Factors related to the consent process, including consenter experience and timing of study enrollment, are significantly associated with or have a trend toward association with markers of patient interest in clinical research.
Abstract
Purpose:
Low rates of participation in cancer clinical trials have been attributed to patient, institutional, and study characteristics. However, few studies have examined factors related to the consent process. We therefore evaluated the impact of consent timing and experience on markers of patient interest in research.
Methods:
We performed a retrospective analysis of patients enrolled in a cancer center tissue repository. During enrollment, patients were asked if they were willing to be contacted in the future to provide medical follow-up information and/or to participate in other clinical research. We analyzed the association between patient responses to these questions and consent process factors using univariate analysis and multivariate logistic regression.
Results:
Of 922 patients evaluated, 85% agreed to be contacted to provide follow-up information, and 83% agreed to be contacted to participate in future research studies. In univariate analysis, willingness to be contacted for future research was associated with consenter experience (P = .01) and had a trend toward association with the timing of enrollment in relation to diagnosis (P = .08), but it was not associated with patient sex, race, or diagnosis. In multivariate analysis, responses remained associated with consenter experience (P = .02).
Conclusion:
Factors related to the consent process, including consenter experience and timing of study enrollment, are significantly associated with or have a trend toward association with markers of patient interest in clinical research. These understudied and potentially modifiable variables warrant further evaluation.
Introduction
Only 1% to 3% of adult patients with cancer in the United States enroll onto clinical trials.1–4 This low participation rate hinders clinical advances and limits research generalizability. Accordingly, much effort has been devoted to identifying the variables that limit and facilitate clinical trial accrual. Cited factors include patient characteristics such as age,5,6 sex,3 race,7–9 and socioeconomic status7,10,11; institutional factors such as clinical trial availability4,9; and study factors such as eligibility criteria.12
However, relatively little research has focused on the role of the consent process in clinical trial participation. Using a university-based tissue repository, we previously evaluated the impact of consenter characteristics on participant interest in clinical research.13 Specifically, we used the surrogate end point of patients' agreeing to be contacted for future research studies. In that study, willingness to be contacted for future research was associated with the total number of participants consented by an individual consenter and with participant-consenter sex discordance. In the present study, we analyze the associations between consent timing, experience, and markers of patient interest in clinical research. We chose to focus on these variables because, in contrast to inherent patient characteristics and study eligibility criteria, they represent factors in the research process that might be readily modified.
Methods
Data Source and Research Setting
The University of Texas Southwestern (UT Southwestern) Tissue Resource is a university-based tissue repository. Since its inception in 2000, the tissue resource has stored tissue and blood samples from consenting participants undergoing a biopsy or surgical procedure for suspected or confirmed cancer. Tissue and/or blood samples, along with corresponding clinical data, are collected and archived prospectively for future research studies. Participants enrolling in the tissue resource are asked the following two questions on the consent form: one, “May members of the University of Texas Southwestern Medical Center Tissue Resource contact you in the future for follow-up information?” and two, “May members of the University of Texas Southwestern Medical Center Tissue Resource contact you in the future to ask you to take part in more research?” Participation in the tissue resource is not dependent on responses to these questions. The consent form is available in both English and Spanish, and participants who speak other languages are enrolled through use of a short-form and translator.
Participants in the UT Southwestern Tissue Resource are recruited from various clinical facilities affiliated with UT Southwestern. These include Parkland Health and Hospital System (968-bed county safety-net hospital and associated outpatient clinics), University Hospital (415-bed tertiary care, inpatient facility), and the Harold C. Simmons Cancer Center, a freestanding National Cancer Center–designated outpatient facility.
Data Collection
This study was approved by the UT Southwestern institutional review board. For each of the patients enrolled in the UT Southwestern Tissue Resource, we obtained the following data: age, sex, race, diagnosis, and responses to questions one and two listed on the consent form. Date of disease diagnosis, date of enrollment in the tissue resource, and identity of the individual who enrolled the patient were also obtained.
Patient race was characterized as non-Hispanic white or other. Race was dichotomized to increase the statistical power of the analysis; in our earlier study of research interest, we found no association when race was characterized as non-Hispanic white, Hispanic, African American, or other.13 Diagnosis was characterized as cancer, benign, or healthy control (generally a family member of a patient in the tissue resource who agreed to provide a blood sample for tissue resource). Timing of enrollment was defined as the interval between date of diagnosis and date of enrollment in the tissue resource. Healthy controls (n = 43; 4% of total participants) were not included in this analysis, because they did not have a date of diagnosis. Consenter identity and date of enrollment were used to determine the order in which patients were enrolled in the tissue resource by individual consenters, which was used as a marker of consenter experience.
Statistical Analyses
Patient responses to questions one and two were analyzed separately. In the univariate analysis, we used the Pearson χ2 test to calculate P values. We obtained odds ratios and 95% CIs from the univariate logistic regression. Enrollment order and timing of enrollment were divided into best-fit quartiles. We fitted a multivariate logistic regression model, including the covariates of dichotomized patient age, sex, enrollment timing, and enrollment order. All reported P values are two sided. All statistical analyses were implemented by SAS 9.1 for Windows (SAS Institute, Cary, NC).
Results
From June 2000 to September 2007, 922 participants were enrolled in the UT Southwestern Tissue Resource. Baseline patient characteristics are listed in Table 1. As previously described,13 67% of patients were female, 75% were age 65 years or younger, and 63% were non-Hispanic white. Enrollment order was determined for 872 patients (95%), who were enrolled by a total of 187 consenters. Among these patients, 187 (21%) were the first patient enrolled by an individual consenter, 186 (21%) were the second or third patient enrolled by an individual consenter, 239 (28%) were the fourth to ninth patient enrolled by an individual consenter, and 260 (30%) were the tenth or later patient enrolled by an individual consenter. The timing of tissue resource enrollment in relation to the date of diagnosis was determined for 899 patients: 217 (24%) were enrolled before diagnosis (typically on day of initial diagnostic procedure), 189 (21%) were enrolled within 1 to 30 days of diagnosis, 239 were enrolled within 31 to 90 days of diagnosis, and 254 (28%) were enrolled more than 90 days after diagnosis.
Table 1.
Baseline Patient Characteristics (N = 922)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| ≤ 65 | 691 | 75 |
| > 65 | 231 | 25 |
| Sex | ||
| Male | 305 | 33 |
| Female | 617 | 67 |
| Race | ||
| White (non-Hispanic) | 578 | 63 |
| Other | 326 | 35 |
| Unknown | 18 | 2 |
| Disease status | ||
| Cancer | 763 | 83 |
| Benign | 116 | 13 |
| Healthy control | 43 | 4 |
The majority of patients agreed to be contacted in the future to provide medical follow-up (question one; 84.9%; 95% CI, 82.5% to 87.2%) and to participate in other research studies (question two; 83.1%; 95% CI, 80.6% to 85.5%). Responses to these questions were highly correlated, with 96% of patients responding either “yes” or “no” to both questions (P < .001).
Table 2 summarizes the association between patient characteristics and responses in univariate analysis. Patient age was significantly associated with willingness to be contacted for medical follow-up. Among patients age 65 years or younger, 86% agreed, compared with 80% of patients age older than 65 years (P = .03). There was a nonsignificant association between patient age and willingness to be contacted for future research (84% v 80%; P = .14). Patient sex, race, and diagnosis were not associated with responses.
Table 2.
Univariate Analysis of Association Between Patient and Disease Characteristics and Patient Responses
| Characteristic | Medical Follow-Up |
Future Research |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| “Yes” |
“No” |
OR* | 95% CI | P | “Yes” |
“No” |
OR* | 95% CI | P | |||||
| No. | % | No. | % | No. | % | No. | % | |||||||
| Age, years | .03 | .14 | ||||||||||||
| ≤ 65 | 580 | 86 | 92 | 14 | Ref | 585 | 84 | 107 | 16 | Ref | ||||
| > 65 | 178 | 80 | 44 | 20 | 0.64 | 0.43 to 0.95 | 177 | 80 | 45 | 20 | 0.75 | 0.51 to 1.10 | ||
| Sex | .19 | .31 | ||||||||||||
| Female | 521 | 86 | 85 | 14 | Ref | 509 | 84 | 97 | 16 | Ref | ||||
| Male | 242 | 83 | 51 | 17 | 0.77 | 0.53 to 1.13 | 238 | 81 | 55 | 19 | 0.83 | 0.57 to 1.12 | ||
| Race | .83 | .40 | ||||||||||||
| White† | 486 | 85 | 85 | 15 | Ref | 479 | 84 | 92 | 16 | Ref | ||||
| Other | 263 | 85 | 48 | 15 | 0.96 | 0.65 to 1.41 | 254 | 82 | 57 | 18 | 0.86 | 0.60 to 1.23 | ||
| Disease | .90 | .30 | ||||||||||||
| Cancer | 627 | 85 | 114 | 15 | Ref | 612 | 83 | 129 | 17 | Ref | ||||
| Benign | 99 | 86 | 16 | 14 | 1.13 | 0.64 to 1.98 | 101 | 88 | 14 | 12 | 1.52 | 0.84 to 2.74 | ||
| Healthy control | 37 | 86 | 6 | 14 | 1.12 | 0.46 to 2.72 | 34 | 79 | 9 | 21 | 0.80 | 0.37 to 1.70 | ||
Abbreviations: OR, odds ratio; Ref, reference.
OR > 1: more likely to answer “yes.”
Non-Hispanic.
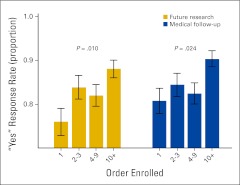
Consenter experience, defined as the order in which a patient was enrolled by an individual consenter, was significantly associated with willingness to provide medical follow-up (P = .024) and to be contacted for future research studies (P = .01; Figure 1). Among individuals who were the first enrolled by an individual consenter for the tissue resource, 81% agreed to be contacted for follow-up, compared with 84% of patients who were the second or third enrolled (P = .35), and 90% of patients who were enrolled tenth or later (P = .004). Regarding contact for future research studies, 76% of patients who were the first enrolled by an individual consenter for the tissue resource agreed, compared with 84% of patients who were the second or third enrolled (P = .06), and 88% of patients who were the tenth or later enrolled (P < .001).
Figure 1.
Association between order of enrollment and patient response.
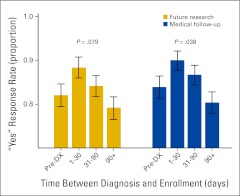
The interval between patient diagnosis and enrollment in the tissue resource was associated with willingness to be contacted for medical follow-up (P = .04) and exhibited a trend toward association with willingness to be contacted for future research studies (P = .08; Figure 2). In both instances, willingness to be contacted was greatest 1 to 30 days after diagnosis. For medical follow-up, 90% of patients enrolled during this time period replied “yes,” compared with 84% of patients enrolled before diagnosis (P = .07). “Yes” responses then declined over time to 80% for those patients enrolled more than 90 days after diagnosis. Similarly, agreement to be contacted for future research studies was greater among those patients enrolled in the tissue resource 1 to 30 days after diagnosis than among patients enrolled before diagnosis (88% v 82%; P = .08). Among patients enrolled in the tissue resource more than 90 days after diagnosis, 79% agreed to be contacted for future research.
Figure 2.
Association between timing of enrollment and patient response. DX, diagnosis.
In multivariate analysis (Appendix Table A1, online only), the order in which a patient was enrolled by an individual consenter remained associated with patient willingness to be contacted for medical follow-up information (P = .01) and future research (P = .008). Age was significantly associated with willingness to be contacted for medical follow-up (P = .01) and exhibited a trend toward association with willingness to be contacted for future research (P = .08).
Discussion
The impact of patient, institutional, and study factors on cancer clinical trial accrual has been examined extensively, but few studies have investigated whether patient interest and participation in clinical research vary by aspects of the research consent process. In this study of patients enrolled in a cancer center tissue repository, we focused on two such potentially modifiable factors: consenter experience and timing of consent. We found that consenter experience was significantly associated with and timing of consent had a clear trend toward association with markers of patient interest in research.
We categorized consenter experience according to the number of participants enrolled in the tissue repository by an individual consenter. Among patients enrolled by first-time consenters, 76% agreed to be contacted in the future to participate in other research studies, compared with 84% of patients who were the second or third participant enrolled by an individual consenter, and 88% of patients who were the tenth or later participant (P = .002). Considered conversely, the proportion of patients declining future contact went from 24% to 12%, a 50% relative decrease.
These results build on our previous work, in which we found that participants enrolled by high-volume consenters were more likely to agree to be contacted for medical follow-up and future research studies.13 In that study, participants consented at any point by study personnel who ultimately enrolled more than the median number of participants per consenter (ie, eight) were more willing to be contacted for future research. With this earlier approach, however, many participants enrolled by an experienced consenter were enrolled at a point when that consenter was not yet experienced, suggesting that other consenter characteristics—such as interest in and enthusiasm for the study—may have contributed to participant response. By examining the specific order in which a patient was enrolled by an individual consenter, the present study provides a more clinically relevant and applicable measure of consenter experience.
Along these lines, prior research has suggested that a supportive and clear physician communication style is associated with patient and family interest in clinical research.14 It has also been shown that direct interaction between study personnel and participants is the most effective means of improving participants' understanding of information disclosed in the informed consent process.15 Other studies have demonstrated that interventions such as workshops and videos can improve research personnel communication skills16–19 and quality of informed consent20 in cancer clinical trials. In the present study, however, consenter experience more likely reflected familiarity with study-specific content and documents than it did general communication skills. First, our data do not account for consenter experience with other research protocols. For some consenters, the first participant enrolled in the tissue resource may have been the first participant he or she enrolled on any research study, whereas others may have participated in numerous prior clinical trials. Second, it is unlikely that consenters' general communication styles would have changed significantly after enrollment of a single participant in the tissue resource.
As clinical research becomes more complex, this ability of research personnel to understand and explain study-specific details and consent documents is likely to become even more important. Over time, consent forms have become longer21,22 and more difficult to comprehend.23,24 The consent form for the UT Southwestern Tissue Resource, the basis of the present study, provides a relevant example. Although study procedures are straightforward—namely, the procurement and storage of clinical information and excess tissue from a procedure performed as standard clinical care—the six-page document covers such complex concepts as compensation for injury, compensation for future commercial developments, and the definition, processing, and storage of DNA.
We also evaluated the timing between diagnosis and invitation to participate in the research study—a factor in the research consent process that has not, to our knowledge, been evaluated previously. Willingness to be contacted for medical follow-up and future research studies was greatest among those patients enrolled between 1 and 30 days after disease diagnosis. A potential explanation is that this interval features less uncertainty and anxiety than the prediagnosis period, during which patients may be more focused on the risks and results of the upcoming diagnostic procedure than on the possibility of future research participation. Furthermore, the early interval after diagnosis is established may represent the height of patients' interest in their disease and treatment, as evidenced by a consistent and significant decline in willingness to be contacted for medical follow-up and future research among patients consented after this period. This observation has particular relevance to those clinical trials, such as secondary prevention studies, that require a waiting period of several months after initial cancer diagnosis and treatment before enrollment.
In this study, patient characteristics predicted responses to a lesser degree than did factors related to the consent process. Willingness to be contacted for medical follow-up and future research was not associated with sex (P = .19 and P = .31, respectively) or race (P = .83 and P = .40, respectively). Numerous studies have demonstrated racial disparities in clinical trial accrual7–9; our results suggest that these differences may in part reflect access to trials or reservations about specific study procedures,25 rather than a general aversion to clinical research. Consistent with previous reports,26,27 older patients in this cohort seemed less willing to be recruited for future research studies.
This study has a number of limitations. Foremost among them is the nature of the primary end point. It is not known if willingness to be contacted for future research opportunities correlates with willingness to participate in research, whether therapeutic or observational. Agreeing to be contacted for future research opportunities is clearly not equivalent to enrolling onto a clinical trial. The former entails minimal commitment or risk, whereas the latter may directly affect treatment selection, toxicities, and clinical outcomes. Nevertheless, patient willingness to consider clinical research represents a critical element of study accrual. We also believe this end point to be more interpretable than clinical trial enrollment, which depends not only on patient preference, but also on external factors such as study eligibility and availability. Although statistically significant, the different results between less and more experienced consenters may on initial consideration seem not clinically meaningful. Indeed, additional experience yielded a relative increase in “yes” responses of only 16%. This result reflects the high baseline rate of agreement to be contacted for future research, which leaves little room for improvement. However, applying the 50% relative decrease in “no” responses we observed to a hypothetical but plausible research scenario in which—perhaps because of difficulties in understanding more complex study content and procedures—a smaller proportion of potential participants agree to participate at baseline, the effects could be substantial.
In addition, aspects of the study population may limit the generalizability of our findings. In our study setting—an academic medical center—patients, staff, and clinicians may have research interests and motivations distinct from those encountered in community practices. This setting may also account for the atypically young age of the study sample (75% ≤ age 65 years), which in turn could bias our results. The 17% of participants who had either benign disease or were healthy controls may have skewed our findings, although disease status was not significantly associated with participant responses in univariate analysis. Finally, the study cohort included only those patients who agreed to participate in the UT Southwestern Tissue Resource. We have no information on those who were never approached to participate in the tissue resource and only limited information on those who were approached but declined. However, we believe our cohort is at least as representative a patient sample as those included in survey- or questionnaire-based studies, in which response rates are generally only 20% to 30%,28,29 and participants may be particularly motivated and interested in research. By contrast, because the UT Southwestern Tissue Resource entails little risk or time commitment, the overwhelming majority of those approached agree to participate. As a result of our interest in this issue, the UT Southwestern Tissue Resource recently started recording these figures; since that time, 514 (92%) of 556 individuals approached have agreed to enroll.
In conclusion, this study demonstrates that factors related to the consent process may affect patient interest in and willingness to consider participation in clinical research. To our knowledge, this is the first study to evaluate these variables quantitatively. Specifically, we found that consenter experience was significantly associated with and timing of consent had a trend toward association with markers of patient interest in research. Consenter experience may be gained as early as the first consenting effort for a particular trial. This experience seems to be study specific and likely reflects familiarity with study procedures and documents. Patients seem most open to the possibility of clinical research shortly after diagnosis, specifically within 30 days. One possible explanation is that before diagnosis, patients are preoccupied by anxiety related to the risks and results of upcoming procedures; as time elapses after diagnosis, patients' interest in their disease and treatment may wane. Patient characteristics including sex, race, and diagnosis were not associated with research interest. Importantly, in this study, the variables most strongly associated with markers of patient interest and potential participation in clinical research—namely, consenter experience and the timing of study enrollment—are readily defined and potentially modifiable. Further study of these factors may improve efforts to increase cancer clinical trial accrual.
Acknowledgment
Presented in abstract form at the 47th Annual Meeting of the American Society of Clinical Oncology, June 4-8, 2010, Chicago, IL. Supported in part by Grant No. KL2 RR024983 from the North and Central Texas Clinical and Translational Research Initiative (D.E.G.).
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: David E. Gerber, Celette Sugg Skinner
Administrative support: David E. Gerber
Provision of study materials or patients: Jennifer R. Sayne
Collection and assembly of data: David E. Gerber, Drew W. Rasco, Jennifer R. Sayne
Data analysis and interpretation: David E. Gerber, Drew W. Rasco, Jonathan E. Dowell, Jingsheng Yan, Yang Xie
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Umutyan A, Chiechi C, Beckett LA, et al. Overcoming barriers to cancer clinical trial accrual: Impact of a mass media campaign. Cancer. 2008;112:212–219. doi: 10.1002/cncr.23170. [DOI] [PubMed] [Google Scholar]
- 2.Viability of cancer clinical research: Patient accrual, coverage, and reimbursement—American Medical Association Council on Scientific Affairs. J Natl Cancer Inst. 1991;83:254–259. doi: 10.1093/jnci/83.4.254. [DOI] [PubMed] [Google Scholar]
- 3.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 4.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 7.Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials: A pilot study. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 8.Shavers VL, Lynch CF, Burmeister LF. Factors that influence African-Americans' willingness to participate in medical research studies. Cancer. 2001;91:233–236. doi: 10.1002/1097-0142(20010101)91:1+<233::aid-cncr10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Movsas B, Moughan J, Owen J, et al. Who enrolls onto clinical oncology trials? A radiation patterns of care study analysis. Int J Radiat Oncol Biol Phys. 2007;68:1145–1150. doi: 10.1016/j.ijrobp.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg ML, Fremont A, Khan DC, et al. Lay patient navigator program implementation for equal access to cancer care and clinical trials: Essential steps and initial challenges. Cancer. 2006;107:2669–2677. doi: 10.1002/cncr.22319. [DOI] [PubMed] [Google Scholar]
- 11.Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–476. doi: 10.1002/cncr.22436. [DOI] [PubMed] [Google Scholar]
- 12.Fuks A, Weijer C, Freedman B, et al. A study in contrasts: Eligibility criteria in a twenty-year sample of NSABP and POG clinical trials—National Surgical Adjuvant Breast and Bowel Program: Pediatric Oncology Group. J Clin Epidemiol. 1998;51:69–79. doi: 10.1016/s0895-4356(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 13.Rasco DW, Xie Y, Yan J, et al. The impact of consenter characteristics and experience on patient interest in clinical research. Oncologist. 2009;14:468–475. doi: 10.1634/theoncologist.2008-0268. [DOI] [PubMed] [Google Scholar]
- 14.Albrecht TL, Eggly SS, Gleason ME, et al. Influence of clinical communication on patients' decision making on participation in clinical trials. J Clin Oncol. 2008;26:2666–2673. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: A systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 16.Fallowfield L, Jenkins V, Farewell V, et al. Efficacy of a Cancer Research UK communication skills training model for oncologists: A randomised controlled trial. Lancet. 2002;359:650–656. doi: 10.1016/S0140-6736(02)07810-8. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins V, Fallowfield L, Solis-Trapala I, et al. Discussing randomised clinical trials of cancer therapy: Evaluation of a Cancer Research UK training programme. BMJ. 2005;330:400. doi: 10.1136/bmj.38366.562685.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razavi D, Merckaert I, Marchal S, et al. How to optimize physicians' communication skills in cancer care: Results of a randomized study assessing the usefulness of posttraining consolidation workshops. J Clin Oncol. 2003;21:3141–3149. doi: 10.1200/JCO.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Back AL, Arnold RM, Baile WF, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med. 2007;167:453–460. doi: 10.1001/archinte.167.5.453. [DOI] [PubMed] [Google Scholar]
- 20.Hietanen PS, Aro AR, Holli KA, et al. A short communication course for physicians improves the quality of patient information in a clinical trial. Acta Oncol. 2007;46:42–48. doi: 10.1080/02841860600849067. [DOI] [PubMed] [Google Scholar]
- 21.Berger O, Gronberg BH, Sand K, et al. The length of consent documents in oncological trials is doubled in twenty years. Ann Oncol. 2009;20:379–385. doi: 10.1093/annonc/mdn623. [DOI] [PubMed] [Google Scholar]
- 22.LoVerde ME, Prochazka AV, Byyny RL. Research consent forms: Continued unreadability and increasing length. J Gen Intern Med. 1989;4:410–412. doi: 10.1007/BF02599693. [DOI] [PubMed] [Google Scholar]
- 23.Baker MT, Taub HA. Readability of informed consent forms for research in a Veterans Administration medical center. JAMA. 1983;250:2646–2648. [PubMed] [Google Scholar]
- 24.Tarnowski KJ, Allen DM, Mayhall C, et al. Readability of pediatric biomedical research informed consent forms. Pediatrics. 1990;85:58–62. [PubMed] [Google Scholar]
- 25.Freimuth VS, Quinn SC, Thomas SB, et al. African Americans' views on research and the Tuskegee Syphilis Study. Soc Sci Med. 2001;52:797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 26.Simon MS, Brown DR, Du W, et al. Accrual to breast cancer clinical trials at a university-affiliated hospital in metropolitan Detroit. Am J Clin Oncol. 1999;22:42–46. doi: 10.1097/00000421-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Tournoux C, Katsahian S, Chevret S, et al. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–270. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 28.Kim SY, Millard RW, Nisbet P, et al. Potential research participants' views regarding researcher and institutional financial conflicts of interest. J Med Ethics. 2004;30:73–79. doi: 10.1136/jme.2002.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comis RL, Miller JD, Aldigé CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]