Abstract
Background
P. vivax infection is characterised by relapsing fever, indicating reinfection by previously hidden parasites in the host. Relapsed infection can lead to the activation of the memory T cell pool, which may lead to protective immunity. This study aims to characterise immune responses in acute P. vivax-infected patients living in an area of central China characterised by only P. vivax infection.
Methodology/Principal Findings
We conducted a cross-sectional immune-phenotypic analysis of adults using the following inclusion criteria: acute P. vivax infection (N = 37), a history of P. vivax infection (N = 17), and no known history of P. vivax infection (N = 21). We also conducted a 2-week longitudinal analysis following acute P. vivax infection, in which PBMC proliferation was measured in response to P. vivax and P. falciparum blood stage lysates. Using flow cytometry, we showed elevated memory T cells in the blood during acute P. vivax infection. The levels of γδ T cells were two-fold higher than those measured in naive controls. This result suggested that in the two populations, memory and γδ T cells promptly responded to P. vivax parasites. Interestingly, P. falciparum antigens stimulated T cells obtained from P. vivax-infected patients during a day 14-convalescence, whereas lymphocytes from the naïve control group responded to a lower degree of convalescence.
Conclusions/Significance
Cell-mediated immunity during the convalescent period of the P. vivax-infected hosts was comprised of T cells that were specifically able to recognise P. falciparum antigens. Although the magnitude of the response was only half that measured after stimulation with P. vivax antigens, the matter of cross-antigenic stimulation is of great interest.
Introduction
Malaria is a common tropical disease associated with mortality among children infected with Plasmodium falciparum, primarily in African countries [1]. In contrast, P. vivax causes relapsing infection and is rarely fatal in most areas to which it is endemic [2]. The underlying factors leading to this opposite outcome between the two malarial infections are still unclear. The unique host-parasite relationship and the immune response to P. vivax have not yet been elucidated. One reason for this lack of understanding is the difficulty in maintaining P. vivax in an in vitro culture [3], [4]. Therefore, the supply of P. vivax and progress in the relevant research is limited. Recent incidences of P. vivax infection have increased gradually in tropical countries [5]. Whether this increased incidence indicates slow development of drug resistance by the P. vivax parasite remains to be verified. Several studies have been conducted in the regions in which P. falciparum is present along with P. vivax. These studies have shown that immunity to P. falciparum parasites readily exists in the host [6], [7], [8], [9], [10], [11], [12]. Thus, the majority of studies analysing immunological parameters and malaria severity have been performed in the regions where P. falciparum and P. vivax co-infection is normally found [13], [14], [15], [16], [17], [18], [19]. Recent studies suggest that immune suppression occurs in response to P. vivax infection because of elevated regulatory T cell levels [20], [21]. The strain-specific and serological cross-reactive immunity between blood stage antigens of P. falciparum and P. vivax has been well-documented [12], [22], [23]. One report has shown no effect on the cell-mediated response [24]. To verify immunity to malaria in P. vivax infection without an interfering immune response caused by P. falciparum, we chose to focus on central China, where P. vivax is the only cause of malaria infection [25]. In this study, we characterise acquired cell-mediated immunity by following P. vivax infection.
Materials and Methods
2.1 Study Populations
This study was approved by the Ethical Approval Committee of the Biomedical Institute of Anhui Medical University. Written informed consent was obtained from each individual before a blood sample was taken.
Blood samples were collected from 37 patients with acute P. vivax infections (AC) at Wuhe County Hospital, Guzhen County Hospital, and The First Hospital of Bengbu in Anhui Province in China. The patients were enrolled sequentially during June and July of 2009 and 2010. All patients enrolled in this study are inhabitants of Wuhe County, Guzhen County or the Bengbu City suburbs. Malaria transmission in this region is non-stable but can lead to malaria endemic in China. In the 1960s to 1970s, there were two malaria epidemics which were primarily caused by the P. vivax parasite. P. falciparum and P. vivax parasites were found together in this region until the end of the 1980s, but P. falciparum has not been found since the early 1990s. During the first decade of this century (from 2000 to 2010), malaria in this and other regions of China was mainly caused by the P. vivax parasite.
Diagnosis of P. vivax malaria infection was based on the examination of Giemsa-stained thick blood films. To prevent the misdiagnosis of mixed infection, confirmation of P. vivax was performed by polymerase chain reaction (PCR) using species-specific primers [26]. Clinical characteristics of the subjects are listed in Table 1. None of the volunteers were treated with a radical cure before blood collection. After collection, all malaria patients were cured by the standard regimen recommended by the Chinese Ministry of Health [27]. All patients were asked to follow up after blood collection; however, only 10 patients consented to give the follow up blood samples on days 7 and 14.
Table 1. Information 0061nd clinical data for P. vivax patients, uninfected malaria-exposed patients, and unexposed controls.* .
| N | Sex | Age | Parasitaemia | Temperature | ||
| M | F | years (range) | (%) | (oC) | ||
| P. vivax infection | 37 | 22 | 15 | 29 (19–47) | 0.15 (0.05–0.50) | 38.5 (37.8–39.0) |
| Uninfected malaria-exposed controls | 17 | 11 | 6 | 33 (19–49) | 0 | 36.5 (36.0–37.0) |
| Unexposed control | 21 | 10 | 11 | 29 (23–41) | 0 | 36.5 (36.0–37.0) |
mean (range)
Blood samples were collected from 17 additional healthy volunteers who were randomly selected from the same P. vivax-endemic area during the same period of time. These subjects, serving as uninfected malaria-exposed controls (UM), had P. vivax infections in the twelve months prior to the time of blood collection. Negative P. vivax parasitaemia was confirmed by PCR with species-specific primers for four strains of human malaria (P. falciparum, P. vivax, P. malariae, and P. ovale) [26]. Twenty-one healthy adults living in the Bengbu city area without previous malaria infection and with no antibodies to malaria parasites, as determined by ELISA, were recruited to serve as unexposed controls (UC).
2.2 Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
Venous blood samples from P. vivax patients, uninfected malaria-exposed controls, and unexposed controls were collected in heparinised tubes, and PBMCs were freshly separated by gradient centrifugation using Ficoll-Hypaque lymphocyte isolation kits (Tian Ji Hao Yao Biological Manufacturer, Tianjing, China) according to the manufacturer’s recommendations. The PBMC pellet was resuspended at 106 cells/mL in RPMI-1640 (GIBCO, Carlsbad, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Hangzhou Sijiqing Organism Engineering Materials, Hangzhou, China). The PBMCs were counted and resuspended in the RPMI-1640 for further experiments.
2.3 Parasite Cultures and Antigen Preparations
P. vivax-infected red blood cells (iRBC) enriched from the blood of acute P. vivax-infected Chinese patients were used as antigens for in vitro stimulation. Briefly, white blood cells were depleted from P. vivax infected blood by filtering through a sterile CF11 cellulose column (Whatman®, Maidstone, UK). Red blood cells were washed with RPMI-1640 by centrifugation at 1,190 g for 5 min. The parasites were cultured for 24–30 hrs at 5% hematocrit in McCoy’s 5A medium (GIBCO) supplemented with 25% human AB serum [4]. P. vivax from all samples was pooled to gain a large number of parasite antigens. For P. falciparum, the infected blood from acute P. falciparum-infected Thai patients was collected, white blood cells were removed, and the parasites were cultured at 5% hematocrit in RPMI-1640 medium supplemented with 10% human serum [28]. The isolated P. falciparum parasites from all samples were pooled to recover a large number of parasite antigens. Both P. vivax and P. falciparum parasites were maintained in an incubator containing 5% CO2, 5% O2, and 90% N2 until the parasites matured to schizont stages (≥6 nuclei). The late stage iRBCs were enriched by gradient centrifugation using 60% Percoll (GE Healthcare, Uppsala, Sweden) at 1,190 g for 10 min. The range of parasite purity was 60–99% for P. vivax and 80–100% for P. falciparum.
The enriched iRBC pellets were sonicated for 40 sec at 150 watts, and the protein concentration was determined by Bradford assay (Bio-Rad, Hercules, USA). The proteins were then aliquoted and stored at −70°C until use. Uninfected RBCs were processed as above, and the RBCs with a protein concentration equal to the malaria antigens were stored at −70°C to be used as a control.
2.4 Determination of Anti-P. vivax or anti-P. falciparum antibodies by Enzyme-linked Immunosorbent Assay (ELISA)
To determine the level of anti-P. vivax or anti-P. falciparum antibodies in P. vivax-infected patients, uninfected malaria-exposed and unexposed control groups, we performed an ELISA as previously reported [21]. Briefly, P. vivax, P. falciparum antigens, or uninfected red blood cell lysates (10 µg/ml) were coated into a 96-well plate. Plasma, at 1∶100 dilutions, was added into duplicate wells and incubated for 2 hrs at 37°C. Horseradish peroxidase-conjugated goat anti-human IgG (Caltag, Burlingame, USA) was added and incubated for 1 hr at room temperature, and 2, 20-azino-di- (3-ethylbenzthiazoline sulphonic acid) containing 50% hydrogen peroxide (Kirkepaard & Perry Laboratories, Gaithersberg, USA) was added and incubated for 30–60 min in the dark at room temperature. Enzyme activity was measured by an automated microplate reader. All values were subtracted from the baseline value obtained using uninfected red blood cell lysate before performing data analyses.
2.5 In vitro Stimulation
PBMCs from ten uninfected malaria-exposed controls, ten unexposed controls and ten P. vivax patients were freshly isolated and used for in vitro stimulation. PBMCs in RPMI-1640 supplemented with 25 mM HEPES, 2 mM glutamine, 40 µg/ml gentamicin and 10% heat-inactivated FCS were cultured at 2×105 cells/well in a round-bottom, 96-well plate (Costar, Corning, USA) for 5 days at 37°C and 5% CO2 in the presence of 1, 10, or 50 µg/ml of either the P. vivax or P. falciparum antigen (AMB47). An equivalent concentration of proteins from nRBC or medium alone was used as baseline stimulation for the lymphocytes. All stimulation values were subtracted from the median percentages of the baseline values (calculated by either values from medium alone or unstimulated RBCs). An anti-CD3 OKT antibody (2 µg/ml) was used as the positive control. All samples were performed in triplicate. After five days of activation, the cells were pulsed with 1 µCi H3-Thymidine for 16–18 hrs. The proliferation of PBMCs was then determined by β-counter FJ-353 (262 Factory, Xian, China), and the data are represented as counts per minute (CPM). The CPM values from the stimulation assay were calculated from the CPM of antigen-stimulated cultures divided by the values from cultures without antigen stimulation.
2.6 Determination of Surface and Intracellular Proteins by Flow Cytometric (FCM) Analysis
Two hundred microliters (µl) of whole blood were collected. RBC were lysed with RBC lysing solution at room temperature for 15 min and the remaining cells were washed with PBS before staining using various combinations of fluorochrome-conjugated mAbs (as shown in Table 2) for 30 min at 4°C. The cell pellets were analysed on a FACSCalibur using CELLQuest software (Becton Dickinson, San Jose, USA).
Table 2. List of the combination of fluorochrome-conjugated anti-human mAbs used for FCM analysis.
| Cell subpopulation | Combination of fluorochrome-conjugated mAbs | |||
| FITC | RPE | RPE-Cy5 | APC | |
| CD4+ MemoryT cells | anti-CD45ROa | anti-CD4a | anti-CD3a | |
| CD8+ MemoryT Cells | anti-CD45ROa | anti-CD8a | anti-CD3a | |
| Gamma deltaT cells | anti-gamma9b | anti-CD3a | ||
| B cells | anti-CD19a | anti-CD3a | ||
| Regulatory T cells | anti-Foxp3c | anti-CD25b | anti-CD3a | anti-CD4a |
| NK and NK T cells | anti-CD56a | anti-CD3a | ||
Caltag, Burlingame, USA;
Immunotech, Marseille, France.
This mAb was conjugated with Alexa fluor® 488 (BioLegend, San Diego, USA).
For intracellular staining, the cells were washed with a permeabilising solution according to the manufacturer’s instructions (BioLegend, San Diego, USA). The cells were incubated in the permeabilising buffer for 20 minutes at room temperature. Alexa fluor® 488-labeled anti-Foxp3 (BioLegend, San Diego, USA) was added and incubated for 30 min at room temperature. The cells were washed with PBS and fixed with 2% paraformaldehyde in PBS for acquisition and analysis on the FACSCalibur.
See the Supplementary Figure S1 for the FACS analysis of CD4+CD25hiFOXP3+ T cell populations.
2.7 Data Analysis
All data were analysed using the SPSS programme (Version 11.5, Chicago, USA). Non-parametric Kruskal-Wallis tests, followed by post tests, were used for statistical comparisons among three groups (Fig. 1 and 2). The data from phenotypic analyses of unstimulated lymphocytes in the results and figures (Fig. 1 and 2) were expressed as medians and interquartile ranges (25th–75th percentile). The mean differences (MD), 95% confidence intervals (CI), and P-values from the statistical analyses are also represented. The results were considered statistically significant when P<0.05 at a 95% confidence interval. The data from the in vitro studies (Fig. 3) are represented as the median ± standard error of the mean (SEM).
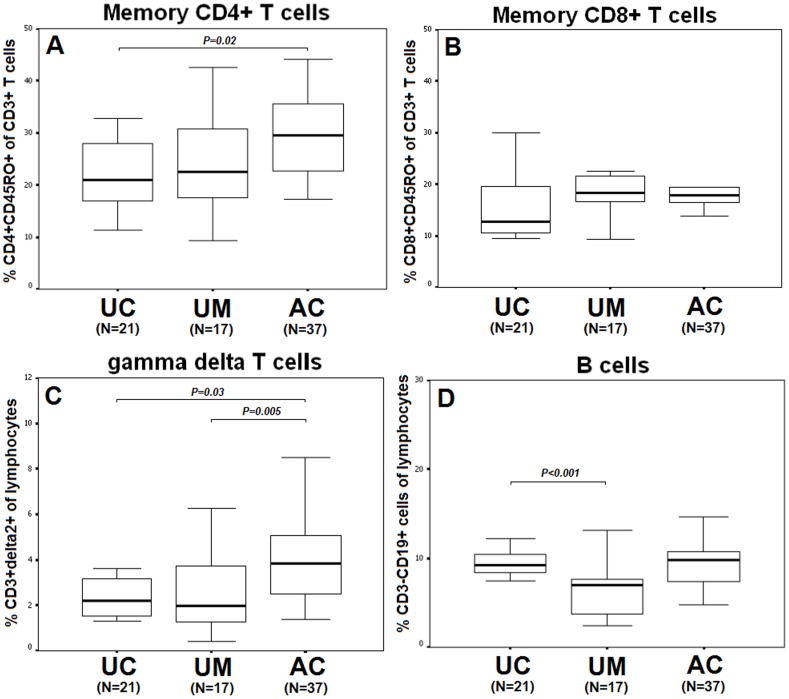
Figure 1. Comparison of memory CD4+ T cell (A) and CD8+ T cell (B), gamma delta T cell (C) and B cell (D) phenotypes between unexposed controls (UC:N = 21), uninfected malaria-exposed subjects (UM: N = 17), and patients with acute P. vivax infection (AC:N = 37).
Data are represented as median, inter-quartile ranges (box plots) and maximum and minimum (upper-lower lines).
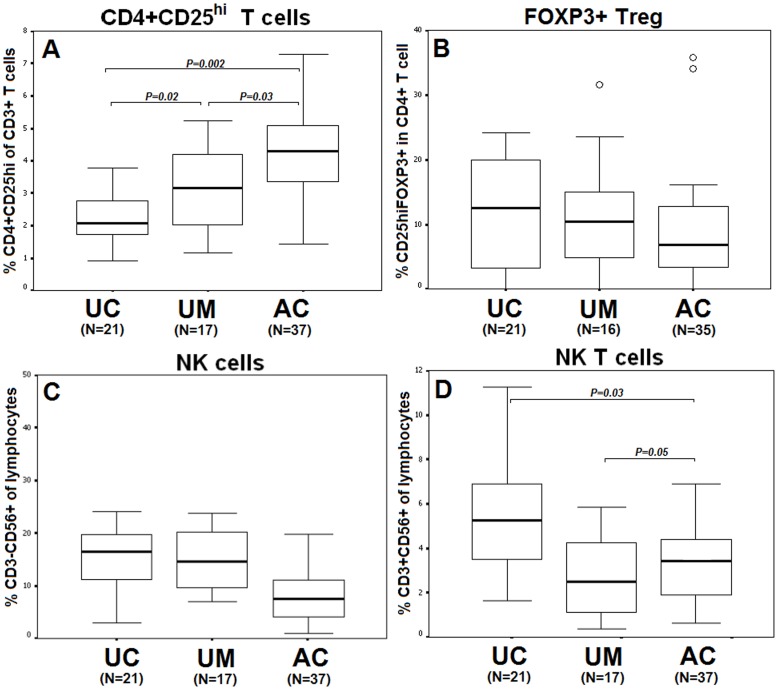
Figure 2. Comparison of regulatory T cell (CD4+CD25hi) (A) and CD4+CD25hiFOXP3+ (B), NK cell (C), and NKT cell (D) phenotypes between unexposed controls (UC:N = 21), uninfected malaria-exposed subjects (UM: N = 17), and patients with acute P. vivax infection (AC:N = 37).
Data are represented as median, inter-quartile ranges (box plots) and maximum and minimum (upper-lower lines).
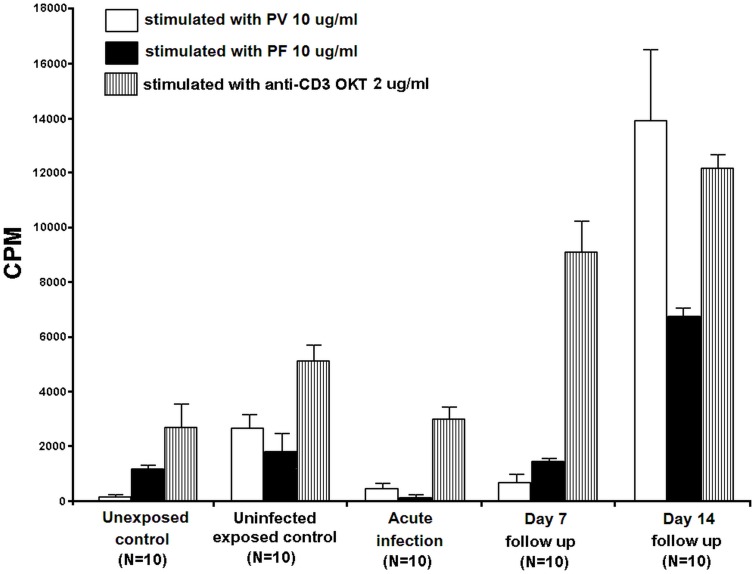
Figure 3. The activation of PBMCs from unexposed controls (N = 10), uninfected malaria-exposed controls (N = 10), and acute P. vivax-infected patients (N = 10), which was followed up on day 7 (N = 10) and day 14 (N = 10) with P. vivax antigens (10 µg/ml) (PV10) and P. falciparum antigens (10 µg/ml) (PF10).
The proliferation values were expressed as counts per minute (CPM). Data are shown in median±SE. All stimulation values were subtracted from the median percentages of baseline value.
Results
Phenotypic Analysis of Unstimulated Lymphocytes
Acute P. vivax infection modulated immune function by altering the reciprocal ratios of the major immune cells in various lymphocyte populations. The percentage of CD4+CD45RO+ memory T cells out of the total number of T cells (27%, N = 37, Fig. 1A) in the acute infection was significantly higher than that of the unexposed controls (20%, N = 21, MD = 8.8, 95%CI = 0.5–17.1, P = 0.02). However, this result was not significant when compared with the data from the uninfected malaria-exposed controls (22%, N = 17, P = 0.1). In contrast, the level of CD8+CD45RO+ memory T cells among patients with acute P. vivax infection (17%, N = 37) was similar to that of the uninfected malaria-exposed controls (18%, N = 17). These levels were higher than, but not significantly different from, the levels observed for the unexposed controls (13%, N = 21, P = 0.2, Fig. 1B).
The median percentage of CD3+δ2+ γδ T cells out of the total number of lymphocytes was significantly higher in the acute P. vivax infection (4%, N = 37) compared to the unexposed controls (2%, N = 21, MD = 2.1, 95%CI = −0.1–4.2, P = 0.03) and the uninfected malaria-exposed controls (2%, N = 17, MD = 3.2, 95%CI = 1.0–5.4, P = 0.005, Fig. 1C).
The median percentage of CD3−CD19+ B cells out of the total number of lymphocytes was significantly lower in the uninfected malaria-exposed group (6%, N = 17) compared to that of the unexposed controls (9%, N = 21, MD = 3.5, 95%CI = 0.9–6.0, P<0.001). During acute P. vivax infection (N = 37), the percentage of B cells was unchanged, showing similar levels to the percentages observed for unexposed controls (9%, N = 21, Fig. 1D).
Among CD3+ T cells, the median percentage of CD4+CD25hi T cells (Fig. 2A) in the uninfected malaria-exposed controls was significantly higher (3%, N = 17) than that of the unexposed controls (2%, N = 21, MD = 0.9, 95%CI = 0.1–1.7, P = 0.02). Moreover, the percentage was significantly elevated due to acute P. vivax infection (4%, N = 37) compared with the percentage observed in the unexposed controls (2%, N = 21, MD = 2.0, 95%CI = 0.7–3.0, P = 0.002) and the uninfected malaria-exposed controls (3%, N = 17, MD = 1.3, 95%CI = 0.9–2.7, P = 0.03).
In contrast to the percentage observed in the CD4+CD25hi T cells, the median percentage of Foxp3+ Treg (CD4+CD25hiFOXP3+ T cells) for the total number of CD4+ T cells among the unexposed controls was high (14%, N = 21) but not significant when compared with the percentage observed for the acute infection group (7%, N = 35, P = 0.2) and the uninfected malaria-exposed controls (10%, N = 16, P = 0.1). Two out of thirty-seven of the acute P. vivax infected patients had 35% FOXP3+ Treg (P = 0.001, compared to P. vivax-infected patients), and 1 out of 17 of the uninfected malaria-exposed controls had an extraordinarily high level (31%) of FOXP3+ Treg (P = 0.001, compared with the uninfected malaria-exposed control group, Fig. 2B).
The median percentage of NK cells in total lymphocytes decreased but was not significant during the acute P. vivax infection (6%, N = 37) compared with the results observed for the uninfected malaria-exposed controls (15%, N = 17) and the unexposed group (16%, N = 26, P>0.05, Fig. 2C). In contrast, the percentage of NKT cells out of total number of lymphocytes (Fig. 2D) was significantly higher during acute infection (3%, N = 37) compared with the uninfected malaria-exposed group (2%, N = 17, MD = 1.4, 95%CI = −0.5–3.0, P = 0.05) and was significantly lower than the results observed for the unexposed control group (5%, N = 21, MD = 1.4, 95%CI = −0.2–3.0, P = 0.03).
Proliferation of T Lymphocytes by Cross Protein Stimulation
Optimisation of PBMC stimulation was performed with PBMCs from 10 malaria patients using various concentrations of P. vivax, P. falciparum, and nRBC antigens. Stimulation with 10 µg/ml of P. vivax or P. falciparum antigens substantially elevated the level of H3-Thymidine uptake compared to cultures without antigen (data not shown). Therefore, further PBMC activation assays were performed using 10 µg/ml of malaria protein.
PBMCs from 10 healthy donors (unexposed) were co-cultured with the nRBC, P. vivax, and P. falciparum antigens to determine the baseline activation as shown in Fig 3. The CPM values showed a low-level response to P. vivax antigens (median±SEM = 152±80 CPM). Higher proliferation was observed when PBMCs were activated with the P. falciparum antigen (1186±247 CPM) compared to their activation by anti-OKT (2800±923 CPM).
PBMCs from 10 uninfected malaria-exposed controls co-cultured with nRBC, P. vivax, and P. falciparum antigens showed a more robust response to P. vivax antigens (2951±452 CPM) than P. falciparum antigen (1963±591 CPM) compared to the activation of PBMCs that was observed using anti-OKT (4968±606 CPM).
PBMCs from 10 acute P. vivax-infected patients and from their follow-up periods on days 7 and 14 were co-cultured with the nRBC, P. vivax, and P. falciparum antigens to determine the cross stimulation between the two malaria species. PBMCs from acute P. vivax-infected patients stimulated with P. vivax antigen showed a higher degree of proliferation (464±417 CPM) compared to PBMC stimulation with the P. falciparum antigen (116±110 CPM) after subtracting the background measured in the nRBC stimulated culture. Higher cell levels of proliferation were observed upon activation by anti-OKT (2916±552 CPM). The 7-day follow up PBMC proliferation assays from these patients showed no significant changes in proliferation upon stimulation with all three proteins. The stimulation by anti-OKT was 9008±1216 CPM. Interestingly, the 14-day follow up PBMC count from the infected patient group showed markedly increased proliferation upon stimulation with P. vivax antigens (13920±4623 CPM) compared to stimulation with P. falciparum antigens (6772±501 CPM, P = 0.03, Fig. 3). The level of stimulation induced by anti-OKT was 12209±450 CPM.
Discussion
People in northern Anhui Province in Central China have had experience with only P. vivax infection. The P. falciparum parasite is absent in this area. Therefore, the existing immunity to malaria infection is shaped exclusively based on exposure to the P. vivax parasites. The T cell phenotypes in people infected with only P. vivax have not been previously investigated. Our major finding was that upon acute P. vivax infection, CD4+ memory T cells and γδ T cells were elevated in the blood. CD8+ memory T cells were only detectable in the acute-infected and uninfected malaria-exposed patients. Moreover, a non-immune suppressive phenotype was found in P. vivax infection, demonstrated by the decreased expression of FOXP3+ Treg cells.
Elevation of CD4+ memory T cells in acute P. vivax-infected patients in China was consistent with a study in Thailand that showed that during both acute infection and the convalescent period, similarly high levels of CD4+ memory T cells were present [21]. However, the levels of both CD4+ and CD8+ memory T cells in acute P. vivax patients from Thailand were higher than the levels of these cells observed in China. The high level of CD4+ and CD8+ memory T cells may be due to frequent exposure to malaria parasites among the Thai population. A previous study has shown IL-4-producing CD4+CD45RO+ T cells in the circulation of people who are exposed to attenuated P. falciparum sporozoites [29]. This finding has been confirmed by recent studies showing the presence of IL-4-producing CD4+ memory T cells (CD45RO+CD27−) induced by attenuated P. falciparum sporozoites. [21], [30]. These memory T cells may also confer protection against P. vivax and long-term protection against P. falciparum infection. However, a recent study has demonstrated that T cells from naïve individuals (without previous exposure to malaria) can differentiate into memory T cells when exposed to cross-reacting malaria antigens. Therefore, the high level of memory T cells observed during acute P. vivax infection suggests memory T cell activation by the parasites or priming by exposure to commensal micro-organisms, pathogens and/or vaccine antigens carrying minimal T cell epitopes that cross-react with malaria proteins [31] . In comparison to the healthy group, the level of CD8+ memory T cells was readily detectable in P. vivax-infected patients. This finding has been confirmed by recent studies showing enhanced effector T-cell populations during acute P. vivax infection [32]. However, cytotoxic T cells have been shown to play a minor role in murine malaria, especially in the pre-erythrocytic stage [33]. Therefore, it is expected that regular exposure to P. vivax parasites could maintain cytotoxic T cells in uninfected malaria-exposed controls. Additionally, exposure to liver-stage antigens of P. vivax during an exo-erythrocytic or a hypnozoite stage could result in the generation of long-lived CD8+ memory T cells in immune individuals.
Elevated γδ T cells have been demonstrated in the current study and in various models of plasmodia infections [21], [34], [35], [36]. A recent study showed that γδ T cells reduce the severity of malaria in humans [13] and control the chronic parasitaemia of P. chabaudi infection in mice [37]. The cytotoxic role of γδ T cells was shown in vitro using P. falciparum [38], [39], [40], [41]. This result suggests that γδ T cells play both cytotoxic and regulatory roles, which presumably lead to protection. However, the role of γδ T cells in protection related to symptom intensity or parasitaemia during malaria infection needs to be investigated further.
Previous findings show the inhibition of B cell phenotypes in both P. falciparum and P. vivax infections [24], [42], [43]. In addition, our results showed the level of B cells in acute P. vivax-infected patients was unchanged. One possible explanation is that Treg may directly suppress B cells [44] or the proliferation of Th cells, resulting in the down-regulation of IL-2 or IL-4 production by responder lymphocytes and the subsequent suppression of B cell differentiation into plasma cells. In addition, we found significantly decreased levels of B cells in P. vivax-uninfected malaria-exposed controls, which was consistent with the low antibody response in P. vivax infection as previously shown [21]. A recent study in P. falciparum-exposed populations of Africa and South America showed significant changes in B cell subsets [42], [45], [46], [47]. The difference in these findings could be consistent with the conventional understanding that P. falciparum is a polyclonal B cell activator that stimulates the production of large amounts of antibodies in the infected host during the convalescent period. However, during P. vivax infection, the patients produce lower levels of antibodies compared to the patients infected by P. falciparum [48].
Treg constitutively express CD25, which is the IL-2/a chain receptor. Co-expression of CD25 with forkhead box protein P3 (FOXP3) dictates the immune-suppressive role of Treg via the release of IL-10 and TGF-β [49], [50]. The unchanged levels of FOXP3+ Treg cells among P. vivax-infected Chinese populations was in contrast to levels found in P. vivax-infected Thai and Brazilian populations, whose levels of FOXP3+ Treg cells were elevated [20], [51], [52]. One reason for this difference could be that patients living in the endemic areas of Thailand are likely to have experienced both P. vivax and P. falciparum infection due to the equal incidence of both species, whereas P. vivax is the only malaria species found in central China. Another reason could be that P. falciparum activates FOXP3+ Treg cells, as shown previously [53]. Taken together, these findings suggest that an activator for FOXP3+ Treg cells in the Thai patients may originate from P. falciparum parasites. However, we found some cases of P. vivax-infected patients and uninfected malaria-exposed controls that had high levels of FOXP3+ Treg cells. This result may be caused by frequent exposure to P. vivax infection among people living in endemic areas.
The reduction of NK cells in P. vivax-infected Chinese populations compared to healthy volunteers was consistent with what was found in P. vivax-infected Thai [54] and Ethiopian populations [24]. The activation of NK cells during acute infection occurred in the host and was not due to geographical region, genetic differences, or level of malaria endemicity. The protective role of NK cells in malaria infection is supported by the finding that these cells destroy P. falciparum-infected erythrocytes via direct contact [34], [55]. Moreover, a recent study showed that NK cells in peripheral blood are the primary producers of IFN-γ upon exposure to the infected erythrocytes in the presence of IL-12 [34]. Therefore, NK cells may be the prime effectors against P. vivax parasites during the acute phase of infection, although only a small change in the level of NK cells was observed. However, the cumulative number of these cells present during the entire course of infection could not be calculated. Therefore, the suppressive effect of Treg and the early activation of NKT cells towards the P. vivax-infected erythrocytes were exerted at the onset of the infection. However, the lower number of NK and NKT cells found during the acute infection period compared to the number of cells observed in the malaria-unexposed controls may be explained by the incidence of lymphopoenia documented during both P. falciparum and P. vivax infections [24], [56], [57].
The results of stimulation of acute P. vivax-infected patients during the 7-day follow up were similar to those of the unexposed controls, suggesting drug resistance or immune suppression caused by the parasites. However, all patients were given chloroquine for 3 days plus primaquine for 8 days for treatment of P. vivax-induced malaria. Several studies have investigated the effect of chloroquine on the human immune system in vivo, with a particular focus on immune suppression [58], [59]; however, the mechanisms underlying this treatment are not thoroughly understood. In our experiments, PBMCs from all day 7 follow up patients were collected 4 days after chloroquine treatment was completed. This finding suggests that there were residual effects of chloroquine on the T cells. However, the response of the patients’ lymphocytes during the convalescent period in this study was not restricted to only P. vivax antigens, although P. vivax is the only cause of malaria infection in this area of central China.
The response of PBMCs from uninfected malaria-exposed controls to P. vivax and P. falciparum antigens suggested that parasites stimulated memory cell pools of P. vivax-specific lymphocytes during infection [21]. The stimulation of uninfected malaria-exposed controls and day 14 follow up PBMCs with P. falciparum antigens also resulted in greater levels of lymphocyte proliferation. This finding suggests antigenic cross-reactivity between P. vivax and P. falciparum, as recently suggested, by using the serological cross-reaction [13], [23] and the accumulation of both P. vivax and P. falciparum-specific memory T cells during the convalescent period, resulting in the robust response to both P. vivax and P. falciparum antigens. This finding sheds light on a possible new strategy for malaria vaccine design, i.e., a combination of antigens derived from P. vivax and P. falciparum parasites, which may attenuate malaria severity. P. vivax parasites do not suppress the immune function of the infected host, which commonly accompanies P. falciparum infection. However, elucidation of the immune cell-parasite interaction during malaria infection using single or mixed species needs to be investigated in depth to gain a better understanding of immunity to malaria infections.
Supporting Information
FACS analysis of the CD4+CD25hiFOXP3+ T cell population (A) represents the CD3+ population. The R1 gate was used for (B), and R2 was the gating population of (C). 20000 of total evens were used for each gate.
(DOCX)
Acknowledgments
The authors wish to thank Dr. Xiu-zhi Ma of Wuhe County Hospital, Dr. Jie Mei of Guzheng County Hospital, and Dr. Mu-shan Zhu of The First Hospital of Bengbu for characterisation and recruitment of malaria patients. We also thank Shoufeng Hu, Department of Parasitology, and Hongta Huang and Jina Jiang, Department of Immunology, Bengbu Medical College, for technical support.
Funding Statement
This work was supported by the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative; a grant [D43TW006571] funded by the Fogarty International Center, NIH; the Commission on Higher Education to KJ [CHE-RES-PD]; the Thailand Research Fund to RU [BRG498009]; The Key Program of Natural Science Foundation of the Anhui Higher Education Institutions (no. KJ2012A200) to HX; and the key research programme [2004sys008] for Key Lab of Provincial University from Anhui Provincial Education Department to BL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sachs J, Malaney P (2002) The economic and social burden of malaria. Nature 415: 680–685. [DOI] [PubMed] [Google Scholar]
- 2. Krotoski WA, Krotoski DM, Garnham PC, Bray RS, Killick-Kendrick R, et al. (1980) Relapses in primate malaria: discovery of two populations of exoerythrocytic stages. Preliminary note. Br Med J 280: 153–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panichakul T, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Cui L, et al. (2007) Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. . Int J Parasitol 37: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 4. Udomsangpetch R, Kaneko O, Chotivanich K, Sattabongkot J (2008) Cultivation of Plasmodium vivax . Trends Parasitol 24: 85–88. [DOI] [PubMed] [Google Scholar]
- 5. Sattabongkot J, Tsuboi T, Zollner GE, Sirichaisinthop J, Cui L (2004) Plasmodium vivax transmission: chances for control? Trends Parasitol 20: 192–198. [DOI] [PubMed] [Google Scholar]
- 6. Achidi EA, Perlmann H, Salimonu LS, Perlmann P, Walker O, et al. (1995) A longitudinal study of seroreactivities to Plasmodium falciparum antigens in Nigerian infants during their first year of life. Acta Trop 59: 173–183. [DOI] [PubMed] [Google Scholar]
- 7. Hviid L (1998) Clinical disease, immunity and protection against Plasmodium falciparum malaria in populations living in endemic areas. Expert Rev Mol Med 1998: 1–10. [DOI] [PubMed] [Google Scholar]
- 8. Hviid L (2005) Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop 95: 270–275. [DOI] [PubMed] [Google Scholar]
- 9. Iriemenam NC, Okafor CM, Balogun HA, Ayede I, Omosun Y, et al. (2009) Cytokine profiles and antibody responses to Plasmodium falciparum malaria infection in individuals living in Ibadan, southwest Nigeria. Afr Health Sci 9: 66–74. [PMC free article] [PubMed] [Google Scholar]
- 10. Osier FH, Polley SD, Mwangi T, Lowe B, Conway DJ, et al. (2007) Naturally acquired antibodies to polymorphic and conserved epitopes of Plasmodium falciparum merozoite surface protein 3. Parasite Immunol 29: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riley EM, Allen SJ, Wheeler JG, Blackman MJ, Bennett S, et al. (1992) Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol 14: 321–337. [DOI] [PubMed] [Google Scholar]
- 12. Doolan DL, Dobano C, Baird JK (2009) Acquired immunity to malaria. Clin Microbiol Rev 22: 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chuangchaiya S, Jangpatarapongsa K, Chootong P, Sirichaisinthop J, Sattabongkot J, et al. (2010) Immune response to Plasmodium vivax has a potential to reduce malaria severity. Clin Exp Immunol 160: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Douglas NM, Nosten F, Ashley EA, Phaiphun L, van Vugt M, et al. (2011) Plasmodium vivax recurrence following falciparum and mixed species malaria: risk factors and effect of antimalarial kinetics. Clin Infect Dis 52: 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kochar DK, Tanwar GS, Khatri PC, Kochar SK, Sengar GS, et al. (2010) Clinical features of children hospitalized with malaria-a study from Bikaner, northwest India. Am J Trop Med Hyg 83: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin E, Kiniboro B, Gray L, Dobbie S, Robinson L, et al. (2010) Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS One 5: e9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin JT, Bethell D, Tyner SD, Lon C, Shah NK, et al. (2011) Plasmodium falciparum gametocyte carriage is associated with subsequent Plasmodium vivax relapse after treatment. PLoS One 6: e18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michon P, Cole-Tobian JL, Dabod E, Schoepflin S, Igu J, et al. (2007) The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg 76: 997–1008. [PMC free article] [PubMed] [Google Scholar]
- 19. Snounou G, White NJ (2004) The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol 20: 333–339. [DOI] [PubMed] [Google Scholar]
- 20. Jangpatarapongsa K, Chootong P, Sattabongkot J, Chotivanich K, Sirichaisinthop J, et al. (2008) Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol 38: 2697–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jangpatarapongsa K, Sirichaisinthop J, Sattabongkot J, Cui L, Montgomery SM, et al. (2006) Memory T cells protect against Plasmodium vivax infection. Microbes Infect 8: 680–686. [DOI] [PubMed] [Google Scholar]
- 22. Woodberry T, Minigo G, Piera KA, Hanley JC, de Silva HD, et al. (2008) Antibodies to Plasmodium falciparum and Plasmodium vivax merozoite surface protein 5 in Indonesia: species-specific and cross-reactive responses. J Infect Dis 198: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diggs CL, Sadun EH (1965) Serological Cross Reactivity between Plasmodium vivax and Plasmodium falciparum as Determined by a Modified Fluorescent Antibody Test. Exp Parasitol 16: 217–223. [DOI] [PubMed] [Google Scholar]
- 24. Kassa D, Petros B, Mesele T, Hailu E, Wolday D (2006) Characterization of peripheral blood lymphocyte subsets in patients with acute Plasmodium falciparum and P. vivax malaria infections at Wonji Sugar Estate, Ethiopia. Clin Vaccine Immunol 13: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Wang L, Fang L, Ma J, Xu Y, et al. (2008) Spatial analysis of malaria in Anhui province, China. Malar J 7: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN (1993) Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol 58: 283–292. [DOI] [PubMed] [Google Scholar]
- 27. Qingjun L, Jihui D, Laiyi T, Xiangjun Z, Jun L, et al. (1998) The effect of drug packaging on patients’ compliance with treatment for Plasmodium vivax malaria in China. Bull World Health Organ 76 Suppl 1 21–27. [PMC free article] [PubMed] [Google Scholar]
- 28. Trager W, Jensen JB (1997) Continuous culture of Plasmodium falciparum: its impact on malaria research. Int J Parasitol 27: 989–1006. [DOI] [PubMed] [Google Scholar]
- 29. Bergmann ES, Ballou RW, Krzych U (1997) Detection of CD4+CD45RO+ T lymphocytes producing IL-4 in response to antigens on Plasmodium falciparum erythrocytes: an in vitro correlate of protective immunity induced with attenuated Plasmodium falciparum sporozoites. Cell Immunol 180: 143–152. [DOI] [PubMed] [Google Scholar]
- 30. Palmer DR, Krzych U (2002) Cellular and molecular requirements for the recall of IL-4-producing memory CD4(+)CD45RO(+)CD27(-) T cells during protection induced by attenuated Plasmodium falciparum sporozoites. Eur J Immunol 32: 652–661. [DOI] [PubMed] [Google Scholar]
- 31. Wipasa J, Okell L, Sakkhachornphop S, Suphavilai C, Chawansuntati K, et al. (2011) Short-lived IFN-gamma effector responses, but long-lived IL-10 memory responses, to malaria in an area of low malaria endemicity. PLoS Pathog 7: e1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salwati E, Minigo G, Woodberry T, Piera KA, de Silva HD, et al. (2011) Differential cellular recognition of antigens during acute Plasmodium falciparum and Plasmodium vivax malaria. J Infect Dis 203: 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsuji MA (2010) retrospective evaluation of the role of T cells in the development of malaria vaccine. Exp Parasitol 126: 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Artavanis-Tsakonas K, Riley EM (2002) Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol 169: 2956–2963. [DOI] [PubMed] [Google Scholar]
- 35. Bordessoule D, Gaulard P, Mason DY (1990) Preferential localisation of human lymphocytes bearing gamma delta T cell receptors to the red pulp of the spleen. J Clin Pathol 43: 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perera MK, Carter R, Goonewardene R, Mendis KN (1994) Transient increase in circulating gamma/delta T cells during Plasmodium vivax malarial paroxysms. J Exp Med 179: 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seixas EM, Langhorne J (1999) gammadelta T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J Immunol 162: 2837–2841. [PubMed] [Google Scholar]
- 38. Behr C, Dubois P (1992) Preferential expansion of V gamma 9 V delta 2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum . Int Immunol 4: 361–366. [DOI] [PubMed] [Google Scholar]
- 39. Farouk SE, Mincheva-Nilsson L, Krensky AM, Dieli F, Troye-Blomberg M (2004) Gamma delta T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. Eur J Immunol 34: 2248–2256. [DOI] [PubMed] [Google Scholar]
- 40. Goodier M, Fey P, Eichmann K, Langhorne J (1992) Human peripheral blood gamma delta T cells respond to antigens of Plasmodium falciparum . Int Immunol 4: 33–41. [DOI] [PubMed] [Google Scholar]
- 41. Troye-Blomberg M, Worku S, Tangteerawatana P, Jamshaid R, Soderstrom K, et al. (1999) Human gamma delta T cells that inhibit the in vitro growth of the asexual blood stages of the Plasmodium falciparum parasite express cytolytic and proinflammatory molecules. Scand J Immunol 50: 642–650. [DOI] [PubMed] [Google Scholar]
- 42. Asito AS, Moormann AM, Kiprotich C, Ng’ang’a ZW, Ploutz-Snyder R, et al. (2008) Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malar J 7: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee HK, Lim J, Kim M, Lee S, Oh EJ, et al. (2001) Immunological alterations associated with Plasmodium vivax malaria in South Korea. Ann Trop Med Parasitol 95: 31–39. [DOI] [PubMed] [Google Scholar]
- 44. Lim HW, Hillsamer P, Banham AH, Kim CH (2005) Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol 175: 4180–4183. [DOI] [PubMed] [Google Scholar]
- 45. Nogaro SI, Hafalla JC, Walther B, Remarque EJ, Tetteh KK, et al. (2011) The breadth, but not the magnitude, of circulating memory B cell responses to P. falciparum increases with age/exposure in an area of low transmission. PLoS One 6: e25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weiss GE, Clark EH, Li S, Traore B, Kayentao K, et al. (2011) A positive correlation between atypical memory B cells and Plasmodium falciparum transmission intensity in cross-sectional studies in Peru and Mali. PLoS One 6: e15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, et al. (2009) Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol 183: 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ribeiro CD (1988) Polyclonal B-cell activation (PBA) what have we hearned from the study of malaria? Mem Inst Oswaldo Cruz 83: 633–648. [DOI] [PubMed] [Google Scholar]
- 49. Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, et al. (2004) IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol 172: 5986–5993. [DOI] [PubMed] [Google Scholar]
- 50. Shevach EM (2002) CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2: 389–400. [DOI] [PubMed] [Google Scholar]
- 51. Bueno LL, Morais CG, Araujo FF, Gomes JA, Correa-Oliveira R, et al. (2010) Plasmodium vivax: induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One 5: e9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goncalves RM, Salmazi KC, Santos BA, Bastos MS, Rocha SC, et al. (2010) CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses? Infect Immun 78: 4763–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walther M, Tongren JE, Andrews L, Korbel D, King E, et al. (2005) Upregulation of TGF-b, Foxp3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23: 287–296. [DOI] [PubMed] [Google Scholar]
- 54. Srisurapanon S, Wiwattanakul S, Apibal S, Suwannuruk R, Sujimanaskul S, et al. (2003) Lymphocyte subpopulations in malaria infected individuals living in an endemic area. Southeast Asian J Trop Med Public Health 34: 310–315. [PubMed] [Google Scholar]
- 55. Mavoungou E, Luty AJ, Kremsner PG (2003) Natural killer (NK) cell-mediated cytolysis of Plasmodium falciparum-infected human red blood cells in vitro. Eur Cytokine Netw 14: 134–142. [PubMed] [Google Scholar]
- 56. Hviid L, Kemp K (2000) What is the cause of lymphopenia in malaria? Infect Immun 68: 6087–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Richards MW, Behrens RH, Doherty JF (1998) Short report: hematologic changes in acute, imported Plasmodium falciparum malaria. Am J Trop Med Hyg 59: 859. [DOI] [PubMed] [Google Scholar]
- 58. Landewe RB, Miltenburg AM, Verdonk MJ, Verweij CL, Breedveld FC, et al. (1995) Chloroquine inhibits T cell proliferation by interfering with IL-2 production and responsiveness. Clin Exp Immunol 102: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schultz KR, Bader S, Paquet J, Li W (1995) Chloroquine treatment affects T-cell priming to minor histocompatibility antigens and graft-versus-host disease. Blood 86: 4344–4352. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FACS analysis of the CD4+CD25hiFOXP3+ T cell population (A) represents the CD3+ population. The R1 gate was used for (B), and R2 was the gating population of (C). 20000 of total evens were used for each gate.
(DOCX)