Abstract
Paraneoplastic neurologic syndrome (pns) is an uncommon manifestation of cancer and may present before any symptoms of malignant disease. This syndrome occurs in fewer than 1 of every 10,000 patients diagnosed with a malignancy. Anti-neural antibodies have been associated with pns, suggesting that this condition may reflect immune mechanisms. Depending on the region of the nervous system that has been affected, pns can have a number of manifestations. Paraneoplastic limbic encephalitis (ple) stems from involvement of the limbic system and may present with seizures and changes in mood, memory, and personality. The present report describes the case of a 55-year-old man presenting with ple in the setting of small-cell lung cancer, with subsequent improvement of his neurologic symptoms. The value of rapid diagnosis and multidisciplinary management of this syndrome are discussed.
Keywords: Paraneoplastic, limbic encephalitis, small-cell lung cancer
1. INTRODUCTION
Paraneoplastic neurologic syndrome (pns) is an uncommon presentation of cancer, occurring in fewer than 1 of every 10,000 patients diagnosed with a malignancy1. It may affect one or more regions of the nervous system and can be categorized based on the resulting clinical manifestation (Table i)2. “Classical syndromes” are those that have documented associations with malignancy. They include encephalomyelitis, subacute cerebellar degeneration, opsoclonus–myoclonus, subacute sensory neuropathy, Lambert–Eaton myasthenic syndrome, and paraneoplastic limbic encephalitis (ple).
TABLE I.
Classification of paraneoplastic neurologic syndromes
| Region | Syndrome |
|---|---|
| Central nervous system | |
| Encephalomyelitisa | |
| Limbic encephalitisa | |
| Brainstem encephalitis | |
| Subacute cerebellar degenerationa | |
| Opsoclonus–myoclonusa | |
| Optic neuritis | |
| Stiff-person syndrome | |
| Necrotizing myelopathy | |
| Motor neuron diseases | |
| Peripheral nervous system | |
| Subacute sensory neuronopathya | |
| Subacute or chronic sensorimotor neuropathies | |
| Neuropathy with vasculitis | |
| Chronic gastrointestinal pseudo-obstruction | |
| Neuromuscular junction and muscle | |
| Myasthenia gravis | |
| Lambert–Eaton myasthenic syndromea | |
| Acquired neuromyotonia | |
| Dermatomyositis | |
| Acute necrotizing myopathy | |
Classical syndromes.
Such syndromes are thought to be a result of immune mechanisms unrelated to the tumour, metastases, or metabolites. The presence of anti-neural antibodies in patients with pns has led to the suggestion that the associated neurologic symptoms are a result of antibody-induced inflammatory reactions3.
Because of the infrequent incidence of ple, there is a paucity of literature discussing its diagnosis and management. Here, we describe a case of ple in a male patient, and we discuss the syndrome’s presentation; the steps involved in diagnosis; the management options available for patients with pns, and ple in particular; and the value of diagnostic efficiency in patients with ple.
2. CASE DESCRIPTION
A 55-year-old previously well man presented to a neurologist in August 2004 with recurring headaches, decreased memory, and visual changes. On examination, he was found to have bilateral papilledema, distal paresthesias of the upper and lower extremities, and difficulties with balance. His social history was significant for a 35 pack–year smoking habit and significant alcohol intake. He had been working as a truck driver until onset of the symptoms, and he was married with two teenage children.
He underwent thorough neurologic assessment consisting of magnetic resonance imaging (mri) and magnetic resonance angiography and venography of the brain, all of which were reported to be negative. A lumbar puncture showed elevated protein (1.27 g) in the cerebrospinal fluid. Cytology was negative. At that time, computed tomography (ct) imaging of the thorax and abdomen were also performed to assess for malignancy, and no notable abnormalities were found.
This individual’s symptoms fluctuated until December 2004, at which time they progressed to include worsening headaches, ascending paresthesias, ataxia, and lower limb pain and hypersensitivity. Subsequent electromyography testing suggested the presence of axonal poly radiculoneuropathy. He was determined to have chronic inflammatory polyneuropathy and was given a dose of intravenous immunoglobulins (ivig), narcotic analgesics, and gabapentin, resulting in some symptomatic relief.
On March 28, 2005, this man presented to the emergency department with worsening memory, ataxia, and significant changes in mood. This symptomatic progression raised the suspicion of ple. Anti-neural antibody testing was positive for anti-Hu antibodies.
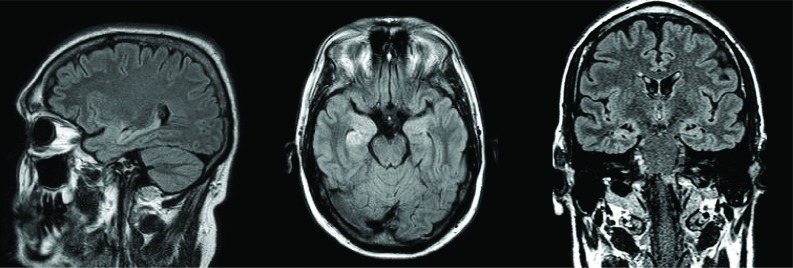
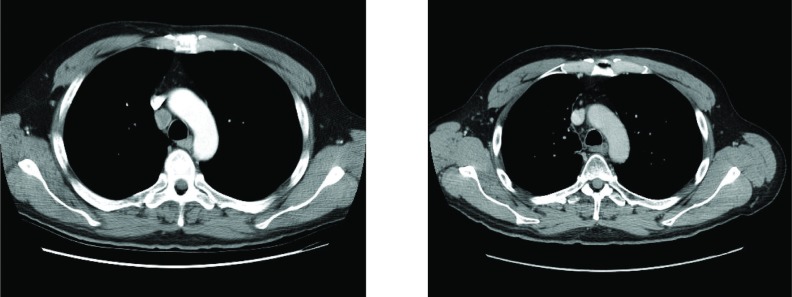
Subsequent brain mri revealed a focus of increased signal in the region of the right insular ribbon, suspicious for ischemia rather than demyelination, with no involvement of the limbic system (Figure 1). Imaging of the thorax by ct revealed the presence of a 2.5-cm paratracheal lymph node with no other signs of disease (Figure 2).
Figure 1.
Magnetic resonance imaging of brain, revealing a focus of increased signal in the region of the right insular ribbon, suspicious for ischemia rather than demyelination, with no involvement of the limbic system. (Images courtesy of Dr. Frank Goldberg, St. Michael’s Hospital, Toronto, ON.)
Figure 2.
(Left panel) Computed tomography imaging of the thorax before treatment shows a 2.5-cm paratracheal right-sided lymph node with no other signs of disease. (Right panel) Computed tomography imaging after completion of concurrent chemoradiation shows resolution of the paratracheal lymph node. (Image courtesy of Dr. Frank Goldberg, St. Michael’s Hospital, Toronto, ON.)
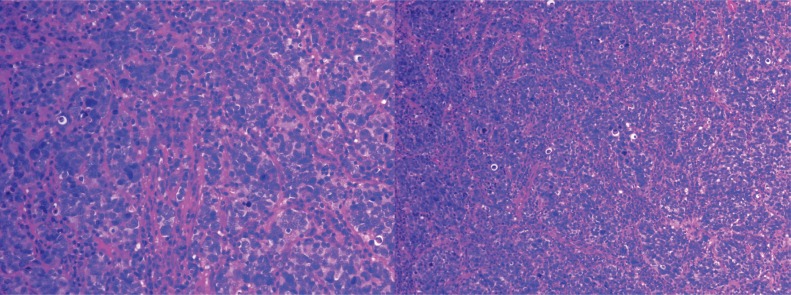
The patient was given a second course of ivig on April 6, 2005, with some improvement in his neurologic symptoms. Biopsy of the mass was performed April 15, 2005. Pathology reports confirmed the presence of anaplastic small-cell carcinoma of intermediate cell size (Figure 3), staged as limiteddisease small-cell lung cancer (sclc).
Figure 3.
Biopsy shows an anaplastic carcinoma characterized by small-to-intermediate–sized cells, often with a fusiform architecture that shows nuclear molding and a hyperchromatic nucleus with no cytoplasm. A very high mitotic rate and patchy nuclear smudging are evident. Immunostaining for the epithelial markers AE1–AE3 and CAM 5.2 shows patchy weak membrane staining; lymphoid markers L26, CD3, and CD5 are negative. Strong cytoplasmic staining with the neuroendocrine marker neuron-specific enolase and weaker staining with synaptophysin are present. Chromogranin staining is negative. The histology and immunoprofile is entirely consistent with anaplastic small-cell carcinoma of intermediate cell size. (Image courtesy of Dr. Geoffrey Gardiner, St. Joseph’s Hospital, Toronto, ON.)
After confirmation of the diagnosis, a multidisciplinary care plan was created. Because of the patient’s poor functional status, it was decided that he undergo a trial of chemotherapy and, depending on response, a subsequent course of concurrent chemoradiation for treatment of the sclc. The patients tolerated the trial well and underwent the first of six 3-week chemotherapy cycles [intravenous cisplatin 80 mg/m2 on day 1 and intravenous etoposide (VP-16) 100 mg/m2 on days 1, 2, and 3] starting April 25, 2005.
Before initiation of the chemotherapy, the patient was found to be alert and oriented to person, place, and time, with insight into his deficit. He had normal reflexes, strength, and sensation. However, he had severe dysarthria and nystagmus and was confined to bed because of his ataxia. His weight at the time of initiation was 82.8 kg. After initiation of chemotherapy, he was found to be tolerating the treatment well, with some immediate improvement of his speech and ataxia. He also began to ambulate with support.
He was discharged to a rehabilitation facility on May 4, 2010. He underwent 15 fractions of externalbeam radiation to the mediastinum to a total of 40 Gy from June 15 to July 6, 2005, concurrent with cycle 3 of his chemotherapy. Prophylactic cranial irradiation was recommended per institutional protocol. The benefits and risks were discussed in detail with the patient and his family. Because of the possible cognitive effects of the prophylactic cranial irradiation, the patient declined that therapy. Complete response of the paratracheal node was shown on ct imaging preformed on July 7, 2005.
The patient completed his last cycle of chemotherapy on September 8, 2005. He then began rehabilitation therapy consisting of physical and memory exercises and a course of Aricept (Eisai, Woodcliff Lake, NJ, U.S.A.). His ataxia improved, enabling him to walk with a cane indoors and a walker outdoors. His peripheral neuropathy improved significantly, affecting only his distal lower limbs. He continues to have slight nystagmus bilaterally; the remainder of his sensory and motor exam is unremarkable.
The patient’s mood has also shown improvement. He has not returned to work, but he regularly attends physiotherapy and rehabilitation, including attendance at a memory clinic. His short-term memory remains poor, although his ability to function in his activities of daily living continues to improve with use of memory aids such as a diary and frequent reminders.
He remains alive with partial recovery of his disabilities 5 years after his initial treatment for sclc. He is closely monitored for signs of metastatic disease, with annual follow-up, including diagnostic imaging of head, neck, thorax, abdomen, and pelvis.
3. DISCUSSION
The diagnosis of malignancy in patients with pns often stems from evaluation of a neurologic complaint4. Because of the infrequent occurrence of pns, a delay of 3–4 months between the presentations of pns and the primary malignancy is not uncommon. The neurologic presentation varies depending on the site of the lesion. Inflammation of the limbic system results in ple, with 60% of limbic encephalitis cases being attributable to a neoplastic cause5.
The limbic system is the primary component of memory, learning, and higher emotion. This system consists of the hippocampus, amygdala, anterior thalamic nuclei, and the limbic cortex. Typically, ple manifests with seizures and changes in mood, memory, and personality. Because neurologic abnormalities often precede the diagnosis of malignancy, all patients presenting with neurologic abnormalities should be investigated to determine the cause of their symptoms, first ruling out nonmalignant conditions. In cases in which risk factors for malignancy are present, any patient with an unexplained neurologic manifestation should be investigated for pns.
Classical pns (such as ple) can be diagnosed on a pathologic or a clinical basis. The clinical diagnosis of ple has four mandatory components2,6:
Clinical symptoms of ple
A cancer diagnosis a maximum of 4 years after onset of the neurologic symptoms
Exclusion of other neurologic causes
- One of
- cerebrospinal fluid analysis showing the presence of inflammation with negative cytology,
- brain mri indicating a lesion in the temporal lobe, and
- epileptic activity in the temporal lobes by electroencephalography analysis
When a neurologic abnormality leads to suspicion of ple, screening for anti-neural antibodies in serum should be conducted. Anti-neural antibodies do not have 100% sensitivity or specificity. The absence of those antibodies should therefore not dissuade a clinician from further investigation for malignant disease2,3,7. Paraneoplastic neurologic syndrome has been associated primarily with anti-Hu and anti-Ma2 antibodies. Anti-Hu–associated ple is often found in patients with sclc; anti-Ma antibodies are often found in testicular and ovarian germ cell tumours6. Anti-Hu antibodies are 82% sensitive and 99% specific for pns8; they are found in almost 50% of patients with ple9. The presence of anti-Hu antibodies in sclc has been shown to be a predictor of treatment response. Patients positive for the antibodies survive longer3.
Our patient underwent a thorough neurologic examination, confirming the clinical symptoms of ple such as changes in mood, memory, and personality, plus sensory abnormalities and gait disturbance. As those symptoms progressed, suspicion of ple was raised, and the patient underwent further testing, including diagnostic imaging and anti-neural antibody screening. The mri findings included a lesion in the temporal lobe, and serum analysis confirmed the presence of anti-Hu antibodies. Those results helped to further confirm the diagnosis of ple among other forms of pns.
Diagnostic imaging may aid in the detection of the primary tumour and may also provide information on the extent of disease. In the event that a tumour is identified but is of a type different from what would be expected, continued investigation is recommended in case a second malignancy is present2. If no evidence of malignancy can be found on ct imaging, positron-emission tomography could be used to identify occult disease4.
Our patient underwent a thorough evaluation, including ct imaging of head, neck, and thorax, which were initially negative. Assessment by positron-emission tomography was not available at his institution at the time of presentation. Subsequent ct imaging then led to the diagnosis of sclc. Thus, this patient met all ple criteria, presenting with a classical clinical syndrome after the exclusion of other causes, with a serum analysis positive for anti-Hu antibodies and a subsequent diagnosis of biopsy-proven sclc.
A multifaceted approach is required for the management of these syndromes, with strong communication between specialists being paramount. These patients require treatment for both the pns and the underlying malignancy. Emphasis should be placed on early intervention, in the hope of a more favourable prognostic outcome, both for the malignant disease and the associated neurologic deficits. Management of the underlying malignancy is vital, possibly delaying activity of the antibodies, with subsequent improvement in neurologic symptoms6.
Our patient was diagnosed with limited-stage sclc and completed a treatment plan consisting of 6 cycles of VP-16 and cisplatin plus external-beam radiation to the mediastinum. His neurologic symptoms began to improve after treatment of his sclc was initiated.
Immune therapies consisting of steroids, plasma exchange, and ivig have been shown to improve the neurologic symptoms in particular types of pns. A distinct benefit of ivig in patients with ple has not yet been shown in the literature4; however, our patient received two courses of ivig, resulting in some improvement of his symptoms. But in the case of our patient, although he had not yet been diagnosed with ple, he received a course of ivig within a few months of the onset of symptoms because of his original diagnosis of chronic inflammatory polyneuropathy. He responded well to the therapy and showed some symptomatic resolution at the time. That early treatment may have contributed to an improved outcome.
All patients should also be considered for other symptomatic treatment to improve quality of life, including cognitive and physical rehabilitation. Our patient underwent rigorous rehabilitation therapy, including physical rehabilitation and attendance at a memory clinic, leading to improved functioning in his activities of daily living. Six years after his original diagnosis, he continues to improve.
4. CONCLUSIONS
Here, we described a 55-year-old man presenting with neurologic abnormalities leading to a diagnosis of ple in the setting of sclc. A delay in the diagnosis of pns can often occur because of the rarity of the condition and the ambiguous presentation. However, these syndromes commonly occur in the early stages of cancer development, and thus detection of pns may lead to early detection of the underlying malignancy and a favourable prognostic outcome. The value of early detection is illustrated in the present case, because sclc has an unfavourable prognosis, with the primary determinant of survival being stage at presentation. Limited-stage sclc has a 5-year survival rate of 10%–13%, compared with a late-stage 5-year survival rate of 1%–2%10. Our patient was diagnosed at an early stage, possibly contributing to an improved outcome. The value of multimodality therapy is also evident here, because the patient has shown continuous symptomatic improvement throughout the management of his primary tumour and neurologic symptoms. A high index of suspicion and a cogent approach to diagnosis and management are required to improve patient outcomes.
5. ACKNOWLEDGMENTS
We express our sincere appreciation to our patient and his family.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis. 2007;2:22. doi: 10.1186/1750-1172-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–40. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graus F, Dalmou J, Reñé R, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 4.Vedeler CA, Antoine JC, Giometto B, et al. on behalf of Paraneoplastic Neurological Syndrome Euronetwork Management of paraneoplastic neurological syndromes: report of an efns Task Force. Eur J Neurol. 2006;13:682–90. doi: 10.1111/j.1468-1331.2006.01266.x. [DOI] [PubMed] [Google Scholar]
- 5.Tüzün E, Dalmau J. Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–71. doi: 10.1097/NRL.0b013e31813e34a5. [DOI] [PubMed] [Google Scholar]
- 6.Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–94. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 7.Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu associated paraneoplastic encephalomyelitis/sensory neuronopathy: a clinical study of 71 patients. Medicine (Baltimore) 1992;71:59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Senties–Madrid H, Vega–Boada F. Paraneoplastic syndromes associated with anti-Hu antibodies. Isr Med Assoc J. 2001;3:94–103. [PubMed] [Google Scholar]
- 9.Alamowitch S, Graus F, Uchuya M, Reñé R, Bescansa E, Delattre JY. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120:923–8. doi: 10.1093/brain/120.6.923. [DOI] [PubMed] [Google Scholar]
- 10.Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P, on behalf of the International Staging Committee and Participating Institutions The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th edition of the TNM Classification of Malignant Tumors and the proposals for the 7th edition. J Thorac Oncol. 2008;3:457–66. doi: 10.1097/JTO.0b013e31816de2b8. [DOI] [PubMed] [Google Scholar]