% reduction in IL-2, a 62% reduction in IFN-γ production, and no significant change in IL-4 production. Both RTL-pretreated T cell clones continued to produce IL-10 upon restimulation with APC/peptide.
While RTL effects on the molecular signal transduction circuitry remains to be completely defined, data from studies with human T cell clones fits well with our previous studies using the LEW rat A1 T cell hybridoma [81]. Of significant importance, our finding with human T cells tie together our data from the SJL/J mouse EAE studies regarding the antigen-specific induction by RTLs of IL-10 secretion, supporting a cytokine switch mechanism. (An overview of the molecular information cascade altered following RTL:TCR engagement is shown in Figure 5). The elevated level of IL-10 induced in human Th1 cells by RTLs has important regulatory implications for autoimmune diseases such as multiple sclerosis because of the known anti-inflammatory effects of this cytokine on Th1 cell and macrophage activation. We are currently in the process of characterizing the signal transduction cascade at the molecular level responsible for the cytokine switch mechanism controlling IL-10 secretion.
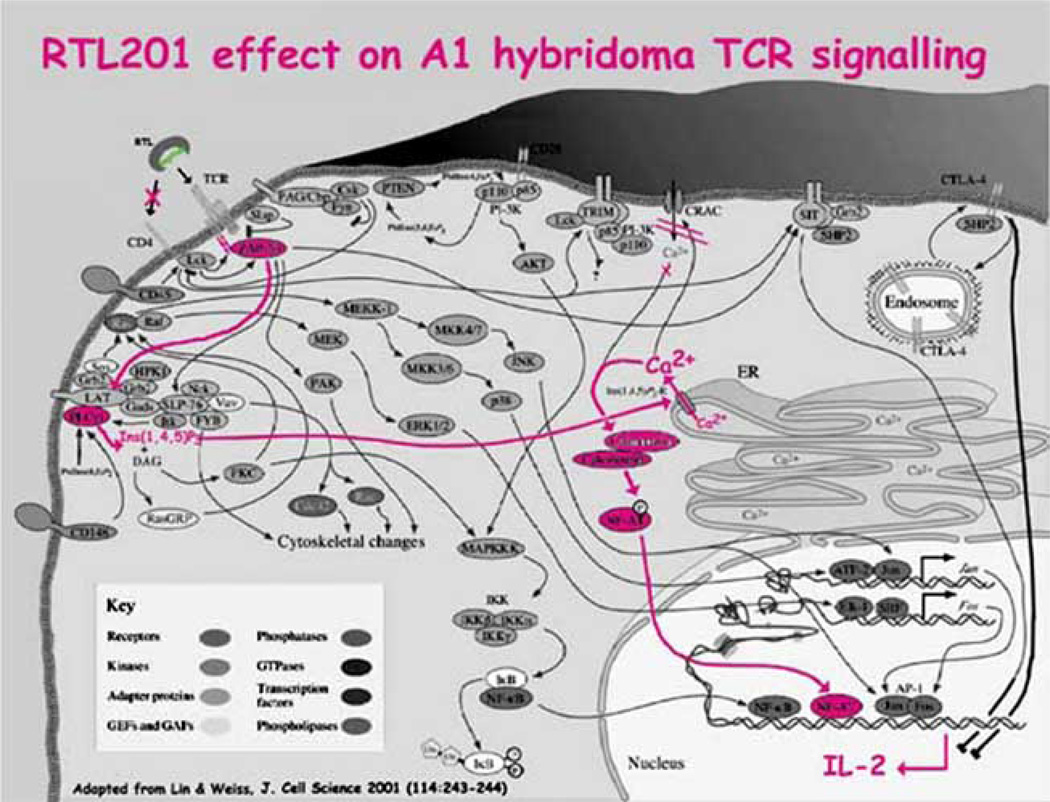
Fig. (5). RTLs initiate a subset of the signals within T cells known to occur upon MHC class II/peptide on APCs binding the TCR.
Here we present schematically the specific pathways documented to be engaged upon RTL:TCR binding. Overall schematic modified from Lin & Weiss [111]. Note that the increased IL-2 induction is short-term.
FUTURE STUDIES IN MS
It is likely that the pathogenesis of MS involves autoreactive Th1 cells directed at one or more immunodominant myelin peptides, including MBP-85-99 and MOG-35-55. Conceivably, RTLs could induce IL-10 production by these T cells, thus neutralizing their pathogenic potential. Moreover, local production of IL-10 after Ag-stimulation in the CNS could result in inhibited activation of bystander T cells that may be of the same or different Ag specificity, as well as macrophages that participate in demyelination. Thus, the findings presented in this review suggest that RTL therapy and its regulatory potential may extend beyond the RTL-ligated neuroantigen specific T cells. SRTL induction of IL-10 in specific T cell populations that recognize CNS antigens could potentially be used to regulate the immune system while preserving the T cell repertoire, and may represent a novel strategy for therapeutic intervention of complex T cell mediated autoimmune diseases such as MS. However, alternative mechanisms explaining the efficacy of RTL therapy in vivo exist that cannot yet be completely ruled out, and before concluding this review a few of these should be mentioned. Our recent studies using HLA-DR2 transgenic mice showed that RTL312, a DR2-derived RTL carrying covalently coupled MOG-35-55 Ag (See Table I), profoundly inhibited T cell responses to the encephalitogenic MOG-35-55 peptide, including blockade of proliferation and secretion of the pro-inflammatory cytokines TNF-α, IFN-γ, and IL-2 [110]. However, the Th2 cytokines IL-4, IL-5, and IL-10 were not detected in mice developing EAE and were not induced after treatment with RTL312. While this may indicate differences in signaling pathways between mouse and human T cells, the data also fit a mechanism of anergy leading to late stage apoptosis proposed by Wucherpfennig, Appel and collaborators [67] used to explain inhibition of human T cells using DR2/MBP-85-99 dimeric constructs. An alternative much less explored mechanism is that the RTL “carrier” protein may represent an efficient vehicle for delivering Ag to tissue and/or compartments where normal processing and re-presentation of the covalently linked Ag by APCs may occur, a variation on the theme of coupling Ag-peptide to a larger carrier protein such as a mAb [32–34]. The RTLs are after all, proteins, and as such, will eventually be metabolized. While these alternative mechanisms have yet to be conclusively ruled out, our working hypothesis is that the profound clinical utility of RTL therapy lies in the specific triggering of a “cytokine switch” in pathogenic CD4+ T cells, intimately connected to the ability of these molecules to directly bind to the TCR of pathogenic T cells. Full elucidation of the novel information cascade engaged uncoupled from costimulatory input that leads to alteration of phenotype in these cells are the goals of future experiments.
ACKNOWLEDGEMENTS
The author wishes to thank collaborators and associates, who contributed hard work and without whom the research presented in this review would not have been possible. These include Dr. Halina Offner, Dr. Arthur Vandenbark, Dr. Yuan Chou, Dr. Dennis Bourdette, Dr. Jianya Huan, Dr. Roberto Meza-Romero, Dr. Chunhe Wang, Dr. Lars Fugger, Jeff Mooney, Cathleen Rich, and Sandhya Subramanian. This work was supported by Grants AI43960, NS41965, and ES10554 from the National Institutes of Health, the Nancy Davis MS Center Without Walls, Virogenomics, Inc., and Grant RG3012 from the National Multiple Sclerosis Society.
REFERENCES
- 1.Lernmark A. J. Clinical Investigation. 2001;108:1091. doi: 10.1172/JCI14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanborg RH. J. Immunol. 1983;130:1503. [PubMed] [Google Scholar]
- 3.Cush JJ, Lipsky PE. Arthritis Rheum. 1988;31:1230. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- 4.Caspi RR. J. Immunol. 1988;140:1490. [PubMed] [Google Scholar]
- 5.Cobbold SP, Nash JA, Prospero TD, Waldham H. Nature. 1988;312:548. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 6.Steinman L. Sci. Am. 1993;269:106. doi: 10.1038/scientificamerican0993-106. [DOI] [PubMed] [Google Scholar]
- 7.Sawyer RT, Maier LA, Kittle LA, Newman LS. Intl. Immunopharm. 2002;2:249. doi: 10.1016/s1567-5769(01)00177-1. [DOI] [PubMed] [Google Scholar]
- 8.Richeldi L, Sorrentino R, Saltini C. Science. 1993;262:242. doi: 10.1126/science.8105536. [DOI] [PubMed] [Google Scholar]
- 9.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Nature Medicine. 1997;3:797. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 10.Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM. Nature Medicine. 1998;4:713. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 11.Sollid LM. Nature Reviews Immunology. 2002;2:647. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 12.Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. J. Exp. Med. 1989;169:345. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjostrom H, Lundin KE, Molberg O, Korner R, McAdam SN, Anthonsen D, Quarsten H, Noren O, Roepstorff P, Thorsby E, Sollid LM. Scandinavian J. Immunol. 1998;48:111. doi: 10.1046/j.1365-3083.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg AD, Wallin JJ, Jones RE, Sullivan TJ, Bourdette DN, Vandenbark AA, Offner H. J. Immunol. 1994;152:4712. [PubMed] [Google Scholar]
- 15.Weinberg AD, Whitham R, Swain SL, Morrison WJ, Wyrick G, Hoy C, Vandenbark AA, Offner H. J. Immunol. 1992;148:2109. [PubMed] [Google Scholar]
- 16.Martin R, McFarland HF. Crit. Rev. Clin. Lab. Sci. 1995;32:121. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 17.Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. J. Immunol. 1989;142:1523. [PubMed] [Google Scholar]
- 18.Ota K, Matsui M, Milford EL, Macklin GA, Weiner HL, Hafler DA. Nature. 1990;346:183. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 19.Chou YK, Bourdette DN, Offner H, Whitham R, Wang R, Hashim GA, Vandenbark AA. J. Neuroimmunol. 1992;38:105. doi: 10.1016/0165-5728(92)90095-3. [DOI] [PubMed] [Google Scholar]
- 20.Steinman L. Cell. 1996;85:299. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 21.KerlerodeRosbo N, Milo R, Lees MB, Burger D, Bernard CC, Ben-Nun A. J. Clin. Invest. 1993;92:2602. doi: 10.1172/JCI116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.KerlerodeRosbo N, Ben-Nun A. J. Autoimmun. 1998;11:287. doi: 10.1006/jaut.1998.0202. [DOI] [PubMed] [Google Scholar]
- 23.Trotter JL, Pelfrey CM, Trotter AL, Selvidge JA, Gushleff KC, Mohanakumar T, McFarland H. J. Neuroimmunol. 1998;84:172. doi: 10.1016/s0165-5728(97)00260-9. [DOI] [PubMed] [Google Scholar]
- 24.Wallstrom E, Khademi M, Andersson M, Weissert R, Linington C, Olsson T. Eur. J. Immunol. 1998;28:3329. doi: 10.1002/(SICI)1521-4141(199810)28:10<3329::AID-IMMU3329>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Madsen LS, Andersson EC, Jansson L, Krogsgaard M, Anderen CB, Engberg J, Strominger L, Svejgaard A, Hjorth JP, Holmdahl R, Wucherpfennig KW, Fugger L. Nature Genetics. 1999;23:343. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 26.Muraro P, Afshar G, McFarland H, Martin R. J. Neuroimmunol. 2002;130:233. doi: 10.1016/s0165-5728(02)00229-1. [DOI] [PubMed] [Google Scholar]
- 27.van Noort JM, van Sechel AC, Bajramovic JJ, el Ouagmiri ME, Polman CH, Lassmann H, Rivid R. Nature. 1995;375:798. doi: 10.1038/375798a0. [DOI] [PubMed] [Google Scholar]
- 28.Chou Y, Burrows GC, LaTocha D, Wang C, Subrmanian S, Bourdette DN, Vandenbark AA. J. Neurosci. Res. 2004;75:516. doi: 10.1002/jnr.20000. [DOI] [PubMed] [Google Scholar]
- 29.Mitchison NA. Immunology. 1968;15:531. [PMC free article] [PubMed] [Google Scholar]
- 30.Matis LA, Glimcher LH, Paul WE, Schwartz RH. Proc. Natl. Acad. Sci. USA. 1983;80:6019. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Critchfield JM, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. Science. 1994;263:1139. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 32.Seddon B, Mason D. European J. Immunol. 1998;28:4313. doi: 10.1002/(SICI)1521-4141(199812)28:12<4313::AID-IMMU4313>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Saoudi A, Simmonds S, Huitinga I, Mason D. J. Exp. Med. 1995;182:335. doi: 10.1084/jem.182.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day MJ, Tse AG, Puklavec M, Simmonds SJ, Mason DW. J. Exp. Med. 1992;175:655. doi: 10.1084/jem.175.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Magistris MT, Alexander J, Coggeshall M, Altman A, Gaeta FC, Grey HM, Sette A. Cell. 1992;68:625. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 36.Windhagen A, Scholz C, Hollsberg P, Fukaura H, Sette A, Hafler DA. Immunity. 1995;2:373. doi: 10.1016/1074-7613(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 37.Vergelli M, Hemmer B, Utz U, Vogt A, Kalbus M, Tranquill L, Conlon P, Ling N, Steinman L, McFarland HF, Martin R. Eur. J. Immunol. 1996;26:2624. doi: 10.1002/eji.1830261113. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson LB, Greer JM, Sobel RA, Lees MB, Kuchroo VK. Immunity. 1995;3:397. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 39.Bielokova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank A, McFarland H, Martin R. Nature Medicine. 2000;6:1167. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 40.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. Nat. Med. 2000;6:1176. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 41.Quill H, Schwartz RH. J. Immunol. 1987;138:3704. [PubMed] [Google Scholar]
- 42.Schwartz RH. J. Exp. Med. 1996;184:1. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nag B, Kendrick T, Arimilli S, Yu SC, Sriram S. Cell. Immunol. 1996;170:25. doi: 10.1006/cimm.1996.0130. [DOI] [PubMed] [Google Scholar]
- 44.Rhode PR, Burkhardt M, Jiao J, Siddiqui AH, Huang GP, Wong HC. J. Immunol. 1996;157:4885. [PubMed] [Google Scholar]
- 45.Ishigami T, White CA, Pender MP. Eur. J. Immunol. 1998;28:1626. doi: 10.1002/(SICI)1521-4141(199805)28:05<1626::AID-IMMU1626>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 46.Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gasgoigne NR, Travers PJ. Immunity. 1999;10:227. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 47.Germain RN, Stefanova I. Annual Review of Immunology. 1999;17:467. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 48.Reichstetter S, Kwok WW, Kochik S, Koelle DM, Beaty JS, Nepom GT. Human Immunology. 1999;60:608. doi: 10.1016/s0198-8859(99)00038-5. [DOI] [PubMed] [Google Scholar]
- 49.Hill JA, Wang D, Jevnikar AM, Cairns E, Bell DA. Arthritis Res. Ther. 2003;5:R40. doi: 10.1186/ar605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LeGuern C. Trends in Immunology. 2003;24:633. doi: 10.1016/j.it.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Matsui K, Boniface JJ, Reay PA, Schild H, Fazekas de St Groth B, Davis MM. Science. 1991;254:1788. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- 52.Lee KH, Wucherpfennig K, Wiley DC. Nature Immunology. 2001;2:501. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 53.Cammarota G, Scheirle A, Takacs B, Doran DM, Knorr R, Bannwarth W, Guardiola J, Sinigaglia F. Nature. 1992;356:799. doi: 10.1038/356799a0. [DOI] [PubMed] [Google Scholar]
- 54.Wang JH, Meijers R, Xiong Y, Liu JH, Sakihama T, Zhang R, Joachimiak A, Reinherz EL. Proc. Nat. Acad. Sci. USA. 2001;98:10799. doi: 10.1073/pnas.191124098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brogdon J, Eckels DD, Davies C, White S, Doyle C. J. Immunol. 1998;161:5472. [PubMed] [Google Scholar]
- 56.Wang Q, Malerbe L, Zhang D, Zingler K, Glaichenhaus N, Killeen N. J. Immunol. 2001;167:4311. doi: 10.4049/jimmunol.167.8.4311. [DOI] [PubMed] [Google Scholar]
- 57.Amigorena S. Journal de la Societe de Biologie. 2001;195:25. [PubMed] [Google Scholar]
- 58.Chaput N, Schartz NE, Andre F, Zitvogel L. Adv. Exp. Med. Bio. 2003;532:215. doi: 10.1007/978-1-4615-0081-0_17. [DOI] [PubMed] [Google Scholar]
- 59.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. J. Cell Science. 2000;113:3365. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 60.Geminard C, de Gassart A, Vidal M. Biocell. 2002;26:205. [PubMed] [Google Scholar]
- 61.Schartz NE, Chaput N, Andre F, Zitvogel L. Current Opinion in Mol. Therapeu. 2002;4:372. [PubMed] [Google Scholar]
- 62.Nag B, Arimilli S, Mukku PV, Astafieva I. J. Biol. Chem. 1996;271:10413. doi: 10.1074/jbc.271.17.10413. [DOI] [PubMed] [Google Scholar]
- 63.Arimilli S, Cardoso C, Mukku P, Baichwal V, Nag B. J. Biol. Chem. 1995;270:971. doi: 10.1074/jbc.270.2.971. [DOI] [PubMed] [Google Scholar]
- 64.Kozono H, White J, Clements J, Marrack P, Kappler J. Nature. 1994;369:151. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- 65.Stewart MA, Dunsavage MB, Islar J, Mukku P, Nag B, Reich EP. J. Allerg. Clin. Immun. 1997;2(1 Pt):S117. [Google Scholar]
- 66.Appel H, Gauthier L, Pyrdol J, Wucherpfennig KW. J. Bio. Chem. 2000;275:312. doi: 10.1074/jbc.275.1.312. [DOI] [PubMed] [Google Scholar]
- 67.Appel H, Seth NP, Gauthier L, Wucherpfennig KW. J. Immunol. 2001;166:5279. doi: 10.4049/jimmunol.166.8.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burrows GG, Bebo BF, Jr, Adlard KL, Vandenbark AA, Offner H. J. Immunol. 1998;161:5987. [PubMed] [Google Scholar]
- 69.Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Wegmann KW, Offner H, Vandenbark AA. Protein Engineering. 1999;12:771. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- 70.Burrows GG, Chou YK, Wang C, Chang JW, Finn TP, Culbertson NE, Kim J, Bourdette DN, Lewinsohn DA, Lewinsohn DM, Ikeda M, Yoshioka T, Allen CN, Offner H, Vandenbark AA. J. Immunol. 2001;167:4386. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- 71.Huan JY, Mooney JL, Chou YK, Edwards DM, Rich C, Link JM, Vandenbark AA, Bourdette DN, Bachinger HP, Burrows GC. J. Chem. Tech. Biotech. 2005;80:2. doi: 10.1002/jctb.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL. Nature. 1993;364:33. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 73.Madden DR, Gorga JC, Strominger JL, Wiley DC. Cell. 1992;70:1035. doi: 10.1016/0092-8674(92)90252-8. [DOI] [PubMed] [Google Scholar]
- 74.Gorga JC, Madden DR, Prendergast JK, Wiley DC, Strominger JL. Proteins. 1992;12:87. doi: 10.1002/prot.340120110. [DOI] [PubMed] [Google Scholar]
- 75.Jardetzky TS, Lane WS, Robinson RA, Madden DR, Wiley DC. Nature. 1991;353:326. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- 76.Madden DR, Gorga JC, Strominger JL, Wiley DC. Nature. 1991;353:321. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- 77.Fremont DH, Hendrickson WA, Marrack P, Kappler J. Science. 1996;272:1001. doi: 10.1126/science.272.5264.1001. [DOI] [PubMed] [Google Scholar]
- 78.Chang JW, Mechling DE, Bachinger HP, Burrows GG. J. Biol. Chem. 2001;276:24170. doi: 10.1074/jbc.M101808200. [DOI] [PubMed] [Google Scholar]
- 79.Burrows GG, Bebo BF, Jr, Adlard KL, Vandenpark AA, Offner H. J. Immunol. 1998;161:5987. [PubMed] [Google Scholar]
- 80.Burrows GG, Adlard KL, Bebo BF, Jr, Chang JW, Tenditnyy K, Vandenbark AA, Offner H. J. Immunol. 2000;164:6366. doi: 10.4049/jimmunol.164.12.6366. [DOI] [PubMed] [Google Scholar]
- 81.Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. J. Immunol. 2003;171(4):1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- 82.McMahan RH, Watson L, Meza-Romero R, Burrows GG, Bourdette DN, Buenafe AC. J. Biol. Chem. 2003;278:30961. doi: 10.1074/jbc.M300628200. [DOI] [PubMed] [Google Scholar]
- 83.Vandenbark AA, Rich C, Mooney JL, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG. J. Immunol. 2003;171(1):127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- 84.Huan J, Subramanian S, Rich C, Mooney J, Jones DNBR, Vandenbark AA, Burrows GG. J. Immunol. 2004;172:4556. doi: 10.4049/jimmunol.172.7.4556. [DOI] [PubMed] [Google Scholar]
- 85.Rhode PR, Burkhardt M, Jiao J, Siddiqui AH, Huang GP, Wong HC. J. Immunol. 1996;157:4885. [PubMed] [Google Scholar]
- 86.Masteller EL, Ferlin WM, Judkowski V, Wilson D, Glaichenhaus N, Bluestone JA. J. Immunol. 2003;171:5587. doi: 10.4049/jimmunol.171.10.5587. [DOI] [PubMed] [Google Scholar]
- 87.Swanborg RH, Gould KE, Stepaniak JA. J. Immunol. 1994;153:2352. [PubMed] [Google Scholar]
- 88.Gould KE, Stepaniak JA, Swanborg RH. J. Neuroimmunol. 1994;54:145. doi: 10.1016/0165-5728(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 89.Stepaniak JA, Gould KE, Sun D, Swanborg RH. J. Immunol. 1995;155:2762. [PubMed] [Google Scholar]
- 90.Begolka WS, Vanderlugt CL, Rahbe SM, Miller SD. J. Immunol. 1998;161:4437. [PubMed] [Google Scholar]
- 91.Gordon EJ, Myers KJ, Dougherty JP, Rosen H, Ron Y. J . Neuroimmunol. 1995;62:153. doi: 10.1016/0165-5728(95)00120-2. [DOI] [PubMed] [Google Scholar]
- 92.Theien BE, Vanderlugt CL, Eager TN, Nickerson-Nutter G, Nazareno R, Kuchroo VK, Miller SD. J. Clin. Invest. 2001;107:995. doi: 10.1172/JCI11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. J. Neurosci. Res. 2001;65:529. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- 94.Ito A, Matejuk A, Hopke C, Drought H, Dwyer J, Zamora A, Subramanian S, Vandenbark AA, Offner H. J. Immunol. 2003;170:4802. doi: 10.4049/jimmunol.170.9.4802. [DOI] [PubMed] [Google Scholar]
- 95.Ploix C, Lo D, Carson MJ. J. Immunol. 2001;167:6724. doi: 10.4049/jimmunol.167.12.6724. [DOI] [PubMed] [Google Scholar]
- 96.Schutyser E, Struyf S, VanDamme J. Cytokine & Growth Factor Rev. 2003;14:409. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 97.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. J. Exp. Med. 1998;187:875. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romagnani P. Molec. Immunol. 2002;38:881. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 99.Matejuk A, Vandenbark AA, Burrows GG, Bebo BF, Jr, Offner H. J. Immunol. 2000;164:3924. doi: 10.4049/jimmunol.164.7.3924. [DOI] [PubMed] [Google Scholar]
- 100.Sharma SD, Nag B, Su XM, Green D, Spack E, Clark BR, Sriram S. Proc. Natl. Acad. Sci. USA. 1991;88:11465. doi: 10.1073/pnas.88.24.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. J. Immunol. 2003;171:1934. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- 102.Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, Kurosaki T. EMBO J. 1995;14:2499. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Science. 1994;263:1136. [PubMed] [Google Scholar]
- 104.Rhee SG. J. Biol. Chem. 1992;267:12393. [PubMed] [Google Scholar]
- 105.Clipstone NA, Fiorentino DF, Crabtree GR. J. Biol. Chem. 1994;269:26431. [PubMed] [Google Scholar]
- 106.Baeuerle PA, Baltimore D. Science. 1988;242:540. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 107.Whitmarsh AJ. J. Mol. Med. 1996;74:589. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 108.Macian F, Lopez-Rodriguez C, Rao A. Oncogene. 2001;20:2476. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 109.Sloan-Lancaster J, Steinberg TH, Allen PM. J. Exp. Med. 1996;184:1525. doi: 10.1084/jem.184.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG. J. Immunol. 2003;171:127. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- 111.Lin J, Weiss A. J. Cell Science. 2001;114:243. doi: 10.1242/jcs.114.2.243. [DOI] [PubMed] [Google Scholar]