Abstract
Purpose
We evaluated the benefit of the current clinical practice of adjuvant chemotherapy for postmenopausal women with early-stage, estrogen- or progesterone-receptor-positive (er/pr+), one-to-three positive axillary lymph node (1–3 ln+), breast cancer (esbc).
Methods
Using the Manitoba Cancer Registry, we identified all postmenopausal women diagnosed with er/pr+ 1–3 ln+ esbc during the periods 1995–1997, 2000–2002, and 2003–2005 (n = 156, 161, and 171 respectively). Treatment data were obtained from the Manitoba Cancer Registry and by linkage with Manitoba administrative databases. Seven-year survival data were available for the 1995–1997 and 2000–2002 populations. Using Cox regression, we assessed the independent effect of the clinical practice of adjuvant chemotherapy on disease-free (dfs) and overall survival (os).
Results
Clinical breast cancer treatments did not differ significantly between the 2000–2002 and 2003–2005 populations. Adjuvant chemotherapy was administered in 103 patients in the 2000–2002 population (64%) and in 44 patients in the 1995–1997 population [28.2%; mean difference: 36%; 95% confidence interval (ci): 31% to 40%; p < 0.0001]. Compared with 1995–1997, 2000–2002 was not significantly associated with an incremental dfs benefit for patients over a period of 7 years (2000–2002 vs. 1995–1997; adjusted hazard ratio: 0.98; 95% ci: 0.64 to 1.4).
Conclusions
The treatment standard of adjuvant chemotherapy in addition to endocrine therapy may not be effective for all women with er/pr+ 1–3 ln+ esbc. There could be a subgroup of those women who do not benefit from adjuvant chemotherapy as expected and who are therefore being overtreated. Further studies with a larger sample size are warranted to confirm our results.
Keywords: Breast cancer, adjuvant chemotherapy, clinical practice patterns
1. INTRODUCTION
In 2011, an estimated 18,600 postmenopausal women were diagnosed with breast cancer in Canada1,2. Approximately 20% of them would have been diagnosed with early-stage estrogen or progesterone receptor–positive (er/pr+), one-to-three positive axillary lymph node (1–3 ln+) breast cancer (esbc)2. Within the last decade, adjuvant chemotherapy has become the treatment standard for those women3,4. That standard is based on several decades of phase iii clinical trials that showed a survival benefit from chemotherapy when added to endocrine therapy alone in postmenopausal women with er/pr+ ln+ breast cancer5–7.
Recent data show that women with er/pr+ ln+ breast cancer, particularly cancers with favorable histopathologic features (1–3 ln+), do not benefit equally from chemotherapy8. The results of subgroup analyses from a phase iii trial by the Southwest Oncology Group have generated interest in the possibility that some er/pr+ ln+ breast cancer patients may not benefit from the addition of chemotherapy to endocrine therapy7,9. In another analysis, a group of postmenopausal women with er+ 1–3 ln+ esbc who were treated with adjuvant endocrine therapy alone were found to have a good prognosis, with a less than 10% risk of distant recurrence at 9 years of followup10,11. Those results challenge the current treatment standard of adjuvant chemotherapy for all women with er/pr+ ln+ breast cancer; however, they were performed on subsets of patients included in clinical trials and therefore may not reflect the experience of the general population.
Avoidance of the toxic effects and costs of adjuvant chemotherapy when it might not be needed is often an important goal in breast cancer treatment4. Most women with er/pr+ 1–3 ln+ esbc—who, compared with women with 4 or more positive nodes, may have higher possibility of avoiding adjuvant chemotherapy—continue to receive such therapy in current clinical practice12. Thus, benefit from the current treatment standard of adjuvant chemotherapy should be evaluated in the general population of women with er/pr+ 1–3 ln+ esbc.
We aimed to evaluate whether current clinical practice that includes adjuvant chemotherapy in addition to endocrine therapy for most postmenopausal women with er/pr+ 1–3 ln+ esbc is associated with an incrementally greater survival benefit than that obtained with the previous clinical practice of adjuvant endocrine therapy alone.
2. METHODS
2.1. Data Source
The Manitoba Cancer Registry (mcr) and Manitoba administrative databases held by Manitoba Health, including the Hospital Discharge Database, the Physician Claims Database, and the Drug Program Information Network (dpin), served as data sources for this analysis. The mcr is a provincial database that contains records for more than 99.5% of all cases of cancer in Manitoba13. Information on breast-cancer staging, based on the American Joint Committee on Cancer version 5 standard, has been routinely collected for breast cancers diagnosed since January 199514. The mcr also collects information on primary tumour location, tumour size, grade differentiation, lymph-node status, er and pr status, age, local recurrence, regional recurrence, distant recurrence, second primary cancer, death, and treatments—including surgery, radiation therapy, endocrine therapy, and chemotherapy—for primary breast cancer or for any recurrence. Patients who move out of the province receive continued surveillance through correspondence with the patient or, in some cases, the appropriate cancer clinic within Canada. The registry has been evaluated by the North American Association of Central Cancer Registries and repeatedly found to be near-complete in the ascertainment of all cancer cases and their follow-up data15. The accuracy and comprehensiveness of those data, especially as they pertain to the ascertainment of crucial points (mortality, recurrence, and second primary) have been established and used for many earlier epidemiology studies16–19, largely because of the multiple sources of ascertainment used for incident cases, disease progression, and mortality, including physician notifications, pathology and hematology reports, hospitalizations, and mortality and autopsy records. In addition, the mcr is linked directly to Manitoba Health’s population registration file, which contains information on vital statistics and migration for almost all residents of the province since 1984.
The Hospital Discharge Database contains inpatient information, including admit date, length of hospital stay, diagnoses, and interventions during the entire hospital stay. The Physician Claims Database contains date, numeric tariff index (a servicespecific code used for physician compensation), and fee for each service provided by physicians. The dpin contains the date, dose, fee, and drug identification number for each drug claim.
Data collection and analysis for the present study was approved by the University of Manitoba’s Health Research Ethics Board.
2.2. Study Population
We used the mcr to identify all postmenopausal women (defined as being at least 50 years of age) diagnosed with er/pr+ 1–3 ln+ esbc during three time intervals: January 1995 to December 1997; January 2000 to December 2002; and January 2003 to December 2005. We selected those periods because they would likely reflect the effects of the 1998 Canadian clinical practice guidelines for the care and treatment of breast cancer20, which recommended the use of adjuvant chemotherapy for postmenopausal women with er/pr+ 1–3 ln+ esbc.
Through the mcr, we also identified breast cancer treatments for those women, including surgery (breast-conserving surgery or mastectomy), radiation therapy, endocrine therapy and chemotherapy for their primary breast cancer. We defined the women as having received any of those treatments for their primary breast cancer if the icd-9-cm (International Classification of Disease, 9th revision, Clinical Modification) or the Canadian Classification of Health Interventions procedure code for any of the foregoing treatments was found within 1 year of diagnosis with esbc before any recurrence, second primary cancer, or death.
Seven-year survival data were available for the 1995–1997 and 2000–2002 populations and were obtained from the mcr. In the present study, we tested our hypotheses using data from the 1995–1997 and 2000–2002 populations so that a relatively long follow-up period would be available. We used treatment data from the 2003–2005 population only to examine whether the clinical practice of breast cancer treatments in the 2000–2002 population would reflect more recent clinical practice.
2.3. Linkage with Manitoba Administrative Databases
We linked the study population identified using the mcr with administrative data held by Manitoba Health, including the Hospital Discharge Database, the Physician Claims Database, and the dpin. To protect confidentiality, linkage was performed on the Scrambled Personal Health Identification Number, using anonymized versions of the databases. Wherever possible, we cross-validated the results using multiple databases. For instance, the surgery data captured by the mcr were validated by linking the study population with the Hospital Discharge Database to identify patients who had received breast cancer surgeries (breast-conserving surgery or mastectomy) as identified by the icd-9-cm procedure codes for those surgeries. Radiation therapy and chemotherapy data captured by the mcr were validated by linking the study population with the Physician Claims Database to identify patients who had received those treatments as identified by the numeric tariff index of the treatment services provided by physicians. Endocrine therapy data captured by the mcr were validated by linking with the dpin to identify patients who had received endocrine therapy (tamoxifen or aromatase inhibitors) as identified by the drug identification numbers of those treatments. In addition, the type of endocrine therapy (tamoxifen or aromatase inhibitors) and chemotherapy (non-anthracycline, anthracycline, or taxane-containing regimens) were identified through the same linkage.
2.4. Comorbidity Index
We determined comorbidities from the Hospital Discharge Database as identified by diagnoses or procedures that were recorded for each patient in the study population during all hospital stays from 1 year before until 6 months after a breast cancer diagnosis. We found at least 1 hospitalization for each patient in our study population. We used comorbid diagnoses coded using the method developed by Charlson et al.21, excluding cancer diagnoses.
2.5. Statistical Analysis
We performed the statistical analyses using the SAS software application (version 9.1: SAS Institute, Cary, NC, U.S.A.). Binary, categorical, and ordinal variables were compared using the Fisher exact test, chi-square test, and Wilcoxon signed-rank test respectively. Distributions of continuous variables were summarized by their means and by standard deviations and were compared using t-tests. All tests for statistical significance were two-sided.
The primary outcome in the analysis was disease-free survival (dfs), defined as survival free from recurrence (local, regional, or distant), second primary breast cancer, and death from any cause. Our secondary outcome was overall survival (os). We used Kaplan–Meier methods for estimation of dfs and os in the two groups. We used the log-rank test to assess differences between the 1995–1997 and 2000–2002 populations with respect to dfs and os. We used Cox proportional hazards models to examine the independent effect of the clinical practice of adjuvant chemotherapy therapy on dfs and os, adjusting for other clinical prognostic factors such as comorbid indices, receipt of radiation therapy, and endocrine therapy. We obtained hazard ratios and 95% confidence intervals from the Cox proportional hazards models.
3. RESULTS
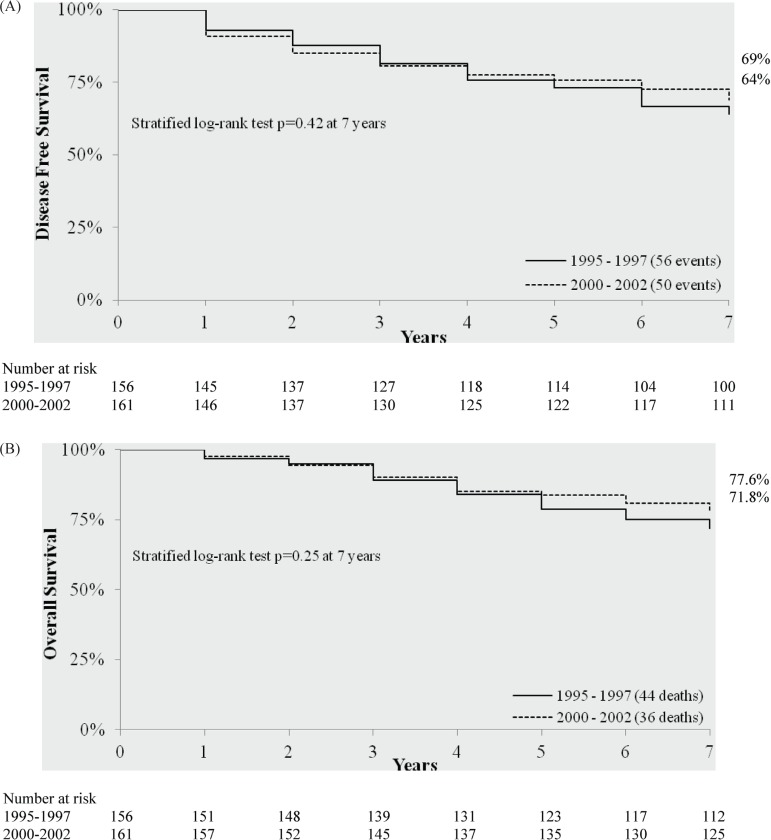
Table i summarizes patient, tumour, and treatment characteristics. We identified 156, 161, and 175 post-menopausal women diagnosed with er+/pr+ 1–3 ln+ esbc during the time periods of 1995–1997, 2000– 2002, and 2003–2005 respectively. We found no significant differences in the annual proportion of the patient population receiving adjuvant chemotherapy during the period 2000–2005 (Table ii). Moreover, with the exception of the type of agents used for endocrine therapy and chemotherapy, we found no significant differences in patient, tumour, and other treatment characteristics between the 2000–2002 and 2003–2005 populations (Table i). Thus, for this analysis, we considered that the 2000–2002 period reflects current clinical practice and the 1995–1997 population reflects earlier clinical practice of adjuvant chemotherapy. Thus, we were able to evaluate a full 7 years of follow-up for both populations. We also found all treatment data to be consistent between the mcr and the Manitoba administrative databases held by Manitoba Health.
TABLE I.
Characteristics of 492 patients being treated for early-stage breast cancer positive for the estrogen or progesterone receptor, with 1–3 positive lymph nodes, by period of diagnosis
| Characteristic |
Period
|
p Valuea
|
|||
|---|---|---|---|---|---|
| (A) 1995–1997 | (B) 2000–2002 | (C) 2003–2005 | (B vs. A) | (C vs. B) | |
| Patients | 156 | 161 | 171 | ||
| Age | |||||
| Mean [se (years)] | 64.4 (0.82) | 64 (0.82) | 64.2 (0.77) | ||
| Range (years) | 50–93 | 50–89 | 50–88 | 0.44 | 0.53 |
| 50–64 years [n (%)] | 88 (56.5) | 94 (58.3) | 102 (59.6) | 0.72 | 0.81 |
| ≥65 years [n (%)] | 68 (43.5) | 67 (41.7) | 69 (40.4) | ||
| Primary tumour size [n (%)] | |||||
| <2 cm | 72 (46.1) | 70 (43.5) | 67 (39.2) | 0.88 | 0.1 |
| 2–5 cm | 76 (48.8) | 82 (50.9) | 83 (48.5) | ||
| >5 cm | 8 (5.1) | 9 (5.6) | 21 (12.3) | ||
| Positive nodes [n (%)] | |||||
| 1 | 84 (53.8) | 96 (59.6) | 106 (62) | 0.55 | 0.89 |
| 2 | 45 (28.8) | 39 (24.2) | 40 (23.4) | ||
| 3 | 27 (17.4) | 26 (16.2) | 25 (14.6) | ||
| Receptor status [n (%)] | |||||
| er+, pr – | 48 (30.7) | 52 (32.3) | 44 (25.7) | 0.27 | 0.35 |
| er –, pr + | 11 (7) | 5 (3.1) | 4 (2.3) | ||
| er +, pr + | 97 (62.3) | 104 (64.6) | 123 (72) | ||
| Tumour grade [n (%)] | |||||
| 1 | 17 (10.9) | 40 (24.8) | 35 (20.4) | 0.13 | 0.64 |
| 2 | 60 (38.5) | 76 (47.2) | 92 (53.8) | ||
| 3 | 31 (19.8) | 37 (23) | 35 (20.5) | ||
| Unknown | 48 (30.8) | 8 (5) | 9 (5.3) | ||
| Stage [n (%)] | |||||
| iia | 82 (52.6) | 77 (47.8) | 77 (45) | 0.73 | 0.72 |
| iib | 63 (40.4) | 71 (44.1) | 75 (43.9) | ||
| iiia | 6 (3.8) | 9 (5.6) | 15 (8.8) | ||
| iiib | 5 (3.2) | 4 (2.5) | 4 (2.3) | ||
| Breast surgeryb [n (%)] | 151(97) | 153 (95) | 161 (94.1) | 0.42 | 0.72 |
| Breast-conserving surgery | 55(36.5) | 76(49.7) | 82 (51) | 0.02 | 0.36 |
| Mastectomy | 96(63.5) | 77(50.3) | 77 (47.8) | ||
| Unknown | 0 | 0 | 2 (1.2) | ||
| Radiotherapy b [n (%)] | 57 (36.5) | 96 (59.6) | 102 (59.6) | <0.0001 | 0.99 |
| Endocrine therapyb [n (%)] | 125 ( 80) | 143 (88.9) | 147 (86) | 0.03 | 0.43 |
| Tamoxifen | 107 ( 85.6) | 81 (56.6) | 64 (43.5) | <0.0001 | <0.0001 |
| ais + tamoxifen | 9 (7.2) | 51 (35.7) | 25 (17) | ||
| ais | 9 (7.2) | 11 (7.7) | 58 (39.5) | ||
| Adjuvant chemotherapyb [n (%)] | 44 (28.2) | 103 (64) | 111 (64.9) | <0.0001 | 0.85 |
| Non-anthracycline-based | 32 (72.7) | 30 (29.1) | 24 (21.6) | <.0001 | <.0001 |
| Anthracycline-based | 8 (18.2) | 66 (64.1) | 51 (46) | ||
| Combination taxane–anthracycline | 2 (4.5) | 4 (3.9) | 32 (28.8) | ||
| Unknown | 2 (4.5) | 3 (2.9) | 4 (3.6) | ||
| Disease-free survival event [n (%)] | 56 (35.9) | 50 (31.1) | — | ||
| Deaths [n (%)] | 44 (28.2) | 36 ( 22.4) | — | ||
| cci score [n (%)]c | |||||
| 0 | 138 (88.5) | 130 (80.7) | 137 (80.1) | 0.05 | 0.99 |
| 1 | 12 (7.8) | 18 (11.3) | 26 (15.2) | ||
| 2 | 4 (2.5) | 9 (5.6) | 5 (2.9) | ||
| 3 | 2 (1.2) | 2 (1.2) | 1 (0.6) | ||
| 4 | 0 | 1 (0.6) | 1 (0.6) | ||
| 5 | 0 | 0 | 0 | ||
| 6 | 0 | 0 | 0 | ||
| 7 | 0 | 0 | 1 (0.6) | ||
| 8 | 0 | 1 (0.6) | 0 | ||
By Fisher exact, chi-square, and Wilcoxon signed-rank test, as appropriate.
Treatments for primary breast cancer were considered to have been received if the International Classification of Diseases, 9th revision, Clinical Modification procedure code or the Canadian Classification of Health Interventions procedure code for the given treatment appeared in the patient record before any recurrence, second primary cancer, or death within 1 year of diagnosis with early-stage breast cancer.
Comorbid diagnoses were considered present if they were found during the period of 1 year before and up to 6 months after a diagnosis of primary breast cancer.
se = standard error; er = estrogen receptor; pr = progesterone receptor; ais = aromatase inhibitors; cci = Charlson comorbidity index.
TABLE II.
Annual proportion of the patient population diagnosed with early-stage breast cancer positive for the estrogen or progesterone receptor, with 1–3 positive lymph nodes, receiving adjuvant chemotherapy during 2000–2005
| Year | Diagnoses (n) | Adjuvant chemo [n (%)] | p Valuea |
|---|---|---|---|
| 2000 | 49 | 29 (59.2) | 0.9 |
| 2001 | 59 | 39 (66) | |
| 2002 | 53 | 35 (66.2) | |
| 2003 | 55 | 34 (62) | |
| 2004 | 62 | 43 (69.3) | |
| 2005 | 54 | 34 (63) |
Chi-square test.
The characteristics of age, clinical tumour size, tumour grade, number of ln+, er and pr status, stage, and use of breast cancer surgery did not differ significantly between the 1995–1997 and the 2000–2002 populations (Table i). Compared with the 1995–1997 population, the 2000–2002 population was more likely to receive chemotherapy, radiation therapy, and endocrine therapy (Table i). Among women who received breast-cancer surgery for their primary esbc, those in the 2000–2002 population were also more likely to receive breast-conserving surgery. Among women who received adjuvant chemotherapy, those in the 2000–2002 population were more likely to receive an anthracycline-based regimen. Among women who received endocrine therapy, those in 2000–2002 population were more likely to receive aromatase inhibitors (Table i).
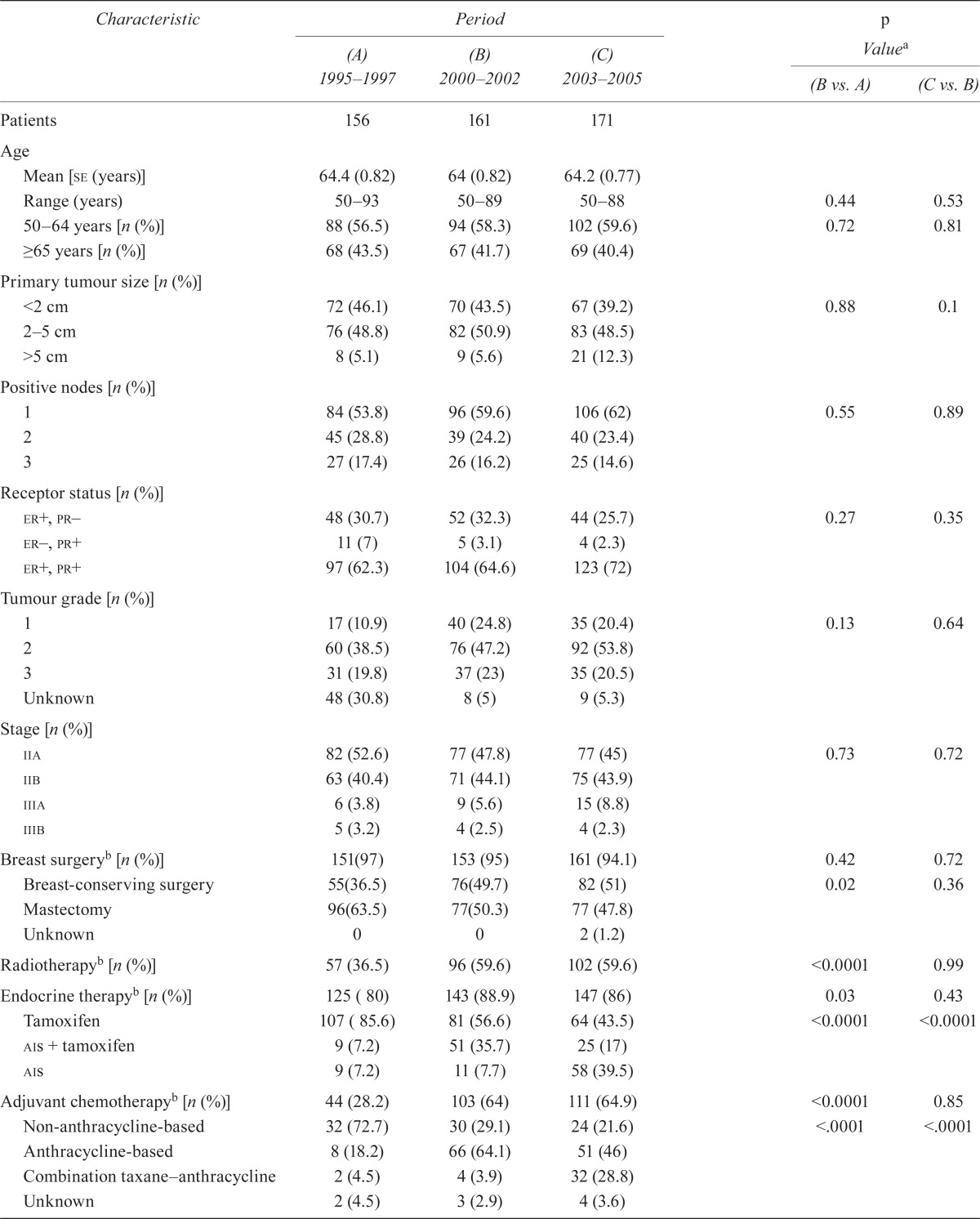
The 7-year Kaplan–Meier estimates of dfs and os did not differ significantly between the 2000–2002 and 1995–1997 populations (Figure 1). In multivariate Cox regression analyses, after adjusting for prognostic factors that were found to differ significantly between the two populations (including indices of comorbidity, and treatment with radiation therapy and endocrine therapy, Table iii), membership in the 2000–2002 population (compared with the 1995–1997 population) was not significantly associated with an incremental dfs benefit over 7 years or with an os benefit over 7 years.
FIGURE 1.
(A) Disease-free survival and (B) overall survival analyses by period of diagnosis. Data are aggregated, with no individual reporting, because of privacy rules with the Manitoba Cancer Registry.
TABLE III.
Multivariate Cox proportional hazards models for estimating the effect of period of clinical practice in 317 patients with early-stage breast cancer positive for the estrogen or progesterone receptor, with 1–3 positive lymph nodes, adjusted for radiation therapy, endocrine therapy, and comorbidity
| Variable |
Survival
|
|||||
|---|---|---|---|---|---|---|
|
Disease-free
|
Overall
|
|||||
| hr | 95% ci | ρ | hr | 95% ci | ρ | |
| Period of clinical practicea (2000–2002 vs. 1995–1997) | 0.98 | 0.648 to 1.472 | 0.91 | 0.86 | 0.54 to 1.37 | 0.51 |
| Adjuvant radiation therapy (yes vs. no) | 0.46 | 0.327 to 0.750 | 0.0009 | 0.43 | 0.26 to 0.7 | 0.0007 |
| Adjuvant endocrine therapy (yes vs. no) | 0.87 | 0.57 to 1.31 | 0.5 | 0.79 | 0.49 to 1.27 | 0.33 |
| Co-morbidity (≥1 vs. 0) | 2.02 | 1.279 to 3.181 | 0.0025 | 2.78 | 1.69 to 4.59 | <0.0001 |
The effect of adjuvant chemotherapy administration independent of the effect of adjuvant radiation therapy, adjuvant endocrine therapy, and comorbidity.
hr = hazard ratio; ci = confidence interval.
In additional analyses, Cox regression models adjusted for indices of comorbidity produced similar results (Table iv). In a post hoc power analysis, we concluded that the study sample size conferred 80% power to detect a 36% decrease in the hazard ratio for dfs for the 2000–2002 population compared with the 1995–1997 population (Table v).
TABLE IV.
Multivariate Cox proportional hazards models for estimating the effect of period of clinical practice in 317 patients with early-stage breast cancer positive for the estrogen or progesterone receptor, with 1–3 positive lymph nodes, adjusted for comorbidity
| Variable |
Survival
|
|||||
|---|---|---|---|---|---|---|
|
Disease-free
|
Overall
|
|||||
| hr | 95% ci | ρ | hr | 95% ci | ρ | |
| Period of clinical practicea (2000–2002 vs. 1995–1997) | 0.79 | 0.54 to 1.64 | 0.23 | 0.68 | 0.43 to 1.06 | 0.09 |
| Co-morbidity (≥1 vs. 0) | 2.1 | 1.33 to 3.3 | 0.0014 | 2.84 | 1.73 to 4.69 | <0.0001 |
The effect of adjuvant therapy administration, including radiation therapy, endocrine therapy, and chemotherapy independent of the effect of comorbidity.
hr = hazard ratio; ci = confidence interval.
TABLE V.
Post hoc power analysis
Calculated using the size of the 1995–1997 population (n = 156) plus the 2000–2002 population (n = 161) at the 5% significance level.
Comparing the 2000–2002 with the 1995–1997 population.
hr = hazard ratio; dfs = disease-free survival.
4. DISCUSSION
Using a population-based analysis, we found that postmenopausal women with er/pr+ 1–3 ln+ esbc did not achieve a statistically significant benefit with respect to dfs and os from the wide use of adjuvant chemotherapy in addition to endocrine therapy that replaced the previous clinical practice of adjuvant endocrine therapy alone. These results suggest that there could be a group of women with er/pr+ 1–3 ln+ esbc who do not achieve a survival benefit from adjuvant chemotherapy in the expected way.
That finding is consistent with earlier analyses. A retrospective analysis that included a subset of patients from the phase iii Southwest Oncology Group 8814 trial9 showed that a group of er/pr+ ln+ breast cancer patients have relatively low risk and do not necessarily benefit from the addition of chemotherapy to endocrine therapy; the analysis also showed that the relevant subgroup can be identified by multi-gene assay. However, participants in a clinical trial typically have a better prognosis than do nonparticipants22,23. In addition, some24,25—although not all26,27—studies suggest that participation in a clinical trial might be associated with improved outcomes independent of the effects of the treatment under investigation.
To our knowledge, our study is the first to evaluate the benefit of wide use of adjuvant chemotherapy in an er/pr+ 1–3 ln+ esbc population using population data that reflect real-world clinical practice. Thus, our finding that there might be a group of patients in the relevant population who are being overtreated with adjuvant chemotherapy is notable.
Current Canadian clinical practice guidelines recommend the use of histopathologic measures to inform adjuvant chemotherapy decisions in women with er/pr+ 1–3 ln+ esbc4. Taken together, our results suggest that those measures may not be accurate predictors of the benefits of adjuvant chemotherapy, further pointing to the need to incorporate accurate prognostic tools such as multi-gene assays to identify women in this specific population who might be spared chemotherapy. Several genetic biomarker analyses—among them, the 70-gene profile8,28 and 21-gene assay9,29,30—have been proposed for use in clinical practice to refine the indications for adjuvant chemotherapy in the er/pr+ 1–3 ln+ esbc population. These multi-gene assays have been shown to predict prognosis and benefit from chemotherapy with greater accuracy than is provided by standard clinical features8,9,29,30. Arguably, when the clinical benefits of chemotherapy are evidence-based, then lives are saved and the side effects of treatment must be accepted on medical, psychological, and fiscal bases. However, side effects are neither medically nor ethically acceptable when a lack of benefit is established. Recently, development of a molecular signature to identify patients who could be spared chemotherapy has been rated as the highest translational research priority for breast cancer31.
Given our observations in the 2003–2005 population (Table i) and current guidelines, women in the 2000–2002 population received adjuvant chemotherapy regimens (anthracycline-based) that might differ from current regimens (a combination of anthracycline- and taxane-based regimens), and a significant number of those women might have received aromatase inhibitors as monotherapy instead aromatase inhibitors plus tamoxifen (aromatase inhibitors after 2–3 years of tamoxifen). In addition, none of the women was treated with trastuzumab. In Manitoba in 2005, trastuzumab became a recommended standard and a publically funded treatment after chemotherapy in the adjuvant setting of human epidermal growth factor receptor 2–positive (her2+) breast cancer. All women in the 2000–2002 population were diagnosed and treated before that standard was established; however, the clinical outcomes (dfs and os) of the 2000–2002 population are still likely to represent current clinical practice in women with er/pr+ 1–3 ln+ esbc. Arguably, several pooled analyses or meta-analyses indicate that modest improvements in dfs and os (approximately 5% and 3% absolute benefit respectively) are seen with taxane-based adjuvant chemotherapy compared with standard anthracycline poly-chemotherapy32–34. Moreover, that efficacy is not well clarified in particular subgroups of patients (defined by traditional biomarkers such as age, er status, and her2 expression) 35. And, compared with tamoxifen alone, aromatase inhibitors as monotherapy or after 2–3 years of tamoxifen have been shown to produce similar lower recurrence rates36. On the other hand, only women with her2+ status (approximately 10%–15% of those with endocrine-positive breast cancers) could benefit from trastuzumab37–41. Thus, only a small proportion of the 2000–2002 population might be candidates for adjuvant trastuzumab after chemotherapy42. In addition, the effect of adjuvant trastuzumab, particularly in women with favorable prognostic features (er+/pr+ 1–3 ln+), such as those in our study population, might be modest and needs to be well defined43,44. When benefits are modest or usage is relatively small, inclusion of particular agents (taxanes, aromatase inhibitors, trastuzumab) is less likely to have a major effect on observed clinical outcomes (dfs and os) of the 2000–2002 population reported here. However, a full analysis of the potential improvements in dfs and os from the use of taxanes, aromatase inhibitors, and trastuzumab could not be examined in the present study because of a lack of data or of statistical power. Future population analyses of er+/pr+ 1–3 ln+ esbc are warranted to examine the survival benefits of those agents in real-world Canadian clinical practice.
Meta-analyses by the Early Breast Cancer Trialists’ Collaborative Group concluded that radiation therapy (after breast-conserving surgery or mastectomy), endocrine therapy, and adjuvant chemotherapy prevent recurrence and increase survival for postmenopausal women with er+/pr+ ln+ esbc5,36,45. According to the latest reports from the Group, the annual proportional reduction in the risks of recurrence and breast cancer mortality were approximately 69% and 17% from radiation therapy45, 40% and 31% from tamoxifen5, and 20% and 10% from non-anthracycline-based adjuvant chemotherapy5. Moreover, their analyses found additional annual proportional reductions in the risk of recurrence and breast cancer mortality of 11% and 16% respectively from the use of anthracycline-based compared with non-anthracycline-based adjuvant chemotherapy regimens5, and 29% and 22% from the use of aromatase inhibitors compared with tamoxifen36.
In our analysis, each of the foregoing therapies was received by a significantly higher proportion of women in the 2000–2002 population than in the 1995–1997 population (Table i). Thus, if the gains from those therapies (radiation therapy, tamoxifen, aromatase inhibitors, anthracycline-based and non-anthracycline-based adjuvant chemotherapy) were additive, then a greater reduction in the hazard ratio for dfs than the 36% that could be detected with 80% power in the present study might be expected to be observed (Table v). We did not observe a reduction of that magnitude; however, our study does not have enough statistical power to rule out a small, but possibly clinically important, dfs benefit for the 2000–2002 clinical practice. Although our data cannot be used to make such definitive conclusions, they do demonstrate that achieving a survival benefit from adjuvant chemotherapy added to endocrine therapy is not definitive in the er/pr+ 1–3 ln+ disease population. Future studies evaluating the use of multi-gene assays compared with clinical and pathologic tumour features in assessing risk and guiding adjuvant chemotherapy decisions in lymph node–negative disease settings should include patients with er/pr+ 1–3 ln+ esbc.
Although several studies have found that the clinical practice patterns and therapies used in the selected time periods in Manitoba reflect practice in other jurisdictions in Canada46–48, the clinical practice used for women with er/pr+ 1–3 ln+ esbc across Canadian provinces may still differ. In addition, outcomes of therapies given in the 2000–2002 population do not necessary reflect the possible benefits of other types of adjuvant therapies or dosing schedules used in current practice. There are limits to what can be ascertained using administrative data. The mcr is a highly accurate source of information about breast cancer13; however, errors in coding can result in incorrect or unrecorded procedures. In the present analysis, we were careful to prevent incorrect ascertainment of breast cancer treatments (including surgery, radiation therapy, endocrine therapy, and chemotherapy) by examining all treatments from two sources—that is, the mcr and the Manitoba administrative databases held by Manitoba Health. There were difficulties in determining the types of chemotherapy agents. We were able to ascertain an anthracycline- or taxane-containing chemotherapy regimen only by linking with the Physician Claims Database and identifying the specific tariff index for services relating to those agents as provided by physicians. Hence, incorrect physician claims may still result in incorrect ascertainment of chemotherapy agents.
5. CONCLUSIONS
The treatment standard of adjuvant chemotherapy in addition to endocrine therapy may not be effective for all postmenopausal women with er/pr+ 1–3 ln+ esbc. It is therefore possible that the members of a relatively large subgroup of those women are currently being overtreated with adjuvant chemotherapy. That finding demonstrates the need for accurate prognostic tools such as multi-gene assays to identify women with er/pr+ 1–3 ln+ esbc who could be spared chemotherapy. Further studies with a greater statistical power are warranted to provide more definitive results.
6. ACKNOWLEDGMENTS
We are indebted to the Department of Epidemiology and the Cancer Registry of Cancer Care Manitoba, and to the Department of Health Information Management of Manitoba Health. Funding was provided by the Canadian Institutes of Health Research Strategic Training Program in Cancer Research and Technology Transfer, by an Academic Development Grant from the University of Western Ontario, and by the Natural Sciences and Engineering Research Council of Canada.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics. Canadian Cancer Statistics 2011. Toronto, ON: Canadian Cancer Society; 2011. [Google Scholar]
- 2.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Fort Washington, PA: NCCN; 2008. Ver 2.2008. [Google Scholar]
- 4.Levine M. Clinical practice guidelines for the care and treatment of breast cancer: adjuvant systemic therapy for node-positive breast cancer (summary of the 2001 update). The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. CMAJ. 2001;164:644–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 6.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–44. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 7.Albain KS, Barlow WE, Ravdin PM, et al. on behalf of the Breast Cancer Intergroup of North America Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:2055–63. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mook S, Schmidt MK, Viale G, et al. The 70-gene prognosissignature predicts disease outcome in breast cancer patients with 1–3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat. 2009;116:295–302. doi: 10.1007/s10549-008-0130-2. [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Barlow WE, Shak S, et al. on behalf of Breast Cancer Intergroup of North America Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a Transatac study. J Clin Oncol. 2010;28:1829–34. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 11.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a Transatac study. J Clin Oncol. 2010;28:1829–34. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Hansen NM, Susnik B. Clinical roundtable monograph: a multidisciplinary approach to the use of oncotype dx in clinical practice. Clin Adv Hematol Oncol. 2009;7:1–7. [PubMed] [Google Scholar]
- 13.Latosinsky S, Fradette K, Lix L, Hildebrand K, Turner D. Canadian breast cancer guidelines: have they made a difference? CMAJ. 2007;176:771–6. doi: 10.1503/cmaj.060854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming ID, Cooper JS, Henson D, et al., editors. AJCC Cancer Staging Manual. 5th ed. Philadelphia, PA: Lippincott–Raven Publishers; 1997. pp. 171–80. [Google Scholar]
- 15.Tang LY, Nugent Z, Demers AA, Singh H. Incidence of right-sided colorectal cancer after breast cancer: a population-based study. Am J Gastroenterol. 2009;104:1213–20. doi: 10.1038/ajg.2009.32. [DOI] [PubMed] [Google Scholar]
- 16.Roos LL, Mustard CA, Nicol JP, et al. Registries and administrative data: organization and accuracy. Med Care. 1993;31:201–12. doi: 10.1097/00005650-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Roos LL, Jr, Nicol JP, Cageorge SM. Using administrative data for longitudinal research: comparisons with primary data collection. J Chronic Dis. 1987;40:41–9. doi: 10.1016/0021-9681(87)90095-6. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JR, Young TK, Roos LL, Gelskey DE. Estimating the burden of disease. Comparing administrative data and self-reports. Med Care. 1997;35:932–47. doi: 10.1097/00005650-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Semenciw R, Kliewer E, Shi Y, Mao Y. Incidence of second primary breast cancer among women with a first primary in Manitoba, Canada. Breast Cancer Res Treat. 2001;67:35–40. doi: 10.1023/A:1010665603732. [DOI] [PubMed] [Google Scholar]
- 20.Adjuvant systemic therapy for women with node-positive breast cancer. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. CMAJ. 1998;158(suppl 3):S52–64. [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Antman K, Amato D, Wood W, et al. Selection bias in clinical trials. J Clin Oncol. 1985;3:1142–7. doi: 10.1200/JCO.1985.3.8.1142. [DOI] [PubMed] [Google Scholar]
- 23.Rahman ZU, Frye DK, Buzdar AU, et al. Impact of selection process on response rate and long-term survival of potential high-dose chemotherapy candidates treated with standard-dose doxorubicin-containing chemotherapy in patients with metastatic breast cancer. J Clin Oncol. 1997;15:3171–7. doi: 10.1200/JCO.1997.15.10.3171. [DOI] [PubMed] [Google Scholar]
- 24.Stiller CA. Centralised treatment, entry to trials and survival. Br J Cancer. 1994;70:352–62. doi: 10.1038/bjc.1994.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol. 2001;54:217–24. doi: 10.1016/S0895-4356(00)00305-X. [DOI] [PubMed] [Google Scholar]
- 26.Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363:263–70. doi: 10.1016/S0140-6736(03)15383-4. [DOI] [PubMed] [Google Scholar]
- 27.Vist GE, Hagen KB, Devereaux PJ, Bryant D, Kristoffersen DT, Oxman AD. Systematic review to determine whether participation in a trial influences outcome. BMJ. 2005;330:1175. doi: 10.1136/bmj.330.7501.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 29.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 30.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from nsabp B-14 and nsabp B-20. J Clin Oncol. 2010;28:1677–83. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowsett M, Goldhirsch A, Hayes DF, Senn HJ, Wood W, Viale G. International Web-based consultation on priorities for translational breast cancer research. Breast Cancer Res. 2007;9:R81. doi: 10.1186/bcr1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bria E, Nistico C, Cuppone F, et al. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer. 2006;106:2337–44. doi: 10.1002/cncr.21886. [DOI] [PubMed] [Google Scholar]
- 33.De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson T, Wilcken N, Vagg R, Ghersi D, Nowak AK. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev. 2007;(4):CD004421. doi: 10.1002/14651858.CD004421.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Bedard PL, Di Leo A, Piccart–Gebhart MJ. Taxanes: optimizing adjuvant chemotherapy for early-stage breast cancer. Nat Rev Clin Oncol. 2010;7:22–36. doi: 10.1038/nrclinonc.2009.186. [DOI] [PubMed] [Google Scholar]
- 36.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 37.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 38.Popescu NC, King CR, Kraus MH. Localization of the human ERBB-2 gene on normal and rearranged chromosomes 17 to bands q12–21.32. Genomics. 1989;4:362–6. doi: 10.1016/0888-7543(89)90343-1. [DOI] [PubMed] [Google Scholar]
- 39.Schechter AL, Hung MC, Vaidyanathan L, et al. The neu gene: an ERBB-homologous gene distinct from and unlinked to the gene encoding the egf receptor. Science. 1985;229:976–8. doi: 10.1126/science.2992090. [DOI] [PubMed] [Google Scholar]
- 40.Slamon DJ, Clark GM. Amplification of c-ErbB-2 and aggressive human breast tumors? Science. 1988;240:1795–8. doi: 10.1126/science.3289120. [DOI] [PubMed] [Google Scholar]
- 41.Huober J, Fasching PA, Barsoum M, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with her2-positive, hormone-receptor-positive metastatic breast cancer—results of the electra trial. Breast. 2012;21:27–33. doi: 10.1016/j.breast.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Drucker A, Skedgel C, Virik K, Rayson D, Sellon M, Younis T. The cost burden of trastuzumab and bevacizumab therapy for solid tumours in Canada. Curr Oncol. 2008;15:136–42. doi: 10.3747/co.v15i3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hind D, Pilgrim H, Ward S. Questions about adjuvant trastuzumab still remain. Lancet. 2007;369:3–5. doi: 10.1016/S0140-6736(07)60004-X. [DOI] [PubMed] [Google Scholar]
- 44.Untch M, Gelber RD, Jackisch C, et al. Estimating the magnitude of trastuzumab effects within patient subgroups in the hera trial. Ann Oncol. 2008;19:1090–6. doi: 10.1093/annonc/mdn005. [DOI] [PubMed] [Google Scholar]
- 45.Clarke M, Collins R, Darby S, et al. on behalf of the Early Breast Cancer Trialists’ Collaborative Group Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 46.Cree M, Tonita J, Turner D, et al. Comparison of treatment received versus long-standing guidelines for stage iii colon and stage ii/iii rectal cancer patients diagnosed in Alberta, Saskatchewan, and Manitoba in 2004. Clin Colorectal Cancer. 2009;8:141–5. doi: 10.3816/CCC.2009.n.023. [DOI] [PubMed] [Google Scholar]
- 47.Baunemann Ott CL, Ratna N, Prayag R, Nugent Z, Badiani K, Navaratnam S. Survival and treatment patterns in elderly patients with advanced non-small-cell lung cancer in Manitoba. Curr Oncol. 2011;18:e238–42. doi: 10.3747/co.v18i5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooke AL, Appell R, Suderman K, Fradette K, Latosinsky S. Radiation treatment waiting times for breast cancer patients in Manitoba, 2001 and 2005. Curr Oncol. 2009;16:58–64. doi: 10.3747/co.v16i5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]