Abstract
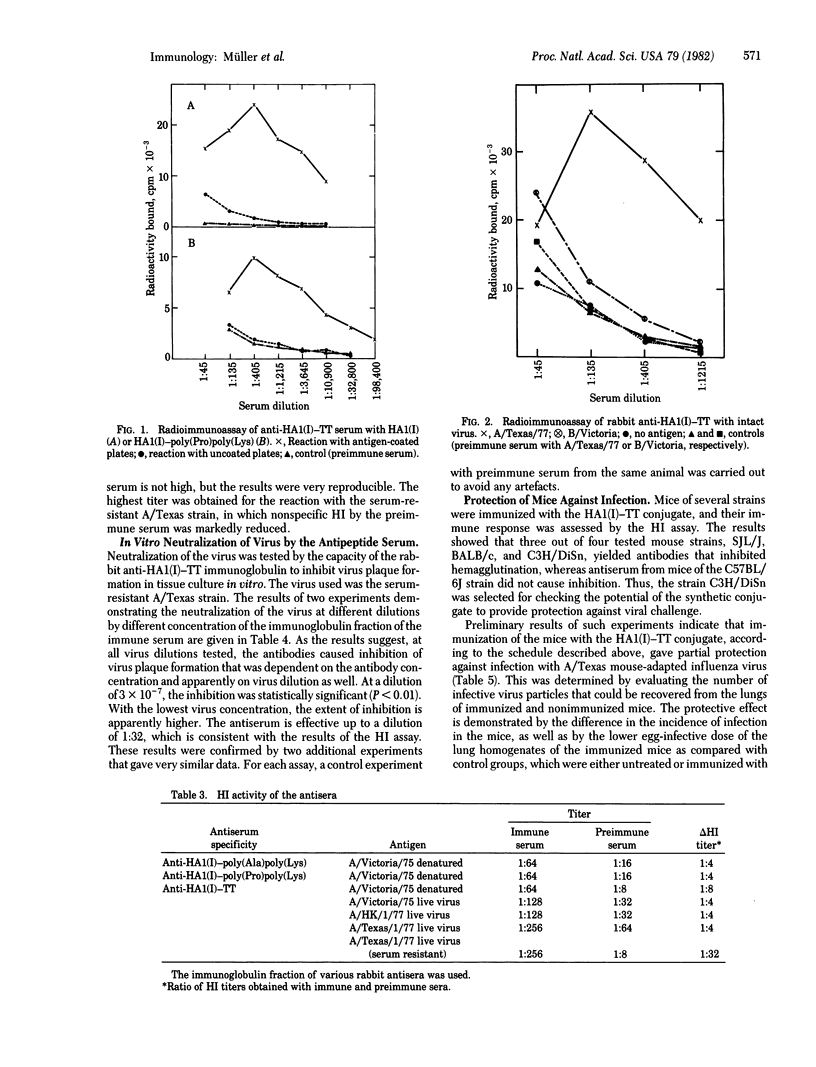
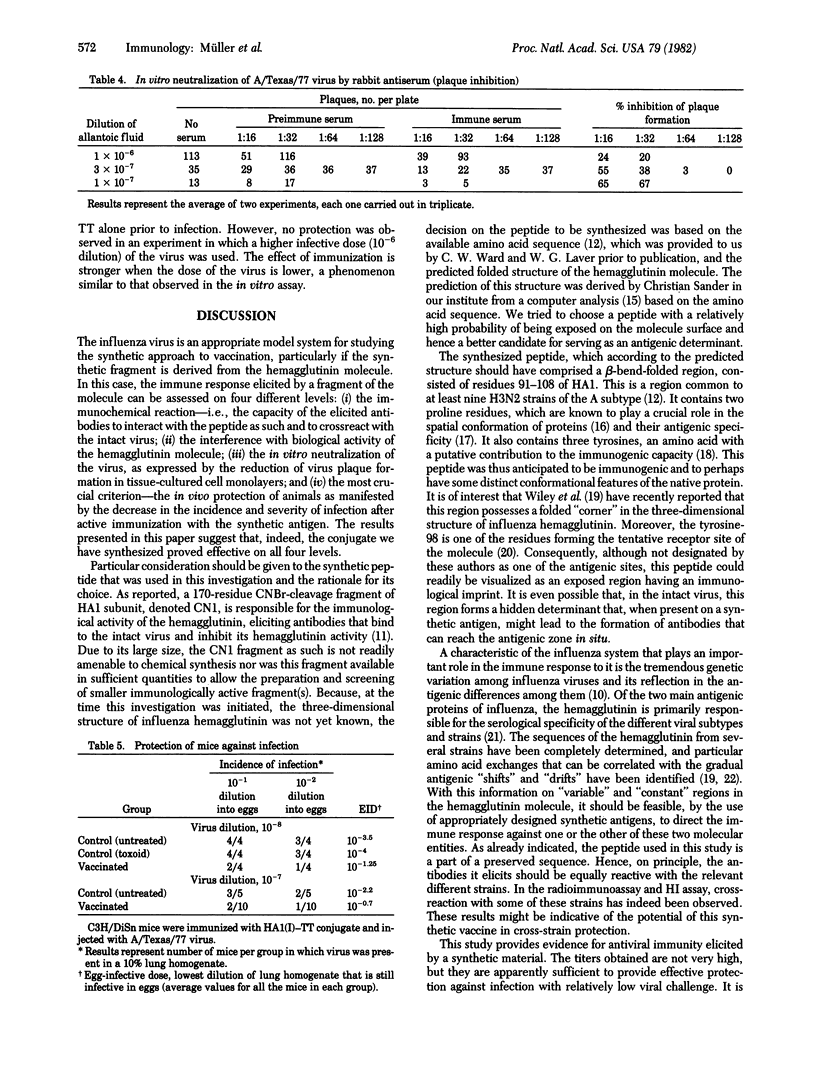
The peptide corresponding to sequence 91--108 of the hemagglutinin of type A H3N2 influenza virus has been synthesized by the solid-phase peptide synthesis method and covalently attached to several macromolecular carriers. The conjugate with tetanus toxoid was used for immunization of rabbits and mice. The immunoglobulin fraction of the rabbit antiserum showed the presence and antipeptide antibodies by both agar gel diffusion and radioimmunoassay. In the latter assay, the antibodies showed marked crossreactivity with the intact virus of the A/Texas/77 strain. The antibodies were also capable of inhibiting the hemagglutination of chicken erythrocytes by the virus; the highest hemagglutination inhibition titer (1:32) was achieved with a serum-resistant strain of A/Texas/77. When the in vitro virus plaque formation assay was used with monolayers of Madin--Darby canine kidney (MDCK) cells, the number of plaques was reduced on interaction with the immunoglobulin fraction of the antiserum, which was effective up to a dilution of 1:32. Preliminary results indicate that C3H/DiSn mice immunized with the peptide--tetanus toxoid conjugate are partially protected against a further challenge with A/Texas mouse-adapted influenza virus. The results are thus indicative of the efficacy of the synthetic material in eliciting anti-influenza immune response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERER F. A. Preparation and properties of an artificial antigen immunologically related to tobacco mosaic virus. Biochim Biophys Acta. 1963 Apr 2;71:246–248. doi: 10.1016/0006-3002(63)91077-1. [DOI] [PubMed] [Google Scholar]
- ARNON R., SELA M. Studies on the chemical basis of the antigenicity of proteins. 2. Antigenic specificity of polytyrosyl gelatins. Biochem J. 1960 Apr;75:103–109. doi: 10.1042/bj0750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzini B., Blass J., Turpin A., Raynaud M. Chemical characterization of tetanus toxin and toxoid. Amino acid composition, number of SH and S-S groups and N-terminal amino acid. Eur J Biochem. 1970 Nov;17(1):100–105. doi: 10.1111/j.1432-1033.1970.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Brown L. E., White D. O., Dopheide T. A., Ward C. W. Antigenic determinants of influenza virus hemagglutinin. IV. Immunogenicity of fragments isolated from the hemagglutinin of A/Memphis/72. J Immunol. 1979 Dec;123(6):2610–2617. [PubMed] [Google Scholar]
- Langbeheim H., Arnon R., Sela M. Antiviral effect on MS-2 coliphage obtained with a synthetic antigen. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4636–4640. doi: 10.1073/pnas.73.12.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Dopheide T. A., Ward C. W. Amino acid sequence changes in the haemagglutinin of A/Hong Kong (H3N2) influenza virus during the period 1968--77. Nature. 1980 Jan 31;283(5746):454–457. doi: 10.1038/283454a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Gerhard W., Ward C. W., Dopheide T. A. Antigenic drift in type A influenza virus: sequence differences in the hemagglutinin of Hong Kong (H3N2) variants selected with monoclonal hybridoma antibodies. Virology. 1979 Oct 15;98(1):226–237. doi: 10.1016/0042-6822(79)90540-3. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Automated synthesis of peptides. Science. 1965 Oct 8;150(3693):178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- Russell R. J., Jackson D. C. Direct solid-phase radioimmunoassay for measuring antigenic differences between the hemagglutinins of influenza viruses. J Immunol Methods. 1978;22(3-4):201–209. doi: 10.1016/0022-1759(78)90028-5. [DOI] [PubMed] [Google Scholar]
- Teicher E., Maron E., Arnon R. The role of specific amino acid residues in the antigenic reactivity of the loop peptide of lysozyme. Immunochemistry. 1973 Apr;10(4):265–271. doi: 10.1016/0019-2791(73)90204-8. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Askonas B. A. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980 May;10(5):396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]



