Differential initiation over time, as well as by age and tumor size, suggests patient preferences and provider recommendations for endocrine therapy vary, despite guideline recommendations.
Abstract
Purpose:
To evaluate tamoxifen and aromatase inhibitor (AI) initiation over time and by patient characteristics among women diagnosed with breast cancer in a community setting.
Methods:
We conducted a retrospective cohort study of 1,501 women age ≥ 18 years diagnosed with stages I to II invasive, hormone receptor–positive breast cancer from 2001 to 2008 in an integrated delivery system. Using automated pharmacy dispensings, we determined endocrine therapy receipt within 12 months of diagnosis. We used generalized linear models to estimate adjusted relative risks (RRs) with 95% CIs for any endocrine therapy use (v none), tamoxifen use (v none), AI use (v none), and AIs first (v tamoxifen). Each model adjusted for age, stage, body mass index, tumor size, lymph node status, comorbidities, other treatment, and diagnosis year.
Results:
Tamoxifen use was at its highest (56.9%) in 2001 and then decreased; AI use was lowest in 2001 (5.5%) and then peaked in 2005 (36.8%). In multivariate models, women age ≥ 65 years were less likely to use any endocrine therapy compared with women age 55 to 64 years (age 65 to 74 years: RR, 0.86; 95% CI, 0.78 to 0.96; age ≥ 75 years: RR, 0.71; 95% CI, 0.61 to 0.81). Women age ≥ 75 years were significantly less likely to begin AIs versus no treatment (RR, 0.46; 95% CI, 0.32 to 0.64) and versus tamoxifen (RR, 0.67; 95% CI, 0.46 to 0.97). Women with tumor sizes 1.0 to 1.9 cm and ≥ 2.0 cm were significantly more likely to use any endocrine therapy compared with women with tumor sizes < 1.0 cm (RR, 1.41; 95% CI, 1.23 to 1.61 and RR, 1.52; 95% CI, 1.27 to 2.81, respectively).
Conclusion:
Differential initiation over time, as well as by age and tumor size, suggests patient preferences and provider recommendations for endocrine therapy vary, despite guideline recommendations.
Introduction
Approximately 80% of women diagnosed with invasive breast cancer have hormone receptor (HR) –positive disease, making a large proportion of women with breast cancer potential candidates for adjuvant endocrine therapy.1,2 Clinical trials consistently demonstrate that tamoxifen or aromatase inhibitor (AI) use significantly improves disease-free survival and overall breast cancer mortality.3,4 Current National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) guidelines recommend AI therapy for 5 years after completion of primary therapy or for 2 to 3 years after 2 to 3 years of tamoxifen for postmenopausal women.1,2,5,6 NCCN guidelines also recommend that adjuvant endocrine therapy be considered for women with HR-positive breast cancer “regardless of menopausal status, age, or HER2 status of the tumor.”2(pMS-25)
We do not know how well these recommendations are currently followed in community practice, even though they stem from the results of large, high-quality clinical trials. Diffusion studies show that AI and tamoxifen were used before the development of guidelines,7–9 so patterns of use in the community may not conform to guidelines. Factors such as age, disease severity, and medical insurance or access to health care—all of which are inherently controlled for in clinical trials—may influence treatment selection in a real-world setting. In fact, a few studies using data before 2005 have shown that endocrine therapy initiation ranges between 15% to 84% in various populations of women with estrogen receptor (ER) –positive tumors.8,10–12 Understanding cancer treatment patterns in the community is critical to evaluating quality of care for women with breast cancer and may provide valuable information for future comparative effectiveness studies. A better understanding of who does and does not use different yet similarly effective treatments may help us more accurately estimate the effectiveness of these drugs in the general population and improve breast cancer survival.
The purpose of this article is to describe women's initiation of endocrine therapy over time and by demographic and tumor characteristics. We hypothesized that overall use of endocrine therapy would increase over time, corresponding to recent increases in AI use. Our study minimized one major factor that might normally bias treatment selection; all women in the study population were insured with comprehensive benefits and full access to health care through Group Health, an integrated health care delivery system in Washington. This provided an opportunity to conduct an unbiased examination of the selection factors that influence adjuvant endocrine therapy initiation.
Methods
Study Setting and Population
We conducted a retrospective cohort study of women age ≥ 18 years who were diagnosed with early-stage invasive breast cancer (American Joint Committee on Cancer Version 5 stage I, IIA, or IIB) from 1990 to 2008. All participants were part of the COMBO (Commonly Used Medications and Breast Cancer Outcomes) study. We obtained a waiver of consent to review electronic data and abstract medical records, and all study procedures were approved by the institutional review board at Group Health.
Women in the COMBO study had been enrolled continuously in Group Health for at least 12 months before and after their breast cancer diagnosis unless they died (n = 4,428) and received care from Group Health–owned and –operated facilities. For this analysis, we limited our sample to women diagnosed from 2001 to 2008 because AIs were not used at Group Health before 2001 (n = 1,927). We excluded women who died within 1 year of diagnosis (n = 28), did not have definitive breast cancer surgery as primary treatment (n = 32), had ER-negative or progesterone receptor (PR) –negative disease (n = 307), or unknown ER and PR status (n = 104). These exclusion criteria were not mutually exclusive, and our final sample for this analysis was 1,501 women. We used age ≥ 55 years as a proxy for postmenopausal status for some analyses, even though age at menopause may vary widely among women undergoing breast cancer treatment (particularly chemotherapy), and thus women younger than 55 years of age may be postmenopausal and eligible for tamoxifen or AI therapy.13
Data Collection
We identified patient cases of breast cancer by linking Group Health enrollees with the Western Washington Surveillance, Epidemiology, and End Results (SEER) registry. Group Health has linked with SEER since 1974 for primary cancer diagnoses and tumor characteristics. We used the detailed electronic administrative data of Group Health on outpatient pharmacy dispensings, enrollment, and demographics to identify our outcomes and covariates for this analysis.
Tamoxifen and AI Use
The outcome for this study was first use of endocrine therapy after diagnosis, either tamoxifen or AIs. All tamoxifen and AI data were collected using electronic pharmacy data, which has been shown to be 97% complete.14,15 Pharmacy use is captured for all Group Health enrollees who fill prescriptions at Group Health–owned and –operated pharmacies and for all Group Health enrollees with a drug benefit who fill prescriptions at outside pharmacies. A recent unpublished survey of women from the COMBO study demonstrates 92% almost always fill their prescriptions at Group Health pharmacies. This also includes Medicare Part D enrollees, starting in 2006, who likely increased capture of pharmacy data because more enrollees were offered a drug benefit through Group Health rather than obtaining prescriptions elsewhere. With a goal of capturing all use and avoiding inclusion of tamoxifen or AI use for recurrence, we defined use as one or more dispensings of one drug within 12 months of diagnosis. Because we were primarily interested in initiation of each drug, using one or more dispensings allowed us to include women who may have stopped using that drug because of intolerable adverse effects. Limiting our definition to 12 months after diagnosis reduced the possibility of misclassifying anyone who used tamoxifen or AIs for breast cancer recurrence instead of their primary diagnosis. This definition is consistent with that in a recent study.16 We defined women as having any endocrine therapy use if they had one or more dispensings of either tamoxifen or AIs within 12 months of diagnosis; nonusers had no dispensings of tamoxifen or AIs. If a woman switched drugs at any point, she was classified as a user of tamoxifen or AIs based on what she filled first (n = 74).
Covariates
Variables of interest included demographic and breast cancer tumor characteristics. These data were collected from electronic administrative databases including enrollment files (age, length of enrollment, comorbidities or Charlson score,17 pharmacy copayment), Group Health Breast Cancer Surveillance project data18 (education, body mass index [BMI]), and SEER data (date of diagnosis, race, ethnicity, stage at diagnosis, tumor size, lymph node status, ER status, PR status, definitive surgical procedure, chemotherapy, radiation therapy).
Statistical Analysis
We used generalized linear models with a log link, Poisson distribution, and robust SEs to estimate rates and relative risks (RRs) of endocrine therapy use. Rates of initiation were estimated for each diagnosis year, and we tested for trends in initiation over time. We estimated the adjusted RR and 95% CI for:
Any endocrine therapy use versus no use of either drug relative to patient demographics and tumor characteristics.
Tamoxifen use first versus no use of either drug relative to demographics and tumor characteristics.
AI use first versus no use of either drug relative to demographics and tumor characteristics; this analysis was limited to women age ≥ 55 years.
Direct comparison of AI use first versus tamoxifen use first, also limited to women age ≥ 55 years; we excluded 164 additional women from this analysis who had one of the following contraindications to AI or tamoxifen use within the 12 months before breast cancer diagnosis: deep vein thrombosis, osteoporotic fracture, pulmonary embolus, or stroke.
All models were adjusted for age, stage, tumor size, lymph nodes, comorbidities, surgical treatment, chemotherapy, radiation therapy, BMI, and diagnosis year, because these were significantly associated with any endocrine therapy use in unadjusted models. Pharmacy copayment, race, ethnicity, and education were not significantly related to endocrine therapy use, so we did not include these variables in the final multivariate models. All analyses were conducted in Stata (StataCorp, College Station, TX), and two-sided P values less than .05 were considered statistically significant.
Results
Among 1,501 women diagnosed with HR-positive breast cancer between 2000 and 2008, 680 (45%) were tamoxifen-first users, 368 (25%) were AI-first users, and 453 (30%) used neither drug (Table 1). Most endocrine therapy nonusers were age 65 to 74 years (28.0%) or 75 years or older (36.4%), diagnosed with stage I breast cancer (79.9%) with tumor sizes < 1.0 cm (38.1%) and/or negative lymph nodes (90.5%). Tamoxifen-first and AI-first users tended to be younger, and a greater proportion were diagnosed with stage IIB breast cancer, had tumors > 2.0 cm in size, and/or had positive lymph nodes compared with nonusers. Surgical treatment did not differ by endocrine therapy use; however, a greater proportion of tamoxifen and AI users received either chemotherapy or radiation therapy compared with nonusers.
Table 1.
Demographic and Clinical Characteristics of Women With Early-Stage Hormone Receptor–Positive Breast Cancer by Endocrine Therapy Initiation Within 12 Months of Diagnosis From 2001 to 2008
| Characteristic | No Tamoxifen or AIs |
Tamoxifen First |
AIs First |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| No.* | % | No.* | % | No.* | % | No.* | % | |
| Total No. of patients | 453 | 680 | 368 | 1,501 | ||||
| Age at diagnosis, years | ||||||||
| < 45 | 18 | 4.0 | 61 | 9.0 | 11 | 3.0 | 90 | 6.0 |
| 45-54 | 52 | 11.5 | 192 | 28.2 | 62 | 16.8 | 306 | 20.4 |
| 55-64 | 91 | 20.1 | 169 | 24.9 | 159 | 43.2 | 419 | 27.9 |
| 65-74 | 127 | 28.0 | 146 | 21.5 | 93 | 25.3 | 366 | 24.4 |
| ≥ 75 | 165 | 36.4 | 112 | 16.5 | 43 | 11.7 | 320 | 21.3 |
| Race | ||||||||
| White | 401 | 88.5 | 589 | 87.3 | 318 | 86.6 | 1,308 | 87.5 |
| Black | 10 | 2.2 | 16 | 2.4 | 12 | 3.3 | 38 | 2.5 |
| American Indian/Alaska Native | 15 | 3.3 | 17 | 2.5 | 15 | 4.1 | 47 | 3.1 |
| Asian | 27 | 6.0 | 53 | 7.9 | 22 | 6.0 | 106 | 6.8 |
| Ethnicity | ||||||||
| Non-Hispanic | 414 | 91.4 | 617 | 90.7 | 344 | 93.5 | 1,375 | 91.6 |
| Hispanic | 39 | 8.6 | 63 | 9.3 | 24 | 6.5 | 126 | 8.4 |
| Education | ||||||||
| High school or less | 88 | 28.4 | 103 | 20.1 | 49 | 17.4 | 240 | 21.8 |
| Some college | 110 | 35.5 | 191 | 37.3 | 118 | 42.0 | 419 | 38.0 |
| College graduate | 60 | 19.4 | 109 | 21.3 | 55 | 19.6 | 224 | 20.3 |
| Postgraduate | 52 | 16.8 | 109 | 21.3 | 59 | 21.0 | 220 | 19.9 |
| BMI, kg/m2 | ||||||||
| < 25.0 | 125 | 36.9 | 168 | 31.8 | 69 | 26.5 | 362 | 32.1 |
| 25.0-29.9 | 102 | 30.1 | 197 | 37.2 | 78 | 30.0 | 377 | 33.4 |
| ≥ 30.0 | 112 | 33.0 | 164 | 31.0 | 113 | 43.5 | 389 | 34.5 |
| Stage at diagnosis | ||||||||
| I | 362 | 79.9 | 396 | 58.2 | 163 | 44.3 | 921 | 61.4 |
| IIA | 66 | 14.6 | 186 | 27.4 | 127 | 34.5 | 379 | 25.2 |
| IIB | 25 | 5.5 | 98 | 14.4 | 78 | 21.2 | 201 | 13.4 |
| Tumor size, cm | ||||||||
| < 1.0 | 169 | 38.1 | 116 | 17.1 | 50 | 13.6 | 335 | 22.5 |
| 1.0-1.9 | 193 | 43.6 | 337 | 49.8 | 175 | 47.7 | 705 | 47.4 |
| ≥ 2.0 | 81 | 18.3 | 224 | 33.1 | 142 | 38.7 | 447 | 30.1 |
| Lymph node status | ||||||||
| Negative | 410 | 90.5 | 494 | 72.6 | 217 | 59.0 | 1,121 | 74.7 |
| Positive | 43 | 9.5 | 186 | 27.4 | 151 | 41.0 | 380 | 25.3 |
| Hormone receptor status | ||||||||
| ER and PR positive | 417 | 93.9 | 619 | 91.8 | 355 | 96.7 | 1,391 | 93.7 |
| ER positive, PR negative | 23 | 5.2 | 45 | 6.7 | 12 | 3.3 | 80 | 5.4 |
| ER negative, PR positive | 4 | 0.9 | 10 | 1.5 | 0 | 0.0 | 14 | 0.9 |
| Definitive surgical procedure | ||||||||
| Breast conserving | 291 | 64.2 | 441 | 64.9 | 228 | 62.0 | 960 | 64.0 |
| Mastectomy | 162 | 35.8 | 239 | 35.1 | 140 | 38.0 | 541 | 36.0 |
| Chemotherapy | ||||||||
| No | 383 | 85.5 | 420 | 62.4 | 202 | 56.3 | 1,005 | 67.9 |
| Yes | 65 | 14.5 | 253 | 37.6 | 157 | 43.7 | 475 | 32.1 |
| Radiation | ||||||||
| No | 195 | 44.1 | 231 | 35.0 | 117 | 34.2 | 543 | 37.6 |
| Yes | 247 | 55.9 | 429 | 65.0 | 225 | 65.8 | 901 | 62.4 |
| Charlson score | ||||||||
| 0 | 308 | 68.0 | 532 | 78.2 | 271 | 73.6 | 1,111 | 74.0 |
| 1 | 85 | 18.8 | 104 | 15.3 | 69 | 18.8 | 258 | 17.2 |
| ≥ 2 | 60 | 13.2 | 44 | 6.5 | 28 | 7.6 | 132 | 8.8 |
| Year of diagnosis | ||||||||
| 2001 | 66 | 14.6 | 114 | 16.8 | 12 | 3.3 | 192 | 12.8 |
| 2002 | 79 | 17.4 | 99 | 14.6 | 31 | 8.4 | 209 | 13.9 |
| 2003 | 62 | 13.7 | 112 | 16.5 | 29 | 7.9 | 203 | 13.5 |
| 2004 | 49 | 10.8 | 78 | 11.5 | 42 | 11.4 | 169 | 11.3 |
| 2005 | 55 | 12.1 | 73 | 10.7 | 75 | 20.4 | 203 | 13.5 |
| 2006 | 61 | 13.5 | 74 | 10.9 | 64 | 17.4 | 199 | 13.3 |
| 2007 | 40 | 8.8 | 71 | 10.4 | 63 | 17.1 | 174 | 11.6 |
| 2008 | 41 | 9.1 | 59 | 8.7 | 52 | 14.1 | 152 | 10.1 |
| Pharmacy copayment (per 30- day supply), $ | ||||||||
| 0-5 | 50 | 15.7 | 107 | 18.4 | 48 | 14.0 | 205 | 16.5 |
| 7-10 | 170 | 53.3 | 306 | 52.5 | 176 | 51.5 | 652 | 52.4 |
| 15-25 | 99 | 31.0 | 170 | 29.2 | 118 | 34.5 | 387 | 31.1 |
Abbreviations: AI, aromatase inhibitor; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor.
Numbers may not add to totals because of missing data.
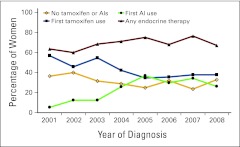
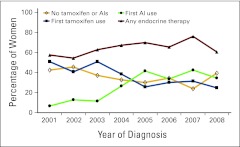
Adjusted rates of first tamoxifen use, first AI use, any endocrine therapy use, and nonuse over time are shown in Figure 1. Users of any endocrine therapy increased from 63.4% in 2001 to 75.1% in 2005 and then decreased to 67.1% in 2008. Tamoxifen use was highest in 2001 (56.9%) but dropped to 34.5% in 2005 and remained fairly steady thereafter. AI use accounted for only 5.5% of women in 2001 but had increased to 36.8% by 2005; it then dropped to 26.3% in 2008. Appendix Figure A1 (online only) shows similar patterns of use over time limited to women age ≥ 55 years. Trend tests for each exposure over time were statistically significant (P < .001) for both figures.
Figure 1.
Proportions of endocrine therapy use within 12 months of diagnosis over time (all women age ≥ 18 years); adjusted for age, stage, tumor size, lymph nodes, body mass index, surgical treatment, chemotherapy, radiation therapy, and comorbidities. AI, aromatase inhibitor.
In multivariable models (Table 2), women age 65 to 74 years and ≥ 75 years were significantly less likely to use any endocrine therapy compared with women age 55 to 64 years (RR, 0.86; 95% CI, 0.78 to 0.96 and RR, 0.71; 95% CI, 0.61 to 0.81, respectively). RR for the association between age ≥ 75 years and first AI use was particularly low (RR, 0.46; 95% CI, 0.32 to 0.64). Women with tumor sizes from 1.0 to 1.9 cm and ≥ 2.0 cm were significantly more likely to use any endocrine therapy compared with women with tumor sizes < 1.0 cm (RR, 1.41; 95% CI, 1.23 to 1.61 and RR, 1.52; 95% CI, 1.27 to 2.81, respectively); a similar pattern was noted for tumor size and tamoxifen use. Women with positive lymph nodes were more likely to use any endocrine therapy (RR, 1.31; 95% CI, 1.11 to 1.54) or tamoxifen (RR, 1.48; 95% CI, 1.18 to 1.86) relative to women with negative lymph nodes. Surgical procedure, chemotherapy, and radiation therapy were not associated with any endocrine therapy use in multivariable analyses.
Table 2.
Multivariate Associations Between Any Endocrine Therapy, Tamoxifen, or AI Initiation During 12 Months After Breast Cancer Diagnosis
| Variable | Any Use v None |
Tamoxifen First v None |
AIs First v None* |
|||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Total No. of patients | 1,048 v 453 | 680 v 453 | 368 v 453 | |||
| Age at diagnosis, years | ||||||
| < 45 | 0.97 | 0.84 to 1.12 | 1.08 | 0.90 to 1.30 | — | — |
| 45-54 | 1.06 | 0.97 to 1.15 | 1.17 | 1.03 to 1.33 | — | — |
| 55-64 | 1 | Reference | 1 | Reference | 1 | Reference |
| 65-74 | 0.86 | 0.78 to 0.96 | 0.86 | 0.73 to 1.01 | 0.76 | 0.62 to 0.94 |
| ≥ 75 | 0.71 | 0.61 to 0.81 | 0.70 | 0.58 to 0.84 | 0.46 | 0.32 to 0.64 |
| BMI, kg/m2 | ||||||
| < 25.0 | 1 | Reference | 1 | Reference | 1 | Reference |
| 25.0-29.9 | 1.11 | 1.01 to 1.22 | 1.14 | 1.01 to 1.28 | 1.28 | 0.98 to 1.66 |
| ≥ 30.0 | 1.04 | 0.94 to 1.15 | 0.98 | 0.86 to 1.13 | 1.18 | 0.91 to 1.52 |
| Stage at diagnosis | ||||||
| I | 1 | Reference | 1 | Reference | 1 | Reference |
| IIA | 0.99 | 0.84 to 1.17 | 0.92 | 0.74 to 1.15 | 1.64 | 0.97 to 2.79 |
| IIB | 0.91 | 0.69 to 1.21 | 0.78 | 0.54 to 1.14 | 1.84 | 0.76 to 4.49 |
| Tumor size, cm | ||||||
| < 1.0 | 1 | Reference | 1 | Reference | 1 | Reference |
| 1.0-1.9 | 1.41 | 1.23 to 1.61 | 1.52 | 1.28 to 1.81 | 1.58 | 1.17 to 2.12 |
| ≥ 2.0 | 1.52 | 1.27 to 1.82 | 1.71 | 1.35 to 2.17 | 1.40 | 0.84 to 2.34 |
| Lymph node status | ||||||
| Negative | 1 | Reference | 1 | Reference | 1 | Reference |
| Positive | 1.31 | 1.11 to 1.54 | 1.48 | 1.18 to 1.86 | 1.16 | 0.71 to 1.87 |
| Definitive surgical procedure | ||||||
| Lumpectomy | 1 | Reference | 1 | Reference | 1 | Reference |
| Mastectomy | 0.95 | 0.85 to 1.06 | 0.94 | 0.80 to 1.11 | 0.98 | 0.75 to 1.28 |
| Chemotherapy | ||||||
| No | 1 | Reference | 1 | Reference | 1 | Reference |
| Yes | 1.03 | 0.95 to 1.12 | 1.04 | 0.92 to 1.17 | 0.99 | 0.80 to 1.22 |
| Radiation therapy | ||||||
| No | 1 | Reference | 1 | Reference | 1 | Reference |
| Yes | 1.05 | 0.94 to 1.18 | 1.08 | 0.91 to 1.27 | 1.16 | 0.89 to 1.52 |
| Charlson score | ||||||
| 0 | 1 | Reference | 1 | Reference | 1 | Reference |
| 1 | 0.99 | 0.89 to 1.10 | 0.96 | 0.83 to 1.11 | 1.18 | 0.93 to 1.50 |
| ≥ 2 | 0.92 | 0.78 to 1.08 | 0.85 | 0.67 to 1.08 | 0.96 | 0.67 to 1.36 |
| Year of diagnosis | ||||||
| 2001 | 1 | Reference | 1 | Reference | 1 | Reference |
| 2002 | 0.97 | 0.85 to 1.11 | 0.93 | 0.79 to 1.09 | 1.62 | 0.88 to 3.00 |
| 2003 | 1.08 | 0.95 to 1.23 | 1.06 | 0.92 to 1.23 | 1.70 | 0.90 to 3.22 |
| 2004 | 1.15 | 0.99 to 1.33 | 1.11 | 0.89 to 1.37 | 2.53 | 1.42 to 4.51 |
| 2005 | 1.14 | 0.99 to 1.31 | 0.94 | 0.76 to 1.16 | 3.52 | 2.00 to 6.18 |
| 2006 | 1.07 | 0.93 to 1.24 | 0.97 | 0.78 to 1.21 | 2.60 | 1.49 to 4.56 |
| 2007 | 1.24 | 1.07 to 1.44 | 1.04 | 0.84 to 1.28 | 3.82 | 2.17 to 6.73 |
| 2008 | 1.10 | 0.94 to 1.29 | 0.95 | 0.76 to 1.18 | 3.06 | 1.72 to 5.47 |
Abbreviations: AI, aromatase inhibitor; BMI, body mass index; RR, relative risk.
All variables in the table are adjusted for one another.
Adjusted RRs for AI use compared with tamoxifen use among women age ≥ 55 years with no contraindications are listed in Appendix Table A1 (online only). Women age 65 to 74 years and ≥ 75 years were significantly less likely to use AIs than tamoxifen compared with women age 55 to 64 years (RR, 0.78; 95% CI, 0.60 to 1.00 and RR, 0.67; 95% CI, 0.46 to 0.97, respectively). Women with BMI ≥ 30.0 were more likely to use AIs than tamoxifen (RR, 1.40; 95% CI, 1.06 to 1.87), as were women diagnosed in 2004 or later; no other associations were statistically significant.
Discussion
Our results show that endocrine therapy, specifically AI use, for early-stage HR-positive breast cancer has increased since 2001. Furthermore, initiation of each type of therapy varies by patient characteristics; older women were least likely to begin endocrine therapy, whereas women with tumor sizes ≥ 1.0 cm and/or positive lymph nodes were most likely. Variation in initiation suggests that certain populations may have different preferences for treatment or may receive different recommendations for treatment from their providers, despite guidelines recommending adjuvant endocrine therapy for all women with HR-positive breast cancer.
Almost one third of women in our study diagnosed with breast cancer as recently as 2008 used no endocrine therapy. Lack of use may reflect patient choice, provider recommendations, and/or perceived recurrence risk. Almost 80% of these women had stage I tumors, and 90% had negative lymph nodes. Using Adjuvant! Online, a woman 65 years of age with average comorbidities, tumor size < 1.0 cm, and negative lymph nodes will reduce her recurrence risk by less than 10% and mortality risk by less than 1% if she uses adjuvant endocrine therapy.19 Although tamoxifen or AI treatment could be beneficial in this case, the potential risks may outweigh the benefits. This follows NCCN guidelines, which make an exception for endocrine therapy treatment in women with tumors < 1 cm and negative lymph nodes.2 A recently published study of discontinuation and adherence to adjuvant hormonal therapy found an even higher proportion of patients with stages I to III HR-positive disease (42% over all study years, 1996 to 2007) did not start therapy within 12 months after diagnosis, although this study did not evaluate initiation by tumor size or lymph node status.16 These results should be verified using prospective data collection, given the possibility of discordance between administrative data and patient self-report.
Older women (age ≥ 65 years) were least likely to use any endocrine therapy, even after adjusting for potential confounders including comorbidities. This result is consistent with those of previous studies showing that older women are less likely to receive any cancer treatment at all, including adjuvant endocrine therapy.12,20–22 The most likely explanation here is that the risks of treating elderly, frail patients largely outweigh the benefits, and providers are unlikely to recommend treatment.23,24 However, it is surprising that women between the ages of 65 and 74 years were less likely to receive any endocrine therapy. Differences in medical coverage among Medicare enrollees may play a role here. Medicare Part D, which covers oral cancer drugs, was not enacted until 2006, and even if women have Part D coverage, costs to patients may be higher if the drugs are not preferred by the health plan or cannot be obtained as generics.25 Therefore, even though all women in our COMBO study population were enrolled in Group Health, prescription drug coverage likely differed among Medicare enrollees, and some may not have had drug coverage at all.
Older age was also the main characteristic associated with decreased AI initiation compared with tamoxifen initiation. Again, this may be related to Medicare prescription drug coverage, but it may also be explained by different adverse effect profiles. For example, older women are at increased risk for osteoporosis, and AIs further increase that risk26,27; therefore, some older women might be more likely to choose tamoxifen. However, other adverse effects, such as increased risk for deep vein thrombosis and stroke, could have influenced some women to begin AIs before tamoxifen. We excluded women with a history of conditions that may have influenced their choice of one drug over the other; however, the potential risk for adverse effects among the remaining women could have influenced their choice.
Women diagnosed with more severe disease (tumor size ≥ 1.0 cm or positive lymph nodes) were more likely to use endocrine therapy than women with smaller tumors or negative lymph nodes. These women were potentially at increased risk for breast cancer recurrence, which may have influenced their treatment preferences and/or provider recommendations. Interestingly, other treatment modalities (surgery, chemotherapy, and radiation) were not associated with endocrine therapy initiation. This is surprising, given that other analyses have suggested that women, especially older women, might be more likely to use endocrine therapy instead of more aggressive adjuvant chemotherapy.10,28,29
The rapid diffusion of AI use between 2001 and 2005 in our study, even before ASCO guidelines were updated to recommend them in 2004,30 is consistent with findings of previous studies.31 Studies show that AI use increased after the ATAC (Arimidex, Tamoxifen, Alone or in Combination) results were first presented at the San Antonio Breast Cancer Symposium in 200132,33 and continued to rise thereafter.7–9 However, our study is the first to our knowledge to investigate diffusion beyond 2005, and we found little change in AI use after the ASCO guidelines30 were published. In fact, AI use in our study decreased slightly after 2005. We speculate this may be because providers gained a better understanding of the potential risks of use and became more selective in their prescribing. In addition, our study showed that the proportion of women who used AIs from 2005 to 2008 was roughly equal to the proportion who used tamoxifen during the same time period. This finding suggests that in recent years, one drug was not necessarily preferred over the other. However, this contradicts evidence published since 2005, which demonstrates greater benefits from AIs and supports the use of AIs over tamoxifen as first-line endocrine therapy.6,34,35 Thus, we may expect use of AIs to increase and tamoxifen to decrease in future years.
Our primary study limitations are that we lacked information on patient preferences for treatment or provider recommendations. Additionally, we had no automated data on whether therapy was prescribed but not filled or number of coprescriptions (which may have affected initiation). We recognize the importance of these factors and their potential effects on treatment decision making. A small study of older adults with breast cancer showed that 29% (most of whom had early-stage disease) refused all or part of their treatment.36 Thus, treatment refusal among older women may partially explain the proportion of women who used no endocrine therapy in our study. The COMBO study is currently abstracting data on reasons for no use, including whether tamoxifen or AIs were recommended and refused, whether therapy was prescribed, and adverse effects of tamoxifen and AI treatment. Although adverse effects are more often associated with treatment adherence, the potential risk for adverse effects may deter some women from starting endocrine therapy. These data will be important to evaluate in future analyses, particularly on discontinuation and adherence.
Our study also has several strengths. We were able to collect accurate, automated data on tamoxifen and AI use through the Group Health administrative pharmacy database. Automated pharmacy data enabled us to evaluate initiation of these drugs over many years within an integrated delivery system, in which disenrollment is uncommon, and prescription drug coverage is not a barrier for most women. Our results are fairly generalizable to women who receive treatment within a community setting (particularly other integrated delivery systems), because we included all women with early-stage breast cancer who would have been eligible for endocrine therapy; this included women with a range of ages and comorbidities.
Our results show that tamoxifen and AI initiation are not independent of patient demographics and tumor characteristics. Differences in age, tumor size, and lymph node status may be explained by patient and provider preferences, potential adverse effect profiles, and changes in Medicare prescription coverage. Overall initiation (70%) was lower than expected for an insured population and strongly related to older age and early-stage cancers. We need a better understanding of the factors that affect patient and provider decision making regarding these drugs, particularly when treating older women with early-stage breast cancer.
Acknowledgment
Supported by Grant No. R01 CA120562 and American Recovery and Reinvestment Act Administrative Supplement No. R01 CA120562 (D.M.B.) and by Grant No. U01 CA063731 (D.S.M.B.) from the National Cancer Institute, National Institutes of Health.
Appendix
Table A1.
Multivariate Associations Between AI Versus Tamoxifen Initiation in Women Age 55 Years and Older
| Variable | AIs First v Tamoxifen First |
|
|---|---|---|
| RR | 95% CI | |
| Age at diagnosis, years | ||
| 55-64 | 1 | Reference |
| 65-74 | 0.78 | 0.60 to 1.00 |
| ≥ 75 | 0.67 | 0.46 to 0.97 |
| BMI, kg/m2 | ||
| < 25.0 | 1 | Reference |
| 25.0-29.9 | 1.11 | 0.80 to 1.54 |
| ≥ 30.0 | 1.40 | 1.06 to 1.87 |
| Stage at diagnosis | ||
| I | 1 | Reference |
| IIA | 1.21 | 0.66 to 2.23 |
| IIB | 2.02 | 0.74 to 5.46 |
| Tumor size, cm | ||
| < 1.0 | 1 | Reference |
| 1.0-1.9 | 1.06 | 0.77 to 1.45 |
| ≥ 2.0 | 0.84 | 0.48 to 1.48 |
| Lymph node status | ||
| Negative | 1 | Reference |
| Positive | 0.89 | 0.50 to 1.59 |
| Definitive surgical procedure | ||
| Breast conservation | 1 | Reference |
| Mastectomy | 1.08 | 0.76 to 1.53 |
| Chemotherapy | ||
| No | 1 | Reference |
| Yes | 0.99 | 0.76 to 1.28 |
| Radiation therapy | ||
| No | 1 | Reference |
| Yes | 0.94 | 0.76 to 1.28 |
| Charlson score | ||
| 0 | 1 | Reference |
| 1 | 1.20 | 0.91 to 1.58 |
| ≥ 2 | 0.87 | 0.58 to 1.28 |
| Year of diagnosis | ||
| 2001 | 1 | Reference |
| 2002 | 1.80 | 0.92 to 3.52 |
| 2003 | 1.32 | 0.65 to 2.39 |
| 2004 | 2.84 | 1.50 to 5.38 |
| 2005 | 4.04 | 2.21 to 7.38 |
| 2006 | 3.77 | 2.06 to 6.88 |
| 2007 | 4.15 | 2.26 to 7.60 |
| 2008 | 4.24 | 2.26 to 7.95 |
Abbreviations: AI, aromatase inhibitor; BMI, body mass index; RR, relative risk.
* All variables in the table are adjusted for each other.
Figure A1.
Proportions of endocrine therapy use within 12 months of diagnosis over time (women age ≥ 55 years); adjusted for age, stage, tumor size, lymph nodes, body mass index, surgical treatment, chemotherapy, radiation therapy, and comorbidities.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Erin J. Aiello Bowles, Diana S.M. Buist, Jessica Chubak, Denise M. Boudreau
Financial support: Diana S.M. Buist, Denise M. Boudreau
Administrative support: Jeanene Johnson
Provision of study materials or patients: Janet Chestnut, Denise M. Boudreau
Collection and assembly of data: Erin J. Aiello Bowles, Diana S.M. Buist, Jeanene Johnson, Denise M. Boudreau
Data analysis and interpretation: Erin J. Aiello Bowles, Diana S.M. Buist, Jessica Chubak, Onchee Yu, Janet Chestnut, Denise M. Boudreau
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Carlson RW, Hudis CA, Pritchard KI, et al. Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: Evolution of NCCN, ASCO, and St Gallen recommendations. J Natl Compr Canc Netw. 2006;4:971–979. doi: 10.6004/jnccn.2006.0082. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology v. 2.2011, Breast Cancer. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [DOI] [PubMed]
- 3.Eisen A, Trudeau M, Shelley W, et al. Aromatase inhibitors in adjuvant therapy for hormone receptor positive breast cancer: A systematic review. Cancer Treat Rev. 2008;34:157–174. doi: 10.1016/j.ctrv.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Buzdar AU, Coombes RC, Goss PE, et al. Summary of aromatase inhibitor clinical trials in postmenopausal women with early breast cancer. Cancer. 2008;112:700–709. doi: 10.1002/cncr.23193. [DOI] [PubMed] [Google Scholar]
- 5.Thuerlimann B, Koeberle D, Senn HJ. Guidelines for the adjuvant treatment of postmenopausal women with endocrine-responsive breast cancer: Past, present and future recommendations. Eur J Cancer. 2007;43:46–52. doi: 10.1016/j.ejca.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiello EJ, Buist DS, Wagner EH, et al. Diffusion of aromatase inhibitors for breast cancer therapy between 1996 and 2003 in the Cancer Research Network. Breast Cancer Res Treat. 2008;107:397–403. doi: 10.1007/s10549-007-9558-z. [DOI] [PubMed] [Google Scholar]
- 8.Svahn TH, Niland JC, Carlson RW, et al. Predictors and temporal trends of adjuvant aromatase inhibitor use in breast cancer. J Natl Compr Canc Netw. 2009;7:115–121. doi: 10.6004/jnccn.2009.0011. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Dong W, Feig BW, et al. Patterns of treatment for early stage breast cancers at the M. D. Anderson Cancer Center from 1997 to 2004. Cancer. 2009;115:2041–251. doi: 10.1002/cncr.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor–positive breast cancer. J Clin Oncol. 2004;22:3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 12.Harlan LC, Clegg LX, Abrams J, et al. Community-based use of chemotherapy and hormonal therapy for early-stage breast cancer: 1987-2000. J Clin Oncol. 2006;24:872–877. doi: 10.1200/JCO.2005.03.5840. [DOI] [PubMed] [Google Scholar]
- 13.Phipps AI, Ichikawa L, Bowles EJ, et al. Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas. 2010;67:60–66. doi: 10.1016/j.maturitas.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudreau DM, Doescher MP, Jackson JE, et al. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38:1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 15.Buist DS, LaCroix AZ, Brenneman SK, et al. A population-based osteoporosis screening program: Who does not participate, and what are the consequences? J Am Geriatr Soc. 2004;52:1130–1137. doi: 10.1111/j.1532-5415.2004.52311.x. [DOI] [PubMed] [Google Scholar]
- 16.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Group Health Research Institute. Breast Cancer Surveillance project. http://www.grouphealthresearch.org/surveillanceproject/default.htm.
- 19.Adjuvant! Online. http://www.adjuvantonline.com/breast.jsp.
- 20.Van Leeuwen BL, Rosenkranz KM, Feng LL, et al. The effect of under-treatment of breast cancer in women 80 years of age and older. Crit Rev Oncol Hematol. 2010;79:315–320. doi: 10.1016/j.critrevonc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Schonberg MA, Marcantonio ER, Li D, et al. Breast cancer among the oldest old: Tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28:2038–2045. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enger SM, Thwin SS, Buist DS, et al. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–4383. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurria A, Wong FL, Villaluna D, et al. Role of age and health in treatment recommendations for older adults with breast cancer: The perspective of oncologists and primary care providers. J Clin Oncol. 2008;26:5386–5392. doi: 10.1200/JCO.2008.17.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster JA, Salinas GD, Mansell D, et al. How does older age influence oncologists' cancer management? Oncologist. 2010;15:584–592. doi: 10.1634/theoncologist.2009-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Medicare & Medicaid Services. http://www.cms.gov.
- 26.Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: A companion study to NCIC CTG MA. 17. J Clin Oncol. 2006;24:3629–3635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 27.Rabaglio M, Sun Z, Price KN, et al. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann Oncol. 2009;20:1489–1498. doi: 10.1093/annonc/mdp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naeim A, Wong FL, Pal SK, et al. Oncologists' recommendations for adjuvant therapy in hormone receptor-positive breast cancer patients of varying age and health status. Clin Breast Cancer. 2010;10:136–143. doi: 10.3816/CBC.2010.n.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silliman RA, Guadagnoli E, Rakowski W, et al. Adjuvant tamoxifen prescription in women 65 years and older with primary breast cancer. J Clin Oncol. 2002;20:2680–2688. doi: 10.1200/JCO.2002.08.137. [DOI] [PubMed] [Google Scholar]
- 30.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor–positive breast cancer: Status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 31.Buzdar A, Macahilig C. How rapidly do oncologists respond to clinical trial data? Oncologist. 2005;10:15–21. doi: 10.1634/theoncologist.10-1-15. [DOI] [PubMed] [Google Scholar]
- 32.Munster PN, Horton J. Tamoxifen vs the aromatase inhibitors: News from San Antonio, 2001. Cancer Control. 2001;8:478–479. doi: 10.1177/107327480100800601. [DOI] [PubMed] [Google Scholar]
- 33.Baum M. The ATAC (Arimidex, Tamoxifen, Alone or in Combination) adjuvant breast cancer trial in post-menopausal (PM) women. Presented at the 24th Annual San Antonio Breast Cancer Symposium; December 10-13, 2001. [Google Scholar]
- 34.Colleoni M, Giobbie-Hurder A, Regan MM, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29:1117–1124. doi: 10.1200/JCO.2010.31.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 36.Puts MT, Monette J, Girre V, et al. Characteristics of older newly diagnosed cancer patients refusing cancer treatments. Support Care Cancer. 2010;18:969–974. doi: 10.1007/s00520-010-0883-0. [DOI] [PubMed] [Google Scholar]