INTRODUCTION
In 2011, the Pan-Canadian Cervical Cancer Screening Initiative (pccsi), supported by the Canadian Partnership Against Cancer, released Cervical Cancer Screening in Canada: Monitoring Program Performance 2006–2008 and for the first time presented information on 12 national quality indicators relating to provincial and territorial cervical cancer screening programs. Among those indicators is the participation rate: the percentage of eligible women who had at least 1 Pap test in a 3-year period. Adequate participation in cervical cancer screening is necessary for screening programs to be successful in reducing mortality from that disease.
CERVICAL CANCER SCREENING IN CANADA
Since the early 1980s, the incidence and mortality of cervical cancer in Canada has declined considerably1. Cervical cancer screening by cervical cytology (Pap test) has been the primary reason for the decline. The goal of cervical cancer screening is to detect and treat precancerous lesions early so as to prevent invasive cervical cancer from developing.
Not being screened for cervical cancer at the recommended time interval is a major risk factor for developing cervical cancer2. A meta-analysis showed that, on average, 53.8% of women diagnosed with invasive cervical cancer had inadequate screening histories, and of the diagnosed women, 41.5% had never been screened3.
In Canada, screening for cervical cancer has been largely opportunistic, although programs are becoming increasingly organized. As of June 2011, organized or partially organized cervical cancer screening programs had been established in 8 provinces, including British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Nova Scotia, Prince Edward Island, and Newfoundland and Labrador. Screening guidelines and the organization of the screening programs occurs at the provincial level, and as such, the specific components of the screening programs vary between the provinces4.
CERVICAL CANCER SCREENING PARTICIPATION
Information on the percentage of eligible women who had at least 1 Pap test in a 3-year period was available for 7 of the 8 provinces with organized or partially organized cervical cancer screening programs. The participation rates for Ontario and British Columbia were adjusted for women who had undergone a total hysterectomy. Women who have undergone a total hysterectomy do not require cervical screening, and so adjusting for hysterectomy provides a more accurate estimate of participation. Adjustment is especially pertinent in older age groups, in which the women are more likely to have undergone hysterectomy. Ontario adjusted for hysterectomy by excluding women who had a prior hysterectomy from the numerator and the denominator. British Columbia adjusted the denominator based on historical hysterectomy rates within the province.
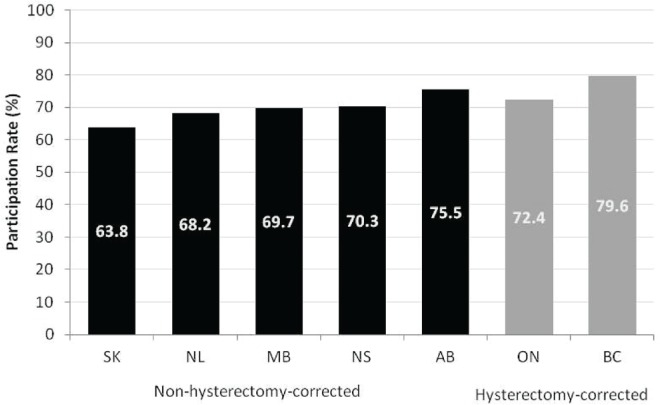
The data show that cervical cancer screening participation is high. From 2006 to 2008, the average percentage of women 20–69 years of age who underwent at least 1 Pap test within a provincial program in a 3-year period was 70.2%. The percentage was slightly higher (74.1%) when corrected for hysterectomy. The percentage of women who underwent at least 1 Pap test over a 3-year period varies between provinces; rates range from 63.8% in Saskatchewan to 75.5% in Alberta (Figure 1). For the two provinces with participation rates corrected for hysterectomy, the participation rates were 72.4% in Ontario and 79.6% in British Columbia.
FIGURE 1.
Percentage of women 20–69 years of age who had at least 1 Pap test in a 3-year period, by province, 2006–2008. Newfoundland and Labrador provided historical data from 2005–2007. Alberta provided data for the areas in which the organized program operated during 2006–2008 (approximately 40% of the population).
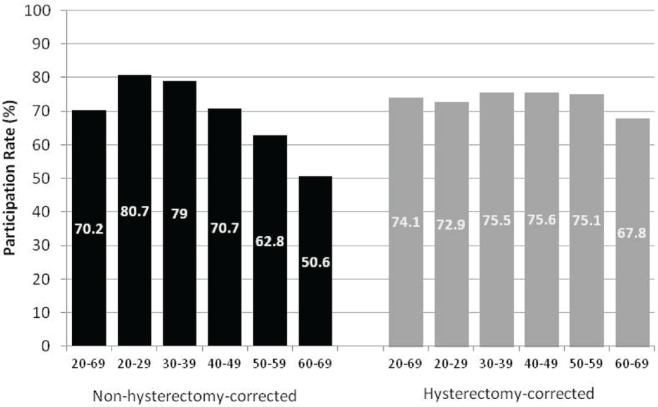
When participation in cervical cancer screening was examined by age group, participation declined with age. Among women 20–29 years of age, 80.7% underwent at least 1 Pap test in a 3-year period; that rate compares with 50.6% among women 60–69 years of age (Figure 2). However, when rates were corrected for hysterectomy, participation varied little across age groups. Participation was lower only for women 60–69 years of age. Provincial and territorial guidelines recommend an end to screening at age 69 (or older in some case). However, guidelines from the U.S. Preventive Services Task Force recommend an end to screening after age 65, provided that women have had adequate recent screening with normal Pap test results5.
FIGURE 2.
Percentage of women 20–69 years of age who had at least 1 Pap test in a 3-year period, by age group, 2006–2008. Newfoundland and Labrador provided historical data from 2005–2007. Alberta provided data for the areas in which the organized program operated during 2006–2008 (approximately 40% of the population).
FUTURE DIRECTIONS
Infection with high-risk types of the human papilloma virus (hpv) causes almost all cases of cervical cancer, with approximately 70% of cases being caused by hpv types 16 and 186. Over the next few years, hpv testing and hpv vaccination programs implemented across the country will have a significant impact on cervical cancer screening guidelines. The pccsi will continue to foster implementation and continuing development of effective cervical cancer screening programs and to focus on integration of screening with hpv vaccination, testing, and surveillance initiatives.
ABOUT THE CERVICAL CANCER SCREENING IN CANADA: MONITORING PROGRAM PERFORMANCE 2006–2008 REPORT
The pccsi serves as a forum for key stakeholders from across the country to discuss and take action on matters related to cervical cancer screening programs and their integration with hpv testing and vaccination initiatives. As part of the pccsi, a working group was formed to coordinate the submission and analysis of baseline cervical cancer screening data and to develop an inaugural report. Cervical Cancer Screening in Canada: Monitoring Program Performance 2006–2008 presents data for 12 screening program performance indicators in women 20–69 years of age and provides baseline information about cervical cancer screening outcomes from across Canada. The 12 program performance indicators were previously developed by the Screening Performance Indicators Working Group under the guidance of the Public Health Agency of Canada’s Steering Committee for the Cervical Cancer Prevention and Control Network. Provincial cervical cancer screening programs contributed data for the inaugural report and reviewed report drafts.
The full report can be viewed at http://www.partnershipagainstcancer.ca/wp-content/uploads/CPAC_Cervical_CS_Report_E_WEB_Final.pdf.
CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2011. Toronto, ON: Canadian Cancer Society; 2011. [Google Scholar]
- 2.Andrae B, Kemetli L, Sparén P, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008;100:622–9. doi: 10.1093/jnci/djn099. [DOI] [PubMed] [Google Scholar]
- 3.Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007;45:93–106. doi: 10.1016/j.ypmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Partnership Against Cancer (cpac) Cervical Cancer Screening in Canada: Monitoring Program Performance 2006–2008. Toronto, ON: CPAC; 2011. [Google Scholar]
- 5.Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–91. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F. Chapter 2: the burden of hpv-related cancers. Vaccine. 2006;24(suppl 3):11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]