Abstract
The blood-testis barrier (BTB) is a unique ultrastructure in the testis which creates a specialized microenvironment in the seminiferous epithelium for post-meiotic germ cell development and to maintain an immunological barrier. In this report, we have demonstrated unequivocally that a functional and intact BTB is crucial for initiation of spermatogenesis in particular differentiation of spermatogonial stem cells (SSCs). It was shown that adult rats (~300 gm body weight, b.w.) treated with adjudin at 50 (low-dose) or 250 (high-dose) mg/kg b.w. by gavage led to germ cell depletion from the seminiferous tubules and >98% of the tubules were devoid of germ cells by ~2-week and rats became infertile in both groups after the sperm reserve in the epididymis was exhausted. While the population of SSC/spermatogonia in the seminiferous tubules from both groups was similar to normal rats, only rats from the low-dose group were capable of re-initiating spermatogenesis by 20-week and by 30-week, greater than 75% of the tubules displayed normal spermatogenesis and the fertility of these rats rebounded. Detailed analysis by dual-labeled immunofluorescence analysis and a functional BTB integrity assay revealed that in both treatment groups, the BTB was disrupted from 6- to 12-week. However, the disrupted BTB “resealed” in the low, but not in the high, dose group. Our findings illustrate that that SSC/spermatogonia failed to differentiate into spermatocytes beyond Aaligned spermatogonia in the high-dose group with a disrupted BTB. In short, these findings illustrate the critical significance of BTB for re-initiation of spermatogenesis besides SSC and spermatogonia.
Keywords: testis, blood-testis barrier, seminiferous epithelial cycle, spermatogonia, adjudin, spermatogonial stem cells
Introduction
Spermatogonial stem cells (SSCs) are a subpopulation (~10%) of Asingle (As) spermatogonia which can be identified based on transplantation analysis (Nakagawa, et al. 2007). During spermatogenesis, As spermatogonia form pairs of cells called Apaired (Apr)-spermatogonia and then chains of cells up to 16 cells known as Aaligned (Aal)-spermatogonia which are connected by intercellular bridges and synchronized with the spermatogenic wave (de Rooij and Russell 2000). It is noted that As, Apr and Aal spermatogonia are called undifferentiated spermatogonia. The Aal spermatogonia then further divide and differentiate to form the primary spermatocytes which enter meiotic prophase (de Rooij and Russell 2000). In rodents, such as mice, one SSC (diploid, 2n) undergoes ten mitotic divisions before entering meiotic prophase to be followed by two meiotic divisions to give rise to 4096 spermatids (haploid, 1n) theoretically (Ehmcke, et al. 2006). However, more than 75% of the spermatids undergo apoptosis to avoid overwhelming the fixed number of Sertoli cells in the seminiferous epithelium in rodent testes (Cheng and Mruk 2010). In short, even though SSCs account for <0.03% of all the germ cells in the mammalian testes (Tegelenbosch and de Rooij 1993), they are capable of sustaining the production of >100 million sperm each day in a typical man from puberty through the entire adulthood via self-renewal, mitosis and meiosis during spermatogenesis. Furthermore, Apr spermatogonia were shown to be capable of switching back to become “true” SSC via de-differentiation (Brawley and Matunis 2004; Nakagawa et al. 2007). Nonetheless, it is expected that if SSCs are all depleted, spermatogenesis would halt, leading to azoospermia and infertility. Indeed, previous studies have shown that the duration of infertility after exposure to irradiation and chemotherapy in humans and rodents correlated with the survival rate of SSCs (Meistrich 1986). However, despite the importance of preservation of SSCs, these progenitor cells alone are not sufficient to maintain spermatogenesis. This was demonstrated in earlier studies in which prolonged azoospermia were found in animals exposed to toxicants even though type A spermatogonia in the seminiferous tubules were persisted in treated animals (Boekelheide and Hall 1991; Meistrich, et al. 1999). It remained unclear why did the existing spermatogonia in the seminiferous epithelium fail to re-initiate spermatogenesis and repopulate the tubules in the testes of these rats.
It is known that SSCs and As, Apr and Aal spermatogonia are found in areas of the seminiferous epithelium that border the interstitial tissue known as the spermatogonial stem cell niche (de Rooij 2009), which are located adjacent to but “outside” of the BTB, nearing the interstitium. In rats, leptotene spermatocytes are known to give rise to zygotene and pachytene spermatocytes to enter meiotic prophase by age 18 post-partum when the BTB is established, illustrating the significance of BTB in spermatogenesis. This possibility is further supported by an earlier study in which treatment of neonatal rats with diethylstilbestrol (DES) that delayed the formation of BTB in rats also delayed differentiation of spermatogonia and thus meiosis (Toyama, et al. 2001). Earlier studies from our laboratory have shown that adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, C15H12Cl2N4O, Mr 335.18, formerly called AF-2364] is a potential male contraceptive drug that exerts its effects by perturbing germ cell adhesion in the seminiferous epithelium, most notably at the apical ectoplasmic specialization (apical ES, a testis-specific adherens junction type). Furthermore, it was shown that when two doses of adjudin at 50 mg/kg b.w. was administered by gavage, transient infertility was induced in rats since fertility rebounded in all the treated rats (Cheng, et al. 2005; Mok, et al. 2011). However, it was also noted that in some rats treated with multiple doses of adjudin at 37.5 – 50 mg/kg b.w. (by gavage), they failed to have the fertility rebounded (Cheng et al. 2005; Mok et al. 2011). These findings thus prompted us to speculate that the adhesion of SSC and/or spermatogonia, and perhaps the BTB integrity, might have disrupted in rats exposed to multiple doses of adjudin, which in turn led to the failure for their fertility to rebound. Herein, we sought to examine the above speculations with high doses of adjudin versus low dose and control rats. Also, if the BTB was disrupted by adjudin, would it be able to re-establish its functionality over time. We also examined the impact of the BTB on the re-initiation of spermatogenesis in the treated rats. These are the subjects of this present report.
Materials and Methods
Animals and antibodies
Sprague-Dawley (outbred) rats were purchased from Charles River Laboratories (Kingston, NY). All animals were housed at the Rockefeller University Comparative Bioscience Center (CBC) with 2 rats per cage. Each rat had free access to rat chow and water ad libitum under controlled temperature (22°C) and constant light-dark cycles (12 hr of light and 12 hr of darkness). The Rockefeller University laboratory animal facilities have been fully accredited by the American Association for Accreditation of Laboratory Animal Care. These animals were maintained in accordance with the applicable portions of the Animal Welfare Act and the guidelines in the Department of Health and Human Services publication Guide for the Care and Use of Laboratory Animals. The use of Sprague-Dawley rats in this reported was approved by the Rockefeller University Institutional Animal Care and Use Committee with Protocol numbers 06018 and 09016. Antibodies were obtained commercially from various vendors with their working dilutions listed in Table 1.
Table 1.
Antibodies used for different experiments in this report
| Antibody | Vendor | Catalog no. | Application | Working dilution for IB or (IF) |
|---|---|---|---|---|
| Mouse anti-Plzf | Calbiochem(San Diego, CA) | OP128 | IF | (1:125) |
| Rabbit anti-Plzf | Santa Cruz Biotechnology(Santa Cruz, CA) | sc-22839 | IB | 1:1000 |
| Rabbit anti-Utf1 | Chemicon/Millipore (Billerica, MA) | AB3383 | IB, IF | 1:1000, (1:800) |
| Goat anti-Oct4 | Santa Cruz Biotechnology | sc-8629 | IB | 1:1000 |
| Mouse anti-Thy-1 | Abcam(Cambridge, MA) | ab225 | IB | 1:1000 |
| Rabbit anti-claudin-11 | Invitrogen(Carlsbad, CA) | 36–4500 | IB, IF | 1:125, (1:100) |
| Rabbit anti-occludin | Invitrogen | 71–1500 | IB, IF | 1:125, (1:100) |
| Rabbit anti-N-cadherin | Santa Cruz Biotechnology | sc-7939 | IB | 1:200 |
| Rabbit anti-β1 integrin | Millipore (Billerica, MA) | AMB1922Z | IB | 1:1000 |
| Rabbit anti-JAM-C | Invitrogen | 40–9000 | IB | 1:150 |
| Mouse anti-ZO-1 | Invitrogen | 33–9188 | IF | (1:100) |
| Goat anti-actin | Santa Cruz Biotechnology | sc-1616 | IB | 1:300 |
Antibodies used in this study cross-reacted with the corresponding rat proteins as indicated by the manufactures. IB, immunoblotting; IF, immunofluorescence microscopy.
Administration of adjudin to adult rats to induce infertility by depleting germ cells from the seminiferous epithelium
A suspension of 20 mg/ml of adjudin was prepared in 0.25% methylcellulose (Sigma-Aldrich) as described (Cheng et al. 2005). A total of three treatment regimens were used in this study: adult rats (~300 gm body weight, b.w.) rats received a single dose of adjudin at 50 (low-dose), 125 or 250 (high-dose) mg/kg body weight (b.w.) via gavage at time 0 as described (Cheng, et al. 2001) (see Table 2). A vehicle control group was also included in which rats received no adjudin (see Table 2). At specified time points with n = 3 to 5 rats for controls, and all treated rats in the three treatment groups, testes were collected and the body and testis weight were recorded. In each animal to be used for morphological and biochemical analysis, one of the testes was fixed in Bouin’s fixative to be used for subsequent histological analysis by hematoxylin and eosin staining using paraffin sections, the other testis was frozen in liquid nitrogen and stored at −80 °C to be used for lysate preparation for immunoblotting and to prepare frozen sections for dual-labeled immunofluorescence analysis. A detailed description of rat usage for the three regimens and the control group is listed in Table 2. It must be noted that in the animal groups at 6-, 20- and 30-wk, while only one rat was dedicated for histological analysis and subjected to hematoxylin and eosin staining following paraffin embedding and sectioning as shown in Fig. 2, one of the testes from each animal used for BTB integrity assay (n = 2 rats) was snap-frozen in liquid nitrogen also used to process for histological analysis but using frozen sections and Mayer’s hematoxylin staining (and also for biochemical analysis) with the other testis used to assess the diffusion of fluorescence tag across the BTB, thus n = 3 rats for these time points for morphological analysis, and data from all three rats were consistent for each time point.
Table 2.
Animal usage for the three treatment regimens versus control group
| Control group | Adjudin at 50 mg/kg b.w. (low dose group) | Adjudin at 125 mg/kg b.w. (high dose group) | Adjudin at 250 mg/kg b.w. (high dose group) | ||||
|---|---|---|---|---|---|---|---|
| Time after treatment | Morphological and biochemical analysis* | Morphological and biochemical analysis* | BTB integrity assay** | Morphological and biochemical analysis* | BTB integrity assay | Morphological and biochemical analysis* | BTB integrity assay |
| Time 0 | 3 | 3 | 2 (4) | 3 | 2 (4) | 3 | 2 (4) |
| 6 hour | 3 | 3 | 3 | ||||
| 9 hour | 3 | 3 | 3 | ||||
| 12 hour | 3 | 3 | 3 | ||||
| 1 Day | 3 | 3 | 3 | ||||
| 2 Day | 3 | 3 | 3 | ||||
| 4 Day | 2 | 3 | 3 | 3 | |||
| 7 Day | 3 | 3 | 3 | ||||
| 2 week | 2 | 3 | 2 (4) | 3 | 2 (4) | 3 | 2 (4) |
| 6 week | 1*** | 2 (4) | 1*** | 2(4) | 1*** | 2(4) | |
| 8 week | 2 | 2 | 2 | ||||
| 12 week | 3 | 3 | 3 | 3 | |||
| 20 week | 1*** | 2 (4) | 1*** | 2 (4) | 1*** | 2 (4) | |
| 24 week | 2 | 2 | 2 | ||||
| 30 week | 3 | 1*** | 2 (4) | 1*** | 2(4) | 1*** | 2(4) |
| Total number of rats used | 13 | 37 | 30 | 37 | 30 | 37 | 30 |
For the testes from rats specified for morphological and biochemical analysis, one of the testes from each rat was snap-frozen immediately in liquid nitrogen and stored at −80 °C until used for: (i) lysate preparation (to quantify the steady-level of spermatogonial markers and other pertinent BTB proteins) and (ii) preparation of frozen sections to score the number of spermatogonia/spermatogonial stem cells, and localization of BTB proteins by dual-labeled immunofluorescence analysis. The other testis of the same rat was fixed in Bouin’s fixative to be used to prepare paraffin sections for histological analysis following hematoxylin and eosin staining. For fertility tests, rats used for this test at specified time points reported in Fig. 1 were obtained from rats in the “Morphological and biochemical analysis” group, and housed with the corresponding female rats (~270 gm b.w.) for 4 days before they were terminated for analysis. In some selected groups, such as rats from 20-, 24- and 30-wk, they were grouped and counted as a single group of 20/24-wk for biochemical analysis, or 24-/30-wk for morphological analysis.
For BTB integrity assay, n = 2 rats for each treatment group, we also included a positive control group (n = 2 rats) wherein rats were treated with CdCl2 at 3 mg/kg b.w. body weight (i.p.) which is known to induce BTB disruption. Also included is a negative untreated control group with n = 2 rats in each BTB integrity assay. Thus, 4 additional rats (in brackets) were used for the two control groups in each BTB integrity assay.
For morphological analysis, a testis from one rat were used for paraffin embedding and sectioning for H&E staining for histological analysis, and the other testis for snap frozen in liquid nitrogen to be used for lysate preparation for immunoblot analysis. However, one testis was also removed from each of the two rats that were used for BTB integrity assay (with the other testis for the BTB integrity assay) and snap frozen in liquid nitrogen, frozen sections were obtained, fixed in Bouin’s fixative, and stained with Mayer’s hematoxylin for histological analysis to assess the status of spermatogenesis; or for lysate preparation for immunoblot analysis. Thus, it is noted that n = 3 in these groups.
Figure 2. Histological analysis of testes in low- and high-dose adjudin treated groups versus controls to monitor changes in the status of spermatogenesis.
Adult rats (~300 g b.w.) were treated with a single low- or high-dose of adjudin at 50 or 250 mg/kg b.w., respectively, at time 0 (controls). Thereafter, rats (n = 3) were terminated at specified time points for histological analysis using paraffin sections by hematoxylin and eosin staining. (A) Cross-sections of testes from the low-dose group with rats terminated at 0- (a), 8- (b), and 12- (c) hour (H); 1- (d), 4- (e), and 7- (f) day (D); and 2- (g), 6- (h), 12- (i), 20- (j), 24- (k), 30- (l) week (W). (B) Cross-sections of testes from the high-dose group. Germ cell sloughing was detected in 6-to 8-hour after adjudin administration at 50 or 250 mg/kg b.w. By 2-week, >98% of the tubules were devoid of germ cells in both treated groups. By 20-week, germ cell repopulation was detected in most tubules in rats from the low-dose group and >75% of the tubules displayed normal spermatogenesis, however, tubules from rats in the high-dose group remained devoid of germ cells by 30-week. Scale bar = 100 μm, which applies to all micrographs in all panels.
BTB integrity assay
The BTB integrity in vivo in rats was assessed by an assay established in our laboratory as earlier described (Li, et al. 2006), which is based on the ability of an intact BTB to block the diffusion of a small fluorescence tag from the basal to the apical compartment of the seminiferous epithelium. Rats were under anesthesia with ketamine HCl (60 mg/kg b.w.) together with an analgesic xylazine (10 mg/kg b.w.), which were administered intramuscularly (i.m.). A small incision, about 1-cm, was made in the area over the jugular vein to expose the blood vessel, about 1.5 mg FITC-conjugated inulin (Mr 4.6 kDa) (Sigma-Aldrich) in 300 μl PBS was administered into the jugular vein with a 28-gauge needle, and animals were allowed to recover for about 30–45 min. Then, rats were euthanized by CO2 asphyxiation. Testes were removed immediately and snap-frozen with liquid nitrogen. Testes were then embedded with Tissue-Tek O.C.T. (optimal cutting temperature) compound (Sakura) and frozen sections (10 μm) were obtained by using a Microm HM500M microtome at −20°C, placed on microscopic slides, air-dried and the distribution of inulin-FITC in the seminiferous epithelium was visualized by using an Olympus BX61 Fluorescence Microscope. Rats treated with CdCl2 at 5 mg/kg b.w. i.p. for 3-day were used for the BTB integrity assay as described above to serve as positive control while normal rat testes served as negative control. Treatment and control groups were processed in the same experimental session for comparison. The distribution of FITC (green fluorescence) in the seminiferous epithelium among tubules was monitored by fluorescence microscopy. BTB was considered damaged when fluorescence signal was no longer confined in the basal compartment but found in the adluminal compartment. The BTB integrity was semi-quantified by the distance traveled by the fluorescence signal from the basement membrane versus the diameter of the seminiferous tubule. Each time point from each treatment group contained 2 rats and at least 90 tubules were scored from each rat.
Immunoblot analysis
Lysates of testes were prepared in IP lysis buffer [50 mM Tris, pH 7.4, at 22 °C, containing 0.15 M NaCl, 1% Nonidet P-40 (vol/vol), 1 mM EGTA, 2 mM N-ethylmaleimide, 10% glycerol (vol/vol)] supplemented with protease inhibitor mixture (Sigma-Aldrich) and phosphatase inhibitor mixture I and II (Sigma-Aldrich) at a dilution of 1:100 to block protease and phosphatase activities as described (Lie, et al. 2010a; Lie, et al. 2010b). Protein concentration was estimated using a BioRad Dc Protein Assay kit and a BioRad Model 680 Spectrophotometry Reader. These lysates were used for immunoblot analysis with ~100 μg protein for each sample. Proteins were transferred to nitrocellulose membranes and detected by immunoblotting with antibodies list in Table 1. Chemiluminescence was performed using ECL kits (GE Healthcare, Waukesha, WI) in a FujiFilm LAS-4000 Mini Luminescent Image Analyzer. Actin served as a protein loading control. All samples within an experimental group were processed simultaneously to avoid inter-experimental variations with n = 3–4 independent experiments.
Dual-labeled immunofluorescence analysis
Frozen sections (7 μm) obtained in a cryostat at −20°C were fixed in either Bouin’s fixative or 4% paraformaldehyde (wt/vol) for 10 min, sections were then permeablized with 0.1% Triton X-100 in PBS (10 mM sodium phosphate, 0.15M NaCl, pH 7.4 at 22°C) (vol/vol) for 4 min. Sections were blocked using 5 % goat serum (vol/vol) with 0.1% or 1% BSA (wt/vol) alone for 30 min, to be followed by an overnight incubation of primary antibodies diluted in corresponding blocking solution at 4 °C or room temperature. Sections were then incubated with Alexa Fluor–conjugated secondary antibodies (Invitrogen; red fluorescence, Alexa Fluor 555; green fluorescence, Alexa Fluor 488) at 1:250 diluted with the corresponding blocking solution at room temperature. Sections were mounted with Vectorshield Antifade mounting media with DAPI for fluorescence microscopy. Fluorescence images were captured by an Olympus DP71 12.5 MPa digital camera interface to an Olympus BX61 fluorescence microscope using the Olympus MicroSuite Five Imaging software package (Version 1226) to obtain images in TIFF format. Image overlay and analysis were performed using PhotoShop in Adobe Creative Suite Design Premium software package (Version 3.0; Adobe Systems). All staining experiments were performed 2–3 times with different sets of testes. The number of Plzf- and Utf1-positive cells per cross-section of seminiferous tubule was quantified as described below. To reduce inter-experimental variations, testes from all time points within a treatment group versus controls were processed simultaneously in a single experimental session. Negative control included the use of mouse or rabbit IgG diluted in PBS to substitute the primary antibody to confirm that the immunofluorescence staining is not the result of an artifact.
Morphometric analysis
Morphometric analysis that scored Plzf and Utf1 positive cells in the seminiferous epithelium of rats from both control and the three treatment groups was performed essentially as earlier described (Bascian, et al. 2008; Forgione, et al. 2010; Pan, et al. 2009; van Bragt, et al. 2008a; Wikström, et al. 2004) with minor modifications. In brief, serial frozen sections (7 μm) of testes were used for scoring the number of Plzf and Utf1 positive cells by dual-labeled immunofluorescence analysis as follows. Each testis was first cut into two halves horizontally at the median line and each tissue block was mounted onto the cryostat. During serial sectioning, every fifth section was removed and collected on microscopic slide inside the cryostat at −20°C, and a total of 5 sections was collected from each testis of a treated or control rat. Thus, the data presented herein for each time point, it was the representative result from a total of 15 cross-sections from 3 testes. Plzf and Utf1 positive cells were scored from randomly selected ~60 to 80 seminiferous tubules in ~20 fields using the 10X Objective in the Olymphus BX 61 fluorescence microscope. The positive Plzf and Utf1 positive cells were further verified using the 40X Objective to confirm the fluorescence staining is not the result of an artifact. We selected only cross-sectioned (i.e., round shaped) seminiferous tubules for scoring the Plzf and Utf1 positive cells instead of obliquely sectioned tubules for scoring in all treatment and control groups.
Fertility tests
Each male rat from the low- and high-dose groups versus controls at specified time point was housed separately with a virgin female rat (~300 g b.w.) for 4–5 days. The female rats were then housed separately for 21-day to allow the completion of gestation period (21-day) with free access to water and standard chow. Number of pups was recorded and any changes in gross morphology were noted.
General Methods and Statistical Analysis
Each experiment was repeated at least three times or each data point has 3 to 5 animals. Statistical analysis of data for Plzf- and Utf1-positive cells scoring was performed with GB-STAT software package (Version 7.0; Dynamic Microsystems) using one one-way ANOVA followed by Dunnett’s test as described (Yan, et al. 2008). Data from immunoblotting and BTB integrity quantification was analysis by two-way ANOVA followed by Newman-Keul’s test.
Results
Differential effects of low- and high-dose adjudin on the resumption of spermatogenesis
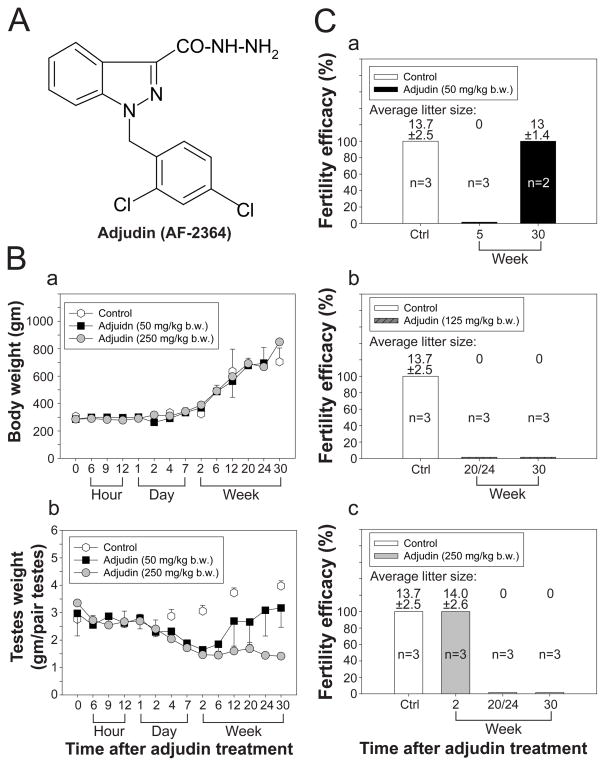
Previous studies have shown that adjudin (Fig. 1A) at 50 mg/kg b.w. (by gavage) induced transient infertility in rats by depleting germ cells from the seminiferous epithelium (Cheng et al. 2005; Mok et al. 2011). Thereafter, spermatogenesis resumed and germ cells gradually repopulated the seminiferous tubules suggested that SSCs were preserved after challenged by adjudin. However, when adjudin was treated at multiple doses, fertility failed to rebound in a small percentage of rats (Cheng et al. 2005; Mok et al. 2011), suggesting that SSCs in some rats might have been depleted and/or other factors which were crucial to spermatogenesis were disrupted. Herein, the effects of a single high-dose of adjudin at 125 or 250 mg/kg b.w. to spermatogenesis versus 50 mg/kg b.w. on fertility were examined. After administration a single dose of 50, 125 or 250 mg/kg b.w. of adjudin to adult rats (~300 gm b.w. at time 0, by gavage) versus controls (normal or vehicle only), a decrease in testis weight, but not body weight, was detected (Fig. 1B:a–b). It is noted that all the rats used in the present study had free access to standard rat chow and water ad libitum without dietary restriction, changes in their body weight over time, such as reaching ~700–750 g at the end of the study period by 30-wk as reported herein is consistent with earlier findings (Hubert, et al. 2000). It was also noted that by 12-week post treatment, testis weight started to rebound gradually in the low- but not the high-dose treated group (Fig. 1B:b), which is likely the result of the resumption of spermatogenesis. These findings are consistent with results of the fertility tests (Fig. 1C) since fertility rebounded in the low- but not in the two high-dose groups (Fig. 1C: a vs. b,c). It is noted that by 2-week post treatment, rats remained fertile in the high-dose group (Fig. 1C:c) because of the sperm reserve in the epididymis, since adjudin had no effects on epididymal sperm (Cheng et al. 2005).
Figure 1. Changes in body weight, testis weight and fertility in rats treated with a low- and high-dose adjudin.
(A) The structural formula of adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, C15H12Cl2N4O, Mr 335.18]. (B) Effects of adjudin on body weight (B:a) and testis weight (B:b) versus control groups in which rats were treated with adjudin at low- (50 mg/kg b.w.) or high- (125 or 250 mg/kg b.w.) dose by gavage. Each bar = mean ± SD of n = 3–6 rats. There was no significant change of body weight among the three groups, but there was a decrease in testis weight in both low- and high-dose groups beginning by 4-day post treatment. Testis weight in the low-dose group, however, started to rebound by 6-week but not in the high-dose group. (C) Results of fertility tests of control group versus low- and high-dose treated groups. The litter size is the number of pups that was counted with a male:female ratio of about 1:1, and no abnormal gross morphology was detected in all pups. Fertility of rats in the low-dose treated group rebounded by 30-week but not in the two high-dose groups.
The results reported in Fig. 1 that illustrate changes in testicular weight and the status of fertility in rats from the low- and high-dose groups versus controls are consistent with findings of histological analysis shown in Fig. 2 which assessed the status of spermatogenesis. Depletion of germ cells following adjudin treatment in the low- (Fig. 2A) and high- (Fig. 2B) dose groups was detected as early as 6- to 8-hr following treatment since immature germ cells are found in the tubule lumen, and by 2-week, the tubules were mostly devoid of germ cells. However, spermatogenesis resumed in the low-dose group beginning 20-week and by 30-week, >75% of the tubules displayed normal spermatogenesis (Fig. 2A:l vs. b–k and a) and fertility also rebounded in these rats (see Fig. 1C:a vs. b, c). However, resumption of spermatogenesis was not detected in the high-dose group by 30-week (Fig. 2B:l vs. b–k and a) and these findings are also consistent with the fertility status of this animal group (Fig. 1C: b, c vs. a).
Adjudin-induced germ cell loss and infertility in both low- and high-dose adjudin treated groups are associated with a surge in the expression of SSC/spermatogonial markers
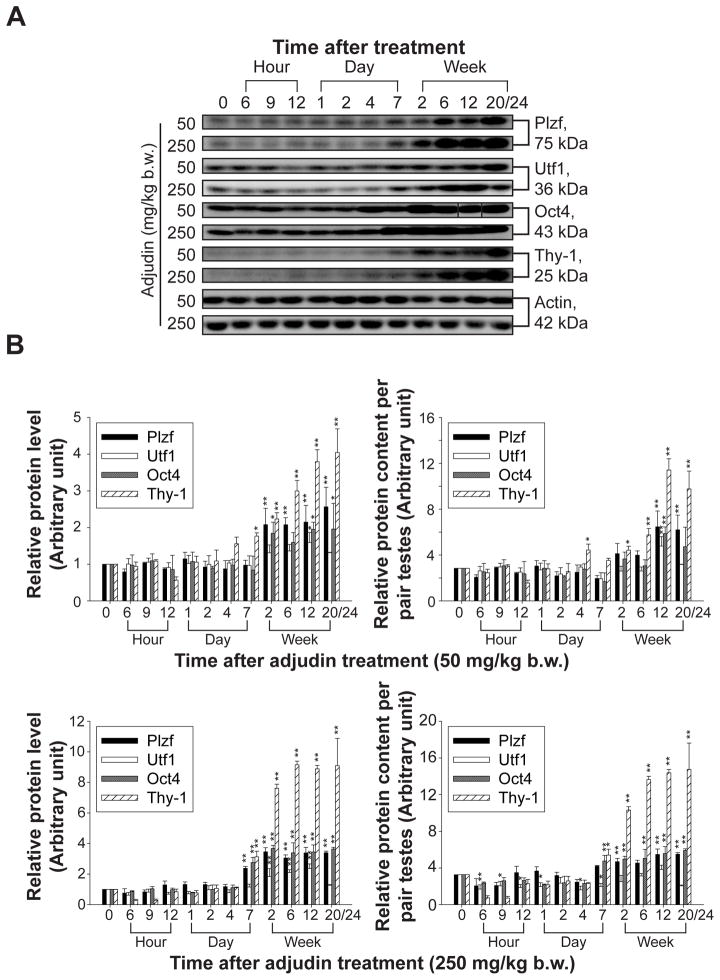
The ability of spermatogenesis recovery in the low-dose treated group suggests that spermatogonia, especially SSCs were not depleted from the epithelium after adjudin treatment. On the other hand, failure of spermatogenesis resumption in high-dose treated group was likely due to a depletion of SSCs and spermatogonia from the epithelium. In order to assess any changes and/or loss in spermatogonia and SSCs, several putative markers for early A spermatogonia which included SSCs were used (Phillips, et al. 2010) for immunoblot analysis. These include promyelocytic leukemia zinc finger protein [Plzf, a transcriptional repressor essential for SSC self-renewal (Buaas, et al. 2004)], undifferentiated embryonic transcription factor 1 [Utf1, a transcription factor involved in embryonic stem cell differentiation (van den Boom, et al. 2007)], octamer-4 [Oct4, a transcription factor essential for maintaining pluripotency and self-renewal properties of SSCs (Dann, et al. 2008)] and thymus cell antigen-1 [Thy-1, a surface antigen of SSCs (Kubota, et al. 2003)]. Immunoblotting results shown in Fig. 3A indicated that in both treatment groups, the steady-state levels of the four spermatogonial markers were significantly induced at the time of adjudin-induced germ cell loss from the seminiferous epithelium (see Fig. 2 vs. Fig. 3). When virtually all the advanced germ cells (e.g., elongated/elongating spermatids, round spermatids, spermatocytes) were depleted from the seminiferous epithelium by 2- to 20/24-week after adjudin treatment (see Fig. 2), results shown in Fig. 3A and Fig. 3B (left panels) could be an over-estimate, which was attributed by an increase in the contribution of proteins from spermatogonia/SSCs being analyzed in these samples. Therefore results shown in the left panels in Fig. 3B based on the scanned data of Fig. 3A were corrected by changes in the testis weight (see Fig. 1B:b) and expressed as protein content per pair testes and shown in the right panels in Fig. 3B. It is noted that the fold of increases in these four spermatogonial markers shown in Fig. 3A could not be accounted for by changes in the cellular composition being analyzed by immunoblottings (Fig. 3B, right panels). Collectively, these findings suggested that spermatogonia/SSCs in the high-dose group are not depleted and may not be significantly different from the low-dose group. The increase in the spermatogonial markers’ expression might be attributed to a physiological response in the testis to maintain the germline stem cell population and at the same time, there is a need for them to differentiate for the resumption of spermatogenesis. Thus, markers pertinent to the maintenance of self-renewal (e.g., Plzf, Oct4) and makers related to differentiation (e.g., Utf1) were up-regulated in both groups, possibly being used to maintain the spermatogonia/SSC population.
Figure 3. Changes in the steady-state protein levels of SSC and spermatogonial markers in low- and high-dose treated group versus normal rats at time 0.
(A) Rats treated with adjudin at 50 or 250 mg/kg b.w. were terminated at specified time points to obtain lysates of testes. Immunoblots of SSC/spermatogonial markers Plzf, Utf1, Oct4 and Thy-1 using lysates from low- and high-dose treated group with actin serve as a loading control. This is a representative set of data from three experiments. (B) Histograms summarizing immunoblot analysis data of (A) are shown on the left panels which are plotted by normalizing each data point against actin. Protein levels at 0 hour were arbitrarily set as 1. Right panels in (B) are histograms plotted by using data sets from the left panels but corrected against the declining testis weight (see Fig. 1B) and expressed as relative protein content per pair testes. Each bar = mean ± SD (n = 3). *P < 0.05; **P < 0.01.
Both low- and high-dose adjudin treated groups maintain similar number of SSC in the testes regardless their ability to re-initiate spermatogenesis
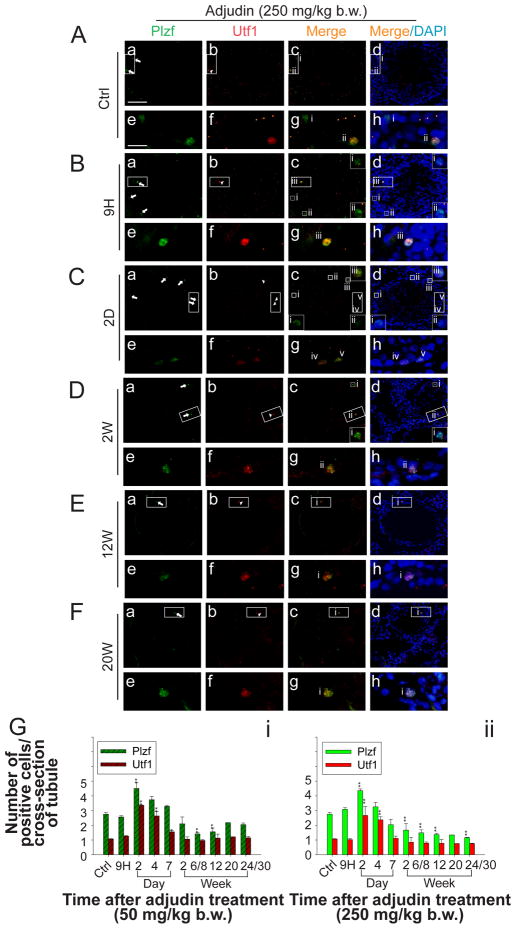
The number of SSCs/spermatogonia in both low- (Fig. S1) and high- (Fig. 4) dose groups versus controls were estimated by dual-labeled immunofluorescence analysis using specific antibodies against Plzf and Utf1 (see Table 1) to score Plzf- and Utf1-positive cells in cross-sections of testes. It is noted that the numbers of Plzf- and Utf1-positive cells were ~3 and ~1 per cross-section of tubules in normal rat testes reported herein (Fig. 4G: i–ii) and this was consistent with an earlier report (van Bragt, et al. 2008b). It is noted that the number of Plzf-positive cells representing As, Apr and long chain of Aal spermatogonia (up to 16 interconnected cells) (Suzuki, et al. 2009) in both groups increased transiently by ~1.5 folds on day 2 post treatment (Fig. S1 and Fig. 4A–G). Thereafter, the number of Plzf-positive cells in the low-dose group declined gradually to ~2 and by week 20 and maintained at a similar level till week 24/30, and this number was not significantly lower than that of the control (~2 versus ~3 Plzf-positive cells per cross-section to tubule between low-dose and control groups) (Fig. 4G:i) Whereas in high-dose group, the number of Plzf-positive cells was significantly reduced to ~1.2 by week 24/30. (Fig. 4G:ii). On the other hand, in both low- and high-dose groups, the number of Utf1-positive cells which were a subpopulation of Plzf-positive cells (van Bragt et al. 2008b) also showed a transient increase (~3-fold) on day 2 post adjudin treatment (Fig. 4G:i vs. ii). Thereafter, the number of Utf1-positive cells in both groups gradually decreased to ~1 which was similar to that of control (Fig. 4G: i, ii). Since Utf1-positive cells represent less advanced spermatogonia which are the As and Apr and short chain of Aal spermatogonia which include the SSCs (Oatley and Brinster 2006), it is likely that the number of SSCs was not reduced in both groups versus control group. Thus, the resumption of spermatogenesis via spermatogonial differentiation to repopulate the tubules detected in the low-dose group is not simply the result of the preservation of SSCs.
Figure 4. Change in the population of SSC/spermatogonia in the testis by scoring Plzf-and Utf1-positive cells per cross-section of tubule in rats from the low-dose versus high-dose adjudin treated group.
(A–F) Representative images illustrating the number of Plzf-(green) and Utf1- (red) positive cells per cross-section of tubule from rats terminated at specified time points after administration of adjudin at 250 mg/kg b.w. Magnified views of rectangular boxed area in (a–d) are shown in (e–h). Square boxes in selected micrographs in (a–d) are magnified and placed in the same panel with corresponding Roman numerals. (G) Number of Plzf- and Utf1-positive cells per cross-section of tubule was counted using frozen sections of testes at specified time points from rats in the low- (G: i) versus high- (G: ii) dose group. A transient increase in the number of Plzf- and Utf1-positive cells was observed in both groups two days after adjudin treatment. Thereafter, the number of Plzf-positive cells decreased gradually to ~2 in G:i and G:ii ~1.2 per tubule (versus ~3 in Ctrl). By 20-week, the number of Plzf-positive cells was not significantly different from Ctrl till 24- to 30-week in the low-dose group (G: i) but remained mildly lower in the high-dose group (G: ii). By 4-day after treatment, the number Utf1-positive cells started to decline gradually in both groups and similar to that of Ctrl until 24- to 30-week after treatment. Each bar = mean ± SD (n = 2–3 rats per time point) and a total of 120–160 tubules were randomly scored from each rat. Scale bar = 100 μm in (A:a) applies to b–d; scale bar = 25 μm in (A:e) which applies to f–h. *P < 0.05; **P < 0.01.
Effects of low- and high-dose adjudin treatment on the expression of constituent proteins at the BTB
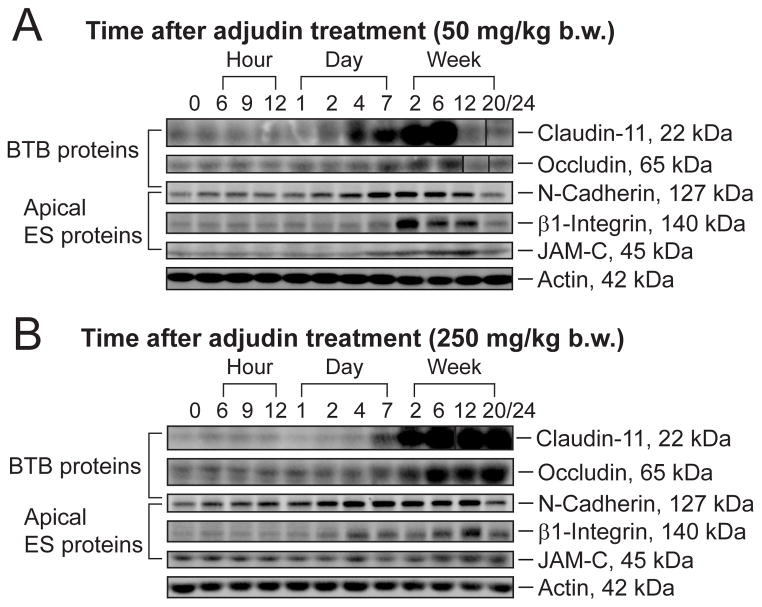
Besides SSCs, earlier studies have demonstrated the significance of the BTB to spermatogenesis (Setchell and Waites 1975), we thus examined the status of the BTB in both groups versus control rats. Immunoblot analysis was used to estimate the steady-state levels of various tight junction (TJ) (e.g., occludin, claudin-11) and basal ectoplasmic specialization (basal ES) (e.g., N-cadherin) proteins which are integral membrane proteins at the BTB for its maintenance. We also examined changes in apical ES proteins (e.g., N-cadherin, β1-integrin and JAM-C) which are restricted to the Sertoli cell-elongating spermatid interface at the apical ES site. It was shown that the steady-state levels of BTB proteins as well as those of the apical ES proteins were up-regulated in both groups versus controls after adjudin administration (Fig. 5A, B and Fig. S2A, B), even when the changes in testicular weight were taken into consideration because of the changes in cellular composition in the samples being analyzed (see right panel in Fig. S2A vs. B). Although there were up-regulations of BTB proteins in both treatment groups, they failed to maintain the BTB integrity (see below).
Figure 5. Change in the steady-state levels of TJ and apical ES proteins in the testes of rats from the low- versus high-dose adjudin treated group.
Rats treated with either 50 (A) or 250 (B) mg/kg b.w. of adjudin were terminated at specified time points to obtain lysates of testes for immunoblot analysis of BTB proteins: occludin, claudin-11, N-cadherin, and apical ES proteins: N-cadherin, β1-integrin, JAM-C, from rats in the low- versus high-dose group with actin serving as a loading control. The results shown here are representative data from three independent experiments. (C) Histograms summarizing results from (A–B) are shown on the left panels in (C) which are plotted by normalizing each data point against actin. Protein levels at 0 hour were arbitrarily set as 1. Right panels are histograms plotted by each data point on the left panels correcting against the decreasing testis weight (see Fig. 1B) and expressed as relative protein level per pair testes. Bars = mean ± SD (n = 3 rats). *P < 0.05; **P < 0.01.
Effects of low- and high-dose adjudin treatment on the localization of constituent proteins at the BTB
Dual-labeled immunofluorescence analysis was used to examine changes in the localization of occludin and ZO-1 in the seminiferous epithelium [note: occludin and ZO-1 is a major cell adhesion complex at the BTB with occludin serves as an integral membrane protein that binds to the adaptor protein ZO-1, which anchors the protein complex to the actin-based cytoskeleton]. In normal rat testes, occludin (red fluorescence) and ZO-1 (green fluorescence) co-localized near the basement membrane consistent with their localization at the BTB (Fig. 6A, B). However, by 6- to 12-week in both low- (Fig. 6A) and high- (Fig. 6B) dose groups, occludin (as well as ZO-1, but to a lesser extent) appeared to become mis-localized, diffusing away from the BTB site. However, the mis-localization of occludin and ZO-1 in the low-dose group was transient since by 20- and 24/30-week post treatment, both BTB proteins redistributed to the original BTB site, making them indistinguishable from control rats (Fig. 6A). Yet in the high-dose group, the mis-localization of both occludin and ZO-1 persisted until 20- to 24/30-week (Fig. 6Bvs. 6A) and failed to recover. This trend was identical when another BTB adhesion protein complex cluadin-11-ZO-1 which was investigated in both the low- and high-dose groups versus controls (see Fig. S3). This pattern of changes in protein localization at the BTB seemed to suggest that there was a transient and irreversible disruption of BTB at the low- and high-dose group, respectively.
Figure 6. Changes in the cellular localization of occludin and ZO-1 in the seminiferous epithelium of rats from the low- and high-dose adjudin treated groups versus normal (control) rats.
(A) and (B) illustrate the co-localization of occludin (red) and ZO-1 (green) in frozen sections of testes obtained from rats treated with adjudin at 50- (low-dose) or 250- (high-dose) mg/kg b.w., respectively. Occludin was found to co-localize with ZO-1 at the BTB in the seminiferous epithelium from tubules in control testes (A:a–d). In both treated groups, occludin and ZO-1 was found to redistribute from its original site and they appeared to become diffused and thickened within ~1-day after treatment. This pattern of changes in redistribution for occludin and ZO-1 became more obvious by 6- to 12-week. However, in the low-dose group, the localization of occludin and ZO-1 was indistinguishable from controls by 20- to 24/30-week. But this shifted redistribution of occludin and ZO-1 persisted until 30-week in testes of rats from the high-dose group. The data shown herein are representative results of an experiment which was repeated three times using samples from different sets of rat testes and yielded similar results. Bar in A:a (and B:a) = 50 μm, which applies to all other micrographs in the panel.
A study by using an in vivo assay to assess the BTB integrity in the low-dose versus high-dose treated group
We hypothesized that the resumption of spermatogenesis in the low-dose group relied on a functional BTB which rebounded following its transient disruption; however, the disrupted BTB in the high-dose group failed to recover, thereby impeding the re-initiation of spermatogenesis. In order to test this hypothesis, an in vivo BTB integrity assay was used to assess the BTB integrity in both treatment groups versus controls. It was noted that by 2-week post adjudin treatment, the BTB in rats from the low-dose treated group remained intact (Fig. 7A), similar to the control, since no fluorescence signal was found in the apical compartment of the epithelium illustrating the BTB integrity (Fig. 7A:a, f vs. c, h). This finding is consistent with earlier results showing that BTB of rats was not perturbed within 2 weeks after adjudin treatment at 50 mg/kg b.w. (Su, et al. 2010). However, in the high-dose groups (rats administered with adjudin at 125 or 250 mg/kg b.w.), BTB was found to be disrupted by 2-week after adjudin treatment. This was demonstrated by the presence of fluorescence signals in the apical compartment of the epithelium beyond the BTB, with some signals even found in the tubule lumen (Fig. 7A: d, i, e, j vs. a, f and b, g), similar to the positive controls in which rats were treated with CdCl2 which is known to disrupt the BTB irreversibly (Fig. 7A) (Setchell and Waites 1970). By week 6, even in the low-dose treated group, BTB was found to be disrupted (Fig. 7B). However, this disruption of BTB in the low-dose group was transient since BTB integrity rebounded by week 20 and persisted to 24/30 week as shown by the ability of BTB at those time points to block the traverse of fluorescence signals from entering the apical compartment, indistinguishable from the controls (Fig. 7C, D vs. B and A). On the other hand, the BTB integrity in the high-dose groups remained disrupted until week 24/30. These data are consistent with findings shown in Fig. 6 and Fig. S3, suggesting that SSCs alone is not sufficient to re-initiate spermatogenesis.
Figure 7. A study to assess the BTB integrity in vivo following treatment of adult rats with adjudin versus controls.
Localization of inulin-FITC (green) in frozen sections of testes following administration of the fluorescence tag at the jugular vein to assess the BTB integrity that blocked its movement from the basal to the apical compartment in the epithelium. (A) Inulin-FITC was administered to rats at the jugular vein in normal rats at time 0 and the diffusion of inulin-FITC was monitored ~45-min thereafter (a, negative control). Rats received CdCl2 (3 mg/kg b.w., via i.p.) at time 0 and used for BTB integrity assay on day 3 were served as positive control (b). Rat received adjudin at 50, 125 or 250 mg/kg b.w. were also used for the BTB integrity assay by 2-, 6-, 20- and 20 to 30- week after treatment and shown in (A) (c–e), (B), (C) and (D), respectively. In A, f–j are the magnified images of the corresponding images shown in a–e; and d–f are the corresponding magnified images in a–c of B–D. White broken-line circles in a–e from (A) and a–c from (B–D) indicate the relative location of the basement membrane in the seminiferous tubule, at the site of the BTB. White brackets indicated the relative distance traveled by inulin-FITC from the BTB. (E) This histogram provides the semi-quantitative data of the BTB integrity assay by quantifying the distance traveled by inulin-FITC from the basement membrane at the BTB site versus the radius of a tubule. Each bar = mean± SD of 90 tubules that were randomly selected and scored from testes of two rats for each time point. Scale bar = 100 μm in a which applies to b–e in (A) and also applies to b–c in (B–D); scale bar = 50 μm in f which applies to g–j in (A), and also applies to d–f in (B–D). *P < 0.05; **P < 0.01.
Discussion
Toxicants that disrupt spermatogenesis and cause infertility do not impede on the population of SSC/spermatogonia, but impair the functional status of the BTB
As reported herein, the testes from rats in both the low- and high-dose adjudin treated groups had virtually all the seminiferous tubules devoid of germ cells, yet the number of Utf1-positive cell population, representing As and Apr and short chain of Aal spermatogonia (van Bragt et al. 2008b), was maintained at a similar level to that of normal adult rats. While the number of Plzf-positive cells in rats from the high-dose group was significantly lower than that of normal rats (reducing from ~2.8 Plzf-positive cells/cross-section of tubule in normal rats to ~1.2 Plzf-positive cells/cross-section of tubule in the high-dose group) by the end of the experimental period at 24/30 week post adjudin treatment. Therefore, we speculated that the type of spermatogonia which had their number reduced was more advanced Aal spermatogonia. Because it is known that Plzf is expressed from As to long chain of Aal spermatogonia (Suzuki et al. 2009) and the Utf1-positive cells which represents less differentiated spermatogonia (van Bragt et al. 2008b) was not reduced. Thus, the reduced population of Plzf-positive cells in the tubules from high-dose treated group versus control and the low-dose groups could be the result of spermatogonia (note: spermatogonia was not depleted in rats from the high-dose group since the population of Utf1-positive cells in the tubules was maintained) that failed to proceed beyond short chain of Aal. This thus hinders the re-initiation of spermatogenesis and we speculate that this is due to the unfavorable microenvironment in the basal compartment, perhaps at the stem cell niche, for spermatogenesis because of the disruption of the BTB. This argument was supported by the observations that the number of Plzf-positive cells in low-dose treated group was also significantly reduced by 6/8-week and 12-week post adjudin treatment but their number rebounded when BTB was “resealed” by 20-week and thereafter. Besides, this postulate is supported by earlier studies reporting that after exposure of rodents to various toxicants, type A spermatogonia were still presence in tubules of infertile animals with azoospermia. These include rats treated with 2, 5-hexanedione (Boekelheide and Hall 1991), irradiation (Kangasniemi, et al. 1996), dibromochloropropane (Meistrich, et al. 2003) and procarbazine (Meistrich et al. 1999). In the above studies, it was shown although the number of spermatogonia was reduced, some of them survived the toxicant exposure and maintained a constant number as manifested by active proliferation of spermatogonia based on mitotic index and proliferation assay (Allrad and Boekelheide 1996; Shuttlesworth, et al. 2000). However, the proliferating spermatogonia rapidly underwent apoptosis and thus failed to differentiate beyond type A spermatogonia (Allrad and Boekelheide 1996; Shuttlesworth et al. 2000). Unfortunately, the integrity of the BTB was not investigated in any of these earlier studies. Collectively, the findings reported herein and those reported earlier thus support the notion that the SSCs/spermatogonia in the testes following toxicant treatment (note: adjudin is a “toxicant” in reference to germ cell adhesion and spermatogenesis) can proliferate and differentiate to reinitiate spermatogenesis in an optimal microenvironment in the epithelium with an intact BTB.
A functional and intact BTB is crucial to re-initiate and maintain spermatogenesis
To the best of our knowledge, this is the first report demonstrating the physiological significance of BTB in re-initiating spermatogenesis after its challenge by toxicants. However, the importance of BTB in spermatogenesis is well established because it provides a specialized microenvironment in the apical compartment for post-meiotic germ cell development behind the immunological barrier by restricting the types and amounts of substances (e.g., ions, nutrients, hormones, electrolytes, biomolecules, water and others) that can have access to developing spermatids via paracellular transport. Besides, BTB also maintains cell polarity and to confer immune privilege status to the testis (Cheng and Mruk 2010; Meinhardt and Hedger 2010). In humans, the BTB has been implicated to affect fertility status (Koksal, et al. 2007; Landon and Pryor 1981). The importance of BTB in fertility is best demonstrated in mouse models with BTB integral membranes proteins (e.g., claudin-11 and occludin) being knocked out. For instance, claudin-11−/− mice were shown to have disorganized Sertoli cell BTB and were infertile (Gow, et al. 1999; Mazaud-Guittot, et al. 2010), and spermatogenesis was later shown to fail to proceed beyond meiosis (Mazaud-Guittot et al. 2010). On the other hand, although occludin−/− mice were fertile in young adult males by 6-week of age, (Saitou, et al. 2000) and the fertility in these occludin−/− mice were maintained up to 10-week postpartum (Saitou et al. 2000; Takehashi, et al. 2007). However, the seminiferous tubules in these occludin−/− mice were devoid of all spermatids and spermatocytes by age 36- to 60-week postpartum and these mice were infertile (Saitou et al. 2000; Takehashi et al. 2007) illustrating meiosis arrest as the result of a loss of occludin-based TJ-fibrils at the BTB.
In this study, it was shown that the BTB integral proteins claudin-11, occludin and N-cadherin were up-regulated during adjudin-induced germ cell loss that led to infertility, yet they failed to strengthen the BTB. It is possible that the up-regulated occludin and claudin-11 proteins were mis-localized as revealed by the unusual thickening and diffusion of the fluorescence from the BTB site based on dual-labeled immunofluorescence analysis. Mis-localization of BTB proteins can be a sign of spermatogenesis and BTB malfunctioning as demonstrated by studies showing that claudin-11 expression was also induced but mis-localized in testes from infertile human with Sertoli cell only syndrome (Nah, et al. 2010) as well as in testes of testicular intraepithelial neoplasia patients with loss of BTB function (Fink, et al. 2009). Besides, there was also study illustrating in patients with carcinoma in situ having perturbed BTB, a diffusion of ZO-1 immunohistochemistry staining in the seminiferous epithelium was found (Fink, et al. 2006), analogous to the our findings in this report. Furthermore, an in vivo BTB integrity assay has demonstrated that the BTB from rats in the high-dose treated group was disrupted at least beginning by 2-week post adjudin treatment, and it was not recovered at the end of this study by 24/30-week. On the other hand, although the BTB in low-dose treated group was once perturbed by 6-week post treatment, it was “resealed” by week 20 and this integrity was maintained at the end of this study by 24/30-week. In summary, while there is no reduction in the population of SSC/spermatogonia in both treatment groups, spermatogenesis failed to resume in the high-dose group because of the disrupted BTB, suggesting that a functional BTB is required to sustain spermatogonial differentiation to re-initiate spermatogenesis.
BTB integrity and infertility in men
In this context, it is of interest to note that following radiotherapy, chemotherapy using drugs such as procarbazine, or exposure to environmental toxicants like dibromochloropropane, prolonged azoospermia is detected in men (Howell and Shalet 2005; Slutsky, et al. 1999). In these disease models, it is likely that the loss of fertility is the result of BTB disruption since spermatogonia are present in these patients. This is analogous to the cadmium model in which rats exposed to CdCl2 leads to irreversible disruption of the BTB, which in turn, perturbs re-initiation of spermatogenesis (Wong, et al. 2004). Also, in both rodents and humans, progression of meiosis and spermiogenesis occur only after the BTB is established by age 15–17 in rodents and following puberty in humans (Cheng and Mruk 2010), illustrating the crucial significance of the BTB to initiation of spermatogenesis. Nonetheless, much research is needed to understand the underlying mechanism(s) that relates a functional BTB and the initiation of spermatogenesis.
Supplementary Material
Acknowledgments
K.W.M. and C.Y.C. performed the research; C.Y.C. designed the research study; W.M.L. contributed essential reagents; K.W.M., D.D.M. and C.Y.C. analyzed and critically evaluated the data; and K.W.M. and C.Y.C. wrote the paper.
Footnotes
This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034, R01 HD056034-02S1 and U54 HD029990 Project 5 to CYC; R03 HD061401 to DDM).
References
- Allrad EK, Boekelheide K. Fate of germ cells in 2,5-hexanedione-induced testicular injury. II. Atrophy persists due to a reduced stem cell mass and ongoing apoptosis. Toxicol Appl Pharmacol. 1996;137:149–156. doi: 10.1006/taap.1996.0067. [DOI] [PubMed] [Google Scholar]
- Bascian S, De Luca G, Dolci S, Brama M, Arizzi M, Mariani S, Rosano G, Spera G, Gnessi L. Platelet-derived growth factor receptor beta-subtype regulates proliferation and migration of gonocytes. Endocrinology. 2008;149:6226–6235. doi: 10.1210/en.2008-0349. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Hall SJ. 2,5-Hexanedione exposure in the rat results in long-term testicular atrophy despite the presence of residual spermatogonia. J Androl. 1991;12:18–26. [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, Lee NPY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, Saso L, Leone MG, Palmery M, Mruk DD. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–2937. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72:580–585. doi: 10.1002/jemt.20699. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Human Reprod Update. 2006;12:275–282. doi: 10.1093/humupd/dmk001. [DOI] [PubMed] [Google Scholar]
- Fink C, Weigel R, Fink L, Wilhelm J, Kliesch S, Zeiler M, Bergmann M, Brehm R. Claudin-11 is over-expressed and dislocated from the blood-testis barrier in Sertoli cells associated with testicular intraepithelial neoplasia in men. Histochem Cell Biol. 2009;131:755–764. doi: 10.1007/s00418-009-0576-2. [DOI] [PubMed] [Google Scholar]
- Fink C, Weigel R, Hembes T, Lauke-Wettwer H, Kliesch S, Bergmann M, Brehm RH. Altered expression of ZO-1 and ZO-2 in Sertoli cells and loss of blood-testis barrier integrity in testicular carcinoma in siu. Neoplasia. 2006;8:1019–1027. doi: 10.1593/neo.06559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgione N, Vogl AW, Varmuza S. Loss of protein phosphatase 1c {gamma} (PPP1CC) leads to impaired spermatogenesis associated with defects in chromatin condensation and acrosome development: an ultrastructural analysisdefects in chromatin condensation and acrosome development: an ultrastructural analysis. Reproduction. 2010;139:1021–1029. doi: 10.1530/REP-10-0063. [DOI] [PubMed] [Google Scholar]
- Gow A, Southwood C, Li J, Pariali M, Riordan G, Brodie S, Danias J, Bronstein J, Kachar B, Lazzarini R. CNS myelin and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005:12–17. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- Hubert MF, Laroque P, Gillet JP, Keenan KP. The effects of diet, ad Libitum feeding, and moderate and severe dietary restriction on body weight, survival, clinical pathology parameters, and cause of death in control Sprague-Dawley rats. Toxicol Sci. 2000;58:195–207. doi: 10.1093/toxsci/58.1.195. [DOI] [PubMed] [Google Scholar]
- Kangasniemi M, Huhtaniemi I, Meistrich ML. Failure of spermatogenesis to recover despite the presence of A spermatogonia in the irradiated LBNF1 rat. Biol Reprod. 1996;54:1200–1208. doi: 10.1095/biolreprod54.6.1200. [DOI] [PubMed] [Google Scholar]
- Koksal IT, Ishak Y, Usta M, Danisman A, Guntekin E, Bassorgun IC, Citftcioglu A. Varicocele-induced testicular dysfunction may be associated with disruption of blood-testis barrier. Arch Androl. 2007;53:43–48. doi: 10.1080/01485010600822606. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon GV, Pryor JP. The blood-testis barrier in men of diverse fertility status: an ultrastructural study. Virchows Arch A Pathol Anat Histol. 1981;392:355–364. doi: 10.1007/BF02155671. [DOI] [PubMed] [Google Scholar]
- Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHY, Siu MKY, Lui WY, Lee WM, Cheng CY. TNFα reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010a;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010b;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaud-Guittot S, Meugnier E, Pesenti S, Wu X, Vidal H, Gow A, Le Magueresse-Battistoni B. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol Reprod. 2010;82:202–213. doi: 10.1095/biolreprod.109.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.03.022. in press. [DOI] [PubMed] [Google Scholar]
- Meistrich ML. Critical components of testicular function and sensitivity to disruption. Biol Reprod. 1986;34:17–28. doi: 10.1095/biolreprod34.1.17. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Wilson G, Huhtaniemi I. Hormonal treatment after cytotoxic therapy stimulates recovery of spermatogenesis. Cancer Res. 1999;59:3557–3560. [PubMed] [Google Scholar]
- Meistrich ML, Wilson G, Porter KL, Huhtaniemi I, Shetty G, Shuttlesworth GA. Restoration of spermatogenesis in dibromochloropropane (DBCP)-treated rats by hormone suppression. Toxicol Sci. 2003;76:418–426. doi: 10.1093/toxsci/kfg237. [DOI] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lie PPY, Lui WY, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction. 2011 doi: 10.1530/REP-10-0464. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah WH, Lee JE, Park HJ, Park NC, Gye MC. Claudin-11 expression increased in spermatogenic defect in human testes. Fertil Steril. 2010;95:385–388. doi: 10.1016/j.fertnstert.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Spermatogonial stem cells. Methods Enzymol. 2006;419:259–282. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- Pan J, Eckardt S, Leu NA, Buffone MG, Zhou J, Gerton GL, McLaughlin KJ, Wang PJ. Inactivation of Nxf2 causes defects in male meiosis and age-dependent depletion of spermatogonia. Dev Biol. 2009;330:167–174. doi: 10.1016/j.ydbio.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke J, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell BP, Waites GMB. The blood-testis barrier. In: Hamilton DW, Greep RO, editors. The Handbook of Physiology. Section 7, Vol. V. Male Reproductive System. Washington, D.C: American Physiological Society; 1975. pp. 143–172. [Google Scholar]
- Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- Shuttlesworth GA, De Rooij DG, Huhtaniemi I, Reissmann T, Russell LD, Shetty G, Wilson G, Meistrich ML. Enhancement of A spermatogonial proliferation and differentiation in irradiated rats by gonadotropin-releasing hormone antagonist administration. Endocrinology. 2000;141:37–49. doi: 10.1210/endo.141.1.7272. [DOI] [PubMed] [Google Scholar]
- Slutsky M, Levin JL, Levy BS. Azoospermia and oligospermia among a large cohort of DBCP applicators in 12 countries. Int J Occup Environ Health. 1999;5:116–122. doi: 10.1179/oeh.1999.5.2.116. [DOI] [PubMed] [Google Scholar]
- Su L, Cheng CY, Mruk DD. Adjudin-mediated Sertoli-germ cell junction disassembly affects Sertoli cell barrier function in vitro and in vivo. Int J Biochem Cell Biol. 2010;42:1864–1875. doi: 10.1016/j.biocel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Takehashi M, Kanatsu-Shinohara M, Miki H, Lee J, Kazuki Y, Inoue K, Ogonuki N, Toyokuni S, Oshimura M, Ogura A, et al. Production of knockout mice by gene targeting in multipotent germline stem cells. Dev Biol. 2007;312:344–352. doi: 10.1016/j.ydbio.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Ohkawa M, Oku R, Maekawa M, Yuasa S. Neonatally administered diethylstilbestrol retards the development of the blood-testis barrier in the rat. J Androl. 2001;22:413–423. [PubMed] [Google Scholar]
- van Bragt MP, Roepers-Gajadien HL, Korver CM, Bogerd J, Okuda A, Eggen BJ, de Rooij DG, van Pelt AM. Expression of the pluripotency marker UTF1 is restricted to a subpopulation of early A spermatogonia in rat testis. Reproduction. 2008a;136:33–40. doi: 10.1530/REP-07-0536. [DOI] [PubMed] [Google Scholar]
- van Bragt MP, Roepers-Gajadien HL, Korver CM, Bogerd J, Okuda A, Eggen BJL, de Rooij DG, van Pelt AM. Expression of the pluripotency marker UTF1 is restricted to a subpopulation of early A spermatogonia in rat testis. Reproduction. 2008b;136:33–40. doi: 10.1530/REP-07-0536. [DOI] [PubMed] [Google Scholar]
- van den Boom V, Kooistra SM, Boesjes M, Geverts B, Houtsmuller AB, Monzen K, Komuro I, Essers J, Drenth-Diephuis LJ, Eggen BJ. UTF1 is a chromatin-associated protein involved in ES cell differentiation. J Cell Biol. 2007;178:913–924. doi: 10.1083/jcb.200702058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström AM, Raivio T, Hadziselimovic F, Wikström S, Tuuri T, Dunkel L. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab. 2004;89:2263–2270. doi: 10.1210/jc.2003-031725. [DOI] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.