Abstract
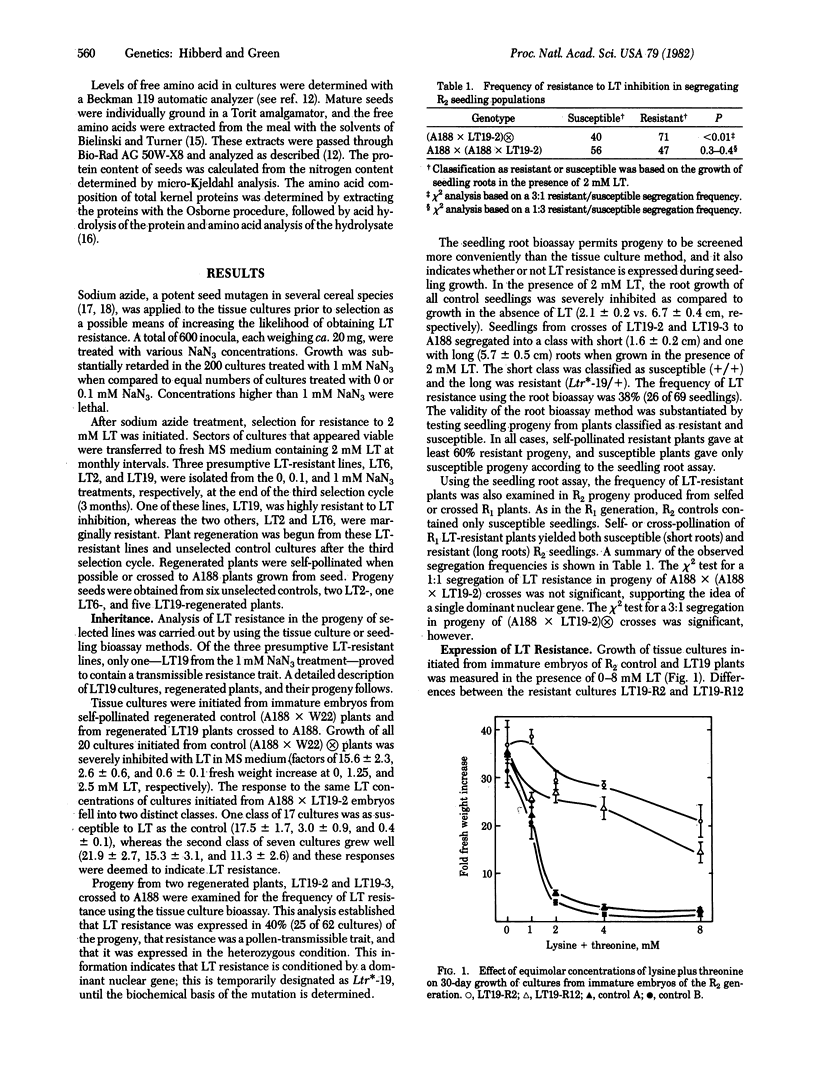
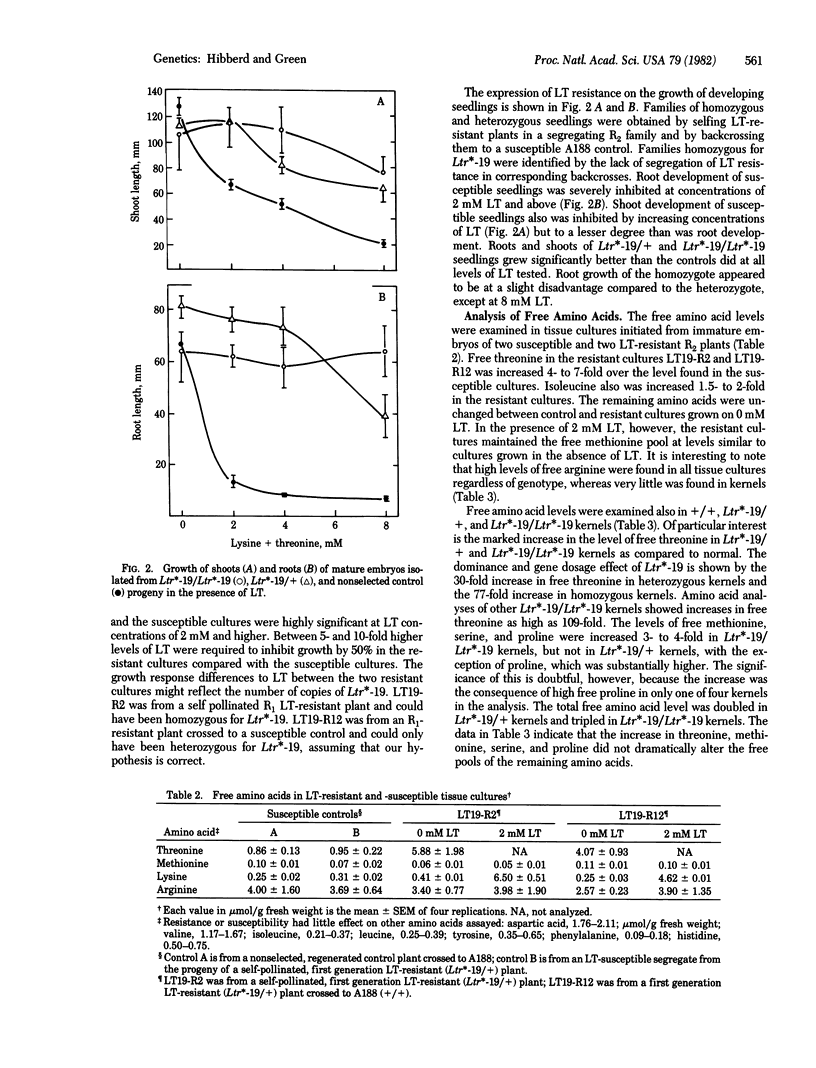
Selection for resistance to growth inhibition by lysine plus threonine in equimolar concentrations (LT) in tissue cultures of maize yielded a stable resistant line, LT19. Genetic analysis of progeny of plants regenerated from LT19 showed that LT resistance was inherited as a single dominant nuclear gene, temporarily designated Ltr*-19. Tissue cultures initiated from resistant embryos required 5-10 times higher levels of LT to inhibit growth than did cultures from LT-sensitive embryos. LT resistance in Ltr*-19 was expressed as much reduced inhibition of root and shoot growth in the presence of LT. The free pool of threonine was increased 6 times in cultures initiated from immature embryos of LT-resistant plants, and 75-100 times in kernels homozygous for Ltr*-19, as compared to cultures and kernels from LT-sensitive embryos and plants, respectively. Overproduction of free threonine increased the total threonine content in homozygous Ltr*-19 kernels by 33-59%. The results demonstrate that LT resistance selected with tissue culture methods is heritable and is expressed in cultures, seedlings, and kernels. Furthermore, they demonstrate a method to obtain amino acid-overproducer mutants in maize, which have the potential to increase substantially specific amino acids in kernels. The capability to increase specifically the nutritionally limiting amino acid(s) could have important nutritional implications for the grain of cereals and other crops.
Keywords: mutants, amino acid overproduction
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieleski R. L., Turner N. A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem. 1966 Nov;17(2):278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Bryan J. K. Studies on the catalytic and regulatory properties of homoserine dehydrogenase of Zea mays roots. Biochim Biophys Acta. 1969 Feb 11;171(2):205–216. doi: 10.1016/0005-2744(69)90154-5. [DOI] [PubMed] [Google Scholar]
- Bryan P. A., Cawley R. D., Brunner C. E., Bryan J. K. Isolation and characterization of a lysine-sensitive aspartokinase from a multicellular plant. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1211–1217. doi: 10.1016/0006-291x(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Carlson P. S. Methionine sulfoximine--resistant mutants of tobacco. Science. 1973 Jun 29;180(4093):1366–1368. doi: 10.1126/science.180.4093.1366. [DOI] [PubMed] [Google Scholar]
- Chaleff R. S., Parsons M. F. Direct selection in vitro for herbicide-resistant mutants of Nicotiana tabacum. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5104–5107. doi: 10.1073/pnas.75.10.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham V. L., Bryan J. K. Synergistic effects of metabolically related amino acids on the growth of a multicellular plant. II. Studies of 14C-amino acid incorporation. Plant Physiol. 1971 Jan;47(1):91–97. doi: 10.1104/pp.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbach B. G., Green C. E., Donovan C. M. Inheritance of selected pathotoxin resistance in maize plants regenerated from cell cultures. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5113–5117. doi: 10.1073/pnas.74.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Marton L Joo F. Non-Mendelian streptomycin-resistant tobacco mutant with altered chlorplasts and mitochondria. Nature. 1975 May 29;255(5507):401–402. doi: 10.1038/255401a0. [DOI] [PubMed] [Google Scholar]
- Saling E., Blücher U., Sander H. Equipment for the recording of tractive power in vaccum extractions. J Perinat Med. 1973;1(2):142–144. [PubMed] [Google Scholar]



