Abstract
Most women with advanced breast cancer will develop bone metastases, which are associated with the development of skeletal-related events (sres) such as pathologic fractures and spinal cord compression. This article reviews the evolving definition and incidence of sres, the pathophysiology of bone metastases, and the key evidence for the safety and efficacy of the currently available systemic treatment options for preventing and delaying sres in the setting of breast cancer with bone metastases.
The bisphosphonates are structural analogues of endogenous pyrophosphate; three of them (clodronate, pamidronate, and zoledronate) are currently approved for use in Canada in the setting of breast cancer with bone metastases. Denosumab is a fully human immunoglobulin G2 monoclonal antibody that binds to human rankl (receptor activator of nuclear factor κB ligand), thereby preventing osteoclast formation, function, and survival, and reducing cancer-induced destruction of bone. Denosumab has recently been approved in Canada for reducing the risk of sres from the bone metastases associated with a variety of malignancies, including breast cancer. How to predict the patients that will benefit most from prophylactic treatment, the agents to select and the timing of switches between agents, the dosing schedules and durations of treatment to choose, the potential utility of the agents in the adjuvant setting, and the utility of additional endpoints such as markers of bone resorption are among the outstanding questions with respect to the optimal use of antiresorptive agents for patients with breast cancer and bone metastases.
Keywords: Breast cancer, bone metastases, skeletal-related events, bisphosphonates, denosumab
1. INTRODUCTION
Bone (including bone marrow) is the most common site of metastasis in advanced breast cancer (all subtypes except basal-like cancers)1. Up to three quarters of women with advanced breast cancer will develop bone metastases during their disease2,3, and because bone metastases are associated with the development of skeletal complications, it is relevant to understand the pathophysiology of skeletal-related events (sres) and the therapies available to reduce their incidence. The present article focuses on sres in patients with advanced breast cancer and metastases to bone, and on the bone-targeted agents that are approved for prevention and delay of sres.
2. SREs: DEFINITIONS, BURDEN OF DISEASE, INCIDENCE, AND PREDICTORS
One of the earliest studies to examine a range of sres and to use a composite of sres as an endpoint was a prospective study in women with breast cancer and osteolytic bone metastases4. It found that oral aminohydroxy propylidene bisphosphonate significantly reduced the incidence of sres, defined as a composite of pathologic fractures, bone pain, hypercalcemia, and palliative radiotherapy to bone. The definition of sres has since evolved such that most subsequent trials have assessed skeletal complications using composite sre endpoints that omit bone pain (and sometimes hypercalcemia), but that add spinal cord compression and the need for orthopedic surgery to bone.
The skeletal complications of bone metastases are responsible for a range of complications and costs and decreased quality of life. Intractable bone pain, fractures, bladder and bowel disturbances, and impairment of mobility lead to declines in functional independence, increased anxiety, and depression5–7.
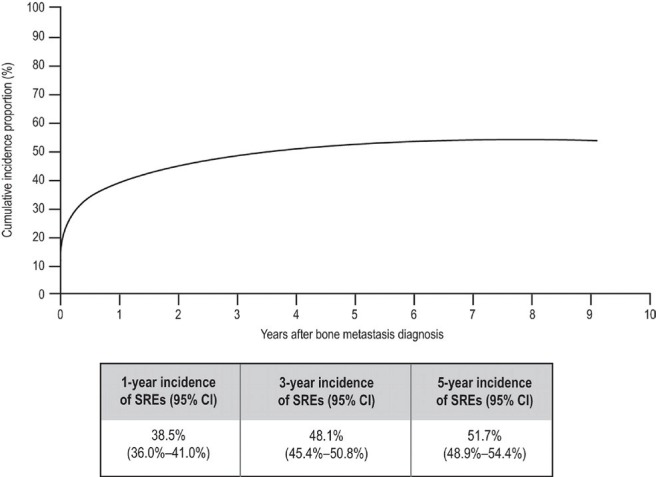
Much of the data on the incidence of sres in advanced breast cancer comes from retrospective cohort studies and randomized trials. One retrospective study of 1049 women who died between 1975 and 1984 (before bisphosphonates came into use) found that 69% had radiologic evidence of bone metastases, and 10% had developed hypercalcemia. Of the 47% of women whose first distant relapse was to bone, 29% developed a skeletal-related complication2. Recently, a Danish population-based estimate of bone metastases and sres in women with newly diagnosed breast cancer from 1999 to 20078 found that 46.4% of those who developed bone metastases developed a sre over a median follow-up of 0.7 years after the bone lesion was diagnosed. The incidence of sres was highest in the first year after diagnosis—not surprising given that metastatic breast cancer often first presents as a sre. The cumulative incidence of sres was 38.5% at 1 year and 51.7% at 5 years (Figure 1).
FIGURE 1.
Cumulative incidence of skeletal-related events (sres) among breast cancer patients with bone metastases8. ci= confidence interval.
In a meta-analysis of two randomized controlled trials comparing pamidronate with placebo in patients receiving either endocrine therapy or chemotherapy (n = 754), the median time from randomization to the first skeletal complication was 7.0 months [95% confidence interval (ci): 6.2 to 8.5 months] in the placebo group and 12.7 months (95% ci: 9.6 to 17.2 months) in the pamidronate group (p = 0.001 by the log-rank test)9. In follow-up data over 24 months, the incidence of any skeletal complication in the placebo group was 68%; of any skeletal complication excluding hypercalcemia of malignancy, 64%; of radiation to bone, 43%; of pathologic fracture, 52%; of surgery to bone, 11%; of spinal cord compression, 3%; and of hypercalcemia, 13%.
Because the risk of an sre varies widely, strategies to calculate individual risk would be useful. However, no validated algorithm to predict sre risk in a patient with bone metastases is available10. Some studies have shed light on potential predictive factors for sres. In a non-trial cohort of 87 patients from two centres who had been treated with pamidronate between 1999 and 2005, a history of osteoporosis and the presence of bone-only metastases increased the risk of developing an sre by a factor of approximately 311. Multivariate analyses12 of data from a phase iii randomized study of zoledronate in breast cancer patients with bone metastases identified an age of 60 years and older, a pain score greater than 3 on the Brief Pain Inventory, a history of a sre before study entry, and predominantly osteolytic lesions as baseline predictors of a first sre. Finally, N-telopeptides (ntx) and C-telopeptides, both degradation products of the collagen matrix of bone, are frequently monitored in clinical trials as markers of bone resorption13. In a retrospective analysis of three phase iii trials, including a large trial comparing zoledronate with pamidronate14, normalized ntx levels after 3 months of treatment were associated with decreased risks of sres and improved overall survival15.
3. THE VICIOUS CYCLE: PATHOPHYSIOLOGY OF BONE METASTASES
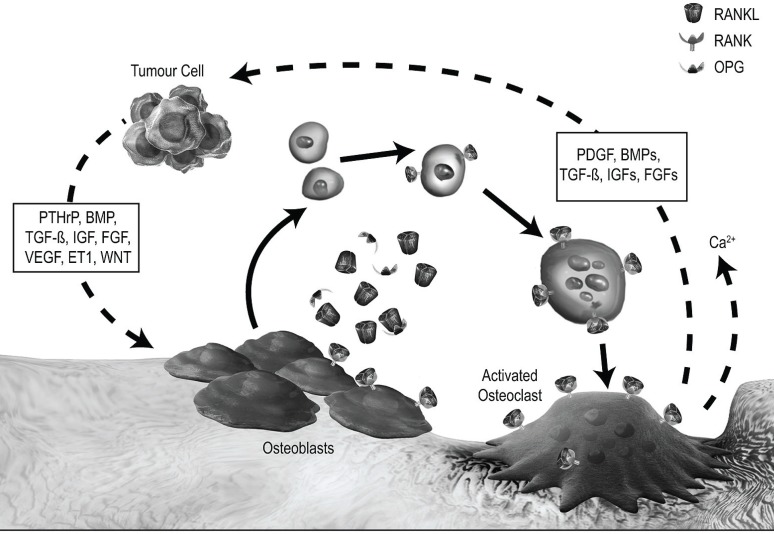
The development of bone metastases depends on the interaction between tumour cells and the microenvironment of the metastatic site. One model of that interaction proposes that a “vicious cycle” develops whereby tumour cells in bone and osteoclasts each release multiple cytokines and growth factors that mutually stimulate growth. Thus, a symbiotic interplay is established between tumour growth and destruction of bone16,17. Figure 2 illustrates in more detail this hypothetical vicious cycle.
FIGURE 2.
The vicious cycle in osteolytic bone metastases. A vicious cycle develops when tumour cells in bone secrete parathyroid hormone–related protein (pthrp) and multiple other cytokines and growth factors that stimulate osteoblasts to produce rankl(receptor activator of nuclear factor κB ligand). In turn, ranklbinds to rank on the surfaces of osteoclast precursors, stimulating their differentiation and activation. The osteoclasts resorb bone, which releases a variety of cytokines and growth factors that complete the cycle by stimulating tumour cells to secrete additional pthrpand other factors. Adapted from Roodman 200417. opg= osteoprotegerin; bmp= bone morphogenic proteins; tgf-β = transforming growth factor β; igf= insulin-like growth factor; fgf= fibroblast growth factor; vegf= vascular endothelial growth factor; et1 = endothelin 1; pdgf= platelet-derived growth factor.
4. CURRENT SYSTEMIC TREATMENT OPTIONS FOR PREVENTING AND DELAYING SREs
4.1. Bisphosphonates
Bisphosphonates are structural analogues of endogenous pyrophosphate that bind to the hydroxyapatite mineral matrix of bone and inhibit the action of osteoclasts through a variety of mechanisms18,19. Bisphosphonates are effective in decreasing the risk of a variety of sres in metastatic breast cancer; Table i summarizes the results of key trials. Three bisphosphonates are currently approved for use in Canada in the setting of breast cancer with bone metastases: clodronate [available in oral and intravenous (IV) forms], IV pamidronate, and IV zoledronate.
TABLE I.
Summary of bisphosphonate trials in breast cancer: agents approved in Canada
| Trial | Patients (n) | Primary endpoints | Results | p Value |
|---|---|---|---|---|
| Clodronate versus placebo | ||||
| Paterson et al., 199320 | 173 | Hypercalcemia, vertebral and non-vertebral fractures, requirement for radiotherapy for bone pain | 218.6 vs. 304.8 events per 100 patient–years | <0.001 |
| Tubiana–Hulin et al., 200121 | 144 | New bone events (hypercalcemia > 3 mmol/L), new or increased bone pain, radiotherapy for control of bone pain, pathologic fractures (including spinal cord compression), or death because of bone metastases | Prolonged time to new bone events: 244 days vs. 180 days | 0.05 |
| Pamidronate versus placebo | ||||
| Hortobagyi et al., 199822 | 382 | Skeletal complications (pathologic fractures, spinal cord compression with vertebral compression fracture, need for surgery for pathologic fractures or spinal cord compression, need for radiation to bone) | Prolonged median time to first skeletal complication: 13.9 months vs. 7.0 months | <0.001 |
| Theriault et al., 199923 | 372 | Skeletal morbidity rate, survival rate | Decreased skeletal morbidity rate at cycles 12, 18, and 24 | 0.028 |
| 0.023 | ||||
| 0.008 | ||||
| Lipton et al., 20009 (combined analysis and 24-month extension of two trials) | 754 | Overall skeletal morbidity rate | ↓ 35% (2.4 events vs. 3.7 events per year) | <0.001 |
| Skeletal complications | ↓ 20% (51% vs. 64%) | <0.001 | ||
| Time to first skeletal complication | 12.7 months vs. 7.0 months | <0.001 | ||
| Zoledronate versus pamidronate | ||||
| Rosen et al., 200114 | 1130a | Proportion of patients with 1 or more on-study skeletal-related events after 13 months | No significant difference in breast cancer patients receiving chemotherapy or hormonal therapy | ns |
| Rosen et al., 200324 (long-term efficacy and safety) | 412b | Proportion of patients with 1 or more on-study skeletal-related events | No significant difference | ns |
Patients with multiple myeloma (n = 158) also entered this trial.
Patients with multiple myeloma (n = 194) also entered this extension phase.
ns = nonsignificant.
4.2. Clodronate
The first large double-blind trial of an oral bisphosphonate for skeletal morbidity compared clodronate 1600 mg daily with placebo in 173 patients with metastatic breast cancer20. In that study (Table i), the clodronate significantly reduced the incidence of hypercalcemia, terminal hypercalcemia, vertebral fractures, vertebral deformity, and all morbid skeletal events (218.6 events vs. 304.8 events per 100 patient–years, p < 0.001). There were also trends in favour of clodronate for nonvertebral fracture rates and the need for palliative radiotherapy to control bone pain. The side-effect profile and overall survival were similar in the two groups. A later randomized trial involving 144 patients with breast cancer and osteolytic bone metastases receiving either oral clodronate or placebo for up to 12 months showed similar results21.
4.3. Pamidronate
Several studies have examined the effect of pamidronate in breast cancer patients with osteolytic bone metastases, including two prospective multicentre double-blind randomized trials, one in women receiving cytotoxic chemotherapy22 and the other in those receiving hormonal therapy23 (Table i). A combined analysis and 24-month extension of those trials (n = 754)9 found that, compared with placebo, pamidronate 90 mg every 3–4 weeks significantly reduced the overall skeletal morbidity rate by 35% (2.4 events vs. 3.7 events per year, p < 0.001) and skeletal complications by 20% (51% vs. 64%, p < 0.001); it also prolonged the time to first skeletal complication (12.7 months vs. 7.0 months, p < 0.001). In addition, pamidronate significantly increased the time to a new pathologic fracture and decreased the proportion of patients whose pain scores increased from baseline. Eastern Cooperative Oncology Group performance status and quality of life scores both worsened in the treatment groups, but less so in the patients taking pamidronate. The bisphosphonate was well tolerated, with similar rates of discontinuation because of adverse events in both treatment arms. Fever related to the study drug was reported more often in the pamidronate group (14% vs. 5%).
4.4. Zoledronate Versus Pamidronate
A large trial compared the effects of zoledronate and pamidronate in 1130 patients with advanced breast cancer and at least 1 metastatic bone lesion (and also in 518 patients with multiple myeloma, whose results are not discussed here)14. Patients were originally randomized to receive either pamidronate 90 mg IV over 2 hours or zoledronate 4 mg or 8 mg IV over 5 minutes every 3–4 weeks. Primarily because of increased creatinine levels with 8 mg zoledronate, the study protocol was amended twice, reducing the dose to 4 mg and increasing the infusion time to 15 minutes; only the original 4-mg arm was used in the analysis of results. For the primary endpoint (the proportion of patients with at least 1 on-study sre after 13 months), no statistical difference was observed between the agents in patients with breast cancer receiving either chemotherapy or hormonal therapy. An extension of that trial to 25 months of follow-up24 included 412 patients with breast cancer. Although the proportion of patients with at least 1 sre was similar in the two treatment groups, multiple-event analysis showed that zoledronate reduced sre risk by 20% over pamidronate [relative risk (rr): 0.799; 95% ci: 0.657 to 0.972; p = 0.025] and also numerically reduced the incidence of individual sres. For time to first sre, skeletal morbidity rate, and risk of skeletal complications, zoledronate was superior to pamidronate in patients receiving hormonal therapy but not chemotherapy25.
4.5. Ibandronate
Ibandronate, a potent third-generation aminobisphosphonate, is not approved for use in Canada. However, phase iii trials have demonstrated its efficacy in both IV and oral formulations. A 24-month trial in 150 patients with bone metastases showed that compared with placebo, IV ibandronate significantly reduced the proportion of patients developing a sre (36% vs. 48%, p = 0.027) and the risk of developing an sre [hazard ratio (hr): 0.69; 95% ci: 0.42 to 0.79; p = 0.003]. It also delayed the median time to a first sre (457 days vs. 304 days, p = 0.007); 64% of ibandronate-treated patients did not experience a new sre during the study26. A pooled analysis of two 96-week studies in a total of 564 patients with breast cancer and bone metastases found that compared with placebo, oral ibandronate reduced the mean number of 12-week periods with new skeletal complications from 1.18 to 0.95 (p = 0.004) and the mean number of events requiring radiotherapy (0.73 vs. 0.98, p < 0.001) or surgery (0.47 vs. 0.53, p = 0.037)27.
5. BISPHOSPHONATES AND SREs: A META-ANALYSIS
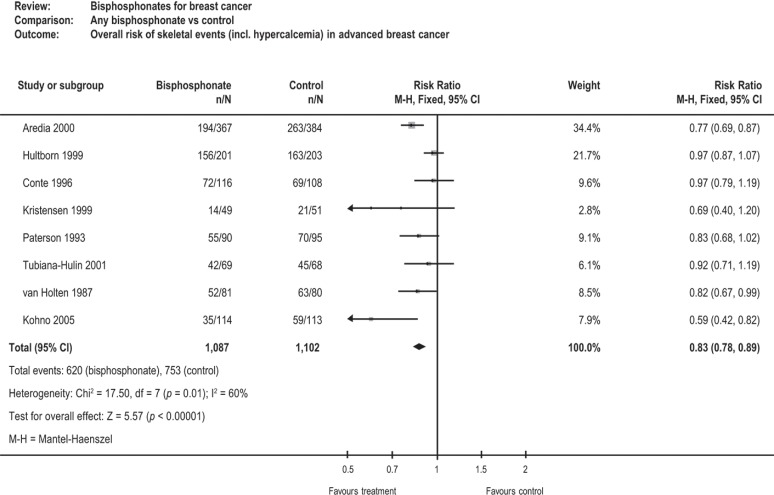
A meta-analysis of 21 randomized studies assessed the effects of bisphosphonates on skeletal events, bone pain, quality of life, and survival in women with early and advanced breast cancer. In the nine studies enrolling women with advanced breast cancer and existing bone metastases (n = 2189), bisphosphonates significantly reduced the risk of sres by 17% (rr: 0.83; 95% ci: 0.78 to 0.89; p < 0.00001; Figure 3). Substantial effects were seen with pamidronate, zoledronate, and oral clodronate. Bisphosphonates did not appear to reduce the incidence of sres in women without bone metastases, nor to improve disease-free survival or overall survival28.
FIGURE 3.
Cochrane meta-analysis of bisphosphonates28. ci= confidence interval.
With respect to bone pain, eleven studies tested the effects of bisphosphonates using a referenced pain scale. Significant reductions in pain were seen in two studies of IV pamidronate (p < 0.001), one study of IV ibandronate (p = 0.0006), one study of oral clodronate (p = 0.01), one study of oral pamidronate (p = 0.007), and pooled studies of oral ibandronate (p = 0.001). Among the studies that examined patient-rated quality of life, only the studies with IV ibandronate (p = 0.004) and oral ibandronate (p = 0 .032) showed a significant improvement in global quality of life29.
5.1. Safety and Tolerability of Bisphosphonates
The safety and tolerability issues of greatest concern with IV aminobisphosphonates are nephrotoxicity, acute-phase reactions, and osteonecrosis of the jaw (onj). Oral agents may cause gastrointestinal toxicity. All these adverse events, which differ between the various bisphosphonates, are summarized in Table ii.
TABLE II.
Adverse events of bisphosphonates
| Event | Route of administration | Patients affected (%) | Potential mechanisms29 |
|---|---|---|---|
| Acute-phase reactions: fever and myalgia | Intravenous | 15%–30%29 | Systemic cytokine flare |
| Gastrointestinal symptoms | Oral | Dose dependent29 | Local toxicity |
| Nephrotoxicity | Intravenous | Creatinine elevations: 2%–8%30 | High drug concentrations in kidneys31 |
| Osteonecrosis of the jaw | Intravenous (primarily) | Intravenous: 1.2%–2.4%31 | Multiple risk factors |
| Hypocalcemia | Intravenous | 4.7%31 | Decreases in serum calcium and urinary calcium excretion |
The 2011 American Society of Clinical Oncology clinical practice guidelines on bone-modifying agents in metastatic breast cancer note that both pamidronate and zoledronate are associated with renal deterioration, especially with prolonged exposure and in patients with pre-existing renal impairment. The guidelines recommend that, if renal function should deteriorate, pamidronate and zoledronate should be withheld until serum creatinine returns to within 10% of baseline32. The product monographs for all three of the commonly used bisphosphonates recommend slow infusion rates, close monitoring of renal status, dose adjustments for those with lesser degrees of renal impairment, and discontinuation if renal function deteriorates33–35. Intravenous pamidronate and zoledronate are not recommended in patients with a creatinine clearance less than 30 mL/min33,34.
First infusions of IV bisphosphonates have been associated with acute-phase reactions in which a non-specific immune-driven release of proinflammatory cytokines is accompanied by flu-like symptoms such as fatigue, nausea, and myalgia, typically subsiding within 72 hours36.
Bisphosphonate-related onj is defined by the American Association of Oral and Maxillofacial Surgeons as exposed bone in the maxillofacial region that has persisted for more than 8 weeks, together with current or previous treatment with a bisphosphonate, without a history of radiation therapy to the jaw37. Key risk factors for the development of onj include the use of high-potency IV aminobisphosphonates, a high cumulative dose of such drugs, concomitant chemotherapeutic agents, and a history of dental disease and invasive dental procedures38,39. In patients with multiple risk factors, the incidence of onj may be as high as 10%39. A preventive dental program has been shown to lower the incidence of onj in patients with solid tumours and bone metastases who have received bisphosphonates40.
5.2. Monoclonal Antibodies (Denosumab)
Denosumab is a fully human immunoglobulin G2 monoclonal antibody with high affinity and specificity for human rankl (receptor activator of nuclear factor κB ligand). By binding to rankl, denosumab inhibits rankl from activating its only receptor, rank, on the surface of osteoclasts and their precursors. Prevention of rankl–rank interaction inhibits osteoclast formation, function, and survival, thereby decreasing bone resorption and interrupting cancer-induced bone destruction. The pharmacokinetics of denosumab do not appear to be affected by sex, age, race, body weight, or disease state. Renal impairment has no effect on the pharmacokinetics and pharmacodynamics of denosumab and therefore dose adjustment for renal impairment is not required. This agent has recently been approved in Canada for reducing the risk of sres in patients with bone metastases from breast cancer, prostate cancer, non-small-cell lung cancer, and other solid tumours41.
A phase iii study42 compared the effects of denosumab with those of zoledronate on sres in 2046 women with breast cancer and evidence of at least 1 metastatic bone lesion (Table iii). Patients were randomized to receive either denosumab 120 mg subcutaneously (SC) with IV placebo or zoledronate 4 mg IV (adjusted for renal function) with SC placebo every 4 weeks. The key efficacy endpoints were time to first on-study sre (tested for non-inferiority and superiority) and time to first and subsequent sres (tested for superiority). The definition of sre was pathologic fracture (excluding major trauma), radiation therapy to bone, surgery to bone, or spinal cord compression. Compared with zoledronate, denosumab significantly delayed time to first on-study sre by 18% (hr: 0.82; 95% ci: 0.71 to 0.95; p < 0.001 by non-inferiority test and p = 0.01 by superiority test). Median time to first on-study sre was 26.4 months for zoledronate; denosumab had not yet reached that endpoint upon publication of the trial results. Further, denosumab was superior with respect to time to first and subsequent on-study sres (rate ratio: 0.77; 95% ci: 0.66 to 0.89; p = 0.001). Denosumab was also superior to zoledronate in the reduction of markers of bone resorption from baseline to the 13-week point. Overall survival and disease progression (exploratory study endpoints) were similar in both groups.
TABLE III.
Denosumab versus zoledronate in patients with breast cancer and evidence of 1 or more metastatic bone lesions
| Reference | Patients (n) | Endpoints | Results (95% ci) | p Value |
|---|---|---|---|---|
| Stopeck et al., 201043 | 2046 | Time (months) to first on-study srea | hr: 0.82 (0.71 to 0.95) | <0.001b |
| 0.01c | ||||
| Time to first and subsequent on-study sre | rr: 0.77 (0.66 to 0.89) | 0.001 |
Defined as pathologic fracture (excluding major trauma), radiation therapy to bone, surgery to bone, or spinal cord compression.
By non-inferiority test.
By superiority test.
ci = confidence interval; sre = skeletal-related event; hr= hazard ratio; rr = relative risk.
The aforementioned phase iii study also assessed the effects of denosumab and zoledronate on pain and quality of life. Among patients with scores of no or mild pain at baseline (n = 1042), a prolonged median time to the development of moderate or severe pain was experienced by those on denosumab compared with those on zoledronate (295 days vs. 176 days; hr: 0.78; 95% ci: 0.67 to 0.92; p = 0.0024)43. Over the 18-month study period, an average of 3.2% more denosumab-treated patients than patients on zoledronate (range: 1%–7%; p < 0.05) experienced a clinically meaningful improvement in health-related quality of life44.
Adverse events were analyzed to identify those with a nominal p < 0.05. Despite dose adjustments, patients in the zoledronate arm had more adverse events potentially related to renal toxicities than did patients in the denosumab arm (8.5% vs. 4.9%). Symptoms related to acute-phase reactions were 2.7 times more frequent with zoledronate (27.3% vs. 10.4%), and denosumab was associated with higher rates of hypocalcemia, usually mild and transient (5.5% vs. 3.4%). The rate of grade 3 or 4 hypocalcemia was similar in the two groups. Finally, the incidence of onj was 2.0% in the denosumab group and 1.4% in the zoledronate group (p = 0.39). Of patients in the denosumab and zoledronate groups respectively, 90% and 71% of those with onj had known risk factors for the condition.
6. DISCUSSION
Key trials of the bisphosphonates clodronate, pamidronate, zoledronate, and ibandronate have all demonstrated efficacy in reducing the risks of a range of sres in metastatic breast cancer. The safety and tolerability issues with these agents include nephrotoxicity, acute-phase reactions, and onj for the IV agents and gastrointestinal side effects for the oral formulations.
The monoclonal anti-rankl antibody denosumab has recently been approved in Canada for reducing the risk of sres in patients with bone metastases from breast cancer, prostate cancer, non-small-cell lung cancer, and other solid tumours. The results of a recent phase iii trial discussed earlier were consistent with those obtained in a trial comparing denosumab with zoledronate for sres in the setting of metastatic prostate cancer45. The subcutaneous administration of denosumab provides an alternative route of administration and eliminates the need for routine testing of renal function before drug administration.
Important questions regarding the optimal use of antiresorptive therapies in the setting of breast cancer with bone metastases remain outstanding. For example, although numerous risk factors for sres have been elucidated, other questions remain entirely unanswered: Precisely how are the patients that are most likely to benefit from prophylactic treatment to be identified? Which agents should be used in specific clinical settings? What are the optimal dosing schedules for each agent? When it is appropriate to discontinue antiresorptive therapy as the patient’s health status declines?
All of the foregoing questions affect potential benefits, health care expenditures, inconvenience, and risk of adverse events. In a survey of 100 medical oncologists across Canada about their use of bisphosphonates for breast cancer patients46 conducted in 2004, very few respondents would stop bisphosphonate treatment because of a sre or disease progression; instead, they would generally either continue the same or switch to an alternative bisphosphonate agent. The agents most frequently used as initial therapy were oral clodronate and IV pamidronate. Availability and funding of agents in specific provinces appear to influence current Canadian practice. Although some trials suggest differences in the delay of sres provided by the various agents, none of the comparative trials have shown a survival benefit, and many Canadian oncologists and funders therefore do not see compelling arguments to abandon funded oral or older agents.
Because patients with bone-only metastatic disease may survive several years, and the risk of adverse events from bisphosphonates increases with cumulative exposure, several ongoing trials are exploring the possibility that more infrequent bisphosphonate doses may be as effective as standard doses47,48. In addition, small phase ii trials have explored the strategy of switching to novel or more potent agents for patients at high risk of further sres on a bisphosphonate49,50. In one of those trials49, 31 patients who experienced either a sre or progressive bone metastases while receiving clodronate or pamidronate were switched to zoledronate, a more potent bisphosphonate; after 8 weeks, pain control had improved significantly. The other trial50 enrolled 111 patients with bone metastases from solid tumours (41% being breast cancers) who were at high risk of a sre by virtue of elevated urinary ntx levels after at least 8 weeks of treatment with an IV bisphosphonate. Compared with patients who continued on bisphosphonates (86% of whom continued on zoledronate), the patients who switched to denosumab experienced significantly improved rates of decreased ntx levels (the primary endpoint) after 12 weeks and also a decreased incidence of sres at 25 weeks (8% vs. 17%; odds ratio: 0.31; 95% ci: 0.08 to 1.18).
Optimal use of bone resorption markers represents an area of interest in clinical practice. Retrospective subset analyses of bisphosphonate trials have investigated the impact of early normalization (at 3 months) of urinary ntx levels in patients receiving bisphosphonates15,51. Compared with persistent elevation of urinary ntx, early normalization of elevated baseline levels was associated with a significantly reduced risk of a first sre, first fracture, surgery to bone, or death. Compared with zoledronic acid, denosumab treatment resulted in greater suppression of bone turnover markers at week 13, resulting in a significantly reduced risk of first and subsequent sres. No differences in progression-free or overall survival were observed between the agents51.
Another novel molecular target of interest is Src, a nonreceptor tyrosine kinase with a key role in osteoclast-mediated bone resorption as well as tumour growth and metastasis. The Src tyrosine kinase inhibitor dasatinib has shown promising preliminary results with respect to tumour response when combined with docetaxel or aromatase inhibitors; ongoing clinical trials are exploring those options52.
Additional future directions that hold promise include explorations of the potential utility of antiresorptive agents in the adjuvant setting, and a greater emphasis on standardizing, measuring, and improving key endpoints besides sres—for example, bone pain, mobility, and other measures with direct impact on quality of life.
8. ACKNOWLEDGMENTS
Expenses associated with the preparation and submission of this manuscript were supported by an unrestricted educational grant from Amgen Inc. The authors did not receive honoraria related to this publication or any other consideration from Amgen or from any other source. The authors thank CTC Communications Corporation and medical writers Dr. Amy Goldwater and Arthur Tan for their assistance in the preparation of this manuscript.
7. CONFLICT OF INTEREST DISCLOSURES
MC has received research funding from Amgen, Novartis, and Roche, and honoraria from Amgen, Novartis, and Roche; he has also participated on advisory boards for Amgen, Novartis, and Roche. KAG has participated on advisory boards for Amgen, AstraZeneca, Novartis, Pfizer, and Roche. KIP has received honoraria from and participated on advisory boards for Amgen, Roche, Novartis, Boehringer–Ingelheim, GlaxoSmithKline, and Pfizer. AHGP has received honoraria from Bayer, Amgen, Roche Diagnostics, and Novartis.
9. REFERENCES
- 1.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–6. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 4.van Holten–Verzantvoort AT, Bijvoet OL, Cleton FJ, et al. Reduced morbidity from skeletal metastases in breast cancer patients during long-term bisphosphonate (apd) treatment. Lancet. 1987;2:983–5. doi: 10.1016/S0140-6736(87)92555-4. [DOI] [PubMed] [Google Scholar]
- 5.LoRusso P. Analysis of skeletal related events in breast cancer and response to therapy. Semin Oncol. 2001;28(suppl 11):22–7. doi: 10.1016/S0093-7754(01)90228-3. [DOI] [PubMed] [Google Scholar]
- 6.Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer. 2008;16:879–89. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 7.Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Pract Oncol. 2009;6:163–74. doi: 10.1038/ncponc1323. [DOI] [PubMed] [Google Scholar]
- 8.Jensen AØ, Jacobsen JB, Nørgaard M, Yong M, Fryzek JP, Sørensen HT. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer. 2011;11:29. doi: 10.1186/1471-2407-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases. Cancer. 2000;88:1082–90. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1082::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Van Poznak C. Predicting skeletal complications in metastatic breast cancer raises challenges. Breast Cancer Res Treat. 2010;123:781–3. doi: 10.1007/s10549-010-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinkaus M, Simmons C, Myers J, Dranatisaris G, Clemons M. Skeletal-related events (sres) in breast cancer patients with bone metastases treated in the nontrial setting. Support Care Cancer. 2010;18:197–203. doi: 10.1007/s00520-009-0645-z. [DOI] [PubMed] [Google Scholar]
- 12.Brown JE, Cook RJ, Lipton A, Costa L, Coleman RE. Prognostic factors for skeletal complications from metastatic bone disease in breast cancer. Breast Cancer Res Treat. 2010;123:767–79. doi: 10.1007/s10549-010-0981-1. [DOI] [PubMed] [Google Scholar]
- 13.Clemons M, Cole DE, Gainford MC. Can bone markers guide more effective treatment of bone metastases from breast cancer? Breast Cancer Res Treat. 2006;97:81–90. doi: 10.1007/s10549-005-9094-7. [DOI] [PubMed] [Google Scholar]
- 14.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase iii, double-blind, comparative trial. Cancer J. 2001;7:377–87. [PubMed] [Google Scholar]
- 15.Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 16.Käkönen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97(suppl 3):834–9. doi: 10.1002/cncr.11132. [DOI] [PubMed] [Google Scholar]
- 17.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 18.Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4:30–4. doi: 10.1186/bcr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporosis Int. 2008;19:733–59. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 20.Paterson AH, Powles TJ, Kanis JA, McCloskey E, Hanson J, Ashley S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11:59–65. doi: 10.1200/JCO.1993.11.1.59. [DOI] [PubMed] [Google Scholar]
- 21.Tubiana–Hulin M, Beuzeboc P, Mauriac L, et al. Double-blinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastases [French] Bull Cancer. 2001;88:701–7. [PubMed] [Google Scholar]
- 22.Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate, Protocol 19, Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16:2038–44. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 23.Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846–54. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 24.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–44. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 25.Lipton A, Small E, Saad F, et al. The new bisphosphonate, Zometa (zoledronic acid), decreases skeletal complications in both osteolytic and osteoblastic lesions: a comparison to pamidronate. Cancer Invest. 2002;20(suppl 2):45–54. doi: 10.1081/CNV-120014886. [DOI] [PubMed] [Google Scholar]
- 26.Heras P, Kritikos K, Hatzopoulos A, Georgopoulou AP. Efficacy of ibandronate for the treatment of skeletal events in patients with metastatic breast cancer. Eur J Cancer Care (Engl) 2009;18:653–6. doi: 10.1111/j.1365-2354.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 27.Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase iii studies. Br J Cancer. 2004;90:1133–7. doi: 10.1038/sj.bjc.6601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2005;(3):CD003474. doi: 10.1002/14651858.CD003474.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Dunstan CR, Felsenberg D, Seibel MJ. Therapy insight: the risks and benefits of bisphosphonates for the treatment of tumor-induced bone disease. Nat Clin Pract Oncol. 2007;4:42–55. doi: 10.1038/ncponc0688. [DOI] [PubMed] [Google Scholar]
- 30.Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19:420–32. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 31.Novartis Pharmaceuticals Canada . Zometa (Zoledronic Acid) Dorval, QC: Novartis Pharmaceuticals Canada; 2009. [product monograph]. [Google Scholar]
- 32.Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29:1221–7. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 33.Bayer Inc. Bonefos (Clodronate Disodium) Toronto, ON: Bayer Inc.; 2010. [product monograph]. [Google Scholar]
- 34.Novartis Pharmaceuticals Canada . Aredia (Pamidronate Disodium) Dorval, QC: Novartis Pharmaceuticals Canada; 2009. [product monograph]. [Google Scholar]
- 35.Novartis Pharmaceuticals Canada . Aclasta (Zoledronic Acid) Dorval, QC: Novartis Pharmaceuticals Canada; 2009. [product monograph]. [Google Scholar]
- 36.Olson K, Van Poznak C. Significance and impact of bisphosphonate-induced acute phase responses. J Oncol Pharm Pract. 2007;13:223–9. doi: 10.1177/1078155207080806. [DOI] [PubMed] [Google Scholar]
- 37.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws: 2009 update. J Oral Maxillofac Surg. 2009;67(suppl 5):2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Gebara SN, Moubayed H. Risk of osteonecrosis of the jaw in cancer patients taking bisphosphonates. Am J Health Syst Pharm. 2009;66:1541–7. doi: 10.2146/ajhp080251. [DOI] [PubMed] [Google Scholar]
- 39.European Medicines Agency (emea) CHMP Assessment Report on Bisphosphonates and Osteonecrosis of the Jaw. London, U.K.: EMEA; 2009. [Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/01/WC500051428.pdf; cited May 18, 2011] [Google Scholar]
- 40.Ripamonti CI, Maniezzo M, Campa T, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20:137–45. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]
- 41.Amgen Canada . Xgeva (Denosumab) Mississauga, ON: Amgen Canada; 2011. [product monograph]. [Google Scholar]
- 42.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 43.Stopeck A, Fallowfield L, Patrick D, et al. Effects of denosumab versus zoledronic acid (za) on pain in patients (pts) with metastatic breast cancer: results from a phase iii clinical trial [abstract 1024] J Clin Oncol. 2010;28 doi: 10.1200/JCO.2010.29.7101. [Available online at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=74&abstractID=44468; cited July 17, 2012] [DOI] [PubMed] [Google Scholar]
- 44.Fallowfield L, Patrick D, Body J, et al. Effects of denosumab versus zoledronic acid (za) on health-related quality of life (hrql) in metastatic breast cancer: results from a randomized phase iii trial [abstract 1025] J Clin Oncol. 2010;28 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=74&abstractID=49593; cited July 17, 2012] [Google Scholar]
- 45.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma S, Kerr–Cresswell D, Dranitsaris G, et al. Bisphosphonate use for the management of breast cancer patients with bone metastases: a survey of Canadian medical oncologists. Support Care Cancer. 2004;12:852–8. doi: 10.1007/s00520-004-0671-9. [DOI] [PubMed] [Google Scholar]
- 47.Fornier MN. Denosumab: second chapter in controlling bone metastases or a new book? J Clin Oncol. 2010;28:5127–31. doi: 10.1200/JCO.2010.31.0128. [DOI] [PubMed] [Google Scholar]
- 48.Bouganim N, Dranitsaris G, Amir E, Clemons M. Optimising the use of bone-targeted agents in patients with metastatic cancers: a practical guide for medical oncologists. Support Care Cancer. 2011;19:1687–96. doi: 10.1007/s00520-011-1230-9. [DOI] [PubMed] [Google Scholar]
- 49.Clemons MJ, Dranitsaris G, Ooi WS, et al. Phase ii trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J Clin Oncol. 2006;24:4895–900. doi: 10.1200/JCO.2006.05.9212. [DOI] [PubMed] [Google Scholar]
- 50.Fizazi K, Lipton A, Mariette X, et al. Randomized phase ii trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–71. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 51.Lipton A, Cook RJ, Major P, Smith MR, Coleman RE. Zoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activity. Oncologist. 2007;12:1035–43. doi: 10.1634/theoncologist.12-9-1035. [DOI] [PubMed] [Google Scholar]
- 52.Saad F, Lipton A. Src kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2010;36:177–84. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]