Abstract
MicroRNAs (miRNAs) are small noncoding RNA that play an important role in posttranscriptional regulation of mRNA. Genetic variations in miRNAs or their target sites have been shown to alter miRNA function and have been associated with risk for several diseases. Previous studies have focused on the most abundant type of genetic variations, single nucleotide polymorphisms (SNPs) that affect miRNA-mRNA interactions. Here, we systematically identified small insertions and deletions (indels) in miRNAs and their target sites, and investigated the effects of indels on miRNA targeting. We studied the distribution of indels in miRNAs and their target sites and found that indels in mature miRNAs, experimentally supported miRNA target sites and PAR-CLIP footprints have significantly lower density compared to flanking regions. We identified over 20 indels in the seed regions of miRNAs, which may disrupt the interactions between these miRNAs and their target genes. We also identified hundreds of indels that alter experimentally supported miRNA target sites. We mapped these genes to human disease pathways to identify indels that affect miRNA targeting in these pathways. We also used the results of genome-wide association studies (GWAS) to identify potential links between miRNA-related indels and diseases.
Introduction
MicroRNAs (miRNAs) are short non-coding RNAs that function as post-transcriptional regulators of genes, repressing mRNA translation and causing mRNA decay [1]. Initial miRNA transcripts are processed in the nucleus to produce ∼100 nt long hairpin precursors, which are exported to the cytoplasm and processed into ∼22 nt long mature miRNA sequences that act on their mRNA targets within the RNA-induced silencing complex (RISC) [1]. MicroRNA target recognition is highly dependent on interactions between complementary sequences in miRNA seed regions and target sites in mRNAs [2], [3], [4], [5]. Therefore, miRNA targeting and function can be affected by sequence polymorphisms in miRNAs and their target sites. Over the last several years, several association studies have identified polymorphisms in miRNA genes and their target sites that are linked with risk for several diseases, including schizophrenia [6], nonsyndromic progressive hearing loss [7], cancer [8], [9], [10], [11], [12], [13], [14], [15], Parkinson’s disease [16], and stroke [17]. While experimental evidence providing a direct, functional role for these polymorphisms in disease development remains weak for most of these associations, some recent experiments have investigated how disease-associated polymorphisms impact the expression or function of miRNAs [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. For example, a single nucleotide polymorphism rs2910164 in the pre-miRNA of miR-146a has been associated with increased risk for several types of cancer [8], [9], [10], [11], [12]. To supplement these association study results, further experimental investigation of miR-146a and rs2910164 has shown that the polymorphism results in variation of expression of miR-146a [8], [9], [10], [11], [12] and its targets [18], including BRCA1 [8], and that miR-146a promotes cell proliferation and colony formation in the NIH/3T3 cell line [10]. In another study, a “TTCA” deletion (rs3783553) in the 3′ UTR of IL1A, a gene that induces antitumor cell immunity, was shown to be associated with hepatocellular carcinoma risk, and subsequent experiments showed that the deletion enhances binding of miR-122 and miR-378 to IL1A, reducing its in vivo expression [15].
As links between miRNA-related polymorphisms and human diseases have been identified, there has been increasing interest in systematic evaluations of polymorphisms within miRNAs and their target sites. Saunders et al. performed one of the first analyses of polymorphisms in miRNAs and found that there was a relatively low level of variation within miRNAs compared to surrounding regions or miRNA target sites [19]. The low level of variation in miRNAs, particularly within mature miRNA sequences and miRNA seed regions, has been subsequently confirmed by further investigation of polymorphisms in humans [20], [21] and targeted sequencing of miRNAs in Arabidopsis [22]. Bao et al [23] developed a database, PolymiRTS, to systematically characterize SNPs in microRNA target sites and link them with complex traits. This database has been recently updated to integrate new data, including SNPs in miRNAs, experimentally supported miRNA target sites, and the results of genome-wide association studies (GWAS) of human diseases [24].
With the recent advances in sequencing technologies, there has been a rapid increase in the number of small indels identified in the human genome [25], [26], [27]. Genome wide analysis has shown that indels are the second most common type of genetic variants after SNPs and that they comprise approximately 18% of known variants [26], [28]. While previous analysis of polymorphisms has focused mainly on SNPs and a handful of indels within miRNAs and their target sites that have been identified [19], [22], a full investigation of how these newly identified indels may impact miRNA function has yet to be performed. There has also been a rapid growth in the number of experimentally supported miRNA target sites in humans [29], reducing the reliance on computational methods for miRNA target prediction. To a large extent, this growth is due to recent experiments, designated HITS-CLIP [30] and PAR-CLIP [31], that identified the specific mRNA sequences that interact with miRNAs in Ago protein-RNA complexes in the mouse brain and human embryonic kidney cells (HEK294), respectively. While large scale evaluation of miRNA targeting based on these experiments is still incomplete because they have only been performed for two specific cell and tissue types, the experiments have greatly expanded the number of experimentally determined miRNA target sites. Additionally, there have been a significant number of miRNA targets identified by low-throughput experiments utilizing, for example, luciferase reporter assays [32].
Here, we created a comprehensive collection of indels in miRNAs and their target sites in the human genome and analyzed the potential functional impact of these indels. We determined polymorphic miRNAs that have been previously associated with the risk of diseases and investigated how indels in miRNA target sites may alter miRNA regulation in human disease pathways. We also identified potential links between the indels altering miRNA targeting and human diseases using the results of association studies.
Results
Distribution of INDELs in 3′ UTRs and miRNAs
We collected all indel variations in dbSNP (build 135) that were located within either miRNAs or the 3′ UTRs of genes, the genetic regions that are believed to harbor the majority of functional miRNA target sites. Among the ∼6 million indels in dbSNP (build 135), 181 indels were located within 124 pre-miRNAs, including 51 in the mature sequence of 43 miRNAs and 26 in the seed region of 22 miRNAs. Additionally, 56,724 indels were located within 3′ UTRs of 9,420 genes, potentially affecting miRNA binding to these genes. Following the classification system provided by Mills et al [25], indels were categorized into three classes, namely (i) single-base-pair indels, (ii) repeat expansions consisting of repeated sequences of one or more nucleotides, and (iii) an “other” class of multiple-base-pair indels other than repeat expansions. The majority of indels within both miRNAs (55%) and 3′ UTRs (57%) were single-base-pairs, while there were fewer repeat expansions (10% of indels within miRNAs, 14% of indels in 3′ UTRs) and indels in the “other” class (35% of indels within miRNAs, 28% in 3′ UTRs). The distribution of indels in 3′UTRs and miRNAs was similar to the distribution across the entire genome (Figure S1).
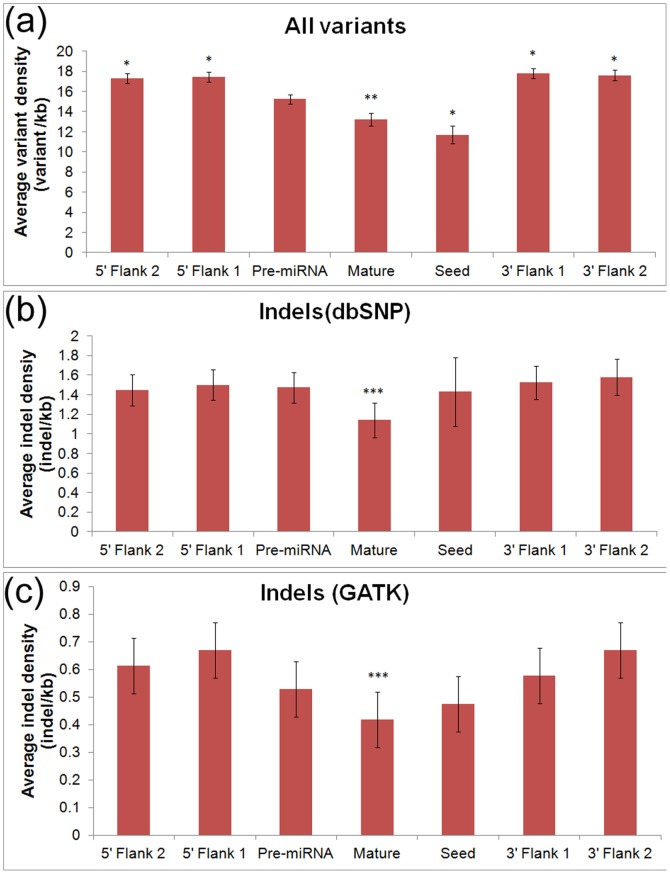
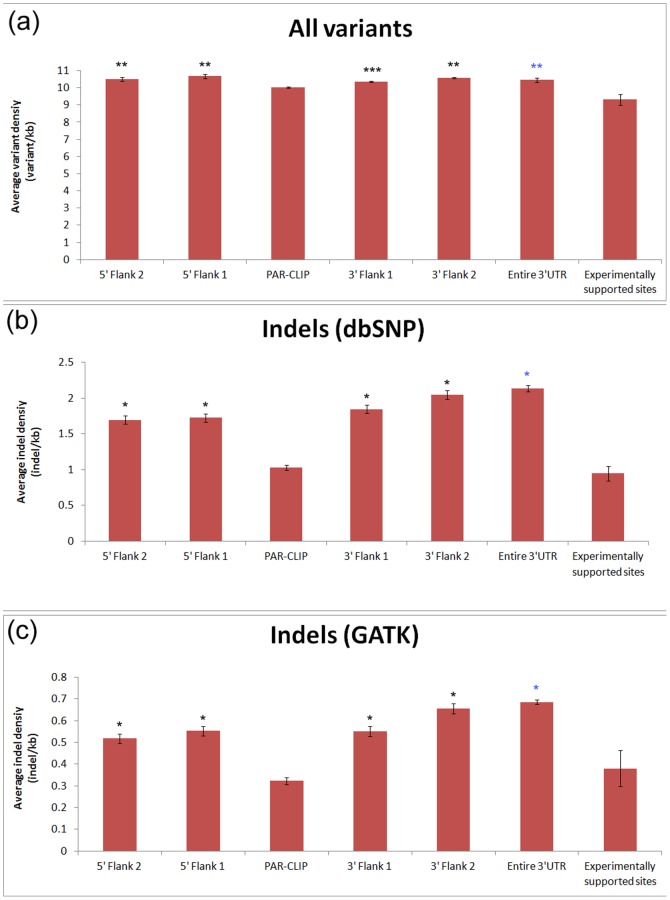
MicroRNAs and their target sites have been shown to have lower polymorphism density than their surrounding regions [19], [21]. Furthermore, the level of variation within mature miRNA sequences and their seed regions has been found to be lower than that in complete precursor miRNA sequences. To determine whether indels have a similar distribution pattern in these regions, we calculated the average density for indels and all types of polymorphisms both within miRNAs and in the genomic regions flanking miRNAs (Figure 1). The average density of all polymorphisms matched the distribution patterns that have been found previously [19], [21] (Figure 1a); the density of all polymorphisms in mature and seed sequences is significantly lower than the polymorphism density within pre-miRNAs, while the density of flanking regions was significantly higher than that of the pre-miRNAs. In contrast, the density of indels in miRNAs compared with flanking regions does not show the same pattern (Figure 1b), as the indel density in pre-miRNAs is similar to the indel density in miRNA seed regions and flanking regions. For further investigation of this finding, we collected a set of high-confidence indels data from the GATK resource bundle (described in the Materials and Methods section) and identified their density in miRNAs (Figure 1c). Several features observed for the indel density within miRNAs and their flanking regions for the GATK indels (Figure 1c) were similar to those found using all indels in dbSNP (Figure 1b). However, we did find that the density of indels in the miRNA seed regions identified from the GATK data, while not as low as the density of indels in the entire mature sequences, was slightly lower than the indel density of flanking regions. One significant feature was maintained for the density of indels from dbSNP and GATK as well as the density of all polymorphisms, as the polymorphism density was significantly lower in mature miRNAs than in the flanking regions. We also investigated the density of indels in the miRNA target sites. We calculated the average density for indels and all types of variants in experimentally supported miRNA target sites, the entire 3′UTRs, PAR-CLIP footprints and their flanking regions (Figure 2). We found that the density of indels (both dbSNP and GATK indels) in PAR-CLIP footprints and experimentally supported miRNA target sites are significantly lower than those of flanking regions and entire 3′UTRs (p<10−12). The density distribution of all variants has a similar distribution pattern, but with less significant p-values (Figure 2a). These observations could suggest that there is selective pressure against genetic variants in miRNAs and their target sites and the selective pressure against indels in miRNA target sites is particularly strong (Figure 2b and Figure 2c).
Figure 1. Density of all genetic variants (a) and indels (b) in dbSNP 135 as well as indels (c) from the GATK resource bundle in pre-miRNAs, mature miRNAs, miRNA seed regions, and flanking regions.
Flanking regions 1 and 2 represent successive sequences adjacent to pre-miRNAs that were equal to the length of the pre-miRNA (∼100 bp). Error bars indicate the standard error. The density of all genetic variants (a) in pre-miRNAs was significantly different from the density in flanking regions, mature miRNAs, and seed regions (*p<0.01, **0.01<p<0.05). The density of indels in mature sequences (b) and (c) was significantly different than the density calculated by averaging across all four flanking regions (***0.01<p<0.05).
Figure 2. Density of all genetic variants (a) and indels (b) in dbSNP 135 as well as indels (c) from the GATK resource bundle in PAR-CLIP footprints and flanking regions, entire 3′ UTR and experimentally validated target sites.
Flanking regions 1 and 2 represent successive sequences adjacent to PAR-CLIP footprints that were equal to the length of the footprints (∼41 bp). Error bars indicate the standard error. The density of all genetic variants (a) in PAR-CLIP footprints was significantly different from the density in flanking regions (**10−5<p<10−3, ***p = 0.04). The density of indels in PAR-CLIP footprints (b) and (c) was significantly different from the density in flanking regions (*p<10−12). The density of all genetic variants (a) in experimentally validated targets was significantly different from the density in entire 3′ UTR regions (**p = 5.7×10−7). The density of indels in experimentally validated targets (b) and (c) was significantly different from the density in 3′ UTR regions (*p<10−12).
Indels in miRNAs
Polymorphisms within miRNAs have been shown to alter miRNA mediated gene regulation and contribute to disease pathogenesis [8], [9], [10], [11], [12], [18]. They may impact miRNA function through two main mechanisms, by either altering miRNA biogenesis and expression or by affecting the binding of the miRNA to its mRNA targets, potentially disrupting the targeting to its original targets and creating a new set of targets. While polymorphisms throughout the entire miRNA precursor sequence are likely to act through only the first mechanism, those within mature miRNA sequences, particularly within miRNA seed regions, can change miRNA function through both mechanisms. In total, we identified 144 miRNAs containing indels, including 25 miRNAs with indels in the seed region. Many of the miRNAs that contain indels in their precursor sequence have been previously linked with diseases. We identified indels in the pre-miRNA sequences of several miRNAs that have been linked to cancers. For example, the precursor of miR-558, which has been previously linked with aggressive neuroblastoma [33], contained 5 indels, and an indel (rs34385807) is located the pre-miRNA sequence of miR-141, which is involved in cancer proliferation [34], [35], [36], [37] and has been shown to target the tumor suppressor PTEN [38]. Additional precursor sequences of miRNAs containing indels include miR-520h [39], [40], [41], miR-486 [42], miR-489 [43], miR-223 [44], miR-373 [45], miR-630 [46] and miR-1233 [17], which have been shown to be involved in cancer development, and miR-631, which is associated with risk of coronary artery disease [47].
We also identified all experimentally supported targets of the miRNAs containing indels by searching PAR-CLIP results [31] as well as experimental targets contained in miRecords [48], TarBase 5.0 [49] and miTarBase [50] (Table S1). We are most interested in the potential functional impact of indels within mature miRNA sequences and seeds and will focus our discussion only on miRNAs with indels in their mature sequences. The mature sequence of miR-940, which has been shown to target the signaling gene SEMA3F [21], contains an indel (rs3536504) that may disrupt the binding of miR-940 to SEMA3F and other targets. Indels within miRNA seed regions may be particularly deleterious as complementarity between this region and the mRNA target is crucial for miRNA target recognition. One miRNA which has an indel in its seed region is miR-513a-1, a miRNA known to post-transcriptionally regulate B7-H1 [51]. This indel, the single nucleotide insertion rs35027589, may disrupt the targeting of B7-H1 by miR-513a and have downstream effects on the B7-H1/PD-1 pathway, a critical pathway for modulating immune responses to cancer [52]. Similarly, miR-562 contains an indel, the 18 bp deletion rs140596642, removing a large portion of the miRNA including the seed region, which may play a critical role in the development of Wilms’ tumor by both causing increased expression of miR-562 and dysregulation of its targets including EYA1 [53]. Another indel, the five nucleotide deletion rs138461304 in the seed region of miR-559, may disrupt targeting of ERBB2 by miR-559 [54], resulting in overexpression of ERBB2, an abnormality that has been associated with cancer [54], [55]. A four nucleotide long deletion indel included in the GATK resource bundle is located in the seed region of miR-302c, potentially disrupting regulation of its targets which include ESR1 [56] and CCND1 [57]. A previous study has associated targeting of ESR1 by miR-302c with a role in breast cancer [56]. Another investigation established that miR-302 simultaneously suppressed both the cyclin E-CDK2 and cyclin D-CDK4/6 pathways to inhibit human pluripotent stem cell tumorigenicity [57].
Indels in miRNA Target Sites
To determine indels that may impact experimentally supported miRNA target sites, we analyzed data from two sources: mRNA sequences that have been shown to interact with miRNAs in PAR-CLIP experiments and miRNA:mRNA target pairs in miRecords [48], miTarBase [50], and TarBase 5.0 [49]. The PAR-CLIP experiments provide the specific mRNA sequences that are targeted by miRNAs and we, therefore, identified all indels that were located in PAR-CLIP footprint regions. Typically, polymorphisms that alter complementary between the mRNA target and the seed region of the miRNA are believed to be the most deleterious, and we therefore determined how indels within the PAR-CLIP footprints alter this complementarity. In total, 152 indels were located within the PAR-CLIP footprint regions, and each indel disrupted or created at least a 6mer match to a miRNA seed (Table S2). In contrast with PAR-CLIP, most low-throughput methods used to identify miRNA target sites only provide miRNA:mRNA target pairs that interact, not the specific target sequences. Therefore, to identify indels that alter this type of experimentally determined target, we collected 4,074 known mRNA:miRNA target pairs from the sources listed above. We then identified all indels in the 3′ UTR of each mRNA in these pairs and scanned the sequence surrounding these indels to determine if they disrupted or created a 6mer or longer sequence complementary to the seed region of the targeting miRNA. We found that 197 experimentally identified mRNA:miRNA pairs had a putative target site that was altered by an indel ().
Integrated Analysis of SNPs and INDELs Altering miRNA Targeting in Disease Pathways
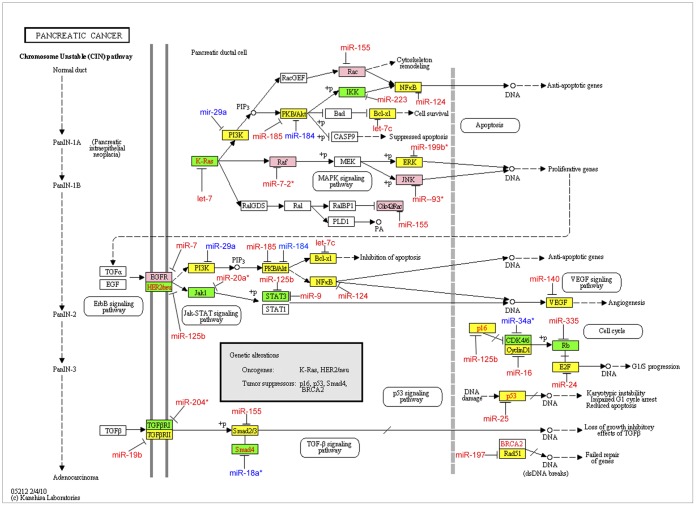
We found indels (Table S2 and S3) in experimentally supported miRNA target sites of 213 genes. To investigate the functional impact of these indels, these 213 genes were mapped to human disease pathways in the KEGG database [58]. Five pathways with ten or more genes with indels in miRNA target sites were selected for further analysis. (Table 1 and Table S4). We also identified SNPs in experimentally supported target sites of the genes in these pathways. Table 1 summarizes the number of genes with indels and SNPs in experimentally supported target sites in each pathway. Figure 3 shows genes containing indels, SNPs, or both in experimentally supported miRNA target sites, along with the miRNAs that target these genes, in the pancreatic cancer (hsa05212) pathway. For example, an indel (rs78669011) is located in the 3′ UTR of EGFR that disrupts a site complementary to the seed of miR-7. The targeting of EGFR by miR-7 has been experimentally supported by several experiments [43], [44] and both the gene and miRNA are known to play a role in cancer [59]. Similar figures for the pathways in cancer (hsa05200), prostate cancer (hsa05215), colorectal cancer (hsa05210) and the ErbB signaling (hsa04012) pathway are shown in Figures S2,S3,S4,S5.
Table 1. Selected human disease pathways containing genes with indels and SNPs in miRNA target sites.
| KEGG Pathway | Number of geneswith indels | Percentage of geneswith indels | Number of geneswith SNPs | Percentage of geneswith SNPs |
| Pathways in cancer | 25 | 7.62 | 77 | 23.48 |
| Pancreatic cancer | 13 | 18.57 | 26 | 37.14 |
| Prostate cancer | 12 | 13.48 | 28 | 31.46 |
| Colorectal cancer | 11 | 17.74 | 24 | 38.71 |
| ErbB signaling pathway | 11 | 12.64 | 15 | 17.24 |
Figure 3. Genes in the pancreatic cancer pathway containing SNPs and indels that altered experimentally supported target sites.
Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles. The miRNAs that have been shown to target these genes are shown with red text for disrupted sites and blue text for created sites.
Identifying Potential Links between Indels that Alter miRNA Targeting with Human Diseases Using Results from Association Studies
Genome-wide association studies have identified a large number of genomic locations harboring genetic variants associated with various diseases. We attempted to integrate the results of these association studies with indels that impact miRNA targeting. We identified all indels in miRNA sequences or experimentally supported target sites that were located within linkage disequilibrium (LD) blocks of any high scoring markers associated with human diseases and traits from GWAS results collected in dbGaP and the NHGRI GWAS Catalog [60] (Table 2).
Table 2. Indels in miRNAs and miRNA target sites in linkage disequilibrium block for high-scoring markers from association studies.
| Indel | Location | miRNA or Target Gene | GWAS Marker, p-value and Location | LD block boundaries: Left, Right | Disease/trait |
| rs34922018 | Chr2:25166699 | DNAJC27 | rs713586, 6×10−22, 25158008 | 25150296, 25182193 | Body Mass Index |
| rs35589685 | Chr5:110464398 | WDR36 | rs2416257, 1×10−6, 110435490 | 110404185, 110467499 | Plasma eosinophil count |
| rs34611972 | Chr2:97498908 | CNNM3 | rs9948, 6×10−6, 97500800 | 97489870, 97525099 | Erectile Dysfunction |
| rs34621455 | Chr1:113213486 | CAPZA1 | rs17030613, 8×10−6, 113190807 | 113110548, 113234456 | Blood Pressure |
| rs71737257 | Chr3:12625276 | RAF1 | rs3729931, 7×10−7, 12626516 | 12624070, 12626516 | Cardiomegaly |
| rs71717337 | Chr3:12625275 | RAF1 | rs3729931, 7×10−7, 12626516 | 12624070, 12626516 | Cardiomegaly |
| rs5745925 | Chr15:75645967 | miR-631 | rs8028182, 3×10−6, 75718669 | 75632867, 75815758 | Sudden cardiac arrest |
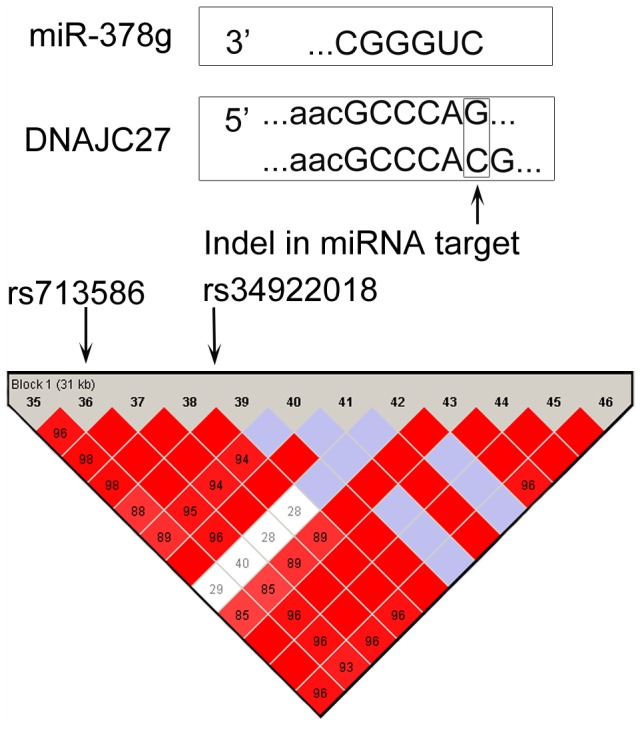
Figure 4 shows an indel in a PAR-CLIP footprint region (rs34922018) that is within a LD block with the GWAS marker rs713586, which was found to have a significant association with “Body Mass Index” (p = 6×10−22) in a Genetic Investigation of Anthropometric Traits (GIANT) study of over 249,796 individuals of European ancestry [61]. This indel, within DNAJC27, may disrupt a binding site for miR-378g, resulting in dysregulation of the gene. Similarly, the indel rs5745925 in miR-631 is within the same LD block as the association study marker SNP rs8028182 in the CEU population. rs5745925 is an insertion of CT that has been found to have a frequency of 0.93 in 90 individuals selected for individual screening in the NIH Polymorphism Discovery Resource (NIHPDR) [62]. The marker SNP rs8028182 was found to be associated with sudden cardiac arrest in patients with coronary artery disease [63]; SULT1A1, an experimentally validated target of miR-631 [47], has been associated with the risk factor of coronary artery disease [64].
Figure 4. Disruption of a target site of miR-378g in the 3′ UTR of DNAJC27 by an indel rs34922018 that is in linkage disequilibrium with a high-scoring marker rs713586 from a body mass index study.
Discussion
Recent advances in genome sequencing and association studies, as well as knowledge of disease pathways, have provided the resources to understand the impact of genetic variations that affect miRNA function. It has become possible to identify genetic polymorphisms altering miRNA targeting through large scale data integration. Although indels constitute the second most abundant type of genetic variants, there has not been a systematic analysis of indels in miRNAs and their target sites. In this work, we integrated multiple types of data to identify indels in miRNAs and in experimentally supported miRNA target sites and investigated the impacts of indels on miRNA targeting and function.
Previous investigations of genetic variations that impact miRNA targeting have mainly relied on computational algorithms for predicting miRNA target sites due to the lack of experimental data. These algorithms predict miRNA target sites based on complementarity between miRNA seeds and the target site, along with additional criteria including evolutionary conservation of binding sites [65], structural accessibility [66], or the nucleotide composition of the region containing the target [67]. However, these computational predictions are limited by high false positive rates and by the difficulty in assessing their false negative rates [68]. We, therefore, limited our analysis to those experimentally supported target sites, an approach that is becoming feasible mainly due to the recent advances in high throughput miRNA target identification such as PAR-CLIP experiments [31].
The integrated analysis of indels altering miRNA targeting and human disease pathways and GWAS results provided insights into the functional impacts of these genetic variants. We found that many genes in cancer pathways contain indels altering experimentally supported target sites (Figure 3, Figures S2,S3,S4,S5 and Table S4). For example, conserved helix-loop-helix ubiquitous kinase (CHUK), which is also known as IKK1, a protein kinase that plays an important role in pancreatic cancer and prostate cancer by regulating the NF-kB transcription factor, has two indels that may disrupt targeting by miR-223; RB1 contains one indel in target site of miR-335; STAT3 contains two indels in target sites of miR-9 and miR-125b; and FOXO1 and EFGR have indels in target sites of miR-9 and miR-7 respectively. We further extended our analysis by integrating the indels altering miRNA targeting with the results of GWAS. Several miRNA-related indels were in linkage disequilibrium with high scoring markers of GWAS, including an indel rs34922018 in linkage disequilibrium with high scoring marker rs713586 from a body mass index study. The indel rs34922018 found to disrupt a target site of miR-378g in the 3′ UTR of DNAJC27 by an insertion of ‘C’ nucleotide (Figure 4).
Materials and Methods
INDELs in Pre-miRNA and Flanking Regions
The genomic locations of all miRNAs were obtained from miRBase release 18 [69]; the locations of pre-miRNAs were obtained from the genome coordinates file, while the locations of mature miRNAs were obtained from the ftp download section of miRBase. The start and end locations of 3′ and 5′ flanking regions around the pre-miRNA were determined by, depending on its transcriptional orientation, either adding or subtracting the length of each pre-miRNA from its location. All variants and only indels within miRNAs and their flanking regions were collected from dbSNP build 135 by setting the appropriated filter functions in the UCSC table browser [70], [71]. We also collected indels from the GATK resource bundle [72], a collection of standard files for working with human resequencing data, which includes a set of high-confidence indels for use with local realignment. Variants in miRNAs and flanking regions were then identified by comparing their chromosome locations. T-tests were used to compare the density of all variants and only indels among different regions of miRNA sequences or with that of the flanking regions.
Indels in miRNA Target Sites
The start and end locations of 41 nt long PAR-CLIP footprints were obtained from Supplementary Table 7 of Hafner et al. [31]. We used the liftover tool in the Galaxy web-server [73] to convert the list of genomic locations presented in PAR-CLIP mRNA:miRNA interaction map to the GRCh37/hg19 assembly of the human genome. All variants and indels in the PAR-CLIP footprint regions were identified by comparing the locations of the footprints with the locations of variants in dbSNP build 135 downloaded from UCSC. We also identified all variants and indels in the 3′ UTRs of mRNAs using a similar procedure. Indels in the 3′ UTR of all mRNAs were also used to calculate the percentage of three indel classes, namely, single-base-pair, repeat expansions, and “other”. We also created a second list of indels that included only those indels in 3′ UTRs of mRNAs that are the experimentally supported target of at least one miRNA by collecting the mRNA-miRNA target pairs contained in miRecords [48], miTarBase [50], and TarBase 5.0 [49]. We used Galaxy [73] to extract 100 base pair long sequences surrounding the genomic location of each indel. From these 100 base pairs long sequences we made two set of sequences, the reference sequences and the mutation sequences that contained insertions and deletions. We then scanned these sequences to determine locations complementary to any of the six miRNA seed types as described by Ellwanger et al. [68]. Target sites found in the reference sequences but not in the mutant sequences, were marked as disrupted by the indel, while target sites found in the mutant sequences but not in the reference sequences were sites created by the indel. For indels within PAR-CLIP footprints, we determined how the indels created and disrupted putative target sites for any miRNA. For indels within 3′ UTRs of mRNAs in experimentally supported mRNA-miRNA pairs, we limited the search to only those miRNAs that have been found to target the mRNA. We also used indels from GATK resource bundle to find disrupted and created miRNA sites in PAR-CLIP footprint region and 3′UTRs of known miRNA target sites. T-tests were used to compare the density of all variants and only indels among different regions shown in Figure 2.
Linking Indels with Pathway and Associations Studies
Genes with indels in the experimentally supported target sites of miRNAs were compared against the list of genes in KEGG pathways to select the five most enriched pathways using the DAVID annotation tools [74]. SNPs in experimentally supported miRNA target sites of the genes in these pathways were also identified using the same procedure for indels. KEGG Mapper, a tool for changing color schema in KEGG pathways was then used to represent genes with indels and/or SNPs in miRNA target sites [58].
Indels in miRNAs or experimentally supported target sites were linked with the results of association studies. High ranking markers for association studies were collected from dbGaP [75] and the NHGRI GWAS Catalog [60]. All indels in miRNAs or experimentally supported target sites within 100 kb of any of the these markers were then tested for linkage disequilibrium (LD) by using Haploview [76] software. In Haploview, we have selected the Gabriel et al [77] algorithm with its default parameter settings to define strong LD blocks. Confidence interval minima have been set with upper at 0.98 and lower at 0.7, while upper confidence interval maximum for strong recombination is set to 0.9 and all the markers with MAF value below 0.05 are excluded from block.
Supporting Information
Comparison of the percentage of indels that are single nucleotide indels, repeat expansions, or other types of indels among indels in miRNAs, 3′ UTRs, and the entire genome.
(TIF)
Genes in the cancer pathway containing SNPs and indels that altered experimentally supported target sites. Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles.
(TIF)
Genes in the prostate cancer pathway containing SNPs and indels that altered experimentally supported target sites. Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles.
(TIF)
Genes in the colorectal cancer pathway containing SNPs and indels that altered experimentally supported target sites. Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles.
(TIF)
Genes in the ErbB signaling pathway containing SNPs and indels that altered experimentally supported target sites. Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles.
(TIF)
List of indels in miRNAs.
(XLS)
Indels in PAR-CLIP data found to disrupt or create sites for miRNA.
(XLS)
Indels in the experimentally supported target sites for miRNA.
(XLS)
Indels and SNPs from experimentally supported miRNA targets in KEGG pathway.
(XLS)
Funding Statement
This work was supported by The University of Tennessee Center for Integrative and Translational Genomics and Department of Defense grant W81XHW-05-01-0227. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Huntzinger E, Izaurralde E (2011) Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12: 99–110. [DOI] [PubMed] [Google Scholar]
- 2. Li L, Xu J, Yang D, Tan X, Wang H (2010) Computational approaches for microRNA studies: a review. Mamm Genome 21: 1–12. [DOI] [PubMed] [Google Scholar]
- 3. Hammell M (2010) Computational methods to identify miRNA targets. Semin Cell Dev Biol 21: 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dai Y, Zhou X (2010) Computational methods for the identification of microRNA targets. Open Access Bioinformatics 2: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia DM, Baek D, Shin C, Bell GW, Grimson A, et al. (2011) Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol 18: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, et al. (2011) Genome-wide association study identifies five new schizophrenia loci. Nat Genet 43: 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mencia A, Modamio-Hoybjor S, Redshaw N, Morin M, Mayo-Merino F, et al. (2009) Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet 41: 609–613. [DOI] [PubMed] [Google Scholar]
- 8. Shen J, Ambrosone CB, DiCioccio RA, Odunsi K, Lele SB, et al. (2008) A functional polymorphism in the miR-146a gene and age of familial breast/ovarian cancer diagnosis. Carcinogenesis 29: 1963–1966. [DOI] [PubMed] [Google Scholar]
- 9. Yue C, Wang M, Ding B, Wang W, Fu S, et al. (2011) Polymorphism of the pre-miR-146a is associated with risk of cervical cancer in a Chinese population. Gynecol Oncol 122: 33–37. [DOI] [PubMed] [Google Scholar]
- 10. Xu T, Zhu Y, Wei QK, Yuan Y, Zhou F, et al. (2008) A functional polymorphism in the miR-146a gene is associated with the risk for hepatocellular carcinoma. Carcinogenesis 29: 2126–2131. [DOI] [PubMed] [Google Scholar]
- 11. Xu B, Feng NH, Li PC, Tao J, Wu D, et al. (2010) A functional polymorphism in Pre-miR-146a gene is associated with prostate cancer risk and mature miR-146a expression in vivo. Prostate 70: 467–472. [DOI] [PubMed] [Google Scholar]
- 12. Jazdzewski K, Liyanarachchi S, Swierniak M, Pachucki J, Ringel MD, et al. (2009) Polymorphic mature microRNAs from passenger strand of pre-miR-146a contribute to thyroid cancer. Proc Natl Acad Sci U S A 106: 1502–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saetrom P, Biesinger J, Li SM, Smith D, Thomas LF, et al. (2009) A risk variant in an miR-125b binding site in BMPR1B is associated with breast cancer pathogenesis. Cancer Res 69: 7459–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng N, Xu B, Tao J, Li P, Cheng G, et al. (2012) A miR-125b binding site polymorphism in bone morphogenetic protein membrane receptor type IB gene and prostate cancer risk in China. Mol Biol Rep 39: 369–373. [DOI] [PubMed] [Google Scholar]
- 15. Gao Y, He Y, Ding J, Wu K, Hu B, et al. (2009) An insertion/deletion polymorphism at miRNA-122-binding site in the interleukin-1alpha 3′ untranslated region confers risk for hepatocellular carcinoma. Carcinogenesis 30: 2064–2069. [DOI] [PubMed] [Google Scholar]
- 16. Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, et al. (2008) Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet 82: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wulfken LM, Moritz R, Ohlmann C, Holdenrieder S, Jung V, et al. (2011) MicroRNAs in renal cell carcinoma: diagnostic implications of serum miR-1233 levels. PLoS One 6: e25787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jazdzewski K, Murray EL, Franssila K, Jarzab B, Schoenberg DR, et al. (2008) Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc Natl Acad Sci U S A 105: 7269–7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saunders MA, Liang H, Li WH (2007) Human polymorphism at microRNAs and microRNA target sites. Proc Natl Acad Sci U S A 104: 3300–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quach H, Barreiro LB, Laval G, Zidane N, Patin E, et al. (2009) Signatures of purifying and local positive selection in human miRNAs. Am J Hum Genet 84: 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong J, Tong Y, Zhang HM, Wang K, Hu T, et al. (2012) Genome-wide identification of SNPs in microRNA genes and the SNP effects on microRNA target binding and biogenesis. Hum Mutat 33: 254–263. [DOI] [PubMed] [Google Scholar]
- 22. Ehrenreich IM, Purugganan MD (2008) Sequence variation of MicroRNAs and their binding sites in Arabidopsis. Plant Physiol 146: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bao L, Zhou M, Wu L, Lu L, Goldowitz D, et al. (2007) PolymiRTS Database: linking polymorphisms in microRNA target sites with complex traits. Nucleic Acids Res 35: D51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ziebarth JD, Bhattacharya A, Chen A, Cui Y (2012) PolymiRTS Database 2.0: linking polymorphisms in microRNA target sites with human diseases and complex traits. Nucleic Acids Res 40: D216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mills RE, Luttig CT, Larkins CE, Beauchamp A, Tsui C, et al. (2006) An initial map of insertion and deletion (INDEL) variation in the human genome. Genome Res 16: 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mullaney JM, Mills RE, Pittard WS, Devine SE (2010) Small insertions and deletions (INDELs) in human genomes. Hum Mol Genet 19: R131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mills RE, Pittard WS, Mullaney JM, Farooq U, Creasy TH, et al. (2011) Natural genetic variation caused by small insertions and deletions in the human genome. Genome Res 21: 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dawson E, Chen Y, Hunt S, Smink LJ, Hunt A, et al. (2001) A SNP resource for human chromosome 22: extracting dense clusters of SNPs from the genomic sequence. Genome Res 11: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vergoulis T, Vlachos IS, Alexiou P, Georgakilas G, Maragkakis M, et al. (2012) TarBase 6.0: capturing the exponential growth of miRNA targets with experimental support. Nucleic Acids Res 40: D222–D229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chi SW, Zang JB, Mele A, Darnell RB (2009) Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 460: 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, et al. (2010) Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141: 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Papadopoulos GL, Reczko M, Simossis VA, Sethupathy P, Hatzigeorgiou AG (2009) The database of experimentally supported targets: a functional update of TarBase. Nucleic Acids Res 37: D155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shohet JM, Ghosh R, Coarfa C, Ludwig A, Benham AL, et al. (2011) A genome-wide search for promoters that respond to increased MYCN reveals both new oncogenic and tumor suppressor microRNAs associated with aggressive neuroblastoma. Cancer Res 71: 3841–3851. [DOI] [PubMed] [Google Scholar]
- 34. Du Y, Xu Y, Ding L, Yao H, Yu H, et al. (2009) Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. J Gastroenterol 44: 556–561. [DOI] [PubMed] [Google Scholar]
- 35. Morales-Prieto DM, Schleussner E, Markert UR (2011) Reduction in miR-141 is induced by leukemia inhibitory factor and inhibits proliferation in choriocarcinoma cell line JEG-3. Am J Reprod Immunol 66 Suppl 1: 57–62. [DOI] [PubMed] [Google Scholar]
- 36. Stratmann J, Wang CJ, Gnosa S, Wallin A, Hinselwood D, et al. (2011) Dicer and miRNA in relation to clinicopathological variables in colorectal cancer patients. BMC Cancer 11: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, et al. (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 105: 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang L, Deng T, Li X, Liu H, Zhou H, et al. (2010) microRNA-141 is involved in a nasopharyngeal carcinoma-related genes network. Carcinogenesis 31: 559–566. [DOI] [PubMed] [Google Scholar]
- 39. Scapoli L, Palmieri A, Lo Muzio L, Pezzetti F, Rubini C, et al. (2010) MicroRNA expression profiling of oral carcinoma identifies new markers of tumor progression. Int J Immunopathol Pharmacol 23: 1229–1234. [DOI] [PubMed] [Google Scholar]
- 40. Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, et al. (2011) Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328, -519c and -520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol 81: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang F, Xue X, Wei J, An Y, Yao J, et al. (2010) hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer 103: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mees ST, Mardin WA, Sielker S, Willscher E, Senninger N, et al. (2009) Involvement of CD40 targeting miR-224 and miR-486 on the progression of pancreatic ductal adenocarcinomas. Ann Surg Oncol 16: 2339–2350. [DOI] [PubMed] [Google Scholar]
- 43. Kikkawa N, Hanazawa T, Fujimura L, Nohata N, Suzuki H, et al. (2010) miR-489 is a tumour-suppressive miRNA target PTPN11 in hypopharyngeal squamous cell carcinoma (HSCC). Br J Cancer 103: 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Guo Y, Liang X, Sun M, Wang G, et al. (2012) MicroRNA-223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol. [DOI] [PubMed]
- 45. Lee KH, Goan YG, Hsiao M, Lee CH, Jian SH, et al. (2009) MicroRNA-373 (miR-373) post-transcriptionally regulates large tumor suppressor, homolog 2 (LATS2) and stimulates proliferation in human esophageal cancer. Exp Cell Res 315: 2529–2538. [DOI] [PubMed] [Google Scholar]
- 46. Huang Y, Chuang A, Hao H, Talbot C, Sen T, et al. (2011) Phospho-DeltaNp63alpha is a key regulator of the cisplatin-induced microRNAome in cancer cells. Cell Death Differ 18: 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu X, Dhakal IB, Beggs M, Edavana VK, Williams S, et al. (2010) Functional genetic variants in the 3′-untranslated region of sulfotransferase isoform 1A1 (SULT1A1) and their effect on enzymatic activity. Toxicol Sci 118: 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiao F, Zuo Z, Cai G, Kang S, Gao X, et al. (2009) miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res 37: D105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sethupathy P, Corda B, Hatzigeorgiou AG (2006) TarBase: A comprehensive database of experimentally supported animal microRNA targets. RNA 12: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hsu SD, Lin FM, Wu WY, Liang C, Huang WC, et al. (2011) miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res 39: D163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gong AY, Zhou R, Hu G, Li X, Splinter PL, et al. (2009) MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol 182: 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Flies DB, Sandler BJ, Sznol M, Chen L (2011) Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J Biol Med 84: 409–421. [PMC free article] [PubMed] [Google Scholar]
- 53. Drake KM, Ruteshouser EC, Natrajan R, Harbor P, Wegert J, et al. (2009) Loss of heterozygosity at 2q37 in sporadic Wilms’ tumor: putative role for miR-562. Clin Cancer Res 15: 5985–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen H, Sun JG, Cao XW, Ma XG, Xu JP, et al. (2009) Preliminary validation of ERBB2 expression regulated by miR-548d-3p and miR-559. Biochem Biophys Res Commun 385: 596–600. [DOI] [PubMed] [Google Scholar]
- 55. Vermeer PD, Bell M, Lee K, Vermeer DW, Wieking BG, et al. (2012) ErbB2, EphrinB1, Src Kinase and PTPN13 Signaling Complex Regulates MAP Kinase Signaling in Human Cancers. PLoS One 7: e30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leivonen SK, Makela R, Ostling P, Kohonen P, Haapa-Paananen S, et al. (2009) Protein lysate microarray analysis to identify microRNAs regulating estrogen receptor signaling in breast cancer cell lines. Oncogene 28: 3926–3936. [DOI] [PubMed] [Google Scholar]
- 57. Lin SL, Chang DC, Ying SY, Leu D, Wu DT (2010) MicroRNA miR-302 inhibits the tumorigenecity of human pluripotent stem cells by coordinate suppression of the CDK2 and CDK4/6 cell cycle pathways. Cancer Res 70: 9473–9482. [DOI] [PubMed] [Google Scholar]
- 58. Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M (2012) KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40: D109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Duex JE, Comeau L, Sorkin A, Purow B, Kefas B (2011) Usp18 regulates epidermal growth factor (EGF) receptor expression and cancer cell survival via microRNA-7. J Biol Chem 286: 25377–25386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, et al. (2009) Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A 106: 9362–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, et al. (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Collins FS, Brooks LD, Chakravarti A (1998) A DNA polymorphism discovery resource for research on human genetic variation. Genome Res 8: 1229–1231. [DOI] [PubMed] [Google Scholar]
- 63. Aouizerat BE, Vittinghoff E, Musone SL, Pawlikowska L, Kwok PY, et al. (2011) GWAS for discovery and replication of genetic loci associated with sudden cardiac arrest in patients with coronary artery disease. BMC Cardiovasc Disord 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Halloran AM, Patterson CC, Horan P, Maree A, Curtin R, et al. (2009) Genetic polymorphisms in platelet-related proteins and coronary artery disease: investigation of candidate genes, including N-acetylgalactosaminyltransferase 4 (GALNT4) and sulphotransferase 1A1/2 (SULT1A1/2). J Thromb Thrombolysis 27: 175–184. [DOI] [PubMed] [Google Scholar]
- 65. Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E (2007) The role of site accessibility in microRNA target recognition. Nat Genet 39: 1278–1284. [DOI] [PubMed] [Google Scholar]
- 67. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, et al. (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ellwanger DC, Buttner FA, Mewes HW, Stumpflen V (2011) The sufficient minimal set of miRNA seed types. Bioinformatics 27: 1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kozomara A, Griffiths-Jones S (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 39: D152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Karolchik D, Hinrichs AS, Furey TS, Roskin KM, Sugnet CW, et al. (2004) The UCSC Table Browser data retrieval tool. Nucleic Acids Res 32: D493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fujita PA, Rhead B, Zweig AS, Hinrichs AS, Karolchik D, et al. (2011) The UCSC Genome Browser database: update 2011. Nucleic Acids Res 39: D876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goecks J, Nekrutenko A, Taylor J (2010) Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol 11: R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57. [DOI] [PubMed] [Google Scholar]
- 75. Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, et al. (2007) The NCBI dbGaP database of genotypes and phenotypes. Nat Genet 39: 1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 77. Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, et al. (2002) The structure of haplotype blocks in the human genome. Science 296: 2225–2229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the percentage of indels that are single nucleotide indels, repeat expansions, or other types of indels among indels in miRNAs, 3′ UTRs, and the entire genome.
(TIF)
Genes in the cancer pathway containing SNPs and indels that altered experimentally supported target sites. Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles.
(TIF)
Genes in the prostate cancer pathway containing SNPs and indels that altered experimentally supported target sites. Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles.
(TIF)
Genes in the colorectal cancer pathway containing SNPs and indels that altered experimentally supported target sites. Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles.
(TIF)
Genes in the ErbB signaling pathway containing SNPs and indels that altered experimentally supported target sites. Genes containing only indels (pink), only SNPs (yellow), and both SNPs and indels (green) in target sites are within colored rectangles.
(TIF)
List of indels in miRNAs.
(XLS)
Indels in PAR-CLIP data found to disrupt or create sites for miRNA.
(XLS)
Indels in the experimentally supported target sites for miRNA.
(XLS)
Indels and SNPs from experimentally supported miRNA targets in KEGG pathway.
(XLS)