The intensity of post-treatment surveillance performed by ASCO members caring for patients with breast cancer varies markedly despite evidence from well-designed, adequately powered randomized controlled trials.
Abstract
Purpose:
To determine how physicians monitor their patients after initial curative-intent treatment for breast carcinoma.
Methods:
A custom-designed survey instrument with four idealized patient vignettes (TNM stages 0 to III) was e-mailed to the 3,245 members of ASCO who had identified themselves as having breast cancer as a major focus of their practice. Respondents were asked how they use 12 specific follow-up modalities during post-treatment years 1 to 5 for each vignette. Mean, median, standard deviation, and range of the intensity of use for each modality were calculated for the four vignettes.
Results:
Of the 3,245 ASCO members surveyed, 1,012 (31%) responded. Of these, 915 (90%) were evaluable and were included in our analysis. Office visit, mammogram, complete blood count, and liver function tests were the most commonly recommended surveillance modalities. There was marked variation in surveillance intensity. For example, office visit was recommended 4.1 ± 2.2 times (mean ± SD) in year 1 after curative treatment of a patient with stage III breast cancer. Similar variation was observed for all modalities.
Conclusions:
The intensity of post-treatment surveillance performed by ASCO members caring for patients with breast cancer varies markedly despite evidence from well-designed, adequately powered randomized controlled trials. Many modalities not recommended by ASCO guidelines are used routinely, which constitutes evidence of overuse. The lack of consensus is likely due to multiple factors and constitutes an appealing target for interventions to rationalize surveillance.
Introduction
Breast carcinoma is the most common cancer (excluding nonmelanoma skin cancer) and the second leading cause of cancer-related death in American women. The incidence is estimated at 123 per 100,000 women per year in the United States, with an age-adjusted mortality rate of 24 per 100,000 women per year.1 The increasing population in the United States means the absolute number of women diagnosed with breast cancer continues to increase yearly, with an estimated 209,060 new cases of invasive breast cancer and 54,010 new cases of in situ breast cancer in 2010.1 This, in combination with decreasing mortality rates, has led to an increase in the number of breast cancer survivors who need long-term surveillance.2
Since the large, well designed trials of the GIVIO (Gruppo Interdisciplinare Valutazione Interventi in Oncologia) investigators and Roselli del Turco et al,3,4 there has been little debate over what tests should be obtained, how often they should be obtained, and how long surveillance should be continued. The National Comprehensive Cancer Network (NCCN) guidelines recommend interval history and physical examination every 4 to 6 months for 5 years and then annually thereafter, with a mammogram every 12 months (the first post- treatment mammogram to be obtained 6 to 12 months after completion of chemotherapy). For women receiving tamoxifen or aromatase inhibitors, annual gynecologic examinations are recommended. Regular bone mineral density examinations are recommended for women receiving aromatase inhibitors. No other routine laboratory and/or imaging modalities are currently recommended for routine surveillance. Further testing is recommended only when abnormalities are detected by history, physical examination, or mammography, as such testing has not been shown to improve overall or disease-free survival.5,6
NCCN surveillance recommendations are not stratified on the basis of cancer stage at time of initial diagnosis or treatment. The ASCO guidelines are similar: history and physical examination every 3 to 6 months for the first 3 years, every 6 to 12 months for years 4 and 5, and then annually thereafter, with further testing only if symptoms arise; annual mammography, gynecologic examination, and bone mineral density examination recommendations mirror the NCCN guidelines.7 The NCCN and ASCO guidelines are more intensive than the preferred (low-intensity) strategy in both large clinical trials.3–7
Previous studies have investigated the impact of more intensive surveillance versus the symptom-driven approach of the NCCN and ASCO guidelines.8–11 The results have generally supported the minimalist strategies. There is also evidence that nurses and primary care physicians can carry out surveillance, with guidance from an oncologist.8,9 However, little is known about current actual practice patterns of expert clinicians and whether they adhere to or deviate from guideline recommendations. We sought to determine the current follow-up patterns of a large number of highly experienced, credentialed oncologists who provide care for patients with breast cancer and monitor them after treatment. We created a survey instrument to accomplish this and chose ASCO members as survey participants.
Methods
We devised four idealized vignettes depicting patients with breast carcinoma who were otherwise healthy. In each vignette, the patient described had curative-intent initial treatment, but each featured a different American Joint Commission on Cancer (AJCC) stage, burden of disease, and/or biomarker profile. The four vignettes featured generally healthy patients with stage 0 (TisN0M0) estrogen receptor (ER) –positive, progesterone receptor (PR) –positive ductal carcinoma in situ (DCIS); stage IIA (T2N0M0) ER-positive, PR-positive, and HER2/neu-nonamplified invasive ductal cancer; stage IIA (T1N1M0) ER-negative, PR-negative, and HER2/neu-nonamplified invasive ductal cancer; and stage IIIA (T3N2M0) ER-positive, PR-positive, and HER2/neu-amplified invasive ductal cancer. We created a questionnaire based on these vignettes to quantify the surveillance practices of our target population. We compiled an e-mail directory of all 3,245 ASCO members who identified themselves as having breast cancer as a major focus of their practice. A cover e-mail outlined the purpose of the survey and the motivation behind it. Links to the four vignettes and the survey instrument were provided in the e-mail. SurveyMonkey.com was used to conduct the survey, and the time to complete the survey was estimated to be approximately 5 to 15 minutes. The first questions in the survey asked whether the member treated patients with breast cancer and whether they also participated in their long-term follow-up care. Only those who both treated breast cancer patients and provided long-term follow-up care at the time of the e-mail survey were asked to complete the entire survey. The others were asked only to complete the portion of the survey dealing with demographic details (age, gender, membership in other medical societies, type of practice [private, academic, government, other], the percentage of his or her practice composed of breast cancer care, and the like).
In the remainder of the survey, we asked each ASCO member to describe his or her surveillance schedule after appropriate curative-intent treatment for each patient described in the four idealized vignettes. Each was asked to indicate the number of annual office visits and surveillance tests he or she recommended in the first 5 years after completion of initial therapy. The list of surveillance tests was compiled after a thorough review of the current pertinent literature and an informal survey of local oncologists who care for patients with breast cancer confirmed that the list contained all tests in current use. In addition to office visit, surveillance tests included complete blood count, liver function tests, serum CA 15-3 level, serum carcinoembryonic antigen level, diagnostic mammogram, diagnostic breast ultrasonography, magnetic resonance imaging (MRI) of the breast, computed tomography (CT) of chest, CT of abdomen/pelvis, radionuclide bone scan, and whole-body positron emission tomography (PET) or PET-CT. The cover letter, the four vignettes, and the survey instrument are available on request from the corresponding author. Reminder e-mails were sent every week for 5 weeks to each individual ASCO member who had not responded to a previous request.
On receipt of the responses, data from all vignettes were compiled into a single database and analyzed. Mean, median, range, and standard deviation were calculated for each surveillance modality in each post-treatment year for each vignette and for all vignettes grouped together. A literature search was conducted to identify all previously published articles on the topic of surveillance practices of expert clinicians after curative-intent treatment for breast cancer. We searched PubMed, Scopus, Ovid, and Google on August 10, 2010. We completed three different searches in each of the four search engines to identify all previously published reports on this topic. The terms for the three searches were “breast cancer” plus “survey” plus “surveillance,” “breast cancer” plus “follow-up testing” plus “survey, ” and “breast cancer” plus “survey” plus “posttreatment testing.” Our multiple computer searches revealed no articles in the English language literature other than those cited herein.
Results
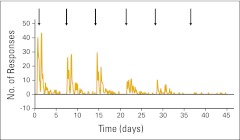
Of the 3,245 ASCO members surveyed, 1012 (31%) responded. Figure 1 illustrates the response rates after the initial e-mail and each subsequent reminder e-mail. There were 97 of the 1,012 (10%) whose responses were excluded because the physicians were retired, did not complete the survey, did not perform long-term follow-up for their patients, and/or represented an extreme outlier (responses were unintelligible). The remaining 915 (90%) completed the survey instruments and were considered evaluable. Demographic data are shown in Table 1. The responses for all four vignettes combined are presented in Tables 2 and Appendix Table A1 (online only). Table 2 describes the survey responses using mean ± one standard deviation. Table A1 (online only) describes the same data using median and range (minimum, maximum). Data were combined across the four vignettes for ease of data presentation; similar variation was observed for each individual vignette. Office visit, mammogram, complete blood count, and liver function tests were the most commonly recommended methods of surveillance. There was marked variation in surveillance intensity regardless of the stage of disease presentation. For example, office visit was used 4.1 (± 2.2) times in year 1 after curative treatment of a stage III breast cancer. The corresponding median and range were 4 and 1 to 12. Similar variation was observed for all modalities.
Figure 1.
Frequency of survey responses to initial and five subsequent e-mail reminders (dates denoted by arrows).
Table 1.
Demographic Characteristics of Survey Responders (N = 915)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| < 30 | 0 | 0 |
| 30-39 | 124 | 13.6 |
| 40-49 | 273 | 29.8 |
| 50-59 | 328 | 35.9 |
| 60-69 | 164 | 17.9 |
| ≥ 70 | 26 | 2.8 |
| Primary practice type | ||
| Private | 479 | 52.4 |
| Academic | 421 | 46.0 |
| Government | 15 | 1.6 |
| Specialty | ||
| Surgical oncology | 87 | 9.5 |
| Radiation oncology | 43 | 4.7 |
| Medical oncology | 644 | 70.4 |
| Other | 141 | 15.4 |
| Percentage of practice devoted to breast cancer | ||
| < 1% | 0 | 0 |
| 1%–5% | 10 | 1.1 |
| 6%–10% | 59 | 6.5 |
| 11%–25% | 205 | 22.4 |
| 26%–50% | 197 | 21.5 |
| > 50% | 444 | 48.5 |
Table 2.
Mean and Standard Deviation Follow-Up Practice Patterns of ASCO Members, Stratified by Post-Treatment Year
| Modality | Post-Treatment Year |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Office visit | 3.4 | 1.7 | 2.9 | 1.2 | 2.4 | 1.2 | 2.2 | 1.3 | 2.1 | 1.3 |
| Complete blood count | 2.0 | 2.2 | 1.7 | 1.8 | 1.4 | 1.7 | 1.3 | 1.6 | 1.3 | 1.6 |
| Liver function tests | 2.1 | 2.1 | 1.7 | 1.7 | 1.5 | 1.6 | 1.4 | 1.6 | 1.3 | 1.6 |
| Serum CA 15-3 antigen level | 0.7 | 1.5 | 0.6 | 1.3 | 0.5 | 1.2 | 0.5 | 1.1 | 0.5 | 1.0 |
| Serum CEA level | 0.4 | 1.2 | 0.4 | 1.1 | 0.3 | 1.0 | 0.3 | 0.9 | 0.3 | 0.9 |
| Diagnostic mammogram | 1.6 | 1.4 | 1.4 | 1.6 | 1.3 | 1.7 | 1.3 | 1.7 | 1.3 | 1.7 |
| Diagnostic ultrasonography | 0.3 | 0.7 | 0.2 | 0.6 | 0.2 | 0.7 | 0.2 | 0.8 | 0.2 | 0.8 |
| Breast MRI | 0.2 | 0.8 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.6 |
| CT chest | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.5 |
| CT abdomen | 0.1 | 0.4 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.5 |
| Bone scan | 0.1 | 0.5 | 0.1 | 0.6 | 0.1 | 0.6 | 0.1 | 0.6 | 0.1 | 0.6 |
| PET scan | 0.1 | 0.4 | 0.0 | 0.3 | 0.0 | 0.2 | 0.0 | 0.2 | 0.1 | 0.6 |
NOTE. All four clinical vignettes are grouped. Data are displayed as the number of times the modality is requested each year. This method of displaying the data gives a conservative impression of the variability among practices.
Abbreviations: CEA, carcinoembryonic antigen; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Although the median use of the more advanced imaging studies (CT chest, CT abdomen/pelvis, bone scan, and PET scan) was 0, the range varied, with 7% to 15% of respondents using each of these tests at least once per year. In contrast, more than 80% of the respondents used complete blood counts and/or liver function tests at least once per year.
Discussion
The intensity of routine surveillance testing in current clinical practice for patients treated for breast cancer with curative intent has been previously studied in well-designed randomized clinical trials.6,7 These and other reports challenge the value of intensive surveillance because there appears to be no survival benefit in diagnosing recurrence before symptoms occur.12–14 Traditionally, physicians have considered breast cancer recurrence to occur most commonly within the first 5 years after initial treatment. Therefore, as suggested by the NCCN and ASCO guidelines, many breast cancer survivors have had intensive follow-up with de-escalation after 5 years. However, recently published data suggest that women with hormone receptor–positive breast cancer have a slower (and/or lower) rate of recurrence over a 10-year period, raising the question of whether surveillance strategies should be based on tumor biology rather than stage at diagnosis.15,16 Currently, practice guidelines do not stratify surveillance intensity on the basis of tumor biology, but rather by the extent of disease at the time of diagnosis and treatment.
The most important finding in the current study is that there is wide variation in surveillance practice, even among practicing oncologists who care for patients with breast cancer and who presumably have more insight into appropriate surveillance strategies than any other physicians. It is not surprising that office visit was the most commonly used modality. Even though the office visit is limited in its ability to detect asymptomatic recurrent disease, it provides excellent evidence about general health status, psychological problems, and similar concerns that cannot easily be obtained in any other way. Clinical breast and/or chest wall examination and regional lymph node examination can detect recurrent disease, a second primary breast cancer, and some treatment-related adverse effects. Our survey revealed that the mean frequency with which oncologists recommended an office visit was 3.4 times in post-treatment year 1, decreasing to 2.1 times per year in year 5.
Complete blood count and liver function tests were also commonly used, despite evidence demonstrating that a protocol of frequent laboratory tests does not improve survival or influence health-related quality of life.6 This finding indicates that a fully minimalist protocol has not been implemented in practice. We believe that the data derived from our survey more accurately indicate the surveillance strategies in current use than evaluation of test frequency in clinical datasets or databases. For example, physicians caring for breast cancer survivors often obtain a diagnostic test when they are uncertain about a clinical complaint or concern, even though they may be fully aware of its limitations in terms of sensitivity, specificity, and predictive value. They may order a test at the office visit scheduled for surveillance to investigate an apparently unrelated symptom. In addition, physicians may be influenced by the patient's expectations. Most patients report a personal preference for more intensive follow-up. However, the intensity of diagnostic testing has not been shown to affect emotional well-being or quality of life.6
As expected, mammography was used by respondents regardless of the post-treatment year of surveillance. The only recurrent breast cancer scenario associated with cure or long-term disease-free survival is local-regional recurrence within an intact breast after breast-conserving therapy or a metachronous axillary lymph node metastasis without evidence of distant spread. Yearly mammographic surveillance of women who have undergone curative treatment and have a good prognosis has been shown to be highly cost effective because these patients are at increased risk for developing a second primary breast cancer.17,18 Further, several studies have demonstrated that such mammographic surveillance is associated with improved survival.10,19,20 No such data exist for surveillance breast ultrasonography or MRI; the rate of use of these modalities was very low in this study.
Grunfeld et al21 recently reported the patterns of follow-up care for breast cancer survivors in Canada. As in our study, substantial variation in adherence to guideline recommendations was observed. They observed both overuse and underuse of surveillance tests and visits. Half of the women had more imaging studies than recommended for detection of metastatic disease, whereas one quarter of the women had fewer than the recommended number of surveillance mammograms. This finding illustrates the need for improved education regarding survivorship care plans.22
The authors recognize that all surveys such as the one used here have limitations. The response rate was only 31%, which is much lower than the preferred 60% to 70% but typical for a survey of this type. This raises the possibility that the results presented here may not be representative of the practices of the entire ASCO membership. The survey results are clearly not generalizable to low-income countries and are probably not generalizable to physicians practicing in remote areas. We did not specifically ask respondents to comment on their country of practice, and we recognize that responses may vary according to different health care delivery systems. Recall bias, the risk of inaccuracy when survey respondents are asked to quantify their own practices, is likely to have occurred. The modalities that respondents indicate that they choose, and the frequency of their use, may not match their actual practice. A potential bias may occur if the oncologist requests a test for what is thought to be an unrelated indication (eg, CT of abdomen/pelvis to investigate abdominal pain) that has the potential to diagnose recurrent cancer because that test may cause the clinician to defer or omit a scheduled test (CT of abdomen/pelvis in this example) aimed at detecting recurrent cancer. Another important limitation is that the survey was sent only to members of ASCO. Other physicians, such as primary care physicians and gynecologists, also monitor breast cancer patients after treatment. Their practices are not represented in this study. The majority of ASCO members practice in the United States, but surveillance schedules may be different in other countries, particularly those without a national health care system or with many low-income citizens. Even in the United States, variation in financial resources among citizens is one of the most important causes of variation in the use of indicated diagnostic measures and therapeutic interventions.23,24
Despite these limitations, this survey provides valuable information regarding the current surveillance practices of oncologists caring for patients with breast cancer who have undergone prior curative treatment. We believe ours is the only recent report about actual practice by oncologists who are actively monitoring patients who have undergone previous curative-intent treatment for breast cancer. Although there is general agreement in the literature about the most appropriate surveillance strategy as defined by well-controlled, adequately powered trials, the intensity of post-treatment surveillance performed by ASCO members varies markedly. The observed lack of consensus is likely due to multiple factors. Improved medical education is needed to inform clinicians and trainees about evidence from existing trials to avoid overuse, underuse, and misuse of scarce medical resources.
The published trials are dated, and there is a need for additional trials that incorporate new diagnostic modalities. Our data provide information about actual practice, on the basis of which such trials can be designed. One important reason that such trials may have been so uncommon is the lack of funding from relevant funding agencies, such as the National Institutes of Health, whose mandate is focused primarily on treatment-related trials rather than post-treatment surveillance trials. Because there are currently more than 10 million cancer survivors in the United States alone, including more than 1 million 20-year survivors, the need for such trials for most types of cancer is great. Although surveillance trials require significant time and money to conduct, the potential impact of such results on the allocation of limited resources is profound. Professional societies, advocacy groups, political organizations, and other entities will presumably have to demand that funding be provided in order to change the status quo. Anything less than robust evidence is not likely to reduce the remarkably large variation in clinical practice that we have documented here. The community of clinicians who treat patients with breast cancer should strongly advocate for such trials. The United States Patient Protection and Affordable Care Act of 2010 provides for a nonprofit Patient-Centered Outcomes Research Institute that is charged with conducting comparative effectiveness research. This mechanism appears ideal for the trials we envision.
Appendix
Table A1.
Median Follow-Up Practice Patterns of ASCO Members, Stratified by Post-Treatment Year
| Modality | Post-Treatment Year |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | |
| Office visit | 4 | 0-12 | 3 | 0-12 | 2 | 0-12 | 2 | 0-12 | 2 | 0-12 |
| Complete blood count | 2 | 0-12 | 1 | 0-12 | 1 | 0-12 | 1 | 0-12 | 1 | 0-12 |
| Liver function tests | 2 | 0-12 | 2 | 0-12 | 1 | 0-12 | 1 | 0-12 | 1 | 0-12 |
| Serum CA 15-3antigen level | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 |
| Serum CEA level | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 |
| Diagnostic mammogram | 1 | 0-12 | 1 | 0-12 | 1 | 0-12 | 1 | 0-12 | 1 | 0-12 |
| Diagnostic ultrasonography | 0 | 0-6 | 0 | 0-6 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 |
| Breast MRI | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 |
| CT chest | 0 | 0-6 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 |
| CT abdomen | 0 | 0-6 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 |
| Bone scan | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 | 0 | 0-12 |
| PET scan | 0 | 0-6 | 0 | 0-10 | 0 | 0-12 | 0 | 0-2 | 0 | 0-10 |
NOTE. All four clinical vignettes are grouped. Data are displayed as the number of times the modality is requested each year. This depiction of the data emphasizes the variability among practices.
Abbreviations: CEA, carcinoembryonic antigen; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Julie A. Margenthaler, Emad Allam, Katherine S. Virgo, Udayan Mayur Kulkarni, Anand P. Patel, Frank E. Johnson
Administrative support: Julie A. Margenthaler, Emad Allam, Katherine S. Virgo, Frank E. Johnson
Provision of study materials or patients: Julie A. Margenthaler, Katherine S. Virgo, Frank E. Johnson
Collection and assembly of data: Julie A. Margenthaler, Emad Allam, Katherine S. Virgo, Frank E. Johnson
Data analysis and interpretation: Julie A. Margenthaler, Emad Allam, Ling Chen, Katherine S. Virgo, Udayan Mayur Kulkarni, Frank E. Johnson
Manuscript writing: Julie A. Margenthaler, Katherine S. Virgo, Frank E. Johnson
Final approval of manuscript: All authors
References
- 1.American Cancer Society. Atlanta, GA: American Cancer Society; 2010. Cancer Facts & Figures 2010. [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; 2008. SEER Cancer Statistics Review, 1975-2005. seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 3.Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The GIVIO Investigators. JAMA. 1994;271:1587–1592. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- 4.Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer Follow-up. JAMA. 1994;271:1593–1597. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology, Breast. 2009;Volume 1 http://www.nccn.com/files/cancer-guidelines/breast/index.html. [Google Scholar]
- 6.Rojas MP, Telaro E, Russo A, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005;4:CD001768. doi: 10.1002/14651858.CD001768. [DOI] [PubMed] [Google Scholar]
- 7.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: A comparison of family physician versus specialist care. J Clin Oncol. 2006;24:848–855. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 9.Grunfeld E, Mant D, Yudkin P, et al. Routine follow-up of breast cancer in primary care: Randomized trial. BMJ. 1996;313:665–669. doi: 10.1136/bmj.313.7058.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paszat L, Sutradhar R, Grunfeld E, et al. Outcomes of surveillance mammography after treatment of primary breast cancer: A population-based case series. Breast Cancer Res Treat. 2009;114:169–178. doi: 10.1007/s10549-008-9986-4. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery DA, Krupa K, Cooke TG. Follow-up in breast cancer: Does routine clinical examination improve outcome? A systematic review of the literature. Br J Cancer. 2007;97:1632–1641. doi: 10.1038/sj.bjc.6604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomin R, Donegan WL. Screening for recurrent breast cancer – its effectiveness and prognostic value. J Clin Oncol. 1987;5:62–67. doi: 10.1200/JCO.1987.5.1.62. [DOI] [PubMed] [Google Scholar]
- 13.Dewar JA, Kerr GR. Value of routine follow-up for women treated for early carcinoma of the breast. BMJ (Clin Res Ed) 1985;291:1464–1467. doi: 10.1136/bmj.291.6507.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly J, Mack P, Donaldson LA. Follow-up of breast cancer: Time for a new approach? Int J Clin Pract. 2001;55:431–433. [PubMed] [Google Scholar]
- 15.Pagani O, Price KN, Gelber RD, et al. International Breast Cancer Study Group (IBCSG) Patterns of recurrence of early breast cancer according to estrogen receptor status: A therapeutic target for a quarter of a century. Breast Cancer Res Treat. 2009;117:319–324. doi: 10.1007/s10549-008-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists' Collaorative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: An overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 17.Lindfors KK, Rosenquist CJ. The cost-effectiveness of mammographic screening strategies. JAMA. 1995;274:881–884. [PubMed] [Google Scholar]
- 18.Cady B. Cost-effective preoperative evaluation, operative treatment, and postoperative follow-up in the breast cancer patient. Surg Clin North Am. 1996;76:25–34. doi: 10.1016/s0039-6109(05)70419-9. [DOI] [PubMed] [Google Scholar]
- 19.Lash TL, Fox MP, Buist DS, et al. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol. 2007;25:3001–3006. doi: 10.1200/JCO.2006.09.9572. [DOI] [PubMed] [Google Scholar]
- 20.Lash TL, Fox MP, Silliman RA. Reduced mortality rate associated with annual mammograms after breast cancer therapy. Breast J. 2006;12:2–6. doi: 10.1111/j.1075-122X.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- 21.Grunfeld E, Hodgson DC, Del Giudice ME, et al. Population-based longitudinal study of follow-up care for breast cancer survivors. J Oncol Pract. 2010;6:174–181. doi: 10.1200/JOP.200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganz PA, Hahn EE. Implementing a survivorship care plan for patients with breast cancer. J Clin Oncol. 2008;26:759–767. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]
- 23.Schootman M, Jeffe DB, Reschke AH, Aft R. Disparities related to socioeconomic status and access to medical care remain in the United States among women who never had a mammogram. Cancer Causes Control. 2003;14:419–423. doi: 10.1023/a:1024941626748. [DOI] [PubMed] [Google Scholar]
- 24.Chu KC, Miller BA, Springfield SA. Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. J Natl Med Assoc. 2007;99:1092–1098. [PMC free article] [PubMed] [Google Scholar]