Abstract
Primary adenocarcinoma of the oropharynx most often arises from the minor salivary glands, and primary squamous cell carcinoma is more commonly seen arising from the tongue. Few cases of adenocarcinoma not otherwise specified of the tongue have been reported in the literature, and none found on the dorsum of the tongue. Successful treatment strategies have therefore not been defined.
We report a case of adenocarcinoma located on the dorsum of the posterior one third of the tongue adjacent to the circumvallate papillae in a woman presenting with globus sensation and mild dysphagia. Treatment consisted of transoral laser excision and postoperative external-beam radiotherapy, resulting in disease-free survival at her 5-year follow-up. The goals of this report are to present a case of adenocarcinoma arising from the minor salivary gland located on the dorsum of the tongue, to discuss previous reports of similar cases, and to suggest that surgery with or without radiotherapy be used as the mainstay of treatment.
Keywords: Adenocarcinoma, base of tongue, radiation therapy, minor salivary gland
1. CASE DESCRIPTION
In December 2005, a 66-year-old woman presented to the otolaryngology clinic at our institution with mild dysphagia and a globus sensation, with no constitutional symptoms, odynophagia, or referred otalgia. The patient was a lifetime nonsmoker with nonsignificant consumption of alcohol. A family history of ovarian cancer (sister), breast cancer (sister), and skin cancer (brother) was reported, but no history of head-and-neck cancer.
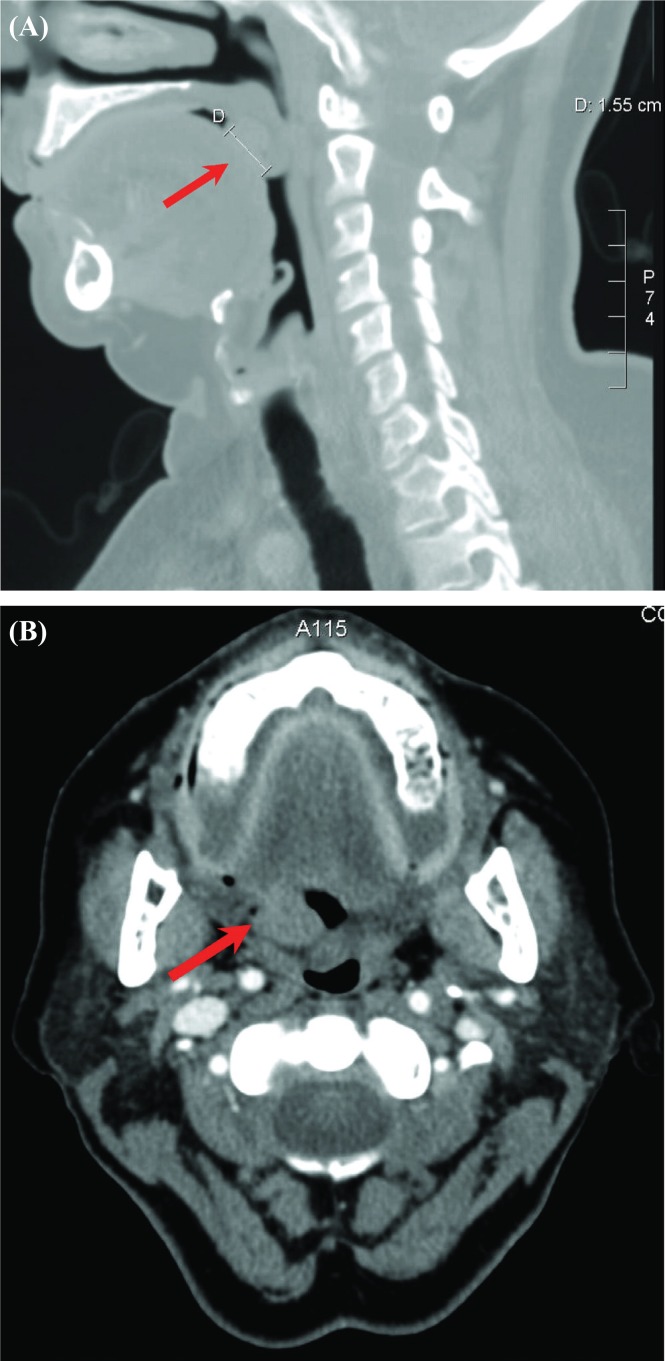
On physical exam, a firm 2×1-cm pedunculated exophytic mass on the dorsum of the tongue was observed, with no ulceration or evidence of local invasion. No cervical lymphadenopathy was noted. Imaging by computed tomography with intravenous contrast was performed to evaluate the primary lesion and the regional lymphatics. This imaging demonstrated a 1.6×1.3×1.5-cm superficial nodular lesion on the dorsum of the posterior aspect of the tongue [Figure 1(A)], with no major compromise of the airway. No involvement of the deep structures of the tongue [Figure 1(B)], nor any involvement of regional lymph nodes, epiglottis, or vallecula was noted. Laser excision of the mass was performed under general anesthetic with tracheostomy in January 2006.
FIGURE 1.
Computed tomography imaging performed at the time of diagnosis. Red arrows indicate the location of the lesion. (A) Sagittal view. Superficial nodular lesion visible at base of tongue extending to the adjacent uvula. D = measured diameter of lesion (1.55 cm). (B) Axial view. Subtly enhancing lesion at right base of tongue, extending to the anterior aspect of the uvula, with no apparent invasion into the deep structures of the tongue. No significant lymphadenopathy noted.
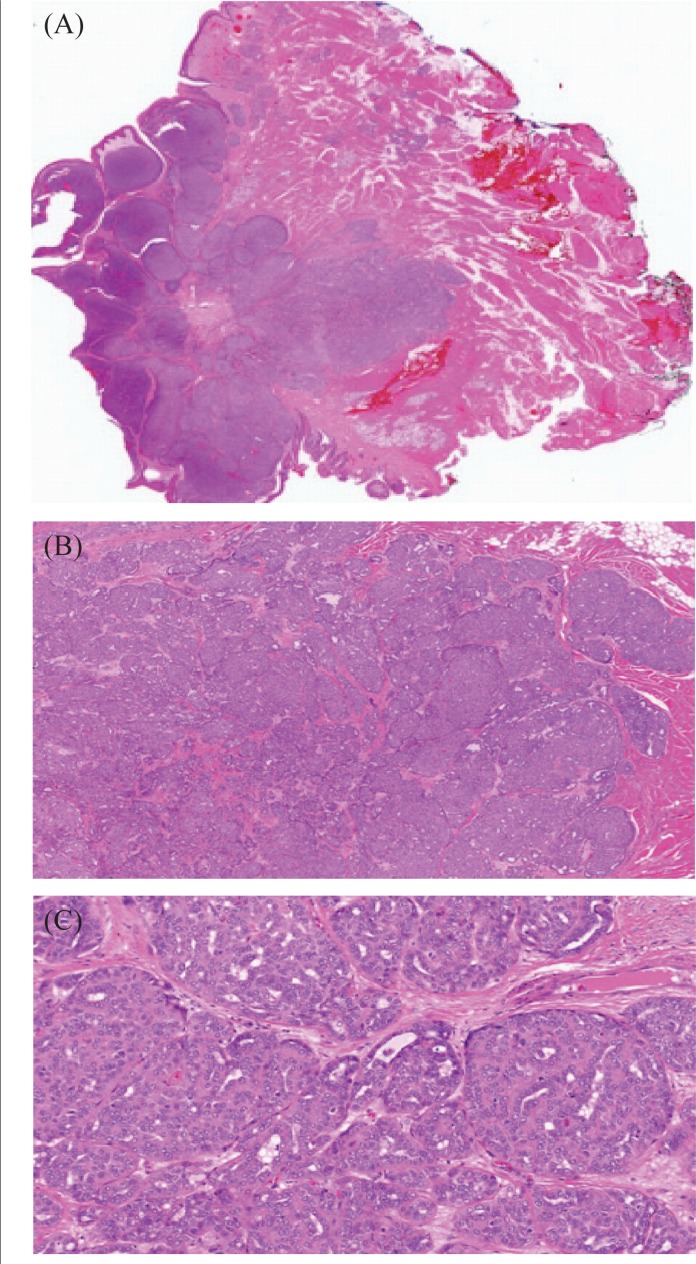
Pathology examination of the tumour revealed a 1.7×1.5×1.4-cm exophytic lobular submucosal nodular lesion with a pushing border and “basaloid-like” architecture [Figure 2(A)]. The neoplastic cells were present in cribriform nests and sheets with focal tubule formation [Figure 2(B)]. The neoplastic cells were cuboidal in shape, with large nuclei having small nucleoli and a clear chromatin pattern [Figure 2(C)]. Squamous differentiation and features of an adenoid cystic carcinoma were not identified. The malignant cells were positive for cytokeratin 7 and S-100. Staining was negative for cytokeratins (34bE12, 8/18, and 20), smooth muscle actin, and smooth muscle myosin. The mass was staged as T1N0 adenocarcinoma not otherwise specified (nos), located on the tongue base and of minor salivary gland origin. The initial resected area showed negative margins (7 mm anterior, 5 mm from the left, 8 mm posterior, 1 mm from the right, and 3 mm deep). Additional resection was performed at the left and right margins, which were negative for malignancy.
FIGURE 2.
Histology sections of adenocarcinoma not otherwise specified of minor salivary gland origin. (A) Low-power photomicrograph of the lesion, demonstrating its well-circumscribed nature in the submucosa. (B) Medium-power photomicrograph demonstrating arrangement of the neoplastic cells in cribriform nests, sheets, and focal tubules. (C) High-power photomicrograph of the neoplasm, demonstrating its cytologic features.
At 1 month post excision, no evidence of local recurrence or lymphadenopathy was obvious, but a firm area (induration) on the posterior tongue base lacking exophytic or ulcerative lesions was noted. Magnetic resonance imaging of the head and neck confirmed this area of asymmetry and irregularity involving the dorsum of the tongue in the midline and slightly to the right. Endoscopy and biopsy of the surgical bed was performed to rule out malignancy. Pathology examination revealed lingual tissue with fibro-inflammatory changes, negative for malignancy.
Postoperative radiotherapy was initiated because postoperative assessment revealed induration of the surgical bed and the abnormality already documented on magnetic resonance imaging, which led to a high index of suspicion for residual disease at the primary site. Moreover, the primary tumour was located at the center of the base of tongue, which contains profuse lymphatics. Postoperative radiotherapy was delivered to the oropharynx, with a boost to the surgical bed; comprehensive prophylactic radiotherapy to the regional lymphatics was also offered.
The patient was treated to a total dose of 60 Gy in 30 fractions, using a standard single isocentric 3-field technique. The isocentre was placed 3 cm deep at the junction of the lateral parallel-opposed fields and the single anterior lower neck field. The lower neck field received 50 Gy in 25 fractions, and the surgical bed received a boost of 10 Gy in 5 fractions. A spinal cord block was placed after 40 Gy to limit the cord dose to less than 45 Gy. Afterwards, the posterior neck lymphatics were treated with a 6 MeV electron boost of 10 Gy in 5 fractions.
At 6 years, the patient has remained asymptomatic with no clinical or radiologic evidence of locoregional recurrence or metastasis.
2. DISCUSSION
2.1. Oropharyngeal Cancer—Tongue and Minor Salivary Gland Tumours
For men and women respectively, head-and-neck cancers represent 2.5% and 1.5% of all newly diagnosed cancers in Canada1. Cancer of the tongue is the second most common type of cancer arising in the upper digestive tract, with approximately 40% arising from the tongue base2. In addition, lesions located in the base of tongue are more likely to be malignant (85%) than those located in the oral tongue (20%)3. Most tongue cancers (more than 95%) are histologically characterized as squamous cell carcinoma; adenocarcinoma of the tongue, often arising from minor salivary glands, occurs in fewer than 2% of all tongue malignancies2.
Minor salivary gland malignancies are themselves rare, representing only 5%–10% of all malignancies in the head-and-neck region. Many histologic subtypes of minor salivary gland tumours in the oropharynx have been documented, mucoepidermoid and adenoid cystic carcinoma being the most common, followed by the more rare subtypes such as adenocarcinoma and acinic cell carcinoma3,4. Adenocarcinoma has been estimated to make up 20% of all lingual minor salivary gland tumours5,6.
A number of risk factors for the development of upper aerodigestive tract cancers have been identified, including positive smoking history, alcohol abuse, body mass index greater than 30 kg/m2 or less than 18.4 kg/m2, and lack of oral health practices9. Several risk factors have also been identified with respect to the development of oropharyngeal cancer, including smoking and alcohol consumption10 and a diet deficient in fruits and vegetables11. However, infection with the human papilloma virus has recently emerged as a major contributor to the development of squamous cell oropharyngeal cancer, including base-of-tongue cancers12, particularly in younger populations13. The same association has not been made with adenocarcinoma of the oropharynx.
Presentation of oropharyngeal adenocarcinoma varies and includes symptoms such as swelling, mass sensation, pain, dysphagia, or odynophagia; however, it is often completely asymptomatic, resulting in a delay in diagnosis6,14. The prognosis for adenocarcinoma is more favourable than that for squamous cell carcinoma15,16, and reported 3-, 5-, and 10-year survival rates are approximately 50%6,17,18. However, survival varies greatly depending on tumour stage, grade, and treatment modalities used.
2.2. Literature Review and Recommended Treatment Paradigm
We are the first to report a case of adenocarcinoma nos arising from the minor salivary glands located on the dorsum of the tongue, near the foramen cecum, in which none of the risk factors mentioned earlier was identified.
Approximately 20 cases of adenocarcinoma nos located in the oropharynx have been reported to date (Table i)3,4,19, but the specific description of anatomic location of those lesions is lacking. They are often classified as either “base of tongue,” “palate,” or “tonsil”4. The lesions classified as “base of tongue” are most often located near the epiglottis and posterior pharynx3,19–21. Pertinent details of the location, size, and histopathologic features of the tumour, as well as relevant facts about the patient, staging, and treatment given, are missing in many published case reports, precluding a comprehensive review or comparison of the cases. Combined with the low incidence of adenocarcinoma of the tongue14, this paucity of comparable data has thus far prevented formulation of a standard treatment paradigm for this entity.
TABLE I.
Summary of the literature: adenocarcinoma of the posterior and base of tongue, minor salivary gland origin
| Reference | Cases (n) | Tumour classification | Site | Age (years) | Sex | Stage/grade | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Burbank et al., 19595 | 9 | |||||||
| Elliott and Pearl, 198120 | 1 | Adenocarcinoma | Base of tongue, extending to the vallecula | 68 | Female | T2N0 | Surgery | Unknown |
| Goepfert et al., 198318 | 4 | Adenocarcinoma | Base of tongue | 15 | Female | T3N2b | Radiation and surgery | ned at 3 years |
| 67 | Female | T4N0 | Radiation and surgery | ned at 3 years | ||||
| 72 | Male | T4N2b | Surgery | Postoperative death; distant metastasis lung | ||||
| 55 | Male | T3N2b | Surgery | Died of distant metastases at 33 months | ||||
| Ballard et al., 198621 | 1 | Adenocarcinoma | Posterior left side, extending to mandible, retromolar trigone and floor of mouth to vallecula | 72 | Male | T3N0 | Radiation | ned at 2 years |
| de Vries et al., 198719 | 1 | Adenocarcinoma nos |
Base of tongue | 50 | Female | T4N3 | Radiation, unresectable tumour | Died of disease at 8 months |
| Goldblatt and Ellis, 19873 | 10 (6 with follow-up data) | Adenocarcinoma nos |
nr | nr | Both | Variable | Surgery | ned at 5 years |
| Surgery | ned at 14 years | |||||||
| Not specified | ned at 15 years | |||||||
| Radiation and surgery | ned at 19 years | |||||||
| Radiation | ned at 1.5 years | |||||||
| Not specified | Died of disease at 6 years | |||||||
| Iyer et al., 20104 | 9 | Adenocarcinoma nos |
nr | nr | Both | T1/2N0 (n=3) | Chemotherapy, with or without radiation, with or without surgery | 10-Year survival: 63% |
| T1/2N+ (n=3) | ||||||||
| T3/4N0 (n=1) | ||||||||
| T3/4N+ (n=2) | ||||||||
| Present report | 1 | Adenocarcinoma nos |
Base of tongue, near circumvallate papillae | 66 | Female | T1N0 | Radiation and surgery | ned at 5 years |
ned = no evidence of disease; nos = not otherwise specified; nr = not reported.
The mainstay of treatment of oropharyngeal adenocarcinoma of minor salivary gland origin includes surgical excision with or without adjuvant radiotherapy22. Complex reconstruction is often required after radical excision because of the location of the lesions4. The use of postoperative radiotherapy for the treatment of these neoplasms varies across centres and depends on surgical margins, extent of nodal involvement, presence or absence of extranodal extension, locoregional invasion, and tumour grade3,4. In a number of studies, albeit with very low patient numbers, patients treated with combined surgery and radiotherapy had a lower incidence of local recurrence6,18,23 and also better long-term survival than did those treated with surgery alone6,18.
In addition, the use of chemotherapy (cyclophosphamide, doxorubicin, and cisplatin) has been reported in a limited number of cases of advanced disease. One study reported an average response rate of 64%, with 28% achieving complete remission24. Another report suggests the use of uft [a mixture of 1-(2-tetrahydrofuryl)-5-fluorouracil and uracil in a molar ratio of 1:4], the authors having achieved a 50% response rate in tumours of the oropharynx and a 75% response rate in tumours classified as adenocarcinoma25; however, the latter neoplasms were not exclusively of minor salivary gland origin.
Although discussions of tumour grade have proved useful for certain histologic subtypes, including adenocarcinoma nos, mucoepidermoid carcinoma, and adenoid cystic carcinoma26, it has been suggested that stage and not grade of salivary gland tumours be considered the major factor, with a 4-cm “rule” suggested as a guideline for directing treatment decisions26. The rule suggests that tumours less than 4 cm in size have a better prognosis independent of the histologic subtype or grade of the tumour, with less locoregional or distant metastasis27. Given that one of the main indications for postoperative radiotherapy is locoregional invasion, the authors propose that tumours larger than 4 cm in size be considered an absolute indication for postoperative radiotherapy, independent of other factors26,27.
Overall, based on our experience and the limited data available in the literature to date, we suggest that the standard of care remain surgical excision (if possible), with or without additional radiation therapy based on presence or absence of the adverse factors discussed earlier that increase the probability of locoregional recurrence.
3. CONCLUSIONS
In this report, we document the successful treatment of adenocarcinoma nos located on the dorsal aspect of the posterior one third of the tongue near the circumvallate papillae. Trans-oral excision was followed by external-beam radiotherapy. Based on clinical and radiologic surveillance, the patient remains disease-free with minimal toxicity after 6 years of follow-up.
4. ACKNOWLEDGMENTS
Verbal consent for this report was obtained from the patient.
BV obtained relevant details from the patient’s chart, performed the literature search, and was the primary author of the manuscript. AL and VV aided by collecting additional patient information and contributing to preparation of the manuscript. JF performed the surgical excision and aided in preparation of the manuscript. KK was the pathologist on the case and provided images and histopathologic details for the manuscript. VMV was the radiation oncologist on the case and aided with the literature search and preparation of the manuscript.
5. CONFLICT OF INTEREST DISCLOSURES
The authors have no competing or conflicts of interest to declare.
6. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee . Canadian Cancer Statistics 2010. Toronto, ON: Canadian Cancer Society; 2010. [Google Scholar]
- 2.Muir C, Weiland L. Upper aerodigestive tract cancers. Cancer. 1995;75(suppl 1):147–53. doi: 10.1002/1097-0142(19950101)75:1+<147::AID-CNCR2820751304>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Goldblatt LI, Ellis GL. Salivary gland tumors of the tongue. Analysis of 55 new cases and review of the literature. Cancer. 1987;60:74–81. doi: 10.1002/1097-0142(19870701)60:1<74::AID-CNCR2820600113>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Iyer NG, Kim L, Nixon IJ, et al. Factors predicting outcome in malignant minor salivary gland tumors of the oropharynx. Arch Otolaryngol Head Neck Surg. 2010;136:1240–7. doi: 10.1001/archoto.2010.213. [DOI] [PubMed] [Google Scholar]
- 5.Burbank PM, Dockerty MB, Devine KD. A clinicopathologic study of 43 cases of glandular tumors of the tongue. Surg Gynecol Obstet. 1959;109:573–82. [PubMed] [Google Scholar]
- 6.Roper PR, Wolf PF, Luna MA, Goepfert H. Malignant salivary gland tumors of the base of the tongue. South Med J. 1987;80:605–8. doi: 10.1097/00007611-198705000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Bennett BR, Lamba MA, Elson HR. Analysis of peripheral doses for base of tongue treatment by linear accelerator and helical TomoTherapy imrt. J Appl Clin Med Phys. 2010;11:3136. doi: 10.1120/jacmp.v11i3.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalilur R, Hayashi K, Shibuya H. Brachytherapy for tongue cancer in the very elderly is an alternative to external beam radiation. Br J Radiol. 2011;84:747–9. doi: 10.1259/bjr/23130739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macfarlane TV, Macfarlane GJ, Oliver RJ, et al. The aetiology of upper aerodigestive tract cancers among young adults in Europe: the arcage study. Cancer Causes Control. 2010;21:2213–21. doi: 10.1007/s10552-010-9641-3. [DOI] [PubMed] [Google Scholar]
- 10.Evans M, Powell NG. The changing aetiology of head and neck cancer: the role of human papillomavirus. Clin Oncol (R Coll Radiol) 2010;22:538–46. doi: 10.1016/j.clon.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Warnakulasuriya S. Causes of oral cancer—an appraisal of controversies. Br Dent J. 2009;207:471–5. doi: 10.1038/sj.bdj.2009.1009. [DOI] [PubMed] [Google Scholar]
- 12.Attner P, Du J, Näsman A, et al. Human papillomavirus and survival in patients with base of tongue cancer. Int J Cancer. 2011;128:2892–7. doi: 10.1002/ijc.25625. [DOI] [PubMed] [Google Scholar]
- 13.Rischin D. Oropharyngeal cancer, human papilloma virus, and clinical trials. J Clin Oncol. 2010;28:1–3. doi: 10.1200/JCO.2009.24.9045. [DOI] [PubMed] [Google Scholar]
- 14.Clark SK, Yarington CT., Jr Lingual malignant disease of minor salivary gland origin. Am J Otolaryngol. 1980;1:181–5. doi: 10.1016/S0196-0709(80)80013-5. [DOI] [PubMed] [Google Scholar]
- 15.Biller HF, Lawson W, Baek SM. Total glossectomy. A technique of reconstruction eliminating laryngectomy. Arch Otolaryngol. 1983;109:69–73. doi: 10.1001/archotol.1983.00800160003001. [DOI] [PubMed] [Google Scholar]
- 16.Dupont JB, Guillamondegui OM, Jesse RH. Surgical treatment of advanced carcinomas of the base of the tongue. Am J Surg. 1978;136:501–3. doi: 10.1016/0002-9610(78)90269-6. [DOI] [PubMed] [Google Scholar]
- 17.Frazell EL. Observations on the management of salivary gland tumors. CA Cancer J Clin. 1968;18:235–40. doi: 10.3322/canjclin.18.4.235. [DOI] [PubMed] [Google Scholar]
- 18.Goepfert H, Luna MA, Lindberg RD, White AK. Malignant salivary gland tumors of the paranasal sinuses and nasal cavity. Arch Otolaryngol. 1983;109:662–8. doi: 10.1001/archotol.1983.00800240028005. [DOI] [PubMed] [Google Scholar]
- 19.de Vries EJ, Johnson JT, Myers EN, Barnes EL, Jr, Mandell–Brown M. Base of tongue salivary gland tumors. Head Neck Surg. 1987;9:329–31. doi: 10.1002/hed.2890090604. [DOI] [PubMed] [Google Scholar]
- 20.Elliott E, Pearl RM. Adenocarcinoma of the tongue—a dialogue case report. Ann Plast Surg. 1981;7:233–5. [PubMed] [Google Scholar]
- 21.Ballard JL, Kerner E, Tyson J, Ashford J, Rees R. Adenocarcinoma of the tongue complicated by a hemimandibulectomy: soft tissue support for a tongue prosthesis in an edentulous glossectomy patient. J Prosthet Dent. 1986;56:470–3. doi: 10.1016/0022-3913(86)90391-4. [DOI] [PubMed] [Google Scholar]
- 22.Fu KK, Leibel SA, Levine ML, Friedlander LM, Boles R, Phillips TL. Carcinoma of the major and minor salivary glands: analysis of treatment results and sites and causes of failures. Cancer. 1977;40:2882–90. doi: 10.1002/1097-0142(197712)40:6<2882::AID-CNCR2820400618>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Kessler DJ, Mickel RA, Calcaterra TC. Malignant salivary gland tumors of the base of the tongue. Arch Otolaryngol. 1985;111:664–6. doi: 10.1001/archotol.1985.00800120058006. [DOI] [PubMed] [Google Scholar]
- 24.Dreyfuss AI, Clark JR, Fallon BG, Posner MR, Norris CM, Jr, Miller D. Cyclophosphamide, doxorubicin, and cisplatin combination chemotherapy for advanced carcinomas of salivary gland origin. Cancer. 1987;60:2869–72. doi: 10.1002/1097-0142(19871215)60:12<2869::AID-CNCR2820601203>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka J, Inuyama Y, Fujii M, Takaoka T, Hosoda H, Saito S. Clinical trials on uft in the treatment of head and neck cancer. Auris Nasus Larynx. 1985;12(suppl 2):S261–6. doi: 10.1016/s0385-8146(85)80070-5. [DOI] [PubMed] [Google Scholar]
- 26.Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8:229–40. doi: 10.1034/j.1601-0825.2002.02870.x. [DOI] [PubMed] [Google Scholar]
- 27.McGurk M, Renehan AG, editors. Controversies in the Management of Salivary Gland Disease. New York, NY: Oxford University Press; 2001. [Google Scholar]