Abstract
Insulin-dependent diabetes induced in susceptible strains of mice by multiple, low-dose streptozotocin treatment has been proposed to entail a thymus-dependent, autoimmune destruction of beta cells. In this study, thymectomized and genetically athymic mice have been tested for susceptibility to streptozotocin. Thymectomy was performed on newborn (day 1) to 3-day-old C57BL/KsJ mice. At 8 wk of age, thymectomized and sham-operated mice of both sexes were tested for susceptibility to diabetes induction by multiple, low-dose streptozotocin treatment (35 mg/kg of body weight per day for 6 consecutive days). Thymectomy failed to block susceptibility of males to induction of severe hyperglycemia. Beta cell necrosis and inflammatory cell infiltrates (insulitis) were consistent histopathological features. In general, females—both thymus-intact and thymectomized—were less susceptible than males to streptozotocin-induced hyperglycemia, and females exhibited an equally severe insulitis by experimental day 14; thus, the detection of an underlying insulitis did not predict the development of a more severe hyperglycemia because most streptozotocin-treated females at experimental day 35 continued to show only a modest hyperglycemia (about 200 mg/dl) compared to males (>400 mg/dl). That streptozotocin-induced hyperglycemia could occur in the absence of an intact thymus was further demonstrated in genetically athymic C57BL/6J NIcrOu nu/nu males and thymus-intact +/? littermate controls. C57BL/6J mice were resistant to streptozotocin-induced insulitis. This study shows that the presence of insulitis does not necessarily presage onset of severe hyperglycemia (e.g., C57BL/KsJ females), and conversely, the presence of severe hyperglycemia after low-dose streptozotocin treatment is not necessarily diagnostic of an underlying insulitis (e.g., C57BL/6J +/? and nu/nu males). These data stress the need for caution in the interpretation of studies of streptozotocin-insulitis sensitivities of nude mice.
Keywords: diabetes, inbred mice, nude mice
Full text
PDF

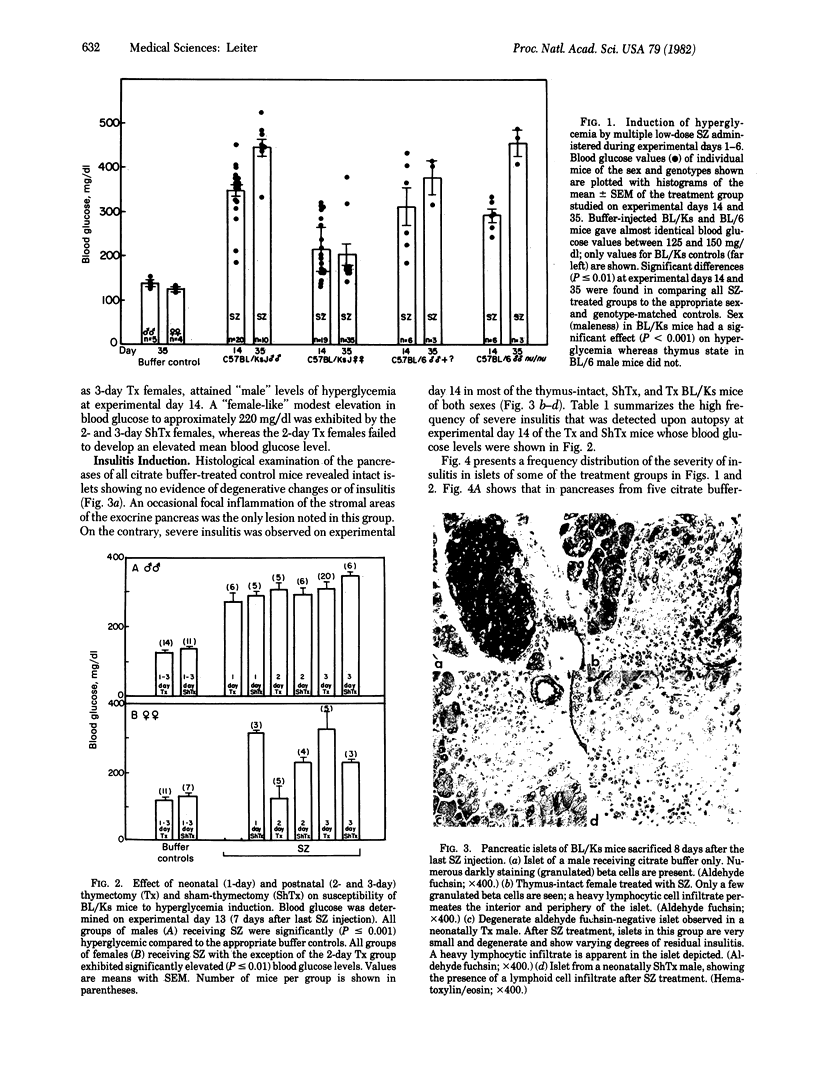
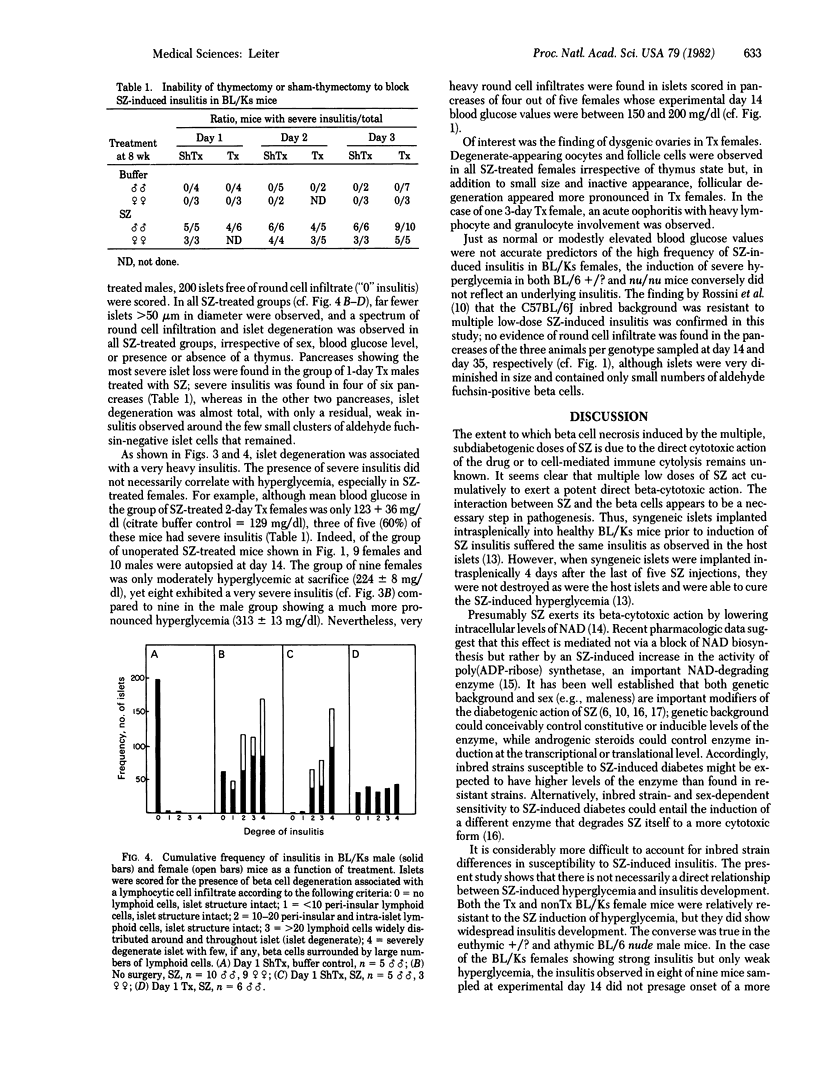

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnevie-Nielsen V., Steffes M. W., Lernmark A. A major loss in islet mass and B-cell function precedes hyperglycemia in mice given multiple low doses of streptozotocin. Diabetes. 1981 May;30(5):424–429. doi: 10.2337/diab.30.5.424. [DOI] [PubMed] [Google Scholar]
- Buschard K., Rygaard J. Is the diabetogenic effect of streptozotocin in part thymus-dependent? Acta Pathol Microbiol Scand C. 1978 Feb;86(1):23–27. doi: 10.1111/j.1699-0463.1978.tb02552.x. [DOI] [PubMed] [Google Scholar]
- Cohn J. A., Cerami A. The influence of genetic background on the susceptibility of mice to diabetes induced by alloxan and on recovery from alloxan diabetes. Diabetologia. 1979 Sep;17(3):187–191. doi: 10.1007/BF01219747. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Hyperinsulinemia in pre-weaning diabetes (db) mice. Diabetologia. 1974 Nov;10 (Suppl):607–610. doi: 10.1007/BF01221993. [DOI] [PubMed] [Google Scholar]
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965 Oct;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Irvine W. J. Immunological aspects of diabetes mellitus. Adv Exp Med Biol. 1979;119:147–155. doi: 10.1007/978-1-4615-9110-8_22. [DOI] [PubMed] [Google Scholar]
- Kiesel U., Freytag G., Biener J., Kolb H. Transfer of experimental autoimmune insulitis by spleen cells in mice. Diabetologia. 1980;19(6):516–520. doi: 10.1007/BF00253178. [DOI] [PubMed] [Google Scholar]
- Kolb H., Freytag G., Kiesel U., Kolb-Bachofen V. Cellular immune reactions against pancreatic islets as a consequence of graft versus host disease. Clin Exp Immunol. 1981 Jan;43(1):121–127. [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Freytag G., Kiesel U., Kolb-Bachofen V. Spontaneous autoimmune reactions against pancreatic islets in mouse strains with generalized autoimmune disease. Diabetologia. 1980 Sep;19(3):216–221. doi: 10.1007/BF00275272. [DOI] [PubMed] [Google Scholar]
- Kromann H., Christy M., Lernmark A., Nerup J. An in vitro, sex dependents, and direct cytotoxic effect of streptozotocin on pancreatic islet cells. Horm Metab Res. 1981 Feb;13(2):120–121. doi: 10.1055/s-2007-1019191. [DOI] [PubMed] [Google Scholar]
- Lazarus S. S., Shapiro S. H. Influence of nicotinamide and pyridine nucleotides on streptozotocin and alloxan-induced pancreatic B cell cytotoxicity. Diabetes. 1973 Jul;22(7):499–506. doi: 10.2337/diab.22.7.499. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Coleman D. L., Eisenstein A. B., Strack I. A new mutation (db3J) at the diabetes locus in strain 129/J mice. I. Physiological and histological characterization. Diabetologia. 1980 Jul;19(1):58–65. doi: 10.1007/BF00258313. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976 Jul 30;193(4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Lipscomb H. L., Gardner P. J., Sharp J. G. The effect of neonatal thymectomy on the induction of autoimmune orchitis in rats. J Reprod Immunol. 1979 Dec;1(4):209–217. doi: 10.1016/0165-0378(79)90001-9. [DOI] [PubMed] [Google Scholar]
- Maclaren N. K., Neufeld M., McLaughlin J. V., Taylor G. Androgen sensitization of streptozotocin-induced diabetes in mice. Diabetes. 1980 Sep;29(9):710–716. doi: 10.2337/diab.29.9.710. [DOI] [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Nakhooda A. F., Like A. A., Chappel C. I., Wei C. N., Marliss E. B. The spontaneously diabetic Wistar rat (the "BB" rat). Studies prior to and during development of the overt syndrome. Diabetologia. 1978 Mar;14(3):199–207. doi: 10.1007/BF00429781. [DOI] [PubMed] [Google Scholar]
- Paik S. G., Fleischer N., Shin S. I. Insulin-dependent diabetes mellitus induced by subdiabetogenic doses of streptozotocin: obligatory role of cell-mediated autoimmune processes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6129–6133. doi: 10.1073/pnas.77.10.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A. A., Appel M. C., Williams R. M., Like A. A. Genetic influence of the streptozotocin-induced insulitis and hyperglycemia. Diabetes. 1977 Oct;26(10):916–920. doi: 10.2337/diab.26.10.916. [DOI] [PubMed] [Google Scholar]
- Rossini A. A., Williams R. M., Appel M. C., Like A. A. Complete protection from low-dose streptozotocin-induced diabetes in mice. Nature. 1978 Nov 9;276(5684):182–184. doi: 10.1038/276182a0. [DOI] [PubMed] [Google Scholar]
- Rossini A. A., Williams R. M., Appel M. C., Like A. A. Sex differences in the multiple-dose streptozotocin model of diabetes. Endocrinology. 1978 Oct;103(4):1518–1520. doi: 10.1210/endo-103-4-1518. [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A. Islet implantation into diabetic mice with pancreatic insulitis. Acta Pathol Microbiol Scand A. 1981 Mar;89(2):107–112. doi: 10.1111/j.1699-0463.1981.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y., Sakakura T., Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980 Jun;40(3):540–553. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Okamoto H. Protection by picolinamide, a novel inhibitor of poly (ADP-ribose) synthetase, against both streptozotocin-induced depression of proinsulin synthesis and reduction of NAD content in pancreatic islets. Biochem Biophys Res Commun. 1980 Jul 16;95(1):474–481. doi: 10.1016/0006-291x(80)90762-7. [DOI] [PubMed] [Google Scholar]