Abstract
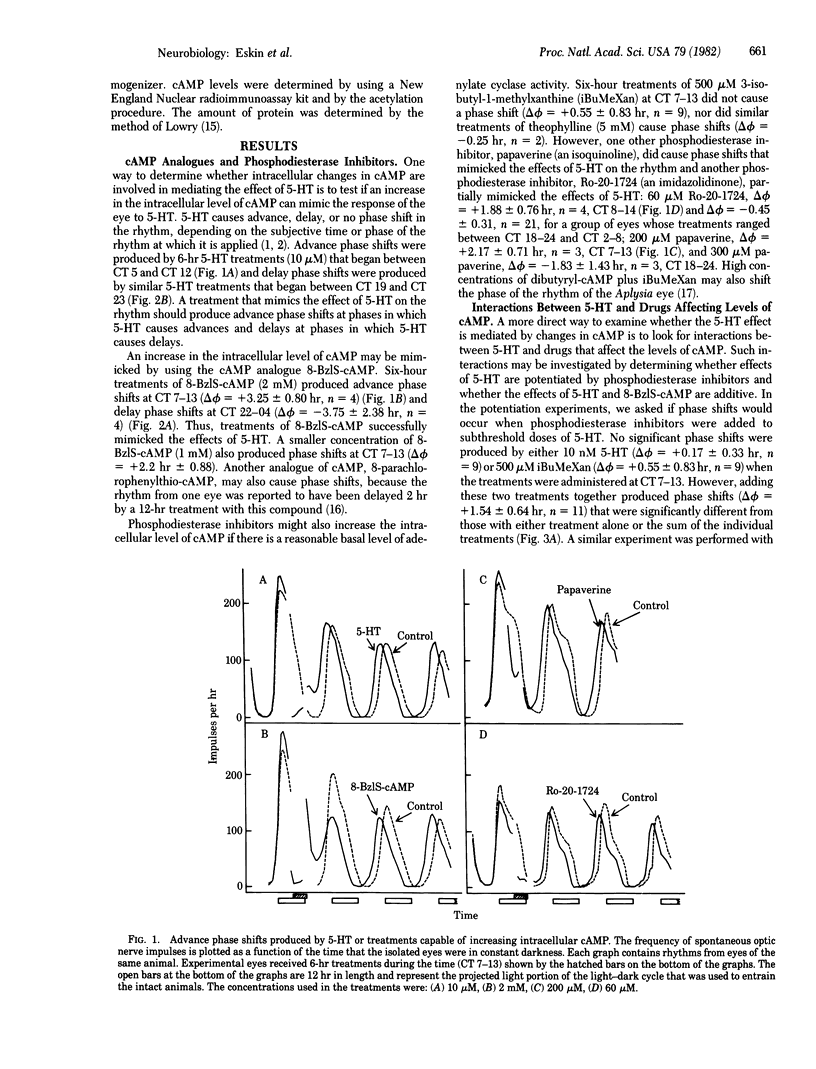
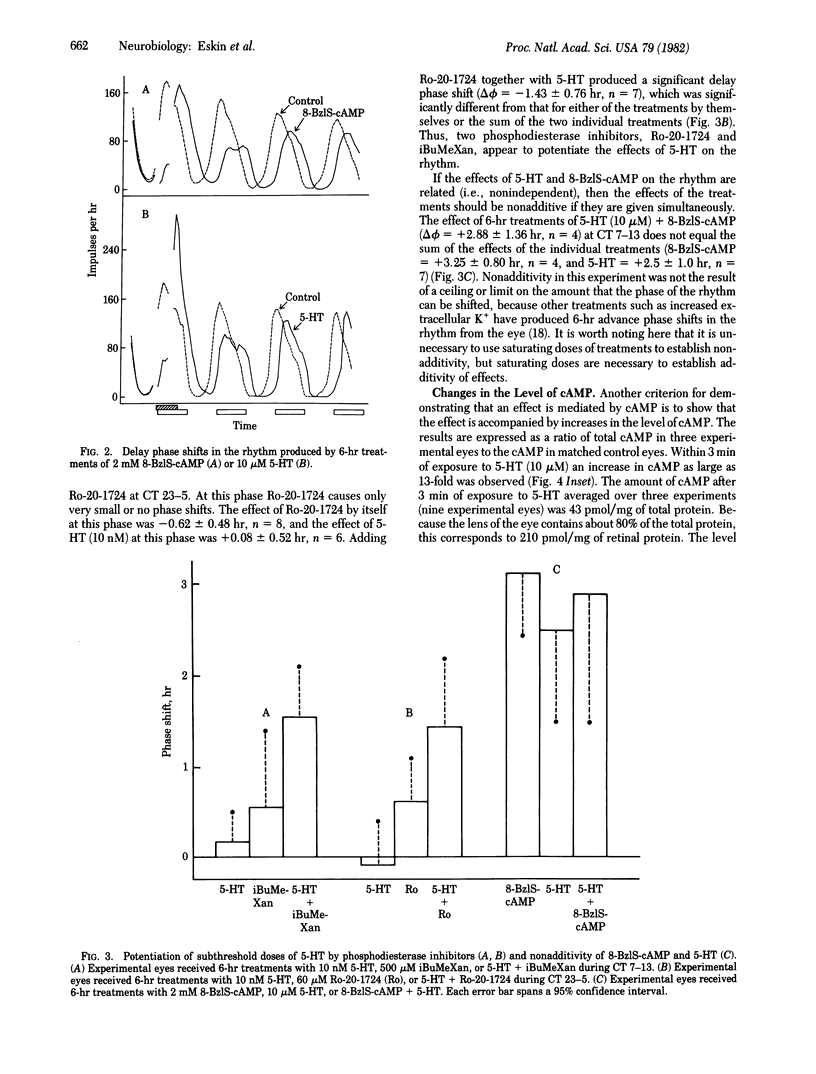
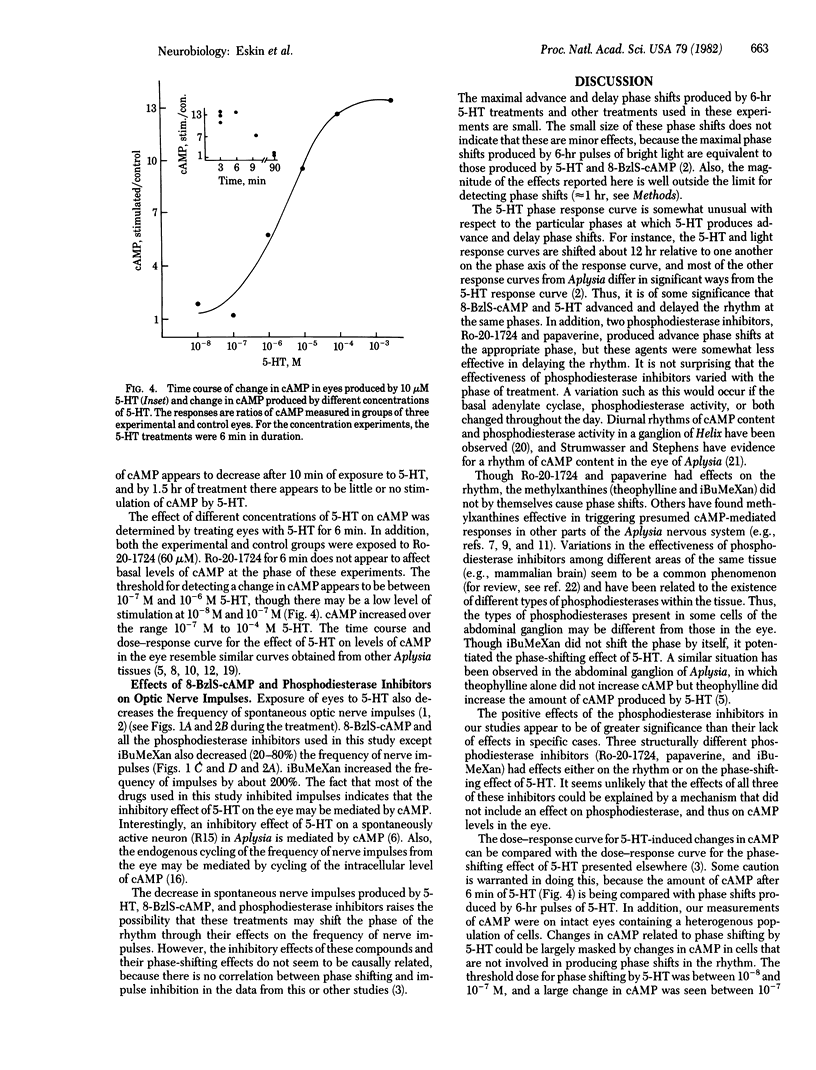
Serotonin (5-hydroxytryptamine, 5-HT) shifts the phase of the circadian rhythm in the eye of Aplysia. We have examined the role of cAMP in mediating the effects of 5-HT on the rhythm. The phase shifts produced by 5-HT are mimicked by treatments that should increase intracellular levels of cAMP. An analogue of cAMP-8-benzylthio-cAMP, advanced and delayed the rhythm at phases in which 5-HT had similar effects on the rhythm. In addition, two phosphodiesterase inhibitors, Ro-20-1724 and papaverine, caused advance phase shifts where 5-HT advances the rhythm. The phosphodiesterase inhibitors Ro-20-1724 and 3-isobutyl-1-methylxanthine each potentiated the effect of subthreshold doses of 5-HT on the rhythm. The effects of 5-HT and 8-benzylthio-cAMP on the rhythm were nonadditive, indicating that 5-HT and 8-benzylthio-cAMP affect the rhythm through a common pathway. Finally, 5-HT produced large changes (13-fold) in the levels of cAMP in the eye. These results indicate that cAMP mediates the effect of 5-HT on the rhythm. There are two possible roles for cAMP in the circadian system. Either the cAMP system is an intracellular step in an entrainment pathway or it is part of the biological clock. Because 5-HT, 8-benzylthio-cAMP, and three phosphodiesterase inhibitors inhibit impulses from the eye, cAMP may also mediate the inhibition produced by 5-HT, or it might be involved in regulating the frequency of spontaneous impulses throughout the day.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson J. A. A cAMP analogue inhibits compound action potential production and phase shifts the circadian clock in the isolated eye of Aplysia californica. Comp Biochem Physiol C. 1980;67C(2):195–198. doi: 10.1016/0306-4492(80)90016-7. [DOI] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Cyclic adenosine monophosphate in the nervous system of Aplysia californica. II. Effect of serotonin and dopamine. J Gen Physiol. 1972 Nov;60(5):570–587. doi: 10.1085/jgp.60.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrent G., McAdoo D. J., Eskin A. Serotonin shifts the phase of the circadian rhythm from the Aplysia eye. Science. 1978 Dec 1;202(4371):977–979. doi: 10.1126/science.309655. [DOI] [PubMed] [Google Scholar]
- Cummings F. W. A biochemical model of the circadian clock. J Theor Biol. 1975 Dec;55(2):455–470. doi: 10.1016/s0022-5193(75)80093-2. [DOI] [PubMed] [Google Scholar]
- Drummond A. H., Benson J. A., Levitan I. B. Serotonin-induced hyperpolarization of an indentified Aplysia neuron is mediated by cyclic AMP. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5013–5017. doi: 10.1073/pnas.77.8.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Tsuruhara T., Horner K. A., Podesta E., Catt K. J. Intermediate role of adenosine 3':5'-cyclic monophosphate and protein kinase during gonadotropin-induced steroidogenesis in testicular interstitial cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3419–3423. doi: 10.1073/pnas.74.8.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp H. S., Steiner A. L. Compartmentalization of cyclic nucleotide-mediated hormone action. Annu Rev Pharmacol Toxicol. 1978;18:431–459. doi: 10.1146/annurev.pa.18.040178.002243. [DOI] [PubMed] [Google Scholar]
- Eskin A. Circadian system of the Aplysia eye: properties of the pacemaker and mechanisms of its entrainment. Fed Proc. 1979 Nov;38(12):2573–2579. [PubMed] [Google Scholar]
- Eskin A. Neurophysiological mechanisms involved in photo-entrainment of the circadian rhythm from the Aplysia eye. J Neurobiol. 1977 May;8(3):273–299. doi: 10.1002/neu.480080310. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K., Strumwasser F. Neurotransmitter modulation, phosphodiesterase inhibitor effects, and cyclic AMP correlates of afterdischarge in peptidergic neurites. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5200–5204. doi: 10.1073/pnas.75.10.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian P. R., Kebabian J. W., Carpenter D. O. Regulation of cyclic AMP in heart and gill of Aplysia by the putative neurotransmitters dopamine and serotonin. Life Sci. 1979 May 7;24(19):1757–1764. doi: 10.1016/0024-3205(79)90064-x. [DOI] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitan I. B., Madsen C. J., Barondes S. H. Cyclic AMP and amine effects on phosphorylation of specific protein in abdominal ganglion of Aplysia californica; localization and kinetic analysis. J Neurobiol. 1974;5(6):511–525. doi: 10.1002/neu.480050604. [DOI] [PubMed] [Google Scholar]
- Mandelbaum D. E., Koester J., Schonberg M., Weiss K. R. Cyclic AMP mediation of the excitatory effect of serotonin in the heart of Aplysia. Brain Res. 1979 Nov 16;177(2):388–394. doi: 10.1016/0006-8993(79)90792-3. [DOI] [PubMed] [Google Scholar]
- Sala G. B., Hayashi K., Catt K. J., Dufau M. L. Adrenocorticotropin action in isolated adrenal cells. The intermediate role of cyclic AMP in stimulation of corticosterone synthesis. J Biol Chem. 1979 May 25;254(10):3861–3865. [PubMed] [Google Scholar]
- Shimahara T., Tauc L. Cyclic AMP induced by serotonin modulates the activity of an identified synapse in Aplysia by facilitating the active permeability to calcium. Brain Res. 1977 May 20;127(1):168–172. doi: 10.1016/0006-8993(77)90389-4. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Terasaki W. L., Brooker G. Cardiac adenosine 3':5'-monophosphate. Free and bound forms in the isolated rat atrium. J Biol Chem. 1977 Feb 10;252(3):1041–1050. [PubMed] [Google Scholar]
- Weinryb I. Cyclic AMP as an intracellular mediator of hormone action: Sutherland's criteria revisited. Perspect Biol Med. 1979 Spring;22(3):415–420. doi: 10.1353/pbm.1979.0010. [DOI] [PubMed] [Google Scholar]
- Weiss B., Fertel R. Pharmacological control of the synthesis and metabolism of cyclic nucleotides. Adv Pharmacol Chemother. 1977;14:189–283. doi: 10.1016/s1054-3589(08)60189-1. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Mandelbaum D. E., Schonberg M., Kupfermann I. Modulation of buccal muscle contractility by serotonergic metacerebral cells in Aplysia: evidence for a role of cyclic adenosine monophosphate. J Neurophysiol. 1979 May;42(3):791–803. doi: 10.1152/jn.1979.42.3.791. [DOI] [PubMed] [Google Scholar]


