Abstract
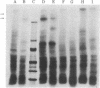
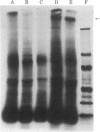
Two monoclonal antibodies directed against Epstein--Barr virus (EBV)-induced membrane antigens (MA) were isolated in this study. On of the monoclonal antibodies, designated 2F5.6, was an IgG2 which, as detected by membrane and fixed cell immunofluorescence, reacted with MA-positive lymphoblastoid cell lines that produced transforming EBV but not with the MA-positive P3HR-1 cell line that produced the lytic, nontransforming strain of this virus. This antibody precipitated the Mr 320,000/350,000 glycoprotein from B-95 virus infected cultures and the Mr 300,000 and 220,000/250,000 glycoproteins from Raji cells superinfected with P3HR-1 virus but did not precipitate any of these EBV-specific glycoproteins from the P3HR-1 cell line. In contrast, the second monoclonal antibody, IgM designated B10.3, reacted with all virus-producing cell lines including the P3HR-1 cell line. The identity of the glycoprotein that serves as the target for this antibody is still unknown. Neither antibody had neutralizing activity against the B-95 or P3HR-1 strain of EBV. These results indicated that the 2F5.6 monoclonal antibody was directed against an antigenic determinant on the major membrane glycoprotein which is common to transforming strains of EBV but absent from the lytic P3HR-1 stain whereas the B10.3 monoclonal antibody was directed against a group-specific EBV-induced membrane determinant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aya T., Osato T. Early events in transformation of human cord leukobytes by Epstain-Barr virus: induction of DNA synthesis, mitosis and the virus-associated nuclear antigen synthesis. Int J Cancer. 1974 Sep 15;14(3):341–347. doi: 10.1002/ijc.2910140307. [DOI] [PubMed] [Google Scholar]
- Delius H., Bornkamm G. W. Heterogeneity of Epstein-Barr virus. III. Comparison of a transforming and a nontransforming virus by partial denaturation mapping of their DNAs. J Virol. 1978 Jul;27(1):81–89. doi: 10.1128/jvi.27.1.81-89.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresen K. O., Cho M. S., zur Hausen H. Recovery of transforming EBV from non-producer cells after superinfection with non-transforming P3HR-1 EBV. Int J Cancer. 1978 Oct 15;22(4):378–383. doi: 10.1002/ijc.2910220403. [DOI] [PubMed] [Google Scholar]
- Gergely L., Klein G., Ernberg I. Host cell macromolecular synthesis in cells containing EBV-induced early antigens, studied by combined immunofluorescence and radioautography. Virology. 1971 Jul;45(1):22–29. doi: 10.1016/0042-6822(71)90108-5. [DOI] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. DNA of Epstein-Barr virus. II. Comparison of the molecular weights of restriction endonuclease fragments of the DNA of Epstein-Barr virus strains and identification of end fragments of the B95-8 strain. J Virol. 1977 Aug;23(2):421–429. doi: 10.1128/jvi.23.2.421-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman G. J., Lazarowitz S. G., Hayward S. D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci U S A. 1980 May;77(5):2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North J. R., Morgan A. J., Epstein M. A. Observations on the EB virus envelope and virus-determined membrane antigen (MA) polypeptides. Int J Cancer. 1980 Aug;26(2):231–240. doi: 10.1002/ijc.2910260216. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Henle G., Henle W. Production of antigens associated with Epstein-Barr virus in experimentally infected lymphoblastoid cell lines. J Natl Cancer Inst. 1971 Jun;46(6):1243–1250. [PubMed] [Google Scholar]
- Pearson G. R., Orr T. W. Antibody-dependent lymphocyte cytotoxicity against cells expressing Epstein-Barr virus antigens. J Natl Cancer Inst. 1976 Mar;56(3):485–488. doi: 10.1093/jnci/56.3.485. [DOI] [PubMed] [Google Scholar]
- Qualtiere L. F., Pearson G. R. Epstein-Barr virus-induced membrane antigens: immunochemical characterization of Triton X-100 solubilized viral membrane antigens from EBV-superinfected Raji cells. Int J Cancer. 1979 Jun 15;23(6):808–817. doi: 10.1002/ijc.2910230612. [DOI] [PubMed] [Google Scholar]
- Qualtiere L. F., Pearson G. R. Radioimmune precipitation study comparing the Epstein-Barr virus membrane antigens expressed on P3HR-1 virus-superinfected Raji cells to those expressed on cells in a B-95 virus-transformed producer culture activated with tumor-promoting agent (TPA). Virology. 1980 Apr 30;102(2):360–369. doi: 10.1016/0042-6822(80)90103-8. [DOI] [PubMed] [Google Scholar]
- Rabin H., Neubauer R. H., Hopkins R. F., Levy B. M. Characterization of lymphoid cell lines established from multiple Epstein-Barr virus (EBV)-induced lymphomas in a cotton-topped marmoset. Int J Cancer. 1977 Jul 15;20(1):44–50. doi: 10.1002/ijc.2910200109. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Sugden B., Summers W. C., Klein G. Nucleic acid renaturation and restriction endonuclease cleavage analyses show that the DNAs of a transforming and a nontransforming strain of Epstein-Barr virus share approximately 90% of their nucleotide sequences. J Virol. 1976 May;18(2):765–775. doi: 10.1128/jvi.18.2.765-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Geilinger K. Monoclonal antibodies against the major glycoprotein (gp350/220) of Epstein-Barr virus neutralize infectivity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5307–5311. doi: 10.1073/pnas.77.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima Y., Marczynska B., Nonoyama M. Transforming activity of Epstein-Barr virus obtained by superinfection of Raji cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2008–2010. doi: 10.1073/pnas.75.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]