Abstract
Introduction
The percentage of time within the target INR range 2.0 to 3.0 (TTR) in patients treated with vitamin K antagonists varies considerably among efficacy-studies of novel anticoagulants. In order to properly asses the quality of anticoagulant control in upcoming cost-effectiveness studies and real life registries this systematic review reports a benchmark of TTR for different treatment durations in patients with venous thromboembolism and discusses ways to calculate TTR.
Methods
Medline and Embase were searched for studies published between January 1990 and May 2012. Randomized controlled trials and cohort studies reporting the TTR in patients with objectively confirmed venous thromboembolism treated with vitamin K antagonists (VKA) were eligible. Duplicate reports, studies only reporting INR during initial treatment or with VKA treatment less than 3 months were excluded. Three authors assessed trials for inclusion and extracted data independently. Discrepancies were resolved by discussion between the reviewers. A meta-analysis was performed by calculating a weighted mean, based on the number of participants in each included study, for each time-period in which the TTR was measured since the confirmation of the diagnosis of VTE.
Results
Forty studies were included (26064 patients). The weighted means of TTR were 54.0% in the first month since the start of treatment, 55.6% in months 1 to 3, 60.0% in months 2 to 3, 60.0% in the months1 to 6+ and 75.2% in months 4 to 12+. Five studies reported TTR in classes. The INR in these studies was ≥67% of time in therapeutic range in 72.0% of the patients.
Conclusion
Reported quality of VKA treatment is highly dependent on the time-period since the start of treatment, with TTR ranging from approximately 56% in studies including the 1st month to 75% in studies excluding the first 3 months.
Introduction
Traditionally, patients with venous thromboembolism (VTE) are treated with low molecular weight heparins (LMWH) and vitamin K antagonists (VKA) such as warfarin, acenocoumarol or phenprocoumon [1], [2]. As with any medical treatment, the weighing of risks and benefits must be carefully balanced. The effect of VKA therapy depends on many factors including variation in dose response between patients, individual variation in pharmacokinetics and pharmacodynamic response, multiple interactions with food, co- medication and finally also by variation in adherence [3], [4]. VKA have a narrow therapeutic index, which needs to be monitored carefully in order to reduce the risk of tromboembolic events as well as bleeding complications [5]. With the large scale clinical testing of novel, direct acting oral anticoagulants, including the thrombin and factor Xa inhibitors dabigatran and rivaroxaban, a new era has been heralded. The main advantage of these new anticoagulants is the lack of a need for laboratory monitoring and dose adjustment due to more stable pharmacokinetics [6]. Several recent large randomized controlled trials have shown non-inferiority in effectiveness and safety of the new anticoagulants compared to VKA treatment [7], [8], [9], [10], [11]. However, the percentage of time within therapeutic range in the VKA-group, representing the quality of the control group, appears to vary considerably among these studies.
The International Normalized Ratio (INR), the ratio of a patient's prothrombin time to a normal (control) sample, raised to the power of the International Sensitivity Index (ISI) value, is established by the World Health Organization (WHO) and the International Committee on Thrombosis and Hemostasis for monitoring the effects of VKA. A target INR range of 2.0 to 3.0 is recommended for the treatment of VTE [3]. The most recognized way to measure the therapeutic effectiveness of VKA over time is to measure the percentage of time in the therapeutic range (TTR). TTR has been shown to strongly correlate with the clinical outcomes of hemorrhage or thrombosis and, thus, TTR is a reliable measure of the quality of anticoagulation management [12].
Dabigatran and rivaroxaban have been recently approved in many countries including the USA, Canada and also in Europe. This development will cause major changes in thrombosis management in the near future. Cost-effectiveness studies and real life registries will be the next step in the implementation of new oral anticoagulants. In order to adequately compare all treatment options, including novel anticoagulants and VKA, and to interpret the relative efficacy and safety of these novel anticoagulants, it is important to properly assess the quality of anticoagulant control, i.e. TTR, in the VKA group. This systematic review tries to provide a benchmark of TTR in patients with VTE receiving VKA and discusses the pros and cons of various ways to calculate TTR. Finally, it emphasizes the need to standardize TTR reporting, thereby contributing to a meaningful comparison among treatment options in studies evaluating novel anticoagulants.
Materials and Methods
Data sources and searches
A systematic search was performed to identify randomized controlled trials and cohort studies reporting the TTR in patients treated with VKA for deep vein thrombosis (DVT) confirmed by a non-compressible venous segment on an ultrasound of the extremities, or pulmonary embolism (PE) confirmed by an arterial filling defect on Computed Tomographic Pulmonary Angiography (CTPA) or a high probability ventilation/perfusion (V/Q) scan, or both (VTE). We searched Medline and Embase for articles in English, French, German, Dutch, Polish, Swedish, Danish, Italian and Spanish. Since the World Health Organization introduced the INR in 1983 [13] and the first studies reporting TTR in VKA in patients with VTE were published in the nineties, we searched for publications between January 1990 and May 2012. See Appendix 1 for detailed information about the search strategy and key words.
Study selection
To be eligible for inclusion, studies had to fulfill the following criteria:
Study population consisted of consecutive adult patients with objectively confirmed DVT or PE.
Patients were treated with VKA for a minimum of three months.
Studies were excluded if they only reported the TTR in the initial treatment period while patients were still on parental medication such as low molecular weight heparin and unfractionated heparin.
Data extraction and management
Three reviewers (PE, HTC, MP) operating in pairs of two extracted independently the following characteristics from each included study: study design, type of study (e.g. evaluation of a new drug, dose-finding, evaluation of duration of anticoagulation), characteristics of the study population (e.g. number of patients treated with VKA, country, inclusion criteria, proportion of patients with a malignancy), initial treatment, type of VKA (e.g. warfarin, acenocoumarol, phenprocoumon or other), initial dose of VKA, treatment duration, INR-monitoring by thrombosis service or self-management, percentage of time below therapeutic range (INR <2), percentage of time within therapeutic range (INR 2.0–3.0), percentage of time above therapeutic range (INR >3), method of calculation TTR and adverse events (e.g. recurrent VTE, major bleeding and mortality). The quality of the included studies was assessed by addressing the following issues: a) were consecutive patients included in the study?, b) did the authors report reasons for exclusion?, c) were incomplete data adequately addressed?, d) did the authors address potential sources of bias?, e) what was the duration of follow-up?, f) how many patients (percentage) were lost to follow-up?. Discrepancies were resolved by discussion. If agreement could not be reached a third reviewer was consulted.
Data synthesis and analysis
A meta-analysis was performed by calculating a weighted mean, based on the number of participants in each included study, for each time-period in which the TTR was measured since the confirmation of the diagnosis of VTE.
Results
Results of the search
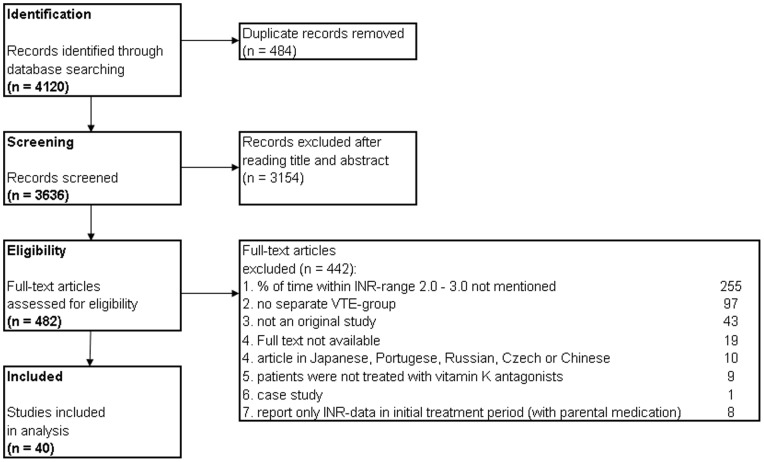
The systematic search yielded 3636 citations. The results were screened and after reading titles and abstracts 3154 articles were excluded. Of the remaining 482 publications the full text was assessed. (Figure 1).
Figure 1. Flow diagram of literature search.
Included studies
In total, 40 studies [8], [9], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51] reporting the TTR in 26064 patients treated with VKA for VTE were included in the analyses.
Most studies included patients with DVT as well as PE. Fifteen studies [9], [14], [16], [19], [20], [21], [22], [25], [31], [32], [33], [36], [39], [42], [47] reported the results from patients with only DVT and eight studies [15], [18], [38], [47], [48], [49], [50], [51] reported the results from patients with only PE. The percentage of cancer ranged from 0% to 100%. The study characteristics of the included studies are presented in Table 1. The quality assessment of each study is shown in Table 2.
Table 1. Study characteristics of included studies.
| Study design/ | % with | Included | Definition | Method | |||
| First author and year: | Type of study: | N: | cancer: | patients: | Treatment: | TR | measurement TTR: |
| Research Committee of | 358 | 3.6% | UFH and Warfarin (4 weeks treatment) | ||||
| British Thoracic Society, 1992 | RCT/duration OAC | 354 | 2.8% | VTE | UFH and Warfarin (3 months treatment) | INR 2.0–3.0 | Classes of %TTR |
| Agnelli, 2001 | RCT/duration OAC | 267 | 0% | DVT | LMWH and Warfarin/Acenocoumarol | INR 2.0–3.0 | Linear Interpolation |
| Agnelli, 2003 | RCT/duration OAC | 326 | 0% | PE | LMWH and Warfarin/Acenocoumarol | INR 2.0–3.0 | Linear Interpolation |
| Agnelli, 2007 | RCT/dose-finding | 126 | <3% | DVT | LMWH and Warfarin/Acenocoumarol | INR 2.0–3.0 | NR |
| 172 | 1% | LMWH and VKA local practice (outpatient treatment) | |||||
| Aujeskey, 2011 | RCT/outpatients vs. inpatients | 172 | 2% | PE | LMWH and VKA local practice (inpatient treatment) | INR 2.0–3.0 | Linear Interpolation |
| Van Bladel, 2010 | Cohort of PE patients | 86 | 14% | PE | LMWH and Acenocoumarol | INR 2.0–3.5 | Linear Interpolation |
| 104 | 100% | ||||||
| Bona, 2000 | Cohort with and without cancer | 208 | 0% | VTE | Warfarin | INR 2.0–3.0 | Linear Interpolation |
| 1103 | 10.2% | Fondaparinux and VKA local practice | |||||
| Büller, 2003 | RCT/medication trial | 1110 | 11.5% | PE | UFH and VKA local practice | INR 2.0–3.0 | Linear Interpolation |
| LMWH/UFH and Warfarin/Aceno- | |||||||
| Büller, 2008 | RCT/dose-finding | 137 | 7.3% | DVT | coumarol/Phenprocoum/Fluindione | INR 2.0–3.0 | Linear Interpolation |
| Caprini, 1999 | Cohort/INR and DVT resolution | 33 | 22.7% | DVT | UFH and Warfarin | INR 2.0–3.0 | NR |
| Cassiopea Investigators, 2012 | RCT/medication trial | 1603 | 6% | PE | LMWH and Warfarin | INR 2.0–3.0 | Linear Interpolation |
| 510 | 23.3% | LMWH and coumarin derivative | |||||
| Columbus Invesigators, 1997 | RCT/medication trial | 511 | 22.1% | VTE | UFH and coumarin derivative | INR 2.0–3.0 | NR |
| Das, 1996 | RCT/medication trial | 55 | 5.5% | DVT | LMWH and Warfarin | INR 2.0–3.0 | NR |
| Daskapoulos, 2005 | RCT/medication trial | 52 | 15.4% | DVT | UFH and Acenocoumarol | INR 2.0–3.0 | NR |
| after discontinuation LMWH | |||||||
| until end of treatment, | |||||||
| Einstein Investigators, 2010 | RCT/medication trial | 1718 | 5.2% | DVT | LMWH and Warfarin/Acenocoumarol | INR 2.0–3.0 | including interruptions |
| after discontinuation LMWH | |||||||
| until end of treatment, | |||||||
| Einstein Investigators, 2012 | RCT/medication trial | 2413 | 4.5% | PE | LMWH and Warfarin/Acenocoumarol | INR 2.0–3.0 | corrected for interruptions |
| Fiesinger, 2005 | RCT/medication trial | 1249 | 13.5% | VTE | LMWH (Enoxaparin) and Warfarin | INR 2.0–3.0 | NR |
| 360 | 22.2% | UFH and Warfarin | |||||
| Galilei Investigators, 2004 | RCT/medication trial | 360 | 21.1% | VTE | LMWH and Warfarin | INR 2.0–3.0 | Classes of %TTR |
| 1452 | 9.5% | DVT | |||||
| Van Gogh Investigators, 2007 | RCT/medication trial | 1120 | 8.4% | PE | LMWH/UFH and Warfarin/Acenonocoumarol | INR 2.0–3.0 | Linear Interpolation |
| Gonzalez-Fajardo, 1999 | RCT/medication trial | 80 | 10% | DVT | UFH and Coumarin | INR 2.0–3.0 | Linear Interpolation |
| Heidinger, 2000 | Cohort/self-management OAC | 622 | NR | VTE | VKA according to local practice | INR 2.0–3.0 | Last 12 values |
| Kearon, 1999 | RCT/duration OAC | 162 | 0% | VTE | LMWH/UFH and Warfarin | INR 2.0–3.0 | Linear Interpolation |
| Kearon, 2003 | RCT/dose-finding | 369 | 0% | VTE | LMWH/UFH and Warfarin | INR 2.0–3.0 | Linear Interpolation |
| Kearon, 2004 | RCT/duration of OAC | 165 | 0% | VTE | LMWH/UFH and Warfarin | INR 2.0–3.0 | Linear Interpolation |
| 355 | 16.6% | UFH and Warfarin | |||||
| Kearon, 2006 | RCT/medication trial | 353 | 15.0% | VTE | LMWH and Warfarin | INR 2.0–3.0 | Linear Interpolation |
| 198 | 18.2% | UFH and VKA local practice | |||||
| Koopman, 1996 | RCT/medication trial | 202 | 16.8% | DVT | LMWH and VKA local practice | INR 2.0–3.0 | Linear Interpolation |
| Levine, 1995 | RCT/duration OAC | 109 | 21.1% | DVT | UFH and Warfarin | INR 2.0–3.0 | NR |
| López-Beret, 2001 | RCT/medication trial | 77 | 23.3% | DVT | LMWH (Nadroparin) and Acenocoumarol | INR 2.0–3.0 | Linear Interpolation |
| Meyer, 2002 | RCT/medication trial | 75 | 100% | VTE | LMWH and Warfarin | INR 2.0–3.0 | NR |
| Monreal, 1998 | Cohort of VTE patients | 244 | 13.5% | VTE | LMWH (Dalteparin) and Coumarin | INR 2.0–3.0 | Linear Interpolation |
| Nielsen, 1993 | RCT/medication trial | 46 | 8.7% | DVT | UFH/Phenprocouman | INR 2.0–3.0 | NR |
| 733 | 0% | ||||||
| Palareti, 2000 | Cohort with and without cancer | 95 | 100% | VTE | Warfarin/Acenocoumarol | INR 2.0–3.0 | Linear Interpolation |
| Pérez-de-Llano, 2010 | RCT/medication trial | 50 | 6% | PE | LMWH (Tinzaparin) and Acenocoumarol | INR 2.0–3.0 | NR |
| Pini, 1994 | RCT/medication trial | 94 | 24.5% | DVT | Heparin and Warfarin | INR 2.0–3.0 | Classes of %TTR |
| RCT computer-assisted OAC | 1560 | NR | VKA local practice (Manual dosage) | ||||
| Poller, 2008 | dosage vs medical staff | 1649 | NR | VTE | VKA local practice (Computer-assisted dosage) | INR 2.0–3.0 | Linear Interpolation |
| Poli, 2007 | Cohort of VTE patients | 182 | 0% | VTE | LMWH/UFH and Warfarin | INR 2.0–3.0 | Median % of time |
| 90 | 16.7% | LMWH/UFH and VKA local practice (stockings) | |||||
| Prandoni, 2004 | RCT/intervention stockings | 90 | 11.1% | DVT | LMWH/UFH and VKA local practice (no stockings) | INR 2.0–3.0 | Classes of %TTR |
| Santamaria, 2006 | Cohort/cost-effectiveness | 116 | 17.2% | VTE | Acenocoumarol/Warfarin | INR 2.0–3.0 | NR |
| Classes of %TTR | |||||||
| Schulman, 1994 | RCT/duration of OAC | 1124 | 0% | VTE | LMWH/UFH and Warfarin | INR 2.0–2.85 | Linear Interpolation |
| Schulman, 2009 | RCT/medication trial | 1265 | 4.5% | VTE | LMWH/UFH and Warfarin | INR 2.0–3.0 | NR |
Abbreviations: RCT, Randomized Controlled Trial; OAC, oral anticoagulation; UFH, Unfractionated Heparin; LMWH, Low Molecular Weight Heparin; VTE, Venous ThromboEmbolism; DVT, Deep Vein Thrombosis; PE, Pulmonary Embolism; TR, Therapeutic Range; INR, International Normalized Ratio; NR, not reported.
Table 2. Quality assessment of the included studies.
| Reasons for | Incomplete data | Efforts to address | ||||
| Consecutive | exclusion | adequately | potential sources | % loss to | ||
| First author and year | patients? | reported? | addressed? | of bias? | Follow-up time | follow-up |
| Research committee of | ||||||
| British Thoracic | Yes | Yes | Yes | Yes | 12 months | 8% |
| Society, 1992 | ||||||
| Agnelli, 2001 | Yes | Yes | Yes | Yes | at least 2 years | 0% |
| Agnelli, 2003 | Yes | Yes | Yes | Yes | at least 1 year | 0% |
| Agnelli, 2007 | Yes | Yes | Yes | Yes | at least 4 | 0% |
| months | ||||||
| Aujeskey, 2011 | Yes | Yes | Yes | Yes | 3 months | 0.2% |
| Van Bladel, 2010 | Yes | Yes | Yes | Yes | 3 months | 1.2% |
| Bona, 2000 | Yes | No | Unclear | Unclear | Unclear | Unclear |
| Büller, 2003 | Yes | Yes | Yes | Yes | 3 months | 0.5% |
| Büller, 2008 | Unclear | No | Yes | Yes | 3 months | 0.7% |
| Caprini, 1999 | Yes | Yes | No | No | 6 months | Unclear |
| Cassiopea, 2012 | Yes | Yes | Yes | Yes | 6–12 months | Unclear |
| Columbus Investigators, 1997 | Yes | Yes | Yes | Yes | 3 months | 0% |
| Das, 1996 | Yes | Yes | Yes | Yes | 3 months | Unclear |
| Daskapoulos, 2005 | Yes | Yes | Yes | Yes | 12 months | 0% |
| Einstein Investigators, 2010 | Yes | Yes | Yes | Yes | 3–12 months | 1% |
| Einstein Investigators, 2012 | Yes | Yes | Yes | Unclear | Unclear | 0.4% |
| Fiesinger, 2005 | Unclear | Yes | Yes | Yes | 6 ½ months | 1.1% |
| Galilei Investigators, 2004 | Yes | Yes | Yes | Yes | 3 months | 0% |
| Van Gogh Investigators, 2007 | Yes | Yes | Yes | Yes | 3 months | 0.8% |
| Gonzalez-Fajardo, 1999 | Yes | Yes | Yes | Yes | 12 months | 1.6% |
| Heidinger, 2000 | No | Yes | No | Unclear | Unclear | Unclear |
| Kearon, 1999 | Yes | Yes | Yes | Yes | Average 10 | Unclear |
| months | ||||||
| Kearon, 2003 | Yes | Yes | Yes | Yes | Average 2.4 | 0.1% |
| years | ||||||
| Kearon, 2004 | Yes | Yes | Yes | Yes | 12 months | 0% |
| Kearon, 2006 | Yes | Yes | Yes | Yes | 3 months | 0% |
| Koopman, 1996 | Yes | Yes | Yes | Yes | 6 months | 1% |
| Levine, 1995 | Unclear | Yes | No | Yes | 12 months | 3.4% |
| López-Beret, 2001 | Yes | Yes | Unclear | Unclear | 12 months | Unclear |
| Meyer, 2002 | Yes | Yes | Unclear | Unclear | 6 months | Unclear |
| Monreal, 1998 | Yes | Yes | Unclear | No | 3–6 months | Unclear |
| Nielsen, 1993 | Yes | No | Unclear | Yes | 3 months | Unclear |
| Palareti, 2000 | Yes | Yes | Yes | Yes | Average 10–11 | Unclear |
| months | ||||||
| Pérez-de-Llano, 2010 | Yes | Yes | Yes | Yes | 6 months | 6% |
| Pini, 1994 | Yes | Yes | Yes | Yes | 9 months | 0% |
| Poller, 2008 | Unclear | Yes | Unclear | Yes | Average 17 | Unclear |
| months | ||||||
| Poli, 2007 | Yes | Yes | Yes | Yes | At least 1 year | 8.1% |
| or until | ||||||
| recurrence | ||||||
| Prandoni, 2004 | Yes | Yes | Yes | Yes | 5 years | 1.7% |
| Santamaria, 2006 | Unclear | Yes | No | No | Median | Unclear |
| 98 days | ||||||
| Schulman, 1994 | Unclear | Yes | Yes | Yes | At least 6 | Unclear |
| months | ||||||
| Schulman, 2009 | Unclear | Yes | Yes | Yes | 6 months | 0.5% |
Methods of calculating TTR
Five studies [24], [39], [42], [43], [45] reported TTR in classes ranging from <33% to ≥75% of time spent within INR-range 2.0 to 3.0. (e.g. 57% of all patients spent 70% of time within therapeutic range). All other TTRs were reported in percentages over time. Two studies [9], [20] reported the TTR in the first month since the start of treatment, thirteen studies [14], [18], [19], [21], [25], [29], [31], [34], [36], [40], [46], [48], [49] reported the TTR measured in months 1 to 3, four studies [20], [28], [32], [44] measured the TTR in months 2 to 3, fifteen studies [8], [9], [17], [22], [23], [26], [33], [35], [37], [38], [41], [45], [47], [50], [51] in months 1 to a minimum of 6 months and four studies [15], [16], [27], [30] reported the TTR in months 4 to at least 12 months since the start of treatment. Twenty (50%) studies [15], [16], [17], [18], [19], [25], [27], [28], [29], [30], [31], [33], [35], [37], [41], [45], [47], [48], [49], [50] reported that they calculated the TTR by using linear interpolation [52]. The method used for calculating TTR was not mentioned in 12 (30.0%) studies [8], [14], [20], [21], [22], [23], [32], [34], [36], [38], [44], [46] (Table 1).
Percentage of time in therapeutic range
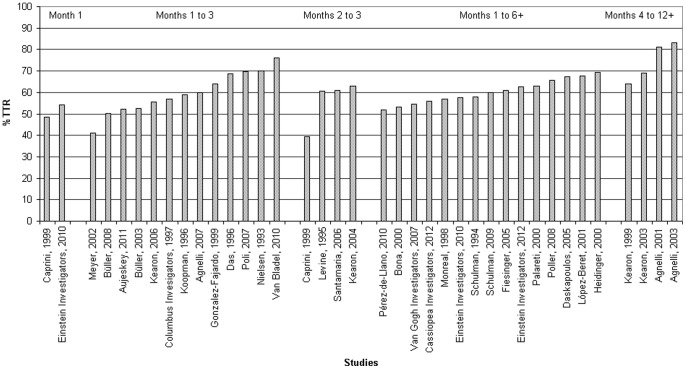
Table 3 presents the percentage of time below, within and above the therapeutic INR range of 2.0 to 3.0 of the individual studies. A histogram with the TTR in each individual study is given in Figure 2.
Table 3. Percentage of time below, within and above the Therapeutic Range of the individual studies.
| Time-Period TTR | TTR | ||||
| First author and year: | N | since diagnosis: | % below TR | % within TR | % above TR |
| Research Committee of | 358 | Month 1 | NR | 37.4% (>67% TTR) | NR |
| The British Thoracic Society, 1992 | 354 | Months 1 to 3 | NR | 35.2% (>67% TTR) | NR |
| Agnelli, 2001 | 267 | Months 4 to 12+ | NR | 81% | NR |
| Agnelli, 2003 | 326 | Months 4 to 12+ | NR | 83% | NR |
| Agnelli, 2007 | 126 | Months 1 to 3 | NR | 60% | NR |
| Aujeskey, 2011 | 172 | 34.7% | 51.8% | 13.5% | |
| 172 | Months 1 to 3 | 32% | 52.5% | 15.6% | |
| Van Bladel | 86 | Months 1 to 3 | 12.1% | 76% | 11.8% |
| 104 | NR | 47.5% | NR | ||
| Bona, 2000 | 208 | Months 1 to 6+ | NR | 56% | NR |
| 1103 | 28% | 53% | 19% | ||
| Büller, 2003 | 1110 | Months 1 to 3 | 28% | 52% | 20% |
| Büller, 2008 | 137 | Months 1 to 3 | 29% | 50.3% | 20.7% |
| At 1 month | 42.4% | 48.5% | 12.1% | ||
| At 3 monhts | 63.6% | 39.4% | 3% | ||
| Caprini, 1999 | 33 | At 6 months | 48.5% | 45.5% | 9.1% |
| Cassiopea Investigators, 2012 | 1603 | Months 1 to 6+ | 25.6% | 55.9% | 18.5% |
| 510 | 22% | 57% | 21% | ||
| The Columbus Invesigators, 1997 | 511 | Months 1 to 3 | 24% | 57% | 21% |
| Das, 1996 | 55 | Months 1 to 3 | NR | 68.8% | NR |
| Daskapoulos, 2005 | 52 | Months 1 to 6+ | 19.1% | 67.2% | 13.6% |
| Month 1 | NR | 54.1% | NR | ||
| The Einstein Investigators, 2010 | 1718 | Months 1 to 6+ | 24.4% | 57.7% | 16.2% |
| The Einstein Investigators, 2012 | 2413 | Months 1 to 6+ | 21.8% | 62.7% | 15.5% |
| Fiesinger, 2005 | 1249 | Months 1 to 6+ | NR | 61% | NR |
| 360 | NR | 72.7% (≥70% TTR) | NR | ||
| The Galilei Investigators, 2004 | 360 | Months 1 to 3 | NR | 70% (≥70% TTR) | NR |
| 1452 | 26.2% | 54.4% | 19.4% | ||
| The van Gogh Investigators, 2007 | 1120 | Months 1 to 6+ | 26.9% | 54.8% | 18.3% |
| Gonzalez-Fajardo, 1999 | 80 | Months 1 to 3 | 15% | 64% | 21% |
| Heidinger, 2000 | 622 | on average at 4.5 months | 22.7% | 69.2% | 8.1% |
| Kearon, 1999 | 162 | Months 4 to 12+ | 22% | 64% | 14% |
| Kearon, 2003 | 369 | Months 4 to 12+ | 20% | 69% | 11% |
| Kearon, 2004 | 165 | Months 2 to 3 | 29% | 63% | 8% |
| 355 | 28% | 55% | 17% | ||
| Kearon, 2006 | 353 | Months 1 to 3 | 25% | 56% | 19% |
| 198 | 18% | 56% | 26% | ||
| Koopman, 1996 | 202 | Months 1 to 3 | 16% | 62% | 22% |
| Levine, 1995 | 109 | Months 2 to 3 | 29.6% | 60.7% | 9.7% |
| López-Beret, 2001 | 77 | Months 1 to 6+ | 22.8% | 67.8% | 9.4% |
| Meyer, 2002 | 75 | Months 1 to 3 | NR | 41% | NR |
| Monreal, 1998 | 244 | Months 1 to 6+ | 25.5% | 56.9% | 17.6% |
| Nielsen, 1993 | 46 | Months 1 to 3 | NR | 70% | NR |
| 733 | 22.5% | 63.6% | 13.9% | ||
| Palareti, 2000 | 95 | Months 1 to 6+ | 23.3% | 58.9% | 17.8% |
| Pérez-de-Llano, 2010 | 50 | Months 1 to 6+ | 41.5% | 51.7% | 6.8% |
| Pini, 1994 | 94 | Months 1 to 3+ | NR | 38% (≥67% TTR) | NR |
| 1560 | NR | 64.9% | NR | ||
| Poller, 2008 | 1649 | Months 1 to 6+ | NR | 66% | NR |
| Poli, 2007 | 182 | Months 1 to 3 | 18.8% | 69.7% | 11.5% |
| 90 | NR | 70% (>70% TTR) | NR | ||
| Prandoni, 2004 | 90 | Months 1 to 3+ | NR | 72.2% (>70% TTR) | NR |
| Santamaria, 2006 | 116 | At 3 months | NR | 61.1% | NR |
| At 12 months | NR | 63% (≥75% TTR) | NR | ||
| Schulman, 1994 | 1124 | Months 1 to 6+ | 25% | 58% | 17% |
| Schulman, 2009 | 1265 | Months 1 to 6+ | 21% | 60% | 19% |
Abbreviations: TTR, Time in Therapeutic Range; TR, Therpeutic Range; INR, International Normalized Ratio; NR, Not Reported.
Figure 2. Time in Therapeutic Range in individual studies.
* A weighted mean is calculated if a study reported more than 1 group; studies that only presented classes of %TTR are not represented here.
Table 4 details the weighted means for different time-periods since objective confirmation of the diagnosis VTE. The reported quality of VKA treatment is highly dependent on the time-period. In the first month the reported TTR is 54.0%. The TTR is 55.6% during the months 1 to 3 and 60.0% during a treatment of at least 6 months including the INRs in the first month. In studies reporting TTR without INRs in the first month, the TTR was 60.0% in months 2 to 3 since the start of treatment and 75.2% in the months 4 to 12 or longer.
Table 4. Weighted mean % of time below, within and above Therapeutic Range INR 2.0–3.0.
| Time-period TTR | |||
| INR 2.0–3.0 | % below TR | % TTR | % above TR |
| since diagnosis | Weighted mean | Weighted mean | Weighted mean |
| Month 1 | |||
| (n studies = 2, n patients = 1751) | 42.4% | 54.0% | 12.1% |
| Months 1 to 3 | |||
| (n studies = 13, n patients = 5473) | 35.0% | 55.6% | 19.2% |
| Months 2 to 3 | |||
| (n studies = 4, n patients = 423) | 32.9% | 60.0% | 8.1% |
| Months 1 to 6+ | |||
| (n studies = 13, n patients = 17338) | 24.1% | 60.0% | 16.7% |
| Months 4 to 12+ | |||
| (n studies = 4, n patients = 1124) | 20.6% | 75.2% | 11.9% |
Abbreviations: TTR, Time in Therapeutic Range; INR, International Normalized Ratio; TR, Therapeutic Range.
Discussion
A strong relationship between TTR and bleeding or thromboembolic rates has been observed across a large number of studies with different patient populations [53]. Since under-anticoagulation gives inadequate protection against thromboembolic events and over-anticoagulation increases the bleeding risk, it is important to report the quality of VKA treatment by using the TTR [54]. The evidence for non-inferiority of new anticoagulants depends on the quality of the VKA control group. The present review provides a benchmark of TTR in patients with VTE receiving VKA and discusses the pros and cons of various ways to calculate TTR.
We included 40 studies with more than 26000 participants and the results indicate that the achieved TTR ranges from approximately 56% to 75%.
The reported quality of VKA treatment was highly dependent on the time-period since the start of treatment. A statistically significant lower TTR was seen in studies reporting a TTR that covers all INRs, including the first month, compared to studies reporting the TTR without the first month. This difference is to be expected because of the difficulty to reach the therapeutic range in the initial treatment period and improvement in TTR during continuation of VKA treatment. Another explanation of the high TTR during longterm treatments is a selection-to-continue bias. Patients with stable INRs are more likely to continue their treatment with VKA than patients who experience problems in reaching the therapeutic range [55]. However, even after 4 to 12 months of treatment with VKA, patients spent 25% of their time outside of the therapeutic range.
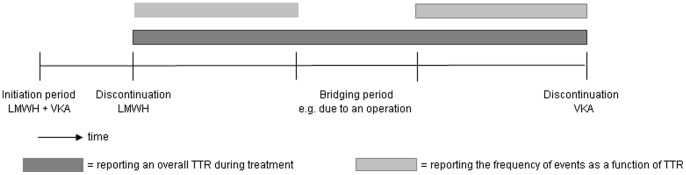
Our review has some limitations that have to be mentioned. First, methods used to calculate TTR differed across the included studies. Fifty percent of the studies used linear interpolation, a few studies reported the percentage of time in a certain TTR class and 30% of the studies did not report the method of TTR calculation at all. Due to missing information about the exact calculation of TTR, we were unable to compare the different methods in a meaningful way. In literature, several methods to assess therapeutic control are described: e.g. the assessment of the number of INR measurements within the target range expressed as a percentage of the total number of INRs obtained, the cross-section-of the-files technique (the fraction of patients in range at one point in time compared to the total number of patients who had an INR at that point in time), equidivision, linear interpolation and the hybrid method [52], [56], [57]. Each approach has its advantages and disadvantages. A disadvantage of the first two methods is that they do not incorporate time and therefore cannot be used to calculate incidence rates of recurrences at different INR levels [54]. Time is incorporated in the method of equidivision, which assumes that the change between two consecutive INR measurements occurred halfway the interval [56]. The time spent in INR ranges can also be estimated by linear interpolation, which assumes that the INR between two measurements varies linearly from the first INR to the second INR [52]. A disadvantage of these last two methods is that extreme out of range INR values may bias overall results [58]. The hybrid method, in addition, takes effects of dosage modifications into account [54]. The results of all of these methods depend on whether an exact (INR 2.0–3.0) or an expanded therapeutic range is used, whether VKA-naïve patients (those just beginning therapy) are included or only patients already on established therapy, whether INRs obtained during invasive procedures when VKA therapy might be interrupted are excluded, and whether different oral anticoagulant preparations (e.g. warfarin or acenocoumarol) are allowed [53]. In a comparison of the equidivision, linear interpolation and hybrid methods, linear interpolation has been suggested as the preferred method as it shows a high validity and reproducibility [54]. We suggest that drug trials and real life registries with a VKA control group report the TTR in a uniform manner, to allow adequate comparison of data. Since linear interpolation has a high validity and was the most common method used to calculate TTR in the present review, we recommend to use linear interpolation in future studies covering the INRs from each patient from the discontinuation of heparin until the end of treatment. In order to avoid complex calculations, we believe that including time-periods with interruptions in VKA treatment in the TTR are acceptable. However, for calculating the relationship between TTR and adverse events, such as major bleeding episodes and thromboembolic events, we would suggest to exclude bridging periods, since the TTR will not represent the quality of anticoagulant treatment during these periods when most patients receive LMWH (Figure 3).
Figure 3. Suggestions for calculating TTR.
Dark grey = reporting an overall TTR during treatment. Light grey = reporting the frequency of events as a function of TTR.
A second important limitation of the present review is that we were not able to investigate the association between TTR and clinical endpoints. Several studies in literature show a strong relationship between TTR and bleeding or thromboembolic events [53]. Unfortunately, data on such clinical endpoints related to TTR was not provided in the included studies.
Additionally, some other interesting sub-analyses were difficult due to small subgroups and the absence of detailed data. Hutten et al. indicated that the therapeutic quality of treatment was decreased when patients were treated with acenocoumarol rather than with warfarin [59]. This might implicate that the use of warfarin is preferable. However, since it is not clear whether these results might be influenced by factors such as frequency of monitoring and comorbidities, we need to be careful with drawing a conclusion. Furthermore, Hutten et al. showed that TTR was decreased in the presence of cancer and in the presence of a pulmonary embolism [59]. The same subgroup analyses in the present review did not show statistically significant results (data not shown). This might be explained by the fact that we did not have individual patient data (IPD). An IPD meta-analysis may give more detailed information for investigating such associations and may be interesting. Hutten et al. also showed a decrease in the therapeutic quality of VKA treatment when more than four changes in co-medication occurred [59]. Unfortunately such data was not available for our review.
The main conclusion of our systematic review is that the reported quality of VKA treatment is highly dependent on the time-period since the start of treatment, with the TTR ranging from approximately 56% in studies including the first month to 75% in studies excluding the first 3 months. The clinical consequences of our findings are not straightforward. However, it needs to be emphasized that the reported quality of VKA treatment should be taken into consideration while interpreting results from trials with new anticoagulants. Assuming an average treatment duration of 6 months, the mean TTR is approximately 60%. We recommend to calculate the TTR by using linear interpolation covering the INRs from each patient from discontinuation of heparin until the end of treatment. Furthermore, TTR is predictive of thromboembolic and bleeding complications for patients on VKA [53]; therefore a proper calculation of TTR in the VKA group is of importance in assessing the adequacy and quality of novel anticoagulants.
Oral anticoagulants are also effective in preventing stroke [60], [61], [62], [63], [64] and prolonging survival rates in patients with atrial fibrillation (AF) [65]. It may be interesting to investigate a benchmark of the TTR in patients treated with VKA in AF in the near future. However, since patients with AF are usually on long-term VKA treatment, selection-to-continue bias will be more evident than in patients with VTE and should be taken into consideration in an analysis in AF patients [55].
Funding Statement
The authors have no support or funding to report.
References
- 1. Hull R, Delmore T, Carter C, Hirsh J, Genton E, et al. (1982) Adjusted subcutaneous heparin versus warfarin sodium in the long-term treatment of venous thrombosis. N Engl J Med 306: 189–194. [DOI] [PubMed] [Google Scholar]
- 2. Hull R, Delmore T, Genton E, Hirsh J, Gent M, et al. (1979) Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 301: 855–858. [DOI] [PubMed] [Google Scholar]
- 3. Buller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, et al. (2004) Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126: 401S–428S. [DOI] [PubMed] [Google Scholar]
- 4. Hirsh J, Fuster V (1994) Guide to anticoagulant therapy. Part 2: Oral anticoagulants. American Heart. Circulation 89: 1469–1480. [DOI] [PubMed] [Google Scholar]
- 5. Hull R, Hirsh J, Jay R, Carter C, England C, et al. (1982) Different intensities of oral anticoagulant therapy in the treatment of proximal-vein thrombosis. N Engl J Med 307: 1676–1681. [DOI] [PubMed] [Google Scholar]
- 6. Eriksson BI, Quinlan DJ, Weitz JI (2009) Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet 48: 1–22. [DOI] [PubMed] [Google Scholar]
- 7. Botticelli-Investigators, Buller H, Deitchman D, Prins M, Segers A (2008) Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost 6: 1313–1318. [DOI] [PubMed] [Google Scholar]
- 8. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, et al. (2009) Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 361: 2342–2352. [DOI] [PubMed] [Google Scholar]
- 9. The-Einstein-Investigators, Bauersachs R, Berkowitz SD, Brenner B, Buller HR, et al. (2010) Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 363: 2499–2510. [DOI] [PubMed] [Google Scholar]
- 10. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 11.ROCKET-AF-Study-Investigators (2010) Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J 159: 340–347 e341. [DOI] [PubMed]
- 12.Phillips KW, Ansell J (2008) Outpatient management of oral vitamin K antagonist therapy: defining and measuring high-quality management. Expert Rev Cardiovasc Ther : 57–70. [DOI] [PubMed]
- 13. WHO Expert Committee on Biological Standardization. Thirty-third report. World Health Organ Tech Rep Ser 687: 1–184. [PubMed] [Google Scholar]
- 14. Agnelli G, Gallus A, Goldhaber SZ, Haas S, Huisman MV, et al. (2007) Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY –7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59–7939 in Patients With Acute Symptomatic Deep-Vein Thrombosis) study. Circulation 116: 180–187. [DOI] [PubMed] [Google Scholar]
- 15. Agnelli G, Prandoni P, Becattini C, Silingardi M, Taliani MR, et al. (2003) Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 139: 19–25. [DOI] [PubMed] [Google Scholar]
- 16. Agnelli G, Prandoni P, Santamaria MG, Bagatella P, Iorio A, et al. (2001) Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 345: 165–169. [DOI] [PubMed] [Google Scholar]
- 17. Bona RD, Hickey AD, Wallace DM (2000) Warfarin is safe as secondary prophylaxis in patients with cancer and a previous episode of venous thrombosis. Am J Clin Oncol 23: 71–73. [DOI] [PubMed] [Google Scholar]
- 18. Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, et al. (2003) Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 349: 1695–1702. [DOI] [PubMed] [Google Scholar]
- 19. Buller HR, Lensing AW, Prins MH, Agnelli G, Cohen A, et al. (2008) A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood 112: 2242–2247. [DOI] [PubMed] [Google Scholar]
- 20. Caprini JA, Arcelus JI, Reyna JJ, Motykie GD, Mohktee D, et al. (1999) Deep vein thrombosis outcome and the level of oral anticoagulation therapy. J Vasc Surg 30: 805–811. [DOI] [PubMed] [Google Scholar]
- 21.Das SK, Cohen AT, Edmondson RA, Melissari E, Kakkar VV (1996) Low-molecular-weight heparin versus warfarin for prevention of recurrent venous thromboembolism: a randomized trial. World J Surg 20: 521–526; discussion 526–527. [DOI] [PubMed]
- 22. Daskalopoulos ME, Daskalopoulou SS, Tzortzis E, Sfiridis P, Nikolaou A, et al. (2005) Long-term treatment of deep venous thrombosis with a low molecular weight heparin (tinzaparin): a prospective randomized trial. Eur J Vasc Endovasc Surg 29: 638–650. [DOI] [PubMed] [Google Scholar]
- 23. Fiessinger JN, Huisman MV, Davidson BL, Bounameaux H, Francis CW, et al. (2005) Ximelagatran vs low-molecular-weight heparin and warfarin for the treatment of deep vein thrombosis: a randomized trial. Jama 293: 681–689. [DOI] [PubMed] [Google Scholar]
- 24. Galilei-Investigators, Prandoni P, Carnovali M, Marchiori A (2004) Subcutaneous adjusted-dose unfractionated heparin vs fixed-dose low-molecular weight heparin in the initial treatment of venous thromboembolism. Arch Intern Med 164: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 25. Gonzalez-Fajardo JA, Arreba E, Castrodeza J, Perez JL, Fernandez L, et al. (1999) Venographic comparison of subcutaneous low-molecular weight heparin with oral anticoagulant therapy in the long-term treatment of deep venous thrombosis. J Vasc Surg 30: 283–292. [DOI] [PubMed] [Google Scholar]
- 26. Heidinger KS, Bernardo A, Taborski U, Muller-Berghaus G (2000) Clinical outcome of self-management of oral anticoagulation in patients with atrial fibrillation or deep vein thrombosis. Thromb Res 98: 287–293. [DOI] [PubMed] [Google Scholar]
- 27. Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, et al. (1999) A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 340: 901–907. [DOI] [PubMed] [Google Scholar]
- 28. Kearon C, Ginsberg JS, Anderson DR, Kovacs MJ, Wells P, et al. (2004) Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J Thromb Haemost 2: 743–749. [DOI] [PubMed] [Google Scholar]
- 29. Kearon C, Ginsberg JS, Julian JA, Douketis J, Solymoss S, et al. (2006) Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. Jama 296: 935–942. [DOI] [PubMed] [Google Scholar]
- 30. Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, et al. (2003) Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 349: 631–639. [DOI] [PubMed] [Google Scholar]
- 31. Koopman MM, Prandoni P, Piovella F, Ockelford PA, Brandjes DP, et al. (1996) Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med 334: 682–687. [DOI] [PubMed] [Google Scholar]
- 32. Levine MN, Hirsh J, Gent M, Turpie AG, Weitz J, et al. (1995) Optimal duration of oral anticoagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thromb Haemost 74: 606–611. [PubMed] [Google Scholar]
- 33. Lopez-Beret P, Orgaz A, Fontcuberta J, Doblas M, Martinez A, et al. (2001) Low molecular weight heparin versus oral anticoagulants in the long-term treatment of deep venous thrombosis. Journal of Vascular Surgery 33: 77–90. [DOI] [PubMed] [Google Scholar]
- 34. Meyer G, Marjanovic Z, Valcke J, Lorcerie B, Gruel Y, et al. (2002) Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med 162: 1729–1735. [DOI] [PubMed] [Google Scholar]
- 35. Monreal M, Roncales FJ, Ruiz J, Muchart J, Fraile M, et al. (1998) Secondary prevention of venous thromboembolism: A role for low-molecular-weight heparin. Haemostasis 28: 236–243. [DOI] [PubMed] [Google Scholar]
- 36. Nielsen HK, Husted SE, Krusell LR, Fasting H, Charles P, et al. (1993) Anticoagulant therapy in deep venous thrombosis. A randomized controlled study. Thromb Res 73: 215–226. [DOI] [PubMed] [Google Scholar]
- 37. Palareti G, Legnani C, Lee A, Manotti C, Hirsh J, et al. (2000) A comparison of the safety and efficacy of oral anticoagulation for the treatment of venous thromboembolic disease in patients with or without malignancy. Thromb Haemost 84: 805–810. [PubMed] [Google Scholar]
- 38. Perez-De-Llano LA, Leiro-Fernandez V, Golpe R, Nunez-Delgado JM, Palacios-Bartolome A, et al. (2010) Comparison of tinzaparin and acenocoumarol for the secondary prevention of venous thromboembolism: A multicentre, randomized study. Blood Coagulation and Fibrinolysis 21: 744–749. [DOI] [PubMed] [Google Scholar]
- 39. Pini M, Aiello S, Manotti C, Pattacini C, Quintavalla R, et al. (1994) Low molecular weight heparin versus warfarin in the prevention of recurrences after deep vein thrombosis. Thromb Haemost 72: 191–197. [PubMed] [Google Scholar]
- 40. Poli D, Antonucci E, Ciuti G, Abbate R, Prisco D (2007) Anticoagulation quality and the risk of recurrence of venous thromboembolism. Thromb Haemost 98: 1148–1150. [PubMed] [Google Scholar]
- 41. Poller L, Keown M, Ibrahim S, Lowe G, Moia M, et al. (2008) An international multicenter randomized study of computer-assisted oral anticoagulant dosage vs. medical staff dosage. J Thromb Haemost 6: 935–943. [DOI] [PubMed] [Google Scholar]
- 42. Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, et al. (2004) Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 141: 249–256. [DOI] [PubMed] [Google Scholar]
- 43. Research-Committee-of-the-British-Thoracic-Society (1992) Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Lancet 340: 873–876. [PubMed] [Google Scholar]
- 44. Santamaria A, Juarez S, Reche A, Gomez-Outes A, Martinez-Gonzalez J, et al. (2006) Low-molecular-weight heparin, bemiparin, in the outpatient treatment and secondary prophylaxis of venous thromboembolism in standard clinical practice: the ESFERA Study. Int J Clin Pract 60: 518–525. [DOI] [PubMed] [Google Scholar]
- 45. Schulman S (1994) Quality of oral anticoagulant control and treatment in Sweden. Duration of Anticoagulation (DURAC) Trial Study Group. J Intern Med 236: 143–152. [DOI] [PubMed] [Google Scholar]
- 46. The-Columbus-Investigators (1997) Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. N Engl J Med 337: 657–662. [DOI] [PubMed] [Google Scholar]
- 47. The-van-Gogh-Investigators, Buller HR, Cohen AT, Davidson B, Decousus H, et al. (2007) Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med 357: 1094–1104. [DOI] [PubMed] [Google Scholar]
- 48. Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, et al. (2011) Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 378: 41–48. [DOI] [PubMed] [Google Scholar]
- 49. van Bladel ER, Agterof MJ, Frijling BD, van der Griend R, Prins MH, et al. (2010) Out of hospital anticoagulant therapy in patients with acute pulmonary embolism is frequently practised but not perfect. Thromb Res 126: 481–485. [DOI] [PubMed] [Google Scholar]
- 50. Cassiopea-Investigators, Buller HR, Gallus AS, Pillion G, Prins MH, et al. (2012) Enoxaparin followed by once-weekly idrabiotaparinux versus enoxaparin plus warfarin for patients with acute symptomatic pulmonary embolism: a randomised, double-blind, double-dummy, non-inferiority trial. Lancet 379: 123–129. [DOI] [PubMed] [Google Scholar]
- 51. The-Einstein-Investigators, Buller HR, Prins MH, Lensin AW, Decousus H, et al. (2012) Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 366: 1287–1297. [DOI] [PubMed] [Google Scholar]
- 52. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E (1993) A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 69: 236–239. [PubMed] [Google Scholar]
- 53. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, et al. (2008) Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133: 160S–198S. [DOI] [PubMed] [Google Scholar]
- 54. Hutten BA, Prins MH, Redekop WK, Tijssen JG, Heisterkamp SH, et al. (1999) Comparison of three methods to assess therapeutic quality control of treatment with vitamin K antagonists. Thromb Haemost 82: 1260–1263. [PubMed] [Google Scholar]
- 55. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, et al. (2008) Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133: 454S–545S. [DOI] [PubMed] [Google Scholar]
- 56. Duxbury BM (1982) Therapeutic control of anticoagulant treatment. Br Med J (Clin Res Ed) 284: 702–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Loeliger EA (1985) Laboratory control, optimal therapeutic ranges and therapeutic quality control in oral anticoagulation. Acta Haematol 74: 125–131. [DOI] [PubMed] [Google Scholar]
- 58. Veeger NJ, Piersma-Wichers M, Tijssen JG, Hillege HL, van der Meer J (2005) Individual time within target range in patients treated with vitamin K antagonists: main determinant of quality of anticoagulation and predictor of clinical outcome. A retrospective study of 2300 consecutive patients with venous thromboembolism. Br J Haematol 128: 513–519. [DOI] [PubMed] [Google Scholar]
- 59.Hutten BA, Riel van I, Buller H, Prins M (2000: 29–39) Treatment with vitamin K antagonists for symptomatic venous thromboembolism: determinants of time spent in the therapeutic range [In thesis: Treatment of Venous Thromboembolism with vitamin K antagonists]. Amsterdam: University of Amsterdam.
- 60. Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation 84: 527–539. [DOI] [PubMed] [Google Scholar]
- 61. EAFT-(European-Atrial-Fibrillation-Trial)-Study-Group (1993) Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 342: 1255–1262. [PubMed] [Google Scholar]
- 62. Ezekowitz MD, Bridgers SL, James KE, Carliner NH, Colling CL, et al. (1992) Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med 327: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 63. Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B (1989) Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet 1: 175–179. [DOI] [PubMed] [Google Scholar]
- 64. The-Boston-Area-Anticoagulation-Trial-for-Atrial-Fibrillation-Investigators (1990) The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med 323: 1505–1511. [DOI] [PubMed] [Google Scholar]
- 65. Currie CJ, Jones M, Goodfellow J, McEwan P, Morgan CL, et al. (2006) Evaluation of survival and ischaemic and thromboembolic event rates in patients with non-valvar atrial fibrillation in the general population when treated and untreated with warfarin. Heart 92: 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]