Abstract
The increasing prevalence of N. gonorrhoeae strains exhibiting decreased susceptibility to third-generation cephalosporins and the recent isolation of two distinct strains with high-level resistance to cefixime or ceftriaxone heralds the possible demise of β-lactam antibiotics as effective treatments for gonorrhea. To identify new compounds that inhibit penicillin-binding proteins (PBPs), which are proven targets for β-lactam antibiotics, we developed a high-throughput assay that uses fluorescence polarization (FP) to distinguish the fluorescent penicillin, Bocillin-FL, in free or PBP-bound form. This assay was used to screen a 50,000 compound library for potential inhibitors of N. gonorrhoeae PBP 2, and 32 compounds were identified that exhibited >50% inhibition of Bocillin-FL binding to PBP 2. These included a cephalosporin that provided validation of the assay. After elimination of compounds that failed to exhibit concentration-dependent inhibition, the antimicrobial activity of the remaining 24 was tested. Of these, 7 showed antimicrobial activity against susceptible and penicillin- or cephalosporin-resistant strains of N. gonorrhoeae. In molecular docking simulations using the crystal structure of PBP 2, two of these inhibitors docked into the active site of the enzyme and each mediate interactions with the active site serine nucleophile. This study demonstrates the validity of a FP-based assay to find novel inhibitors of PBPs and paves the way for more comprehensive high-throughput screening against highly resistant strains of N. gonorrhoeae. It also provides a set of lead compounds for optimization of anti-gonococcal agents.
Introduction
Historically, β-lactams have been highly successful for the treatment of bacterial infections, but the emergence of resistance to these and other antibiotics has markedly limited the treatment options in a number of pathogens. One notable example is Neisseria gonorrhoeae, an obligate human pathogen and cause of the sexually transmitted disease, gonorrhea. For over 40 years, gonorrhea was treated with a single dose of penicillin, but the increasing prevalence of strains exhibiting resistance to penicillin eventually led to its withdrawal in 1986 by the Centers for Disease Control and Prevention (CDC) as a recommended antibiotic for gonococcal infections. With tetracycline and fluoroquinolones [1] being withdrawn for similar reasons, only the extended-spectrum cephalosporins, cefixime and ceftriaxone, remain as recommended treatments in the U.S. However, during the last decade, strains exhibiting decreased susceptibility to cefixime and ceftriaxone have emerged in Japan and Europe [2], [3], [4], [5], [6], [7], and the recent isolation of two distinct strains in Japan and France with high-level cefixime and ceftriaxone resistance (both with minimum inhibitory concentrations (MICs) ≥2 µg/ml)) [8], [9] signals the potential demise of these antibiotics as effective treatments for gonorrhea [10].
The lethal targets for β-lactams are penicillin-binding proteins (PBPs) [11],[12], which are transpeptidases that catalyze the formation of peptide cross-links between adjacent glycan strands during the final stages of peptidoglycan synthesis in bacteria. Peptidoglycan envelops the bacterial cell and is essential for cell growth, division and maintenance of cell shape [13]. PBPs share a common penicillin-binding/transpeptidase domain with an active site that contains three conserved motifs: SxxK, SxN and KTG (where × is a variable residue) [14]. The SxxK motif contains the serine nucleophile that attacks the carbonyl carbon of the penultimate d-Ala of the peptide substrate or amide carbonyl of the β-lactam ring. There are three classes of PBP: Class A PBPs catalyze both glycosyl transferase and transpeptidase activities, Class B catalyze only transpeptidase activity and Class C PBPs are carboxypeptidases and/or endopeptidases. In most bacteria, Class A and B PBPs are essential enzymes, whereas Class C PBPs can often be deleted genetically without significant impact on cell growth or morphology [15]. The genome of N. gonorrhoeae encodes 4 PBPs. PBPs 3 and 4 are Class C PBPs and are non-essential for cell viability [16]. PBP 1 (Class A) and PBP 2 (Class B) are both essential, but given that PBP 2 is inhibited at a 10-fold lower concentration of penicillin than PBP 1, it is the primary clinical target in penicillin-susceptible strains [17], [18].
N. gonorrhoeae develops chromosomally mediated resistance to β-lactams through alteration of the PBP targets, increased expression of the MtrC-MtrD-MtrE efflux pump and mutation of the porin PorB1b that restricts entry into the periplasm [19], [20]. The primary step in this process is the acquisition of mutated forms of PBP 2 that exhibit lowered reactivity with β-lactams and compromise the effectiveness of these agents [21], [22], [23], [24], [25], [26]. PBP 2 is essential for the growth of N. gonorrhoeae and is a validated target for β-lactam antibiotics directed against this organism [18], but its value as a clinical target has been diminished by mutations associated with resistance. In order to develop new treatment options for penicillin- and cephalosporin-resistant strains of N. gonorrhoeae, new inhibitors of PBP 2 are needed. In this study, we report the development of a high-throughput screening (HTS) assay for PBPs that uses fluorescence polarization to detect binding of the fluorescent β-lactam, Bocillin-FL [27]. We used this assay to screen a 50,000 chemical library and identified a number of compounds that inhibited PBP 2 activity in the micromolar range. Of these, seven demonstrated antimicrobial activity against N. gonorrhoeae, including strains exhibiting resistance to penicillin or cefixime.
Materials and Methods
Materials
A soluble construct of wild-type PBP 2 from the penicillin-susceptible strain of N. gonorrhoeae FA19 was expressed and purified as described previously [26]. Bocillin FL™ was obtained from Invitrogen Inc. (Carlsbad, CA). Penicillin G and γ-Globulins from bovine blood (BGG) were purchased from Sigma (St. Louis, MO). Prior to use, all reagents were diluted in an assay buffer comprising 50 mM potassium phosphate, pH 8, and 0.1 mg/ml BGG. The DIVERSet library of 50,080 small lead compounds from ChemBridge Corporation (San Diego, CA) was provided by the MUSC Drug Discovery Core (DDC). Three laboratory strains of N. gonorrhoeae–FA19 [28], penicillin-resistant FA6140 [29], and cephalosporin-intermediate resistant CephI strain 35/02 [30]–were from the laboratory collection of R. Nicholas (UNC-Chapel Hill).
Fluorescence Polarization (FP) Measurements
Measurements of fluorescence and FP were performed on a Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths of 485 nm and 520 nm, respectively, using a 515 nm filter to block residual excitation light and to minimize background interference. Black, shallow 384-well micro plates (ProxiPlate ™ –384 F Plus, PerkinElmer) were used to record data. To minimize the polarization effects from any fluorophore bound to the surface of the well, both excitation and emission data were recorded from the top of the well. Reading time was 100 ms per well. Fluorescence polarization was quantified in millipolarization units (mP) according to the equation, mP = 1000×[(Iv − G.Ih)/(Iv + G.Ih)], where Iv and Ih are parallel and perpendicular emission intensity measurements, respectively, corrected for background (buffer), and G is the instrument-dependent correction coefficient, defined as a ratio of sensitivities of the detection system for vertically and horizontally polarized light. Using the equation G = Iv/Ih and SoftMax® Pro Data Acquisition & Analysis Software (Molecular Devices, Sunnyvale, CA), the G-factor was determined with dilution series of Bocillin-FL-only (free tracer). The assay window (ΔmP) was defined as the difference between protein-tracer sample and free-tracer, i.e. ΔmP = mPs – mPfree, and is a measure of the maximum specific binding.
FP Assay Optimization
To calculate the G-factor, FP was measured in 10 µl reaction volumes for free Bocillin-FL at concentrations of 0.2, 0.5, 1, 2, 3, and 4 µM, where the FP signal of the fluorescent tracer was low and stable. The optimal tracer-to-protein ratio was determined in the binding experiments with increasing concentrations of PBP 2 (0.02–4 µM). FP was recorded after shaking the plate for 2 min followed by 30 min incubation, at which point the reaction reached its steady state (data not shown). Each experiment was performed in quadruplicate at room temperature.
To evaluate the performance of the assay, steady-state concentration-response experiments were carried out using penicillin G in a competition assay with Bocillin-FL. Penicillin G (0.05–1000 µM) was mixed with 1 µM PBP 2 and 1 µM Bocillin-FL, followed by a 1 hr incubation. The positive (Pc) and negative (Nc) controls were defined as the FP of the Bocillin-FL - protein and of the free tracer, respectively, in the absence of penicillin G. The FP of the Bocillin-FL - protein at 100 µM penicillin G was defined as a displaced tracer control (Dc). Since DMSO was used as a solvent in the compound library, the effect of 10% DMSO on the FP-binding assay was also determined. Data points were normalized to the maximum specific binding, which defines complete saturation of PBP 2 by Bocillin-FL in the absence of penicillin G, and IC50 values were determined using non-linear regression analysis using GraphPad Prism version 4.00 for Windows (GraphPad Software, Inc, San Diego, CA).
Assay performance was assessed using the following parameters: the signal-to-noise ratio S/N = (µpc-µnc)/SDnc, Z′ and Z factors. The latter were calculated as Z′ = 1− (3SDpc +3SDnc)/(µpc-µnc) and Z = 1− (3SDpc +3SDdc)/(µpc-µc), where SDpc, SDnc, SDdc are standard deviations and µpc, µnc, µdc are means of recorded polarization values of Pc, Nc, and Dc, respectively [31].
High-throughput Assay and Screening for the Inhibitors
HTS screening against the ChemBridge DIVERSet library was carried out under the following conditions: 1 µl of each compound (10% DMSO final) in duplicate was pre-incubated with 9 µl of PBP 2 for 1 h at room temperature, followed by additional 30 min incubation with 2 µl Bocillin-FL (0.87 µM PBP2∶ 1 µM Bocillin-FL final). In addition to samples with the compounds, each plate also contained 2 background wells (12 µl buffer only), and at least 4 wells each for Pc, Nc, and Dc reactions. All samples used for background measurements or controls included 10% DMSO. Initial screening was carried out with 10 mM cocktails of 10 compounds (giving a final concentration of 100 µM for each compound). Compounds from cocktails that showed 80% or more reduction in ΔmP compared to maximum binding (ΔmPmax = Pc – Nc) were then tested individually. The auto fluorescence of each compound was also tested individually.
Concentration-response Experiments
Concentration-response experiments with the individual compounds that demonstrated ≥50% ΔmP reduction compared to maximum binding were conducted using FP-based and SDS-PAGE-based steady-state binding assays. For the FP experiments, 0.01–200 µM of each potential hit was incubated with PBP 2 (1 µM) for 1 h, followed by an additional 30 min incubation with 1 µM Bocillin-FL to detect the residual activity of the labeled penicillin binding. For the SDS-PAGE-based concentration-response experiments, PBP 2 (1 µM) in 50 mM sodium phosphate, 0.01% Triton X-100, pH 8 was incubated with 0.05–1000 µM of a compound for 1 h, followed by additional 15 min incubation with 10 µM Bocillin-FL. The reaction was stopped by mixing each sample with 5 X SDS-loading buffer, followed by boiling for 2 min. The samples were submitted to electrophoresis on 10% Mini-Protean TGX™ SDS-PAGE gels (Bio-Rad, Hercules, CA) to separate bound PBP 2 from free ligand. At least two independent reactions were performed in duplicate at each concentration of an inhibitor. Gels were visualized by UV using a Kodak EDAS 290 imaging system (Scientific Imaging Systems Eastman Kodak, New Haven, CT, USA) and the protein bands were stained with Coomassie to verify equal loading. Densitometry was performed with ImageJ 1.37v program (National Institutes of Health, USA, http://rsb.info.nih.gov/ij/). In both methods, data points were normalized to the maximum value of the fluorescence intensity, which defines complete saturation of PBP 2 by Bocillin-FL in the absence of compound, and IC50 values of the inhibitors were defined as the concentration of a compound at 50% of the residual activity of Bocillin-FL and calculated using GraphPad Prism (see above).
Antimicrobial Susceptibility Testing
Three strains of N. gonorrhoeae were used in susceptibility tests: 1) FA19, a penicillin-and cephalosporin-susceptible strain; 2) FA6140, a penicillin-resistant but cephalosporin-susceptible strain; and 3) 35/02, a penicillin-resistant and cephalosporin intermediate-resistant strain. The disc diffusion zone method was applied for preliminary testing of “hits” identified in the screening process [32]. For this method, 100 µl of a cell suspension (∼1×107 bacteria) was added to 3 ml of GCB top agar (GC Broth containing 0.75% agar) at 50oC, which was then added to GCB plates and allowed to solidify. Five µl of each compound (5 µg) was added separately to antibiotic susceptibility discs (BBL™, BD, Franklin Lakes, NJ), the discs were placed on top of plates, and the plates were incubated overnight in a 4% CO2 incubator at 37oC. Those compounds that produced a visible zone of inhibition were then tested for their MICs on agar plates as described [33]. Briefly, GCB agar plates containing increasing amounts of the tested compounds were spotted with 5×104 cells. The lowest concentration of the test compound that prevented growth of <5 colonies was defined as the MIC. These experiments were repeated three times, with each experiment producing similar results.
Molecular Modeling of PBP2 Inhibitors
Modeling, simulations and visualizations were performed using Molecular Operating Environment (MOE) Version 2011.10 (Chemical Computing Group Inc., Montreal, Canada). The crystal structure of wild-type N. gonorrhoeae PBP 2 was used as the starting model for docking simulations (PDB:3EQU; [26]). Following protonation of the main chain at pH 7.5 and addition of salt at a concentration of 0.2 M, the structure was energy minimized using the Amber99 forcefield and Born solvation model. Ligands corresponding to the 7 compounds that displayed antimicrobial activity against N. gonorrhoeae were prepared in MOE, and also protonated at pH 7.5 with a salt concentration of 0.2 M. The entire protein surface was used for the simulations, in which PBP 2 was held rigid and the ligand was flexed. Five hundred poses per ligand were derived using triangle-matching placement with London dG scoring. The top 250 poses were then refined using forcefield placement and affinity dG scoring, leading to a final database of 1750 poses for the seven compounds. All poses within the active site were then energy minimized using the Amber99 forcefield and Born solvation model, with both PBP 2 and the ligand allowed to flex.
Results
Development of the Fluorescence Polarization Assay
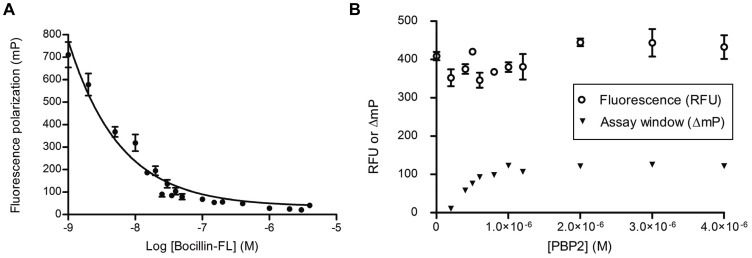
Initial experiments were performed to elucidate the minimal amounts of Bocillin-FL and PBP 2 that give the largest range of specific binding. The mP recorded for free Bocillin-FL in the range of 0.2–4 µM was 40–65 mP (Fig. 1A). Based on the results of steady-state binding experiments using variable concentrations of PBP 2 and Bocillin-FL, the optimal assay window (ΔmP) was 119 mP at a protein-Bocillin-FL ratio of 1∶1 µM. At this ratio, the total fluorescence of Bocillin-FL was not significantly affected by protein binding (Fig. 1B). The average value of the G-factor coefficient used to correct mP measurements for the instrument bias was 1.0±0.01. This was determined within a 0.2–4 µM concentration range of free Bocillin-FL and used in subsequent FP experiments, including the HTS.
Figure 1. Optimization of the fluorescence polarization (FP) assay.
A. Fluorescence polarization (mP) of free Bocillin-FL at various concentrations from 0.002 to 4 µM. B. Binding experiments with Bocillin-FL (1 µM) and PBP 2 at various protein concentrations from 0.2 to 4 µM. Fluorescence of Bocillin-FL – PBP2 was measured in relative fluorescence units (RFU) (open circles). Maximum specific binding (triangles), i.e. assay window (ΔmP), was determined by FP. Assay window is defined as the difference between FP of protein-tracer sample and free-tracer, i.e. ΔmP = mPs – mPfree. In all experiments, the data points represent the mean ± standard deviation of four replicate experiments at each concentration of Bocillin-FL or PBP 2.
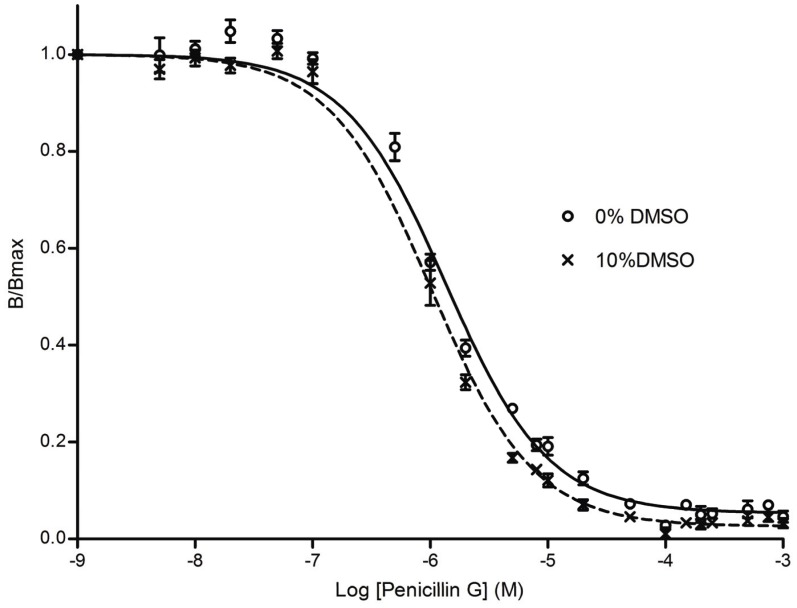
Penicillin G was used to evaluate and validate the assay for HTS. The IC50 values of penicillin G for PBP 2 were determined in a FP-based competition assay with and without 10% DMSO (Fig. 2) and were 1.0 µM (95% confidence interval 0.9–1.2 µM, R2 = 0.99) and 1.4 µM (95% confidence interval 1.2–1.5 µM, R2 = 0.98), respectively. The statistical parameters, including a signal-to-noise ratio >20, and Z′- and Z- factors ≥0.64, indicated the sensitivity of the FP assay and demonstrated its suitability for HTS (Table 1 and Fig. S1).
Figure 2. Inhibition of FP of Bocillin-FL and PBP 2 by increasing amounts of penicillin G.
Assays contained variable concentrations of penicillin G (0.05–1000 µM) with fixed concentrations of PBP 2 (1 µM) and Bocillin-FL (1 µM) with or without 10% DMSO. The solid line represents data for PBP 2 without DMSO and the dashed line is PBP 2 with DMSO. In all experiments, the data points represent the mean ± standard deviation of four replicate experiments at each concentration of penicillin G.
Table 1. Statistical parameters of the FP assay.
| Pc ± SD (mP) | Nc ± SD (mP) | S/N | Z′-factor | Dc ± SD (mP) | Z-factor | |
| Optimization | 172±9 (n = 8) | 53±5 (n = 8) | 23.8 | 0.65 | 55±5 (n = 8) | 0.64 |
| HTS assay | 171±11 (n = 379) | 46±8 (n = 368) | 15.4 | 0.55 | 46.5±9.7 (n = 246) | 0.51 |
The performance of the assay was determined during initial optimization and after the HTS assay. Pc is the mean FP signal of PBP 2-bound tracer control, Nc is the mean FP signal of free Bocillin-FL, and Dc is the FP signal in the presence of 100 µm penicillin G. S/N (signal-to-noise) and the Z factors are as defined in Material and Methods. (n = number of measurements). See also Fig. S1).
High-throughput Assay for Inhibitors of N. gonorrhoeae PBP 2
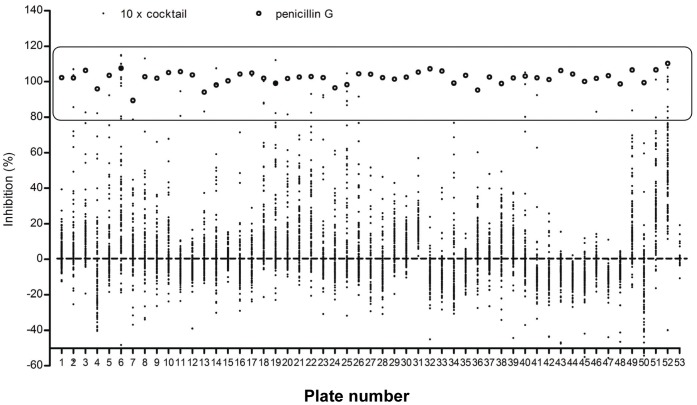
The FP assay was then employed in HTS of the ChemBridge DIVERset chemical library to find potential inhibitors of N. gonorrhoeae PBP 2. This is a diversity library of small molecules filtered for the inclusion of desirable drug-like features and exclusion of non-selective toxic groups. In one published example of HTS against zebra fish embryos using a subset of this library, the toxicity rate was 2% [34] and for the full library we have observed overall toxicity in the range of 3%, even as pooled compounds (YKP, personal observation). In the initial screening of 50,080 compounds (present as cocktails of 10 compounds), 58 cocktails exhibited ≥80% inhibition of Bocillin-FL binding to PBP 2 (Fig. 3). Each of the 580 compounds was then tested individually and 32 of these demonstrated more than 50% inhibition (Tables S1, S2). None of the “hits” were autofluorescent at the wavelengths used for the FP assay, thus paving the way for further characterization.
Figure 3. Initial HTS of 50,080 small lead compounds from DIVERSet ChemBridge Library was performed using cocktails of 10 different compounds (10X cocktails).
Each plate contained duplicate samples of 10X cocktails with PBP 2 and Bocillin-FL. Data points denoted by the black dots represent the percent of inhibition for each 10X cocktail determined using the average of two sample FP measurements, and the means of free Bocillin-FL (Nc) and bound Bocillin–FL (Pc) controls recorded in quadruplicate for each corresponding plate. Data points of the displaced tracer controls (Dc - the FP of the Bocillin-FL - protein at 100 µM penicillin G), denoted by open circles, represent the percent of inhibition based on the average of four FP measurements. Fifty-two plates with 96×10X cocktails each and one plate with eight×10 X cocktails were screened. The box indicates the 58 cocktails that exhibited ≥80% inhibition of Bocillin-FL binding to PBP 2.
Concentration-response Experiments
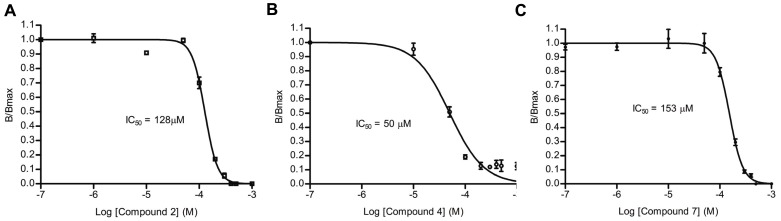
To characterize the inhibitory activity of each of the 32 hits, concentration-response experiments were performed using the FP-based binding assay in which 0.01–200 µM of each compound was incubated with 1 µM of both PBP 2 and Bocillin-FL. Three compounds failed to show a concentration-dependent response and were excluded from further study. A cephalosporin, which had an IC50 of 3 µM (the lowest of all the “hits”), was also excluded since the goal was to identify non-β-lactam inhibitors; however, the successful identification of a β-lactam was validation of the effectiveness of the assay (Fig. S2 and Table S3). IC50 values for 24 of the remaining 28 compounds (four compounds were not available from ChemBridge) were also measured in an SDS-PAGE concentration-response assay using a 0.05–1000 µM concentration range for the inhibitor with 1 µM PBP 2 and 10 µM of Bocillin-FL to confirm their binding activities. Triton X-100 (0.01%) was included in this assay to eliminate “promiscuous” inhibitors (e.g. those that inhibit by non-specific aggregation [35], [36]). Six compounds failed to show concentration-dependent inhibition in the presence of Triton X-100 and were therefore excluded. The remaining 18 compounds had IC50 values in the range of 50–900 µM (Table 2 and Table S3). Representative examples are shown in Fig. 4 and an example of an SDS-PAGE gel used to determine the IC50 for compound 7 is shown in Fig. S3.
Table 2. IC50 values and antimicrobial activities of seven compounds identified by high-throughput screening against PBP 2 from N. gonorrhoeae strain FA19.
| Compound # | IC50(µM; µg/ml) | MIC (µg/ml) | ||
| FA19 | 35/02 | FA6140 | ||
| 1 | 221/135 | 4 | 16 | 16 |
| 2 | 128/38 | 4 | 8 | 16 |
| 3 | 898/208 | 16 | 32 | 32 |
| 4 | 50/20 | 16 | 16 | 16 |
| 5 | 56/20 | 8 | 16 | 16 |
| 6 | 49/38 | 4 | 8 | 8 |
| 7 | 153/54 | 2 | 8 | 8 |
Figure 4. IC50 values of three representative hits from HTS for inhibition of acylation of PBP 2 by Bocillin-FL.
IC50s of the inhibitors were determined by SDS-PAGE-based concentration-response assays using a 0.05–1000 µM concentration range for the inhibitors with 1 µM PBP 2 and 10 µM of Bocillin-FL. Triton X-100 (0.01%) was included to eliminate promiscuous inhibitors. A. Compound 2 (in Table 2). B. Compound 4. C. Compound 7. In all experiments, the data points represent the mean ± standard deviation for two replicate (compounds 4 and two) or four replicate (compound 7) experiments at each concentration of inhibitor.
Antimicrobial Susceptibility Testing
The 18 compounds were then tested for their ability to suppress the growth of N. gonorrhoeae FA19 using the disc diffusion zone method. Eleven compounds did not suppress growth and were excluded (data not shown). For the remaining 7 compounds, the minimal inhibitory concentrations (MICs) were determined against three N. gonorrhoeae strains: FA19, a penicillin-susceptible strain [28]; FA6140, a penicillin-resistant but cephalosporin-susceptible strain [29]; and 35/02, a strain that exhibits intermediate resistance to cephalosporin (CephI)[30]. The IC50 values and MICs of the seven compounds are shown in Table 2 and their respective structures are shown in Fig. 5. The MIC values ranged from 2–32 µg/ml and all except for compound 4 were higher in resistant strains compared to FA19. The best compound overall (7) exhibited an MIC of 2 µg/ml (5.6 µM) against FA19 and 8 µg/ml (23.4 µM) against both FA6140 and 35/02.
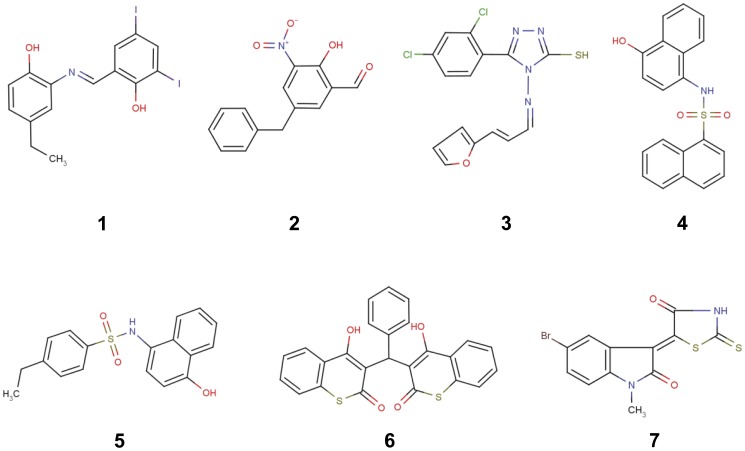
Figure 5. Structures of seven compounds that exhibited antimicrobial activity against N. gonorrhoeae.
Compound numbers correspond with those in Table 2.
Docking Simulations
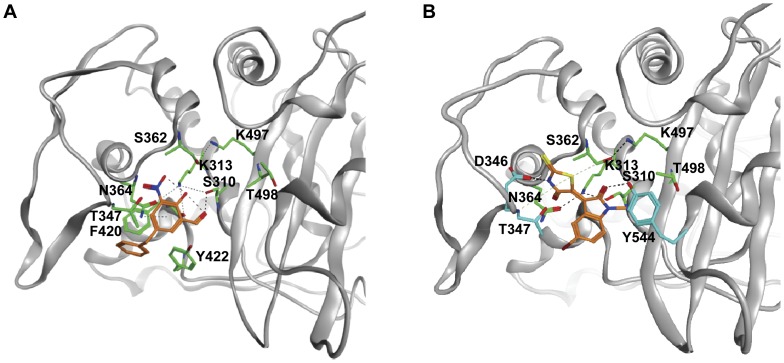
In order to identify potential binding sites and evaluate the mode of interaction between the experimental compounds and PBP 2, we probed the entire surface of the protein with the inhibitors using molecular docking simulations. Five hundred random starting positions for each compound were generated and the best 250 scoring poses were refined and ranked. Top poses within the active site were energy minimized, with both protein and inhibitor bonds allowed to rotate. The presence of a refined pose within the active site of PBP 2 in the top ten poses was taken as a high probability of selective interaction (Fig. S4). Hit rates to the active site varied for each compound as follows: Compound 1 - 5/10, Compound 2 - 1/10, Compound 3 - 1/10, Compound 4 - 7/10, Compound 5 - 6/10, Compound 6 - 4/10, and Compound 7 - 3/10. These data indicate a high propensity for all the active compounds to bind to the active site. For compound 2, the number one pose out of 250 refined poses docked within the active site (Fig. 6A). There are three predicted contacts between this compound and PBP 2: a hydrogen bond between the ligand carbonyl and the hydroxyl of Ser310 (the active site nucleophile), an interaction between the amide nitrogen of Thr347 and the nitrous acid of compound 2, and an arene-hydrogen interaction between the conjugated phenyl ring of compound 2 and Asn364. The phenyl ring interacts with a shallow hydrophobic pocket formed by Phe420 and Tyr422 on one side, but is solvent exposed on the other. Compound 7 was found in the active site in the fifth pose out of 250 refined poses (Fig. 6B). In this model, hydrogen bonds are observed between Asp346 and the secondary amine of the thiazolidine ring, and between the hydroxyl of Tyr544 and the carbonyl of compound 7. Additionally, there are arene-hydrogen interactions from the thiazolidine ring to both Thr347 and Ser362. The bromine atom appears to be totally solvated and does not interact with the protein directly. Overall, the docking results suggest that all of the seven compounds can bind within the active site of PBP 2, although precise details of binding can only be revealed by experimental structural studies.
Figure 6. Docking of two compounds into the structure of N. gonorrhoeae penicillin-binding protein 2.
The main chain of PBP 2 is shown as a grey ribbon, residues of the active site motifs of PBPs are shown with green bonds and additional amino acids that are predicted to form interactions with the ligand are shown in blue. A. compound 2. B. compound 7. Figure prepared with MOE 2011.10 (Chemical Computing Group Inc.).
Discussion
As important targets for β-lactams and against the background of increasing clinical resistance to existing antibiotics, much effort has been directed toward developing new inhibitors of PBPs. Some approaches have sought to adapt the β-lactam moiety by, for instance, incorporating elements of the peptide substrates onto one of the R1 or R2 side chains [37], [38], [39], while others have synthesized compounds that mimic the tetrahedral intermediates of the reaction, including phosphonates and boronates [40], [41], [42], [43]. Given the wealth of structural information for several clinically important PBPs, in silico docking has also been employed in some systems [44]. To contribute to this effort, we have developed a high-throughput assay for PBPs that measures the fluorescence polarization (FP) of Bocillin-FL [27] and used it to screen for potential inhibitors of N. gonorrhoeae PBP 2 from a library of 50,080 compounds. After eliminating those that acted promiscuously, did not demonstrate a concentration-dependent inhibition, or were β-lactams, 18 compounds were examined in more detail, and 7 of these exhibited antimicrobial activity against N. gonorrhoeae, including PenR and CephI strains. To the best of our knowledge, this is the first report of high-throughput screening (HTS) against a PBP.
Development of the assay was made possible by using fluorescence polarization to distinguish between the fluorescent penicillin (Bocillin-FL) remaining in solution from that bound to the PBP target, an approach first suggested by Zhao et al. [27]. The robustness and sensitivity of the assay is supported by the large assay window and Z factor. As a safeguard against any false positives arising from the FP-based assay, an SDS-PAGE competition assay was used to confirm inhibition of PBP 2. The identification of a cephalosporin with an IC50 of 3 µM against PBP2 was demonstration of the validity of the assay.
Out of the seven compounds that exhibited the lowest IC50 values for PBP 2 (∼ 50 µM) and good anti-gonococcal activity, two of these (compounds 4 and 5) contain a sulfonamide group between two aromatic rings. Interestingly, a sulfonamide of similar structure was identified recently as an inhibitor of PBP2a from methicillin-resistant Staphylococcus aureus (MRSA) [45]. Similar to compounds 4 and 5, compound 1 has two aromatic rings joined by a bridging group reminiscent of the sulfonamide inhibitors and showed reasonable antimicrobial activity against FA19, but less so against 6140 and 35/02. Compound 2 also possesses two aromatic rings, one of which contains a potentially reactive hydroxyl nitrobenzaldehyde group. This compound demonstrated moderate antibacterial activity against FA19, with higher MIC values against FA610 and 35/02. It was the most successful compound during the docking simulations and in this model there is an interaction with the serine nucleophile of PBP 2 (Ser310). Compound 6 exhibited the lowest IC50 value (49 µM) with good MICs against FA19 (4 µg/ml) and the two antibiotic-resistant strains (both were 8 µg/ml). Compound 7 showed rather moderate enzyme inhibition (IC50 = 153 µM) but exhibited the highest antimicrobial activity in all N. gonorrhoeae strains. Interestingly, this compound contains a thiazolidine ring, a feature of penicillin antibiotics and arylalkylidene iminothiazolidin-4-ones, which inhibit some PBPs in vitro and shows antimicrobial activity [46]. Compound 7 was also successful in the docking simulations and there are predicted interactions with Ser310. The resemblance of compound 7 to a β-lactam-like compound led us to determine whether there is a covalent interaction with PBP 2, but no increase in mass of the protein was observed by mass spectrometry (data not shown). Finally, compound 3 is probably the least promising candidate at this stage since it has a relatively high IC50 against PBP 2 and its MIC values against the N. gonorrhoeae strains are correspondingly weak.
Overall, there was relatively weak correlation between IC50 and MIC values for the seven compounds, but this is expected for initial hits from HTS because there are a variety of factors that determine the MIC. Since N. gonorrhoeae expresses two essential PBPs, PBP 1 could be the lethal target for a given compound in addition to, or independent of, PBP 2. The outer membrane of Gram-negative bacteria is a barrier to hydrophobic compounds [47] and the lipooligosaccharide structure of N. gonorrhoeae also impacts permeability. In addition, entry of hydrophilic compounds into the periplasm is influenced by porins and the action of efflux systems can remove compounds from the periplasm. Both of the antibiotic resistant strains (FA6140 and 35/02) contain a mutation in the promoter of mtrCDE that increases expression of the MtrC-MtrD-MtrE efflux pump. In addition, both strains harbor an altered porB1B allele encoding the major outer membrane porin, which decreases influx of antibiotics into the periplasmic space [17], [30], [48]. Hence, the ability of the different compounds to permeate porins or to be substrates of the efflux pump can have a profound impact on antibiotic efficacy [19]. It is also possible that some of the compounds with low MICs are cytotoxic to N. gonorrhoeae. Further investigation of the compounds is therefore necessary to establish their in vivo mechanism of antimicrobial activity.
In conclusion, an FP-based HTS has generated several inhibitors of PBP 2 and several show promising antimicrobial activity against PenR and CephI strains of N. gonorrhoeae. Such compounds require further study to confirm their mechanism of action, followed by chemical optimization to improve their efficacy. The development of this assay paves the way to screen using larger compound libraries and against variants of PBP 2 that contribute to third-generation cephalosporin resistance.
Supporting Information
Millipolarization values (mP) of positive control (Pc), negative control (Nc) and displaced tracer control (Dc) for all measurements recorded during the HTS, including the initial optimization. The data points represent mP measurements (n = sample number).
(TIF)
Four of the 32 compounds that were excluded from further study. A. Compounds 30, 31, 32 failed to show a concentration-dependent response in FP-based experiments. IC50s of the compounds were determined by FP-based concentration-response assays using a 0.01–200 µM concentration range for the compounds with 1 µM PBP 2 and 1 µM of Bocillin-FL. In all experiments, the data points represent the mean ± standard deviation over four replicate experiments. B. Compound 29 had an IC50 of 3 µM (the lowest of all the “hits”), but is a cephalosporin.
(TIF)
SDS-PAGE-based analysis of the inhibitory activity of compound 7 against PBP 2 (IC50 = 153 µM). PBP 2 (1 µM) in 50 mM sodium phosphate, 0.01% Triton X-100, pH 8 was incubated with 0.05–1000 µM of compound 7 for 1 h, followed by 15 min incubation with 10 µM Bocillin-FL. The reaction was stopped by mixing with 5 X SDS-loading buffer, followed by boiling for 2 min. 10% SDS-PAGE gels was then used to separate bound PBP 2 from free ligand. At least two independent reactions were performed in duplicate at each concentration of the inhibitor. Gels were visualized by UV (top panel) to measure IC50 and the same gels were stained with Coomassie Brilliant Blue R-250 to verify equal loading of protein (lower panel).
(TIF)
Docking of compounds as surface probes with PBP 2. Depicted are the top 10 poses for each compound (numbered 1–7) from 250 refined poses, as described in the Material & Methods. PBP 2 is displayed as a grey surface and is in the same orientation as Fig. 6. The active site region is colored green and the compounds are displayed in bond format and colored orange.
(TIF)
The layout of the 384-well plates used for the high-throughput screening of the 50,080 compound Chembridge DIVERSet library. Compounds were present as cocktails of 10 compounds each (10X cocktails). In total, fifty-two plates with 96 cocktails and one plate with 8 cocktails were screened. The wells are numbered according to the scheme below, where each well contains one cocktail and each cocktail is present twice for two independent measurements. Rows J-P in each plate were not used. Dc = displaced tracer control, Bk = blank, Nc = negative control and Pc = positive control.
(DOCX)
Analysis of the 58 cocktails showing ≥80% inhibition of Bocillin-FL binding to PBP 2.
(DOCX)
Analysis of 32 individual compounds that exhibited ≥50% inhibition of Bocillin-FL binding to PBP 2.
(DOCX)
Acknowledgments
We thank Dr. Charles D. Smith for technical advice. The MUSC Drug Discovery Shared Resource is supported by the MUSC Hollings Cancer Center.
Funding Statement
This work was supported by the National Institutes of Health grants GM66861 to C.D. and AI36901 to R.A.N. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Centers for Disease Control (CDC) (2007) Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 56: 332–336. [PubMed] [Google Scholar]
- 2. Akasaka S, Muratani T, Yamada Y, Inatomi H, Takahashi K, et al. (2001) Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. J Infect Chemother 7: 49–50. [DOI] [PubMed] [Google Scholar]
- 3. Muratani T, Akasaka S, Kobayashi T, Yamada Y, Inatomi H, et al. (2001) Outbreak of cefozopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimicrob Agents Chemother 45: 3603–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tapsall J, Read P, Carmody C, Bourne C, Ray S, et al. (2009) Two cases of failed ceftriaxone treatment in pharyngeal gonorrhoea verified by molecular microbiological methods. J Med Microbiol 58: 683–687. [DOI] [PubMed] [Google Scholar]
- 5. Golparian D, Hellmark B, Fredlund H, Unemo M (2010) Emergence, spread and characteristics of Neisseria gonorrhoeae isolates with in vitro decreased susceptibility and resistance to extended-spectrum cephalosporins in Sweden. Sex Transm Infect 86: 454–460. [DOI] [PubMed] [Google Scholar]
- 6.Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H (2010) Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill 15: pii: 19721. [DOI] [PubMed]
- 7. Unemo M, Golparian D, Hestner A (2011) Ceftriaxone treatment failure of pharyngeal gonorrhoeae verified by international recommendations, Sweden, July 2010. Euro Surveill 16: 1–3. [PubMed] [Google Scholar]
- 8. Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, et al. (2011) Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis 17: 148–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, et al. (2012) High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56: 1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, et al. (2011) Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55: 3538–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A (2006) Penicillin binding proteins: key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol Rev 30: 673–691. [DOI] [PubMed] [Google Scholar]
- 12. Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P (2008) The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev 32: 234–258. [DOI] [PubMed] [Google Scholar]
- 13. Vollmer W, Blanot D, de Pedro MA (2008) Peptidoglycan structure and architecture. FEMS Microbiol Rev 32: 149–167. [DOI] [PubMed] [Google Scholar]
- 14. Goffin C, Ghuysen JM (1998) Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol Mol Biol Rev 62: 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh AS, Chowdhury C, Nelson DE (2008) Physiological functions of D-alanine carboxypeptidases in Escherichia coli. Trends Microbiol 16: 309–317. [DOI] [PubMed] [Google Scholar]
- 16. Stefanova ME, Tomberg J, Olesky M, Holtje JV, Gutheil WG, et al. (2003) Neisseria gonorrhoeae penicillin-binding protein 3 exhibits exceptionally high carboxypeptidase and beta-lactam binding activities. Biochemistry 42: 14614–14625. [DOI] [PubMed] [Google Scholar]
- 17. Ropp PA, Hu M, Olesky M, Nicholas RA (2002) Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae . Antimicrob Agents Chemother 46: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barbour AG (1981) Properties of penicillin-binding proteins in Neisseria gonorrhoeae . Antimicrob Agents Chemother 19: 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafer WM, Folster JP, Nicholas RA (2010) Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseriae. In: Genco CA, L W, editors. Neisseria: Molecular Mechanisms of Pathogenesis: Horizon Scientific Press. 245.
- 20.Nicholas RA, Davies C (2012) Structural Mechanisms of β-Lactam Antibiotic Resistance in Penicillin-Binding Proteins.. In: Dougherty TJ, Pucci MJ, editors. Antibiotic Discovery & Development: Springer-Verlag 397–425.
- 21. Brannigan JA, Tirodimos IA, Zhang QY, Dowson CG, Spratt BG (1990) Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae . Mol Microbiol 4: 913–919. [DOI] [PubMed] [Google Scholar]
- 22. Dowson CG, Jephcott AE, Gough KR, Spratt BG (1989) Penicillin-binding protein 2 genes of non-beta-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae . Mol Microbiol 3: 35–41. [DOI] [PubMed] [Google Scholar]
- 23. Ameyama S, Onodera S, Takahata M, Minami S, Maki N, et al. (2002) Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother 46: 3744–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, et al. (2005) Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother 49: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ochiai S, Sekiguchi S, Hayashi A, Shimadzu M, Ishiko H, et al. (2007) Decreased affinity of mosaic-structure recombinant penicillin-binding protein 2 for oral cephalosporins in Neisseria gonorrhoeae . J Antimicrob Chemother 60: 54–60. [DOI] [PubMed] [Google Scholar]
- 26. Powell AJ, Tomberg J, Deacon AM, Nicholas RA, Davies C (2009) Crystal structures of penicillin-binding protein 2 from penicillin-susceptible and -resistant strains of Neisseria gonorrhoeae reveal an unexpectedly subtle mechanism for antibiotic resistance. J Biol Chem 284: 1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC (1999) BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob Agents Chemother 43: 1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maness MJ, Sparling PF (1973) Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae . J Infect Dis 128: 321–330. [DOI] [PubMed] [Google Scholar]
- 29. Danielsson D, Faruki H, Dyer D, Sparling PF (1986) Recombination near the antibiotic resistance locus penB results in antigenic variation of gonococcal outer membrane protein. Infect Immun 52: 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindberg R, Fredlund H, Nicholas R, Unemo M (2007) Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob Agents Chemother 51: 2117–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang JH, Chung TD, Oldenburg KR (1999) A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen 4: 67–73. [DOI] [PubMed] [Google Scholar]
- 32. Bauer AW, Perry DM, Kirby WM (1959) Single-disk antibiotic-sensitivity testing of staphylococci; an analysis of technique and results. AMA Arch Intern Med 104: 208–216. [DOI] [PubMed] [Google Scholar]
- 33. Tomberg J, Unemo M, Davies C, Nicholas RA (2010) Molecular and Structural Analysis of Mosaic Variants of Penicillin-Binding Protein 2 Conferring Decreased Susceptibility to Expanded-Spectrum Cephalosporins in Neisseria gonorrhoeae: Role of Epistatic Mutations. Biochemistry 49: 8062–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peterson RT, Link BA, Dowling JE, Schreiber SL (2000) Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A 97: 12965–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng BY, Shoichet BK (2006) A detergent-based assay for the detection of promiscuous inhibitors. Nat Protoc 1: 550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shoichet BK (2006) Screening in a spirit haunted world. Drug Discov Today 11: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Josephine HR, Kumar I, Pratt RF (2004) The perfect penicillin? Inhibition of a bacterial DD-peptidase by peptidoglycan-mimetic beta-lactams. J Am Chem Soc 126: 8122–8123. [DOI] [PubMed] [Google Scholar]
- 38. Josephine HR, Charlier P, Davies C, Nicholas RA, Pratt RF (2006) Reactivity of penicillin-binding proteins with peptidoglycan-mimetic beta-lactams: what’s wrong with these enzymes? Biochemistry 45: 15873–15883. [DOI] [PubMed] [Google Scholar]
- 39. Kumar I, Josephine HR, Pratt RF (2007) Reactions of peptidoglycan-mimetic beta-lactams with penicillin-binding proteins in vivo and in membranes. ACS Chem Biol 2: 620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silvaggi NR, Anderson JW, Brinsmade SR, Pratt RF, Kelly JA (2003) The crystal structure of phosphonate-inhibited D-Ala-D-Ala peptidase reveals an analogue of a tetrahedral transition state. Biochemistry 42: 1199–1208. [DOI] [PubMed] [Google Scholar]
- 41. Pechenov A, Stefanova ME, Nicholas RA, Peddi S, Gutheil WG (2003) Potential transition state analogue inhibitors for the penicillin-binding proteins. Biochemistry 42: 579–588. [DOI] [PubMed] [Google Scholar]
- 42. Nicola G, Peddi S, Stefanova ME, Nicholas RA, Gutheil WG, et al. (2005) Crystal structure of Escherichia coli penicillin-binding protein 5 bound to a tripeptide boronic acid inhibitor: a role for Ser-110 in deacylation Biochemistry. 44: 8207–8217. [DOI] [PubMed] [Google Scholar]
- 43. Inglis SR, Zervosen A, Woon EC, Gerards T, Teller N, et al. (2009) Synthesis and evaluation of 3-(dihydroxyboryl)benzoic acids as D,D-carboxypeptidase R39 inhibitors. J Med Chem 52: 6097–6106. [DOI] [PubMed] [Google Scholar]
- 44. Miguet L, Zervosen A, Gerards T, Pasha FA, Luxen A, et al. (2009) Discovery of new inhibitors of resistant Streptococcus pneumoniae penicillin binding protein (PBP) 2x by structure-based virtual screening. J Med Chem 52: 5926–5936. [DOI] [PubMed] [Google Scholar]
- 45. Turk S, Verlaine O, Gerards T, Zivec M, Humljan J, et al. (2011) New noncovalent inhibitors of penicillin-binding proteins from penicillin-resistant bacteria. PLoS One 6: e19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zervosen A, Lu WP, Chen Z, White RE, Demuth TP, et al. (2004) Interactions between penicillin-binding proteins (PBPs) and two novel classes of PBP inhibitors, arylalkylidene rhodanines and arylalkylidene iminothiazolidin-4-ones. Antimicrob Agents Chemother 48: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nikaido H (1994) Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264: 382–388. [DOI] [PubMed] [Google Scholar]
- 48. Olesky M, Hobbs M, Nicholas RA (2002) Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae . Antimicrob Agents Chemother 46: 2811–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Millipolarization values (mP) of positive control (Pc), negative control (Nc) and displaced tracer control (Dc) for all measurements recorded during the HTS, including the initial optimization. The data points represent mP measurements (n = sample number).
(TIF)
Four of the 32 compounds that were excluded from further study. A. Compounds 30, 31, 32 failed to show a concentration-dependent response in FP-based experiments. IC50s of the compounds were determined by FP-based concentration-response assays using a 0.01–200 µM concentration range for the compounds with 1 µM PBP 2 and 1 µM of Bocillin-FL. In all experiments, the data points represent the mean ± standard deviation over four replicate experiments. B. Compound 29 had an IC50 of 3 µM (the lowest of all the “hits”), but is a cephalosporin.
(TIF)
SDS-PAGE-based analysis of the inhibitory activity of compound 7 against PBP 2 (IC50 = 153 µM). PBP 2 (1 µM) in 50 mM sodium phosphate, 0.01% Triton X-100, pH 8 was incubated with 0.05–1000 µM of compound 7 for 1 h, followed by 15 min incubation with 10 µM Bocillin-FL. The reaction was stopped by mixing with 5 X SDS-loading buffer, followed by boiling for 2 min. 10% SDS-PAGE gels was then used to separate bound PBP 2 from free ligand. At least two independent reactions were performed in duplicate at each concentration of the inhibitor. Gels were visualized by UV (top panel) to measure IC50 and the same gels were stained with Coomassie Brilliant Blue R-250 to verify equal loading of protein (lower panel).
(TIF)
Docking of compounds as surface probes with PBP 2. Depicted are the top 10 poses for each compound (numbered 1–7) from 250 refined poses, as described in the Material & Methods. PBP 2 is displayed as a grey surface and is in the same orientation as Fig. 6. The active site region is colored green and the compounds are displayed in bond format and colored orange.
(TIF)
The layout of the 384-well plates used for the high-throughput screening of the 50,080 compound Chembridge DIVERSet library. Compounds were present as cocktails of 10 compounds each (10X cocktails). In total, fifty-two plates with 96 cocktails and one plate with 8 cocktails were screened. The wells are numbered according to the scheme below, where each well contains one cocktail and each cocktail is present twice for two independent measurements. Rows J-P in each plate were not used. Dc = displaced tracer control, Bk = blank, Nc = negative control and Pc = positive control.
(DOCX)
Analysis of the 58 cocktails showing ≥80% inhibition of Bocillin-FL binding to PBP 2.
(DOCX)
Analysis of 32 individual compounds that exhibited ≥50% inhibition of Bocillin-FL binding to PBP 2.
(DOCX)