Abstract
In immune-mediated diseases, Treg and proinflammatory Th17 cells have been suggested to play either suppressor (beneficial) or effector (detrimental) roles, respectively. Tissue damage in viral infections can be caused by direct viral replication or immunopathology. Viral replication can be enhanced by anti-inflammatory responses and suppressed by proinflammatory responses. However, Tregs could suppress proinflammatory responses, reducing immunopathology, while Th17 cell-induced inflammation may enhance immunopathology. Here, the roles of Treg and Th17 cells depend on whether tissue damage is caused by direct virus replication or immunopathology, which differ depending on the virus, disease stage and host immune background. Although the precise mechanisms of tissue damage in multiple sclerosis and myocarditis are unclear, both viral replication and immune effector cells have been proposed to cause pathogenesis. Personalized medicine that alters the balance between Treg and Th17 cells may ameliorate viral pathology during infections.
Keywords: autoimmunity, Cardiovirus infections, CNS demyelinating disease, coinfection, experimental nervous system autoimmune disease, immunology, inflammation, Picornaviridae infections, regulatory T-lymphocyte, Th17 cells
When the immune response is triggered against a pathogen, the innate immune system directs the adaptive immune system toward the appropriate response against the pathogen to protect the host. The adaptive immune response is composed of specialized effector cells and their products that eliminate pathogens and generate a memory response to establish immunity. Major adaptive host defense effectors include CD8+ cytotoxic T lymphocytes (CTLs) and antibody-producing B cells. IFN-γ and CTLs contribute to clearance of intracellular pathogens, including viruses, while antibodies help to eradicate extracellular pathogens. However, immune responses are not always protective. Sometimes, the immune response triggered against a pathogen is detrimental to the host or insufficient and can result in either tissue damage by immune cells (immunopathology) or incomplete clearance of the pathogen (persistent infection) [1]. An inappropriate immune response can be due to the genetic background of the host or strategies developed by the pathogen to escape clearance by the host.
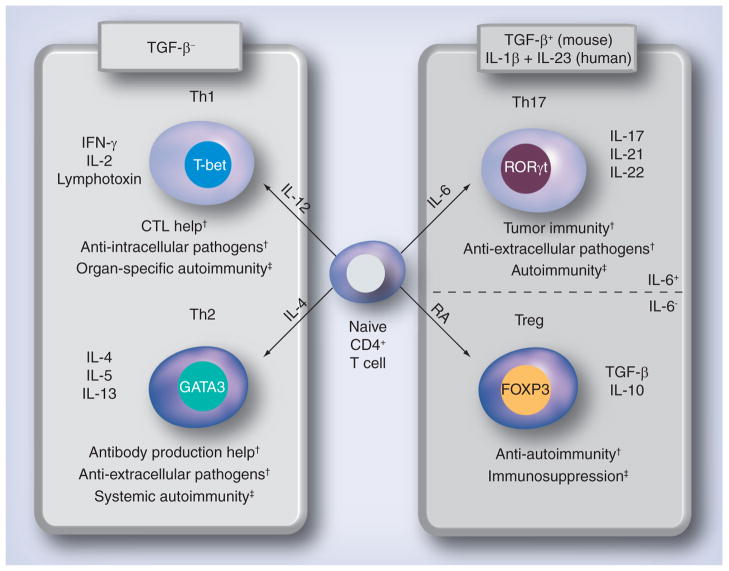
During activation and expansion, CD4+ T cells differentiate into different T-helper (Th) cell subsets that have different cytokine profiles and distinct effector functions. Until recently, CD4+ T cells were thought to diverge into either Th1 or Th2 cells, and were characterized based on their cytokine profiles (Figure 1) [2]; Th1 cells produce IL-2, IFN-γ and lymphotoxin, while Th2 cells produce IL-4, -5 and -13. In general, Th1 and Th2 cells help activation of CTLs and B cells, respectively, while all Th cells can promote production of antibody subsets. Recently, two more subsets of CD4+ T cells have been proposed: Tregs and Th17 cells. While these two subsets share a common lineage and are induced by a common factor in mice, TGF-β (in humans this is somewhat controversial [3–9]), they have quite opposite effects, with one being anti-inflammatory (Tregs) and the other being proinflammatory (Th17).
Figure 1. Differentiation of T-helper subsets.
T-cell subset differentiation is influenced by cytokines released from dendritic cells and other immune cells. Th1 cells help CTL induction and are induced in the presence of IL-12 and the transcription factor T-bet. Th1 cells are also effectors in immunity against intracellular pathogens and organ-specific autoimmunity. Th2 cells promote production of antibody, contributing to the clearance of extracellular pathogens and systemic autoimmunity, and are induced by IL-4 and the transcription factor, GATA3. Th1 and Th2 differentiation is inhibited by TGF-β. Both Treg and Th17 cells are induced by TGF-β: in addition, Tregs require RA and Th17 cells require IL-6. Tregs express the transcription factor FOXP3, and secrete the anti-inflammatory cytokines, TGF-β and IL-10, which can suppress autoimmunity and immune responses to pathogens. Th17 cells express the transcription factor, RORC2/RORγt (humans/mice), and are involved with defense against extracellular pathogens, tumor immunity and autoimmunity.
†Protective role.
‡Detrimental role.
CTL: Cytotoxic T lymphocyte; RA: Retinoic acid.
In autoimmunity, generally, organ-specific autoimmunity is mediated by Th1 and/or Th17 cells and systemic autoimmunity is mediated by autoantibodies, whose production is enhanced by Th2 cells (Table 1). Here, Tregs play a beneficial role by suppressing autoreactive Th cells, while Th17 cells play a detrimental role as effector cells. On the contrary, in cancer, proinflammatory Th1 and Th17 responses can lead to tumor clearance, while suppression of tumor immunity can lead to cancer progression. Here, Tregs play a detrimental role by suppressing antitumor immunity. Th17 cells have been shown to play a beneficial role in tumor clearance in most cases, although Th17 cells may promote angiogenesis, helping tumor growth (Table 1) [10]. In this review, we will discuss how Treg and Th17 cells can play both beneficial and detrimental roles in viral infections. Although Tregs can control antiviral inflammatory responses, preventing immunopathology, the suppression of antiviral immunity by Tregs can enhance viral replication, leading to a persistent viral infection. Th17 cells may play a defensive role in some viral infections; however, Th17 cells often cause immunopathology. The role of Treg and Th17 cells depends on whether tissue damage is caused by viral replication itself or immune cells (immunopathology), which can differ depending on the virus, disease stage and host immune background. As examples of viral-mediated immune disease, we will further discuss the roles of Treg and Th17 cells in multiple sclerosis (MS) and myocarditis.
Table 1.
CD4+ T-helper cell subsets in diseases.
| Autoimmunity | Cancer | Bacterial infection | Viral infection | |
|---|---|---|---|---|
| Treg | Prevent immunopathology† | Suppress tumor immunity‡ | Suppress host defense‡ | Can suppress immunopathology† Suppress CTL responses, promoting viral replication‡ |
| Th17 | Cause immunopathology‡ | Can promote tumor clearance† Can promote angiogenesis‡ |
Promote bacterial clearance† | May prevent secondary complications† Cause immunopathology and can inhibit CTL responses enhancing viral replication‡ |
| Th1 | Cause organ-specific autoimmunity‡ | Promote CTL killing of tumor† | Clearance of intracellular infections† | Promote CTL killing of infected cells† |
| Th2/antibody | Cause systemic autoimmunity‡ Suppress Th1 autoimmunity† |
Can be used to clear some cancers† | Clearance of extracellular infections† | Help neutralizing antibody production† |
Protective role.
Detrimental role.
CTL: Cytotoxic T lymphocyte.
Tregs
Tregs typically produce immunosuppressive cytokines such as IL-10 and TGF-β, while Tregs do not produce IL-2 and thus cannot promote their own expansion. Tregs are potent inhibitors of T-cell immune responses and express CD4, CD25 (IL-2 receptor α chain) and the transcription factor FOXP3 [11]. Two types of Tregs have been classified: natural (nTregs) and induced (iTregs). nTregs differentiate in the thymus and react to self-antigen upon recognition of self-peptide presented by MHC class II [12]. In addition to the naturally occurring population of nTregs, naive CD4+ T cells in the periphery can be induced by TGF-β to express FOXP3 and become iTregs with properties similar to nTregs (Figure 1) [13–15]. iTregs react to both self- and foreign antigens [15–17]. Both self-reactive nTregs and iTregs prevent damage from immunopathology (Table 1). After origination, Tregs are long-lived and constitute approximately 5–10% of the circulating CD4+ T cells in humans (~2–5% in mice). Deficiencies in FOXP3 result in immune dysregulation and severe autoimmune and inflammatory diseases such as immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome in humans and scurfy mice in rodents [18–20]. This demonstrates the necessity of Tregs.
Th17 cells
Th17 cells express the transcription factor RORC2/RORγt (humans/mice) and secrete the proinflammatory cytokines IL-17, IL-21, IL-22 and TNF-α [21]. Naive CD4+ T cells are differentiated into Th17 cells by priming in the presence of TGF-β and IL-6, which induces their hallmark transcription factor RORγt [15]. IL-23 promotes the survival of Th17 cells; conversely, Th1- and Th2-associated cytokines, such as IL-2, IL-4, IL-12 and IFN-γ, inhibit the differentiation of Th17 cells [22]. Since the IL-17 receptor (IL-17R) and IL-22 receptor are present on a broad range of cell types, Th17 cells can promote a widespread reaction that includes the production of IL-6 and other inflammatory cytokines. The release of proinflammatory cytokines from stromal cells results in the expansion of bone marrow cells and the subsequent recruitment of neutrophils to sites of inflammation to phagocytize and eliminate invading pathogens. The immune response generated by Th17 cells has been shown to be involved in the defense against extracellular bacteria and fungi, for example Klebsiella pneumoniae and Candida albicans (Table 1) [23,24]. Deficiencies in IL-17 or the IL-17R lead to increased susceptibility to opportunistic pathogens, such as Staphylococcus aureus and C. albicans [25,26]. In cancer patients, the presence and function of Th17 cells has been correlated with tumor reduction and improved survival in general [27–30]. However, Th17 responses have been reported to help tumor growth depending on the cancer and immune status of the hosts (Table 1) [31]. The release of inflammatory cytokines from Th17 cells can cause severe immunopathology; dysregulation of Th17 cells has been implicated in many immune-mediated diseases ranging from MS to inflammatory bowel disease(s) [32].
Regulation of Treg versus Th17 development
In addition to the different factors that are responsible for the induction of Treg and Th17 cells, FOXP3 and RORγt, the transcription factors that regulate their divergence, antagonize each other. FOXP3 binds to RORγt and blocks its transactivation domain [33]. Conversely, the signals that IL-6 induces override the repression of RORγt by FOXP3 and silence FOXP3 [34].
The development of these antagonizing CD4+ T-cell subsets may have arisen as a strategy to promote homeostasis between the mucosal associated lymphoid tissue and the gut microbiota. During normal conditions in the gut mucosa, induction of Tregs is favored over Th17 cells due to the high levels of TGF-β present in the mucosa and the production of another cofactor for Treg induction, all-trans retinoic acid, by dendritic cells [35]. However, once dendritic cells are activated by microbial antigen in a proinflammatory manner, they begin to secrete IL-6, which, in combination with TGF-β, promotes the induction of Th17 cells [15,36]. Here, the balance between IL-6 and retinoic acid produced by dendritic cells affects the cell type that is predominately induced in the gut and the type of immune response that will be elicited against microbes in the gut.
Tregs in viral infections
In viral infections, the role of Tregs can be either protective or detrimental to the host (Table 2), because Tregs can control inflammation, preventing excessive immunopathology, or inhibit the antiviral immune response, facilitating viral replication. Tissue injury in viral infections can be caused directly by viral propagation in the tissue. This pathology is generally suppressed by the antiviral immune response, which acts to suppress viral replication. CTLs play a key role in the control of intracellular viral replication by killing infected cells, while extracellular virus can be neutralized by antibody. If CTL responses are impaired or the virus alters CTL responses, then the virus can establish a chronic infection [37–40]. The impaired CTL response may be due to immunological genetic background, viral proteins, virally induced genes or a suppressed immune response to avoid excessive immunopathology [41,42]. However, in some viral infections, the pathology caused by immune effector cells is more deleterious than the tissue damage induced by viral propagation [43–45]. Here, it is more favorable for the host to control the immunopathology than the viral replication. A balance between preventing immunopathology by Tregs and allowing the immune system to eradicate viral pathogens is necessary to prevent immunopathology and viral pathology during infections.
Table 2.
Tregs in viral infections.
| Virus | Organism | Comments | Ref. |
|---|---|---|---|
| Possible detrimental role | |||
| Friend virus | Mouse | Suppress CTL responses enhancing viral replication | [39,48–50] |
| TMEV | Mouse | Suppress CTL responses enhancing viral replication | [78] |
| HIV | Human | May suppress CTL responses enhancing viral replication | [52,53] |
| Possible protective role | |||
| WNV | Mouse | Suppress immunopathology | [46] |
| HSV | Mouse | Modulate immune responses and prolong survival | [47] |
| MHV | Mouse | Suppress immunopathology | [76,77] |
| CVB3 | Mouse | Suppress immunopathology | [118] |
CTL: Cytotoxic T lymphocyte; CVB3: Coxsackie virus B3; MHV: Mouse hepatitis virus; TMEV: Theiler’s murine encephalitis virus; WNV: West Nile virus.
During viral infections, the physiological role of Tregs appears to be to control the immune response and reduce immunopathology. This reduction in immunopathology can ameliorate clinical signs when tissue damage is caused by the immune response. In both acute herpes virus and West Nile virus (WNV) infection, low levels of Tregs have been demonstrated to result in worse clinical outcomes. Lanteri et al. conducted a study that linked the development of symptomatic WNV infections in both humans and mice to the Treg response [46]. In humans and mice, asymptomatic individuals infected with WNV had higher levels of Tregs than symptomatic individuals. To confirm the effects of Tregs in WNV infection, transgenic mice expressing the diphtheria toxin receptor under the control of the FOXP3 promoter (DEREG mice) were used to deplete Tregs in vivo. DEREG mice were depleted of Tregs and then infected with WNV; here, Treg-depleted mice had a higher frequency of fatality compared with nondepleted mice and more severe clinical signs and weight loss. The authors suggested that Tregs regulate the immune response and can prevent excess immunopathology. In experimental acute genital herpes virus infection, although ablation of Tregs resulted in increased levels of proinflammatory chemokines in the draining lymph nodes, it reduced proinflammatory cytokines in the genitals [47]. In addition, the viral load increased and fatality was accelerated in Treg-depleted mice. The authors suggested that Tregs facilitate protective immune responses to infection by aiding in the coordination of antiviral responses.
In many viral infections, Tregs act to suppress the antiviral immune response, facilitating persistent infections. Zelinskyy et al. described the relationship of Tregs and CTL responses in Friend virus infection [48,49]. Friend virus infects different lymphoid organs and replicates at different levels in these organs, where increased levels of virus-specific CTLs correlated with higher levels of viral replication. Here, the expansion of Tregs was associated with the levels of CTL responses and viral replication. The expansion of Tregs was found to be tissue-specific, which agrees with a previous finding that Friend virus replication in organs correlated with Treg expansion [50]. To determine whether Tregs could limit the CTL response, DEREG mice were depleted of Tregs, and the CTL responses were examined. In the DEREG mice, enhanced CTL responses reduced viral load significantly, while immunopathology was not increased. In HIV infection, protective CTLs have been demonstrated to avoid suppression by Tregs [51]. Protective CTLs did not express the inhibitory receptor Tim-3, and were able to directly kill Tregs in a granzyme B-dependent manner. It has also been shown in HIV patients that an increased ratio of Treg:Th17 cells was indicative of a worse prognosis than patients who maintain a balanced cell ratio [52–54]. These studies along with others suggest that Tregs can be usurped for the evasion of the immune system by viruses, enabling viral replication.
In viral infections, the role of Tregs appears to be coordinating the immune response as well as preventing excessive immunopathology. The suppression of immune responses can turn deleterious by allowing for further viral replication that may result in a persistent infection. Since the outcome of many viral infections can be influenced by the effects of Tregs, treatments that modify Treg responses, for example the Treg-depleting drug, denileukin diftitox, may be useful therapeutic tools in treating specific infections [55].
Th17 cells in viral infections
In viral infections, the role of Th17 cells is generally considered to be detrimental to the host due to induction of immunopathology (Table 3). In addition, the production of IL-17 can prevent the differentiation of Th1 cells, inhibiting the production of IL-2 and IFN-γ, which have CTL induction and antiviral functions. The inhibition of Th1 cells by Th17 cells could lead to viral persistence. However, in some viral infections, indirect evidence has demonstrated that Th17 cells may be necessary to prevent disease exacerbation.
Table 3.
Th17 cells in viral infections.
| Virus | Organism | Comments | Ref. |
|---|---|---|---|
| Possible detrimental role | |||
| Influenza virus | Mouse | Immunopathology and inhibit CTL responses | [58] |
| MHV | Mouse | Immunopathology | [56] |
| HCV | Human | Immunopathology | [57] |
| HSV | Mouse | Exacerbate immunopathology caused by CTLs | [59] |
| TMEV | Mouse | Inhibit CTL response, leading to a persistent viral infection | [79] |
| CVB3 | Mouse | Immunopathology and possibly inhibit CTL responses | [102,103] |
| Possible protective role | |||
| HIV | Human | Depletion may lead to secondary infections | [61,63] |
| SIV | PT AGM |
Depletion correlates with pathogenesis No observable effects on Th17 cells (these monkeys develop a nonpathogenic chronic infection) |
[62] |
| Influenza virus | Mouse | Tc17 cells can contribute to viral clearance | [66] |
AGM: African green monkey; CTL: Cytotoxic T lymphocyte; CVB3: Coxsackie virus B3; MHV: Mouse hepatitis virus; PT: Pigtailed macaque; Tc: Cytotoxic T; TMEV: Theiler’s murine encephalitis virus; WNV: West Nile virus.
In mouse hepatitis virus (MHV) infection, Th17 cells were found to be responsible for immunopathology in the liver [56]. Here, MHV-infected IFN-γ receptor-knockout (KO) mice mounted enhanced Th17 responses compared with wild-type mice. The increased production of proinflammatory cytokines resulted in more severe liver pathology. Th17 cells have also been shown to contribute to liver damage in other viral infections, such as HCV [57]. Furthermore, in influenza virus infection, compared with wild-type mice, IL-17A-KO mice had reduced levels of inflammation in the lung and yet the mice were still able to clear the virus [1]. In vitro, IL-17 was found to boost the production of the respiratory syncytial virus-induced proinflammatory cytokines IL-6 and IL-8 [58]. This was abrogated if components of the interferon signaling pathway were disrupted. This may explain the immunopathology that results from Th17 cells in viral infections, where IL-17 leads to the production of proinflammatory cytokines, such as IL-8, which results in excessive neutrophil recruitment, causing further immunopathology. In both liver and lung experiments, Th17 cells appear to inhibit Th1-type immune responses, preventing viral clearance and/or causing immunopathology.
On the other hand, Th17 cells have been shown to synergize with Th1 cells, enhancing immunopathology in HSV-1-induced corneal immunopathology [59]. In this system, Th1 cells not only cleared the virus but also caused some initial immunopathology, which was followed by the recruitment of Th17 cells to the site of infection, resulting in increased inflammation and immunopathology. Since the Th1 cells were HSV-1-specific and the Th17 cells were not, the Th17 cells may be activated in response to the corneal self-antigen that became exposed due to the initial immunopathology caused by the Th1 cells (‘epitope spreading’ from viral antigen to self-antigen). The cornea is an immunologically privileged site where self-antigen is not seen by the immune system and thus does not induce self-tolerance; exposure of T cells to the sequestered antigens results in the activation of the T cells [60]. The induction of Th17 immune responses by the corneal self-antigen (autoantigen) that is constantly being released may explain why corneal HSV-1 infection results in chronic pathology.
In HIV infection, the role of Th17 cells is not fully understood, since some researchers have been able to find virus-specific Th17 cells while others have not [61,62]. However, since both the virus and Th17 cells are abundant in the mucosa, Th17 cells may play a beneficial or detrimental role in the pathogenesis of HIV. Since Th17 cells express CD4 molecules, a receptor of HIV, Th17 cells can be infected by HIV. Direct HIV infection may deplete Th17 cells, which would open the door for opportunistic infections of extracellular bacteria and fungi, exacerbating AIDS [63]. The loss of Th17 cells from the gut mucosa could also permit the natural flora of the gut to cross the gut lining, generating inflammation. In an animal model for HIV infection, SIV infection, a depletion of Th17 cells was associated with clinical disease [64]. In symptomatic infections of SIV, the levels of Th17 cells were decreased, while in asymptomatic infections, the levels were unaffected. In viral infections, the maintenance of a healthy population of Th17 cells may be necessary to prevent secondary infections or other secondary effects of an infection, such as tumor development.
While there are no studies that convincingly show a necessity for Th17 cells to clear a viral infection, several reports suggest that Th17 cells or their primary cytokine, IL-17, help in the clearance of viruses. In mice immunized against rotavirus, viral challenge upregulated expression of IL-17 mRNA in intestinal lymphoid cells and production of IL-17 from virus-specific T cells [65]. Although this suggests that IL-17-producing cells participate in rotavirus immunity, immunity to rotavirus was still found in IL-17R-KO mice. A newly discovered subtype of CTL, T cytotoxic (Tc)17 cells, which are CD8+RORγt+ IL-17-secreting T cells, have been linked to protection from lethal influenza virus challenge [66]. Tc17 protection was perforin-independent and associated with an influx of neutrophils. Blocking IL-17 increased weight loss and decreased survival after a lethal virus challenge in mice. Together, these findings suggest that IL-17-secreting cells can contribute to viral clearance.
In virus infections, the role of Th17 cells appears to vary depending on the immunologic background of the host and the virus that is infecting the host. Th17 cells can be detrimental to the host if they suppress Th1 immune responses, leaving viral replication uncontrolled, or if they synergize with Th1-mediated immunopathology. These two scenarios could result in persistent and/or inflammatory disease from viral infections that are normally nonpathogenic in the general population.
While Th17 cells may cause immunopathology in some viral infections, Th17 cells are necessary for host defense against some extracellular bacteria and fungi. Thus, depletion of Th17 cells by viral infections such as HIV may exacerbate the disease caused by extracellular microbes, including viruses. Once Th17 cells are depleted, opportunistic infections in the gut may take hold. The opportunistic infection may itself kill the host or alter the systemic immune responses, causing the viral infection to go unchecked [67]. In these scenarios, the condition of the Th17 cell population may be imperative to the health of the host and indirectly to the resolution of the viral infection.
Treg & Th17 cells in viral models of MS
MS is a chronic inflammatory disease of the CNS, characterized by demyelination and axonal degeneration. Symptoms include visual and sensory impairment, paralysis and other neurological deficits, such as cognitive dysfunction. Approximately 90% of MS patients develop a relapsing–remitting disease, where relapses are associated with inflammatory immune responses (Th1 and Th17) and remissions are associated with anti-inflammatory immune responses (Th2 and Tregs) [68]. The possibility that MS has an infectious trigger has been considered since the initial descriptions of the disease [69]. Many different viruses have been associated with MS, particularly EBV. Up to 95% of the general population are seropositive for EBV, while 99% of MS patients are seropositive [70]. Other viruses, such as human herpes virus 6, human endogenous retrovirus, varicella zoster virus, measles virus and canine distemper virus, have been investigated and linked to MS [71,72]. The presence of oligoclonal IgG bands in the cerebrospinal fluid of MS patients also supports an infection as the cause of MS [73]. Evidence of a potential viral etiology also comes from animals that develop diseases similar to MS. For example, a MS-like disease was discovered in Japanese macaques infected with a gamma herpesvirus [74]. Several viral models of MS are used in mice, including canine distemper virus, MHV and Semliki forest virus infection. The most prominent viral model for MS is Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice. TMEV infection is clinically and pathologically similar to MS, where susceptible mice develop a paralytic chronic progressive disease (Figure 2) [75].
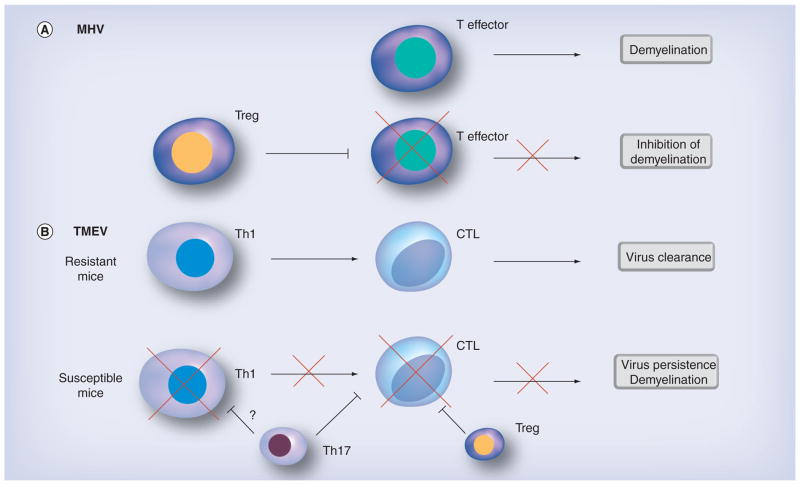
Figure 2. Animal models of virus-induced demyelination.
(A) In MHV infection, Tregs prevent immunopathology caused by T effector cells. (B) TMEV infection in resistant C57BL/6 mice induces a Th1 response that promotes CTL responses and clearance of the virus. In susceptible SJL/J mice, Treg and Th17 cells inhibit CTL responses, resulting in virus persistence and demyelination. CTL: Cytotoxic T lymphocyte; MHV: Mouse hepatitis virus; TMEV: Theiler’s murine encephalomyelitis virus.
In the MHV model of virus-induced demyelinating disease using the neurotropic strain of MHV, adoptive transfer of Tregs was found to decrease immune-mediated demyelination and mortality [76,77]. In this model, demyelination has been suggested to be immune-mediated, since RAG1-KO mice infected with MHV do not develop demyelination unless reconstituted with MHV-immune CD4+ or CD8+ T cells. RAG1-KO mice receiving an adoptive transfer of Tregs concomitantly with MHV-immune splenocytes had decreased T-cell proliferation and proinflammatory cytokine production. In wild-type mice, a more virulent strain of MHV kills 100% of infected mice; however, an adoptive transfer of Tregs cut the case fatality rate in half. Here, Tregs play a beneficial role by blocking immune-mediated demyelination (Figure 2).
In TMEV infection, CD4+ Th1 and CD8+ T cells and antibody have been shown to cause immunopathology [2]. Interestingly, however, Richards et al. suggested that an expansion of Tregs leads to susceptibility in TMEV infection [78]. An elevated Treg to effector cell ratio was found in the spleen and CNS of TMEV-infected SJL/J (susceptible) mice compared with C57BL/6 (resistant) mice. SJL/J mice depleted of Tregs using anti-CD25 monoclonal antibody (mAb) exhibited more potent antiviral immune responses, lower viral loads and decreased clinical signs compared with control mice. In contrast to the MHV model where Tregs appear to prevent immune-mediated demyelination, Tregs blocked antiviral immune responses, facilitating viral replication and demyelination (Figure 2). These two models demonstrate the opposite effects that Tregs can have in viral infections that result in similar disease.
Hou et al. studied the role of Th17 cells in TMEV infection by investigating how Th17 cells affected viral replication and the function of CTLs [40,79]. TMEV-infected mice treated with anti-IL-17 mAb during the acute stage of infection had lower levels of virus persistence and demyelination compared with infected mice treated with control antibody. Anti-IL-17 mAb-treated mice also displayed enhanced antiviral CTL responses. They also found that susceptible SJL/J mice have higher levels of Th17 cells than resistant C57BL/6 mice after TMEV infection [40,79]. This suggests that a genetic predisposition towards different adaptive immune responses may affect the outcome of disease. TMEV-infected SJL/J mice have been shown to have abnormal antiviral CTL responses; the expansion of Th17 immune responses may be a response to this abnormality, as has been observed in other viral infections in mice deficient in CTL responses [80,81]. In addition, a bacterial endotoxin, lipopolysaccharide, which promotes Th17 development, was capable of rendering C57BL/6 mice susceptible to persistent TMEV infection [79]. This implies that additional factors such as adjuvants are capable of influencing the immune response against pathogens and can alter an infection that is normally resolved to become a persistent infection. In this scenario, it may be possible that in a situation where an individual has a polymicrobial infection where the appropriate immune response would be a Th17-type immune response to resolve it, the immune response against the other pathogen could be skewed toward a Th17 immune response. This could result in immunopathology caused by Th17 cells or viral persistence if Th17 cells suppress CTL responses. As a result, a normally benign infection could be altered to an immune-mediated and/or persistent viral disease.
The expansion of Tregs seen by Richards et al. may be a protective response to the expansion of Th17 cells to avoid immunopathology in the CNS, while it is unknown whether Th17 cells can cause immunopathology as effector cells in TMEV infection [78]. On the other hand, it is also possible that Th17 cells that are expanded in response to TMEV infection suppress Th1 responses, leading to a suppression of CTL responses. If this is the case, since Tregs can also suppress CTL responses, Treg and Th17 cells may suppress CTL responses synergistically (Figure 2). The combined suppressive effects of Treg and Th17 cells against CTL responses may be what cause mice to become susceptible. This scenario would be supported if it were found that in normally TMEV-resistant mice treated with lipopolysaccharide, to render them susceptible, the increase of Th17 immune responses was accompanied by an expansion of Tregs. Another possibility is that the expansion of Tregs suppresses the initial CTL responses, leading to the expansion of Th17 cells as an attempt to control viral replication; an expansion of Th17 cells has previously been observed in mice with impaired CTLs [80].
In MS patients, Tregs have been observed to be present at lower levels and to have functional defects [82–84]. This has led to the transfer of Tregs into MS patients being proposed as a therapy. However, due to the dichotomous nature of Tregs in viral infections and the unknown etiology of MS, one must take caution in this approach. If MS is indeed caused by a viral infection or the patient has an ongoing viral infection, Tregs may exacerbate this condition. If MS is solely caused by immunopathology (without the involvement of pathogens), Tregs should act as a therapeutic agent. Since the etiology of MS is unknown, MS may be caused by different pathogens and susceptibility is influenced by host genetic background, as shown in TMEV and MHV models. If this is the case, modulation of Tregs may have similar effects in patients to those seen in the mouse models, making some worse and some better.
MS could be the result of an inappropriate Th17 immune response to a virus, since increased Th17 immune responses have been observed during relapses in MS patients [68]. Here, a common latent pathogen, for example EBV, could be responded to by Th17 cells causing focal inflammatory lesions when the virus reactivates. If virus infection occurs sequentially or simultaneously with a Th17-inducing pathogen, such as extracellular bacteria or fungi (a ‘polymicrobial infection’), it may generate Th17 cells that respond to viral antigen. When the virus reactivates, the release of viral-antigen would result in inflammatory lesions. If the individual had a genetically biased immune response towards Th17 cells, an individual could generate a pathogenic Th17 immune response towards an innocuous pathogen that normally induces protective immune responses. Additionally, a chronic inflammatory condition could be initially caused by Th1 responses followed by Th17 responses, similar to what was found in HSV-1- induced corneal pathology, where the Th17 responses can be aimed at self-antigen. Here, a Th17 response would be generated towards self-antigen, and when normally benign damage occurred in that area, the release of self-antigen would result in inflammation, causing further damage.
Treg & Th17 cells in viral myocarditis
Myocarditis is an inflammation of the myocardium, the heart muscle. The consequences of myocarditis are broad: myocarditis may resolve completely, or result in chest pain, arrhythmia, heart failure or death [85,86]. Since myocarditis is often asymptomatic and only approximately 10% of patients with clinical evidence of myocarditis are estimated to develop symptoms, the exact incidence of myocarditis in the general population is unclear [87]. However, the prevalence of myocarditis has been estimated at 1% in the USA by a necropsy study of more than 12,000 victims of violent or accidental deaths. Viruses have been proposed to contribute to myocarditis induction, such as enterovirus, adenovirus, parvovirus B19, EBV, human herpes virus 6 and CMV [88,89]. Among viruses, picornaviruses, such as Coxsackievirus B (CVB) and echovirus, are known as dominant pathogens of myocarditis [87,90,91]. In North America, nearly 50% of myocarditis results from CVB infection [90]. Coxsackieviruses generally have an infection rate with a relatively low myocarditic potential, and a relatively small percentage of the human population develop clinically visible viral myocarditis in a lifetime [92].
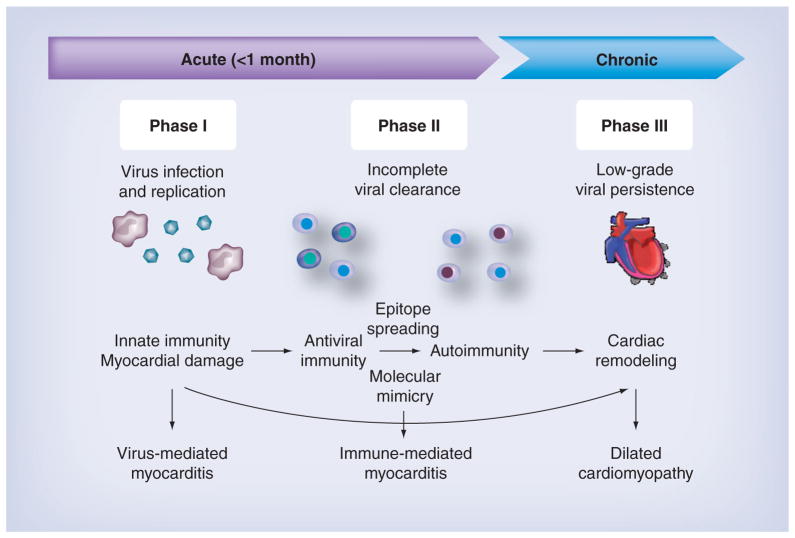
Viral myocarditis can be a triphasic disease, although the exact mechanism is unclear (Figure 3). In phase I, since myocardial damage is caused by viral replication, antiviral therapy with antiviral drugs is the most ideal treatment. In most cases, however, only symptomatic treatment is applied, since the causative viruses are not identified or treatable. In phase II, antiviral immune and/or autoimmune responses contribute to the pathogenesis of viral myocarditis (immunopathology). Here, Th1 cells have been proposed to activate CTLs that kill uninfected cardiomyocytes causing immunopathology, although Th1 immune responses have also been shown to protect against viral myocarditis by decreasing viral replication [93–96]. At this phase, immune suppression is the most appropriate treatment, unless virus persistence occurs [97]. As a result of phase I and II, dilated cardiomyopathy (phase III), characterized by remodeling of cardiac structure and function, has been suggested to progress independent of inflammation. In phase III, the pump function of the heart is impaired and the ventricles are dilated, and some of the cases become progressive. Regardless of the cause, once patients develop dilated cardiomyopathy, the patients are treated with standard therapy for heart failure to improve the heart function with drugs such as angiotensin-converting enzyme inhibitor, diuretics and β-blocker. Some progressive cases require heart transplantation [98]. In phase I, cardiac damage is observed in the absence of substantial host immune responses. In phase III, the progression to dilated cardiomyopathy is determined by cardiac damage inflicted during the previous stages. Thus, Th17 cells and Tregs mainly play roles in phase II.
Figure 3. The three phases of viral myocarditis.
During phase I, a virus infects and replicates in the heart and induces innate immune responses. Myocardial damage induced by viral replication or antiviral immunity can lead to induction of autoantibodies and autoimmune T-cell responses via epitope spreading or molecular mimicry during phase II. Low-grade viral persistence and tissue damage during phases I and II can lead to cardiac remodeling and contribute to dilated cardiomyopathy during phase III.
Th17 cells have been proposed to play a pathogenic role in experimental autoimmune myocarditis [99,100]. Yuan et al. demonstrated that both the frequency of Th17 cells in peripheral blood mononuclear cells and the levels of IL-17 in the serum were higher in patients with acute viral myocarditis (AVMC) than in healthy subjects [101]. Similar to the disease in humans, the percentages of Th17 cells and the levels of serum IL-17 have been reported to increase in a viral model for myocarditis: CVB3 infection of mice [102,103]. In the CVB3 model, IL-17 neutralization with anti-IL-17 mAb injection ameliorated clinical signs and decreased viral replication in the heart [102,104]. However, the treatment did not completely prevent viral myocarditis. It is unclear how Th17 cells could favor CVB3 replication; however, Th17 cells may inhibit Th1 cells and CTLs, both of which play a central role in CVB3 clearance.
Some viruses encode antigenic determinants that are similar to heart proteins, including adenine nucleotide translocator (ANT), myosin heavy chain, β1-adrenergic receptor and M2-cholinergic receptor [105–109]. Because of this ‘molecular mimicry’ between the virus and heart proteins, immune responses to these viruses cannot only attack viruses, but cross-react with heart tissue. Indeed, antiheart autoantibodies for these four proteins have frequently been detected in the serum from patients with AVMC [107,110]. Recently, there have been some reports that Th17 cells not only contribute to inflammation, but also promote pathogenic autoantibody production from B cells [111,112]. Yuan et al. demonstrated that the levels of IL-17R expression on B cells in peripheral blood mononuclear cells are higher in patients with AVMC than in healthy subjects. Additionally, in patients with AVMC, the activation of B cells isolated from blood was positively correlated with the levels of serum IL-17 [101]. Similarly, in the CVB3 model, treatment with anti-IL-17 mAb ameliorated clinical signs with decreased serum anti-ANT IgG in AVMC mice. B cells from anti-IL-17 antibody-treated mice had reduced proliferation and anti-ANT antibody production in vitro [113]. Thus, Th17 cells may contribute to the development of viral myocarditis by the enhancement of antiheart autoantibody production by IL-17R-expressing B cells.
Although Tregs have been proposed to control autoimmune models for myocarditis, the role of Tregs in viral myocarditis is still controversial [114–116]. Galectin-9, one of the β-galactoside-binding lectins, has been shown to promote the induction of Tregs and suppress Th1 and Th17 cells. Lv et al. demonstrated that the upregulation of Tregs by galectin-9 injection ameliorated CVB3-induced myocarditis [117]. In this study, treatment with galectin-9 increased the levels of IL-10 in the heart. The adoptive transfer of Tregs reduced viral replication and inflammatory cell infiltration in the heart by enhancing levels of cardiac TGF-β in AVMC mice [118]. Similarly, TGF-β-transgenic mice developed mild CVB3-induced myocarditis with decreased antiheart antibody production and migration of inflammatory cells into the heart compared with wild-type mice [119]. Thus, Tregs may suppress viral myocarditis via anti-inflammatory cytokine production.
However, Tregs may not always be beneficial in viral myocarditis. Tregs have been proposed to play a detrimental role in some viral infections, since Tregs suppress antiviral immune responses, which are important for viral clearance [48–50]. In the CVB3-induced AVMC model, Xie et al. demonstrated an increased frequency of both Treg and Th17 cells in the spleen and levels of TGF-β and IL-17A in the heart [104]. The neutralization of IL-17 by anti-IL-17 mAb injection decreased the frequency of not only Th17 cells, but also Tregs. Anti-IL-17 antibody treatment also decreased inflammation of CVB3-infected mice and lowered levels of viral RNA. These results suggest that both Treg and Th17 cell functions are intimately associated with inflammation and viral replication in viral myocarditis.
Conclusion
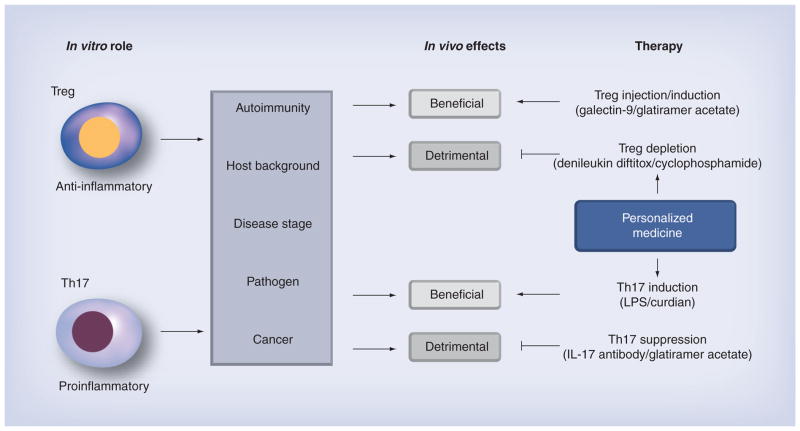
In vitro Tregs produce anti-inflammatory cytokines and suppress T-cell proliferation, while Th17 cells produce inflammatory cytokines. In vivo, these functions can result in different outcomes, depending on interactions with other immune cells, pathogens and the pathophysiological conditions (Figure 4). For example, Treg and Th17 cells play protective and detrimental roles in autoimmunity, respectively, while Treg and Th17 cells can play the opposite roles in tumor immunity (Table 1). In viral infections, Treg and Th17 cells can have diverse effects on viral infections ranging from exacerbating to preventing disease. Tregs can exacerbate disease by inhibiting CTLs and facilitating viral replication. On the other hand, by modulating the immune response, Tregs can both facilitate viral clearance and prevent immunopathology. Although Th17 cells are categorized as proinflammatory cells, in many viral infections, it appears that Th17 cells promote viral replication by inhibiting Th1 immune responses. Th17 cells also contribute to immunopathology by enhancing the inflammatory response. In both MS and myocarditis, Th17 cells appear to play a detrimental role by promoting inflammation, and possibly by inhibiting antiviral Th1 immune responses. In viral models for MS and myocarditis, Tregs can be beneficial by suppressing inflammation, and there is some evidence that Tregs may enhance viral replication and thus promote further tissue damage; this should be considered when Tregs are suggested as a therapeutic agent in virus-induced disease.
Figure 4. The anti-inflammatory and proinflammatory effects of Tregs and Th17 cells, respectively, can have different outcomes in different settings.
Depending on the host background (genetic, immunological, age and sex), disease stage, pathogen (virus, bacteria or fungus) and type of disease (autoimmunity, cancer or microbial), the effects of Treg and Th17 cells can be either beneficial or detrimental in disease. Developing personalized medicines that takes these factors into account may be an effective way to treat these diseases. For example, Tregs can play a beneficial role in autoimmune diseases, in which injection of Treg-inducing drugs will be effective. On the other hand, in infection, Tregs can play a detrimental immunosuppressive role, where Treg depletion is the most appropriate treatment. Th17 cells can play a protective role in cancer where Th17 cell induction may be applied, while Th17 cells can play detrimental effector roles in autoimmunity that may be treated by a Th17 suppressive therapy.
LPS: Lipopolysaccharide.
Future perspective
Several reagents have been shown to modulate the populations of Treg and Th17 cells, including glatiramer acetate (Copaxone®), an immunomodulatory drug composed of random tetramers of four amino acids found in myelin basic protein, which is used for the treatment of MS [120,121]. Modulating the populations of Treg and Th17 cells using drugs could be applicable in virus-induced diseases (Figure 4). Here, it is imperative that the exact role of these cell populations is elucidated in each disease, due to the different roles they may play in disease (beneficial or detrimental), and the adverse effects that could result. Since the stage of disease, immunologic background and other factors determine the effects of Treg and Th17 cells, drugs that can modulate Treg and Th17 cells should be used with consideration of these factors in individual patients (personalized medicine) (Figure 4). For example, drugs that deplete Tregs can have adverse effects similar to what is observed in cyclophosphamide treatment that depletes Tregs as a side effect and enhances contact hypersensitivity [122].
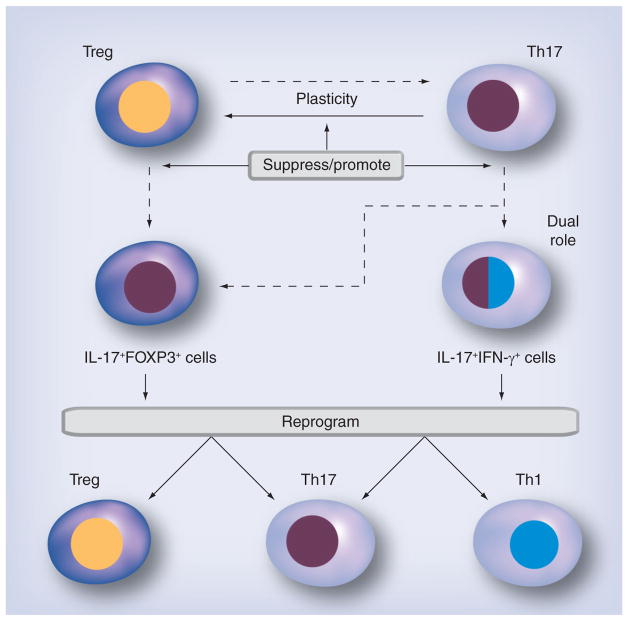
While Treg and Th17 cells appear to be novel targets for treating virus-induced disease, there are some emerging issues of phenotypic plasticity that could complicate treatments targeting Treg and/or Th17 cells (Figure 5). Typically, the different Th cell subsets are considered to secrete specific cytokines and be terminally differentiated. However, it has recently been discovered that the cytokine secretion profiles of the subsets can overlap and that Th cell subsets can be ‘reprogrammed’ into other subsets (plasticity) [123]. Treg and Th17 cells have been found to switch to phenotypes that resemble each other’s or a Th1 phenotype, while Th1 cells have not been found to switch to a Treg or Th17 phenotype. For example, human Th17 cells have been shown to differentiate into Tregs at the clonal level [123]. It is still controversial whether Tregs are also susceptible to conversion into other Th cells [124]. This raises the possibility that the local cytokine environment can reprogram cells and can overcome ‘predetermined’ programming. One intriguing complication to the Treg/Th17 paradigm is the discovery of IL-17+FOXP3+CD4+ T cells [125]. These cells suppress T-cell activation while stimulating inflammatory cytokine production from tissue. In addition, Th cells that produce cytokines from two different Th subsets have been reported, including Th1/Th2, Th2/Treg and Th1/Th17 cytokines. This raises the issue that transfers of specific cell types may be ineffective and possibly detrimental to disease, if they are converted to pathogenic subsets or to cells that secrete two opposing cytokines.
Figure 5. Future therapeutic strategies against phenotype plasticity and overlap in Treg and Th17 cells.
Treg and Th17 cells may switch to phenotypes that resemble each other (‘plasticity’) or that have dual roles of two T-helper subsets (IL-17FOXP3+ cells, IL-17+IFN-γ+ cells). Depending on the disease conditions, personalized medicines can be applied to each patient, such as drugs that can suppress or promote the plasticity between Th17 cells and Tregs or drugs that reprogram the cells with dual roles to redifferentiate into a single phenotype.
The future therapeutic strategy to treat these diseases may be with drugs that can suppress or alter the Th subset programming of effector cells (Figure 5). For example, in autoimmunity, drugs that can promote plasticity from Th17 cells to Tregs will be effective, while the plasticity of cells should be suppressed in infections with extracellular pathogens to preserve antimicrobial Th17 responses. In cases where the cell type has an overlapping phenotype (IL-17+IFN-γ+ cells) that may play a detrimental role in autoimmunity, two therapeutic strategies can be applied: suppression of conversion from Th17 cells to dual phenotypes (Th1/Th17); and reprogramming drugs that redifferentiate dual phenotypes (Th1/Th17) into a single phenotype, either Th1 or Th17. On the other hand, in cancer, IL-17+IFN-γ+ cells can play a beneficial role, while IL-17+FOXP3+ cells may be ineffective because of their potential immunosuppressive nature. Here, reprogramming IL-17+FOXP3+ cells into Th17 cells will be an appropriate treatment.
Executive summary.
Treg versus Th17 cells
Tregs suppress other effector cells of the immune system.
Th17 cells are proinflammatory effector cells that are involved in tumor immunity and host defense against extracellular pathogens.
Tregs and Th17 cells antagonize the development of each other.
Tregs in viral infections
The immunosuppressive effects of Tregs may facilitate viral replication.
Regulation of the immune response may prevent immunopathology.
Th17 cells in viral infections
Th17 cells may inhibit cytotoxic T lymphocyte responses, preventing viral clearance.
Th17 cells can cause immunopathology.
Viral theory of multiple sclerosis
Tregs can suppress immunopathology or enhance viral replication.
Th17 cells can cause immunopathology; this may be due to abnormal immune responses or polymicrobial infections.
Viral myocarditis
Tregs can suppress immunopathology.
Th17 cells can cause immunopathology and may facilitate viral replication by inhibiting cytotoxic T lymphocyte responses.
Future perspective
Drugs that influence the phenotype of effector T cells may be key in treating viral diseases.
The plasticity of T-helper subsets adds to the potential difficulty of treating immune dysregulation in viral infections.
Acknowledgments
The authors thank Sadie Faith Pearson and Lesya Ekshyyan for their excellent technical assistance.
Footnotes
Financial & competing interests disclosure
This project was supported by fellowships (F Sato and S Omura) from the Malcolm Feist Cardiovascular Research Endowment, LSU Health Sciences Center-Shreveport, and grants (I Tsunoda) from the National Center for Research Resources (5P20RR018724-10) and the National Institute of General Medical Sciences COBRE Grant (8 P20 GM103433-10). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Crowe CR, Chen K, Pociask DA, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183(8):5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato F, Omura S, Martinez NE, Tsunoda I. Animal models of multiple sclerosis. In: Minager A, editor. Neuroinflammation. Elsevier; Amsterdam, The Netherlands: 2011. pp. 55–80. [Google Scholar]
- 3.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 4.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 5.Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+ Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci USA. 2010;107(45):19402–19407. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghoreschi K, Laurence A, Yang XP, et al. Generation of pathogenic Th17 cells in the absence of TGF-beta signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human Th-17 responses. Nat Immunol. 2008;9(6):650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human Th17 cells. Nature. 2008;454(7202):350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou W, Restifo NP. Th17 cells in tumour immunity and immunotherapy. Nat Immunol. 2010;10(4):248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Karlsson F, Robinson-Jackson SA, Gray L, Zhang S, Grisham MB. Ex vivo generation of regulatory T cells: characterization and therapeutic evaluation in a model of chronic colitis. Method Mol Biol. 2011;677:47–61. doi: 10.1007/978-1-60761-869-0_4. Demonstrates a method for generating approximately 20 million highly purified Tregs from one mouse spleen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Immunol. 2005;5(10):772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 13.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 14.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of FOXP3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101(13):4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Naturally arising FOXP3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 17.Schwele S, Fischer AM, Brestrich G, et al. Cytomegalovirus-specific regulatory and effector T cells share TCR clonality-possible relation to repetitive CMV infections. Am J Transplant. 2012;12(3):669–681. doi: 10.1111/j.1600-6143.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 18.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187(5):2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma R, Ju ST. Genetic control of the inflammatory T-cell response in regulatory T-cell deficient scurfy mice. Clin Immunol. 2010;136(2):162–169. doi: 10.1016/j.clim.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 21.Unutmaz D. RORC2: the master of human Th17 cell programming. Eur J Immunol. 2009;39(6):1452–1455. doi: 10.1002/eji.200939540. [DOI] [PubMed] [Google Scholar]
- 22.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18(3):349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202(6):761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190(3):624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 25.Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandiyan P, Conti HR, Zheng L, et al. CD4+CD25+FOXP3+ regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34(3):422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals Th17 and Treg skewing. Clin Cancer Res. 2008;14(11):3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JG, Xia JC, Liang XT, et al. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. Int J Biol Sci. 2011;7(1):53–60. doi: 10.7150/ijbs.7.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye ZJ, Zhou Q, Gu YY, et al. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185(10):6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 31.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 33.Ichiyama K, Yoshida H, Wakabayashi Y, et al. FOXP3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283(25):17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced FOXP3 inhibits Th17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of FOXP3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Fogle JE, Tompkins WA, Tompkins MB. CD4+CD25+ T regulatory cells from FIV+ cats induce a unique anergic profile in CD8+ lymphocyte targets. Retrovirology. 2010;7:97. doi: 10.1186/1742-4690-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogle JE, Mexas AM, Tompkins WA, Tompkins MB. CD4(+)CD25(+) T regulatory cells inhibit CD8(+) IFN-gamma production during acute and chronic FIV infection utilizing a membrane TGF-beta-dependent mechanism. AIDS Res Hum Retroviruses. 2010;26(2):201–216. doi: 10.1089/aid.2009.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietze KK, Zelinskyy G, Gibbert K, et al. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc Natl Acad Sci USA. 2011;108(6):2420–2425. doi: 10.1073/pnas.1015148108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin Y-H, Kang HS, Mohindru M, Kim BS. Preferential induction of protective T cell responses to Theiler’s virus in resistant (C57BL/6 x SJL)F1 mice. J Virol. 2011;85(6):3033–3040. doi: 10.1128/JVI.02400-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kriegs M, Burckstummer T, Himmelsbach K, et al. The hepatitis C virus non-structural NS5A protein impairs both the innate and adaptive hepatic immune response in vivo. J Biol Chem. 2009;284(41):28343–28351. doi: 10.1074/jbc.M109.038877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kijak GH, Walsh AM, Koehler RN, et al. HLA class I allele and haplotype diversity in Ugandans supports the presence of a major east African genetic cluster. Tissue Antigens. 2009;73(3):262–269. doi: 10.1111/j.1399-0039.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 43.Mccormick S, Shaler CR, Small CL, et al. Control of pathogenic CD4 T cells and lethal immunopathology by signaling immunoadaptor DAP12 during influenza infection. J Immunol. 2011;187(8):4280–4292. doi: 10.4049/jimmunol.1101050. [DOI] [PubMed] [Google Scholar]
- 44.Matter MS, Hilmenyuk T, Claus C, et al. Destruction of lymphoid organ architecture and hepatitis caused by CD4+ T cells. PLoS One. 2011;6(9):e24772. doi: 10.1371/journal.pone.0024772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fulton RB, Meyerholz DK, Varga SM. FOXP3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J Immunol. 2010;185(4):2382–2392. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.Lanteri MC, O’Brien KM, Purtha WE, et al. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J Clin Invest. 2009;119(11):3266–3277. doi: 10.1172/JCI39387. Tregs prevent immunopathology, which causes the clinical signs in West Nile virus infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪▪.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320(5880):1220–1224. doi: 10.1126/science.1155209. Tregs modulate the immune response and increase its effectiveness in a viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Zelinskyy G, Dietze KK, Husecken YP, et al. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009;114(15):3199–3207. doi: 10.1182/blood-2009-03-208736. First publication to demonstrate Treg ablation decreased viral load and did not increase immunopathology. [DOI] [PubMed] [Google Scholar]
- 49.Zelinskyy G, Dietze K, Sparwasser T, Dittmer U. Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathog. 2009;5(8):e1000406. doi: 10.1371/journal.ppat.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers L, Messer RJ, Carmody AB, Hasenkrug KJ. Tissue-specific abundance of regulatory T cells correlates with CD8+ T cell dysfunction and chronic retrovirus loads. J Immunol. 2009;183(3):1636–1643. doi: 10.4049/jimmunol.0900350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elahi S, Dinges WL, Lejarcegui N, et al. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat Med. 2011;17(8):989–995. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanwar B, Favre D, McCune JM. Th17 and regulatory T cells: implications for AIDS pathogenesis. Curr Opin HIV AIDs. 2010;5(2):151–157. doi: 10.1097/COH.0b013e328335c0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandt L, Benfield T, Mens H, et al. Low level of regulatory T cells and maintenance of balance between regulatory T cells and Th17 cells in HIV-1-infected elite controllers. J Acquir Immune Defic Syndr. 2011;57(2):101–108. doi: 10.1097/QAI.0b013e318215a991. [DOI] [PubMed] [Google Scholar]
- 54.He Y, Li J, Zheng Y, et al. A randomized case-control study of dynamic changes in peripheral blood Th17/Treg cell balance and interleukin-17 levels in highly active antiretroviral-treated HIV type 1/AIDS patients. AIDS Res Hum Retroviruses. 2012;28(4):339–345. doi: 10.1089/aid.2011.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salagianni M, Lekka E, Moustaki A, et al. NK cell adoptive transfer combined with Ontak-mediated regulatory T cell elimination induces effective adaptive antitumor immune responses. J Immunol. 2011;186(6):3327–3335. doi: 10.4049/jimmunol.1000652. [DOI] [PubMed] [Google Scholar]
- 56.Yang W, Ding X, Deng J, et al. Interferon-gamma negatively regulates Th17-mediated immunopathology during mouse hepatitis virus infection. J Mol Med. 2011;89(4):399–409. doi: 10.1007/s00109-010-0711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang Q, Wang YK, Zhao Q, Wang CZ, Hu YZ, Wu BY. Th17 cells are increased with severity of liver inflammation in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2012;27(2):273–278. doi: 10.1111/j.1440-1746.2011.06782.x. [DOI] [PubMed] [Google Scholar]
- 58.Ryzhakov G, Lai CC, Blazek K, To KW, Hussell T, Udalova I. IL-17 boosts proinflammatory outcome of antiviral response in human cells. J Immunol. 2011;187(10):5357–5362. doi: 10.4049/jimmunol.1100917. [DOI] [PubMed] [Google Scholar]
- 59.Suryawanshi A, Veiga-Parga T, Rajasagi NK, et al. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187(4):1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 7. Elsevier/Saunders; PA, USA: 2012. [Google Scholar]
- 61.Yue FY, Merchant A, Kovacs CM, Loutfy M, Persad D, Ostrowski MA. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection. J Virol. 2008;82(13):6767–6771. doi: 10.1128/JVI.02550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El Hed A, Khaitan A, Kozhaya L, et al. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J Infect Dis. 2010;201(6):843–854. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5(2):e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smiley KL, McNeal MM, Basu M, Choi AH-C, Clements JD, Ward RL. Association of gamma interferon and interleukin-17 production in intestinal CD4+ T cells with protection against rotavirus shedding in mice intranasally immunized with VP6 and the adjuvant LT(R192G) J Virol. 2007;81(8):3740–3748. doi: 10.1128/JVI.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪.Hamada H, de la Luz Garcia-Hernandez M, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182(6):3469–3481. doi: 10.4049/jimmunol.0801814. Describes IL-17-secreting CD8+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kosiewicz MM, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol. 2011;2:180. doi: 10.3389/fmicb.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peelen E, Damoiseaux J, Smolders J, et al. Th17 expansion in MS patients is counterbalanced by an expanded CD39+ regulatory T cell population during remission but not during relapse. J Neuroimmunol. 2011;240–241:97–103. doi: 10.1016/j.jneuroim.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Murray TJ. The history of multiple sclerosis: the changing frame of the disease over the centuries. J Neuro Sci. 2009;277(Suppl 1):S3–S8. doi: 10.1016/S0022-510X(09)70003-6. [DOI] [PubMed] [Google Scholar]
- 70.Pohl D. Epstein–Barr virus and multiple sclerosis. J Neurol Sci. 2009;286(1–2):62–64. doi: 10.1016/j.jns.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 71.Tselis A. Evidence for viral etiology of multiple sclerosis. Semin Neurol. 2011;31(3):307–316. doi: 10.1055/s-0031-1287656. [DOI] [PubMed] [Google Scholar]
- 72.Lipton HL, Liang Z, Hertzler S, Son KN. A specific viral cause of multiple sclerosis: one virus, one disease. Ann Neurol. 2007;61(6):514–523. doi: 10.1002/ana.21116. [DOI] [PubMed] [Google Scholar]
- 73.Saadatnia M, Najafi MR, Najafi F, Davoudi V, Keyhanian K, Maghzi AH. CD24 gene allele variation is not associated with oligoclonal IgG bands and IgG index of multiple sclerosis patients. Neuroimmunomodulation. 2012;19(3):195–199. doi: 10.1159/000332011. [DOI] [PubMed] [Google Scholar]
- 74▪▪.Axthelm MK, Bourdette DN, Marracci GH, et al. Japanese macaque encephalomyelitis: a spontaneous multiple sclerosis-like disease in a nonhuman primate. Ann Neurol. 2011;70(3):362–373. doi: 10.1002/ana.22449. Monkeys with a naturally occurring multiple sclerosis-like disease were found to be infected with a gammaherpes virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinez NE, Sato F, Omura S, Minigar A, Alexander JS, Tsunoda I. Immunopathological patterns from EAE and Theiler’s virus infection: is multiple sclerosis a homogenous 1-stage or heterogenous 2-stage disease? Pathophysiology. 2012 doi: 10.1016/j.pathophys.2012.03.003. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trandem K, Anghelina D, Zhao J, Perlman S. Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus. J Immunol. 2010;184(8):4391–4400. doi: 10.4049/jimmunol.0903918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anghelina D, Zhao J, Trandem K, Perlman S. Role of regulatory T cells in coronavirus-induced acute encephalitis. Virology. 2009;385(2):358–367. doi: 10.1016/j.virol.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richards MH, Getts MT, Podojil JR, Jin YH, Kim BS, Miller SD. Virus expanded regulatory T cells control disease severity in the Theiler’s virus mouse model of MS. J Autoimmun. 2011;36(2):142–154. doi: 10.1016/j.jaut.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79▪.Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. 2009;206(2):313–328. doi: 10.1084/jem.20082030. Th17 immune responses inhibit Th1 immune responses and facilitate a chronic viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80▪.Intlekofer AM, Banerjee A, Takemoto N, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321(5887):408–411. doi: 10.1126/science.1159806. Mice lacking Th1 immune responses mounted Th17-like immune responses against a viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindsley MD, Thiemann R, Rodriguez M. Cytotoxic T cells isolated from the central nervous systems of mice infected with Theiler’s virus. J Virol. 1991;65(12):6612–6620. doi: 10.1128/jvi.65.12.6612-6620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venken K, Hellings N, Liblau R, Stinissen P. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med. 2010;16(2):58–68. doi: 10.1016/j.molmed.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81(1):45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 84.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199(7):971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bendig JW, O’Brien PS, Muir P, Porter HJ, Caul EO. Enterovirus sequences resembling coxsackievirus A2 detected in stool and spleen from a girl with fatal myocarditis. J Med Virol. 2001;64(4):482–486. doi: 10.1002/jmv.1075. [DOI] [PubMed] [Google Scholar]
- 86.Ward C. Severe arrhythmias in coxsackievirus B3 myopericarditis. Arch Dis Child. 1978;53(2):174–176. doi: 10.1136/adc.53.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitton JL, Feuer R. Myocarditis, microbes and autoimmunity. Autoimmunity. 2004;37(5):375–386. doi: 10.1080/08916930410001713089. [DOI] [PubMed] [Google Scholar]
- 88.Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. Eur Heart J. 2008;29(17):2073–2082. doi: 10.1093/eurheartj/ehn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360(15):1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huber SA, Gauntt CJ, Sakkinen P. Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv Viral Res. 1998;51:35–80. doi: 10.1016/s0065-3527(08)60783-6. [DOI] [PubMed] [Google Scholar]
- 91.Yajima T, Knowlton KU. Viral myocarditis: from the perspective of the virus. Circulation. 2009;119(19):2615–2624. doi: 10.1161/CIRCULATIONAHA.108.766022. [DOI] [PubMed] [Google Scholar]
- 92.Taylor LA, Carthy CM, Yang D, et al. Host gene regulation during coxsackievirus B3 infection in mice: assessment by microarrays. Circulation Res. 2000;87(4):328–334. doi: 10.1161/01.res.87.4.328. [DOI] [PubMed] [Google Scholar]
- 93.Huber SA, Sartini D, Exley M. Vgamma4+ T cells promote autoimmune CD8+ cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4+ Th1 cells. J Virol. 2002;76(21):10785–10790. doi: 10.1128/JVI.76.21.10785-10790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Estrin M, Huber SA. Coxsackievirus B3-induced myocarditis Autoimmunity is L3T4+ T helper cell and IL-2 independent in BALB/c mice. Am J Pathol. 1987;127(2):335–341. [PMC free article] [PubMed] [Google Scholar]
- 95.Yue Y, Gui J, Ai W, Xu W, Xiong S. Direct gene transfer with IP-10 mutant ameliorates mouse CVB3-induced myocarditis by blunting Th1 immune responses. PLoS One. 2011;6(3):e18186. doi: 10.1371/journal.pone.0018186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174(1):261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 97.Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333(5):269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 98.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104(9):1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 99.Rangachari M, Mauermann N, Marty RR, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203(8):2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang H, Hanawa H, Yoshida T, et al. Alteration of IL-17 related protein expressions in experimental autoimmune myocarditis and inhibition of IL-17 by IL-10–Ig fusion gene transfer. Circ J. 2008;72(5):813–819. doi: 10.1253/circj.72.813. [DOI] [PubMed] [Google Scholar]
- 101.Yuan J, Cao AL, Yu M, et al. Th17 cells facilitate the humoral immune response in patients with acute viral myocarditis. J Clin Immunol. 2010;30(2):226–234. doi: 10.1007/s10875-009-9355-z. [DOI] [PubMed] [Google Scholar]
- 102.Yuan J, Yu M, Lin QW, et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol. 2010;185(7):4004–4010. doi: 10.4049/jimmunol.1001718. [DOI] [PubMed] [Google Scholar]
- 103.Fan Y, Weifeng W, Yuluan Y, Qing K, Yu P, Yanlan H. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of coxsackievirus B3-induced viral myocarditis reduces myocardium inflammation. Virol J. 2011;8:17. doi: 10.1186/1743-422X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie Y, Chen R, Zhang X, et al. The role of Th17 cells and regulatory T cells in coxsackievirus B3-induced myocarditis. Virology. 2011;421(1):78–84. doi: 10.1016/j.virol.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 105.Mena I, Perry CM, Harkins S, Rodriguez F, Gebhard J, Whitton JL. The role of B lymphocytes in coxsackievirus B3 infection. Am J Pathol. 1999;155(4):1205–1215. doi: 10.1016/S0002-9440(10)65223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schwimmbeck PL, Bigalke B, Schulze K, Pauschinger M, Kuhl U, Schultheiss HP. The humoral immune response in viral heart disease: characterization and pathophysiological significance of antibodies. Med Microbiol Immunol. 2004;193(2–3):115–119. doi: 10.1007/s00430-003-0217-7. [DOI] [PubMed] [Google Scholar]
- 107.Rose NR. Autoimmunity in coxsackievirus infection. Curr Top Microbiol Immunol. 2008;323:293–314. doi: 10.1007/978-3-540-75546-3_14. [DOI] [PubMed] [Google Scholar]
- 108.Rose NR, Hill SL. The pathogenesis of postinfectious myocarditis. Clin Immunol Immunopathol. 1996;80(3 Pt 2):S92–S99. doi: 10.1006/clin.1996.0146. [DOI] [PubMed] [Google Scholar]
- 109.Rose NR, Hill SL. Autoimmune myocarditis. Int J Cardiol. 1996;54(2):171–175. doi: 10.1016/0167-5273(96)02595-8. [DOI] [PubMed] [Google Scholar]
- 110.Liao YH. Functional analysis of autoantibodies against ADP/ATP carrier from dilated cardiomyopathy. Int J Cardiol. 1996;54(2):165–169. doi: 10.1016/0167-5273(96)02594-6. [DOI] [PubMed] [Google Scholar]
- 111.Hsu HC, Yang P, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9(2):166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 112.Doreau A, Belot A, Bastid J, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10(7):778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 113.Yuan J, Yu M, Lin QW, et al. Neutralization of IL-17 inhibits the production of anti-ANT autoantibodies in CVB3-induced acute viral myocarditis. Int Immunopharmacol. 2010;10(3):272–276. doi: 10.1016/j.intimp.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 114.Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related proteinhigh, FOXP3-expressing CD25+ and CD25- regulatory T cells. J Immunol. 2006;176(8):4748–4756. doi: 10.4049/jimmunol.176.8.4748. [DOI] [PubMed] [Google Scholar]
- 115.Wei L, Wei-Min L, Cheng G, Bao-Guo Z. Upregulation of CD4+CD25+ T lymphocyte by adenovirus-mediated gene transfer of CTLA4Ig fusion protein in experimental autoimmune myocarditis. Autoimmunity. 2006;39(4):289–298. doi: 10.1080/08916930600758035. [DOI] [PubMed] [Google Scholar]
- 116.Wang S, Liu J, Wang M, Zhang J, Wang Z. Treatment and prevention of experimental autoimmune myocarditis with CD28 superagonists. Cardiology. 2010;115(2):107–113. doi: 10.1159/000256660. [DOI] [PubMed] [Google Scholar]
- 117.Lv K, Xu W, Wang C, Niki T, Hirashima M, Xiong S. Galectin-9 administration ameliorates CVB3 induced myocarditis by promoting the proliferation of regulatory T cells and alternatively activated Th2 cells. Clin Immunol. 2011;140(1):92–101. doi: 10.1016/j.clim.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 118.Shi Y, Fukuoka M, Li G, et al. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor beta–coxsackie–adenovirus receptor pathway. Circulation. 2010;121(24):2624–2634. doi: 10.1161/CIRCULATIONAHA.109.893248. [DOI] [PubMed] [Google Scholar]
- 119.Horwitz MS, Knudsen M, Ilic A, Fine C, Sarvetnick N. Transforming growth factor-beta inhibits coxsackievirus-mediated autoimmune myocarditis. Viral Immunol. 2006;19(4):722–733. doi: 10.1089/vim.2006.19.722. [DOI] [PubMed] [Google Scholar]
- 120.Begum-Haque S, Sharma A, Kasper IR, et al. Downregulation of IL-17 and IL-6 in the central nervous system by glatiramer acetate in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;204(1–2):58–65. doi: 10.1016/j.jneuroim.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 121.Haas J, Korporal M, Balint B, Fritzsching B, Schwarz A, Wildemann B. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4+CD25+FOXP3+CD31+ T-cells in patients with multiple sclerosis. J Neuroimmunol. 2009;216(1–2):113–117. doi: 10.1016/j.jneuroim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 122.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci. 2005;39(2):105–112. doi: 10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 123.Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2012.01.014. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miyao T, Floess S, Setoguchi R, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36(2):262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 125.Kryczek I, Wu K, Zhao E, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186(7):4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]