Abstract
Daughters of HIV-positive women are often exposed to the same factors that placed their mothers at risk. This cross-sectional study (N = 176 dyads) examined HIV status, parent-teen sexual risk communication (PTSRC), and daughters’ abstinence and condom use beliefs and intentions. Maternal HIV status was not associated with PTSRC. Path analyses show that maternal depression was associated with PTSRC behavioral and normative beliefs; relationship satisfaction was associated with PTSRC normative and control beliefs. Control beliefs were solely predictive of maternal PTSRC intention. PTSRC was associated with adolescent behavioral and normative beliefs. Abstinence beliefs were associated with abstinence intentions; condom beliefs were associated with condom use intentions. Relationship satisfaction was associated with adolescent control beliefs about both abstinence and condom use. There is a need for interventions that help HIV-positive mothers recognize their daughter’s HIV risk and provide them with relationship building and parent process skills to help reduce these risks.
Keywords: Parent–child sexual risk communication, Adolescent sexual risk, Maternal HIV, Relationship satisfaction
Introduction
Due to improved HIV treatment, there has been a dramatic increase in the number of HIV-positive women who are parenting HIV-negative children. An estimated 390,000 women (above age 15) in the United States are living with HIV; 6,000–7,000 infected women give birth annually [1]. As these children come of age, many face the same environmental risks for HIV that their mothers experienced [2] and have been found to be at increased risk for sexually acquired HIV [3]. Parents can exert a protective influence on their adolescents’ sexual beliefs, intentions, and behaviors through supervision, values transmission, and communication [4–7]. The most influential of these parenting behaviors is parent-teen sexual risk communication (PTSRC) (see review by Markham et al. [8]). However, little is known about how these parenting processes, particularly PTSRC, may differ in families headed by a parent who is HIV-positive. Thus, the purpose of this study was to examine the relationships among maternal HIV status, HIV-protective parenting behaviors (PTSRC), and daughters’ HIV-related sexual risk beliefs and intentions.
PTSRC and Adolescent HIV Risk
PTSRC is an important means through which parents can influence their adolescents’ sexual risk-related attitudes, beliefs, and behaviors [4, 6, 7, 9]. Parent–child communication about sex has been associated with abstinence [10, 11], intentions to abstain or delay initiation [12, 13], and use of birth control [10]. Maternal-child sexual communication is of particular importance; mothers are more likely to communicate with daughters than sons [13–17], daughters report greater comfort with and frequency of sex discussions with mothers [12, 13], and mother–daughter communication covers a wider range of topics than communication with fathers [13].
Relationship Satisfaction
Parent–child connectedness may be an important factor in influencing how parents engage with adolescents (e.g., PTSRC) and the behaviors of adolescents (e.g., intention to have sex). Perceptions of positive parent–child closeness have been found to be associated with adolescent self-esteem [18, 19] as well as decreased depressive symptoms [18, 20]. Connectedness has also been significantly and inversely related to substance use, highlighting the potential for parental opinions to protect against unhealthy behaviors [18]. Connectedness with parents can also buffer engagement in sexual risk behaviors [21]. Ream and Savin-Williams [22] found that a less close parent–child relationship contributed to an increased likelihood of sexual debut and continued sexual activity. Among families with HIV infection, children’s reports of maternal warmness and support have been found to be associated with fewer negative externalizing behaviors [23]. All of these studies highlight the potential importance of not only parental processes (actions) but also parent–child relationship (quality) in understanding the role of parents in HIV risk reduction.
HIV-Infected Families
The majority of HIV-positive women and their children in the United States live in inner cities [24]. These families, often female-headed, single-parent households, are exposed to extremely difficult conditions and affected by racism, classism, urban violence, environmental degradation, poor education systems, and exposure to illegal substance use [24]. In addition, HIV-positive parents face issues unique to having HIV (e.g., fear, physical symptoms, disclosure to children and social networks, and depression) [2, 25, 26]. Children of HIV-positive parents may also experience unique issues, including role changes, caretaking responsibilities, and premature parentification, that have been associated with engagement in risk behaviors [3]. Youth of HIV-positive mothers have been found to engage in the same behaviors that placed their mothers at risk for HIV infection, often at early ages [3]. These youth have been found to initiate sex earlier and report higher rates of unprotected sex and alcohol and illicit substance use [27, 28].
Depression and HIV are tightly intertwined; rates of depression are generally found to be higher among HIV-positive persons compared to their non-infected counterparts [29–31]. Compared to HIV-infected women without depression, HIV-positive women with depression are more likely to report greater HIV health worries and number of symptoms, and poorer perceived social support [29, 32, 33]. This increased risk for depressive symptoms among HIV-positive women not only impacts the women individually but also their families. Depression in HIV-positive mothers is associated with lower levels of family cohesion and family sociability [34]. Findings related to mental health among children of HIV-positive women varies. Although some studies have found no differences in depressive symptoms among children of HIV-positive parents and their non HIV-affected peers [35], others have reported higher rates of depressive symptoms [36, 37], lifetime and recent anxiety disorders [37], and disruptive disorders [38]. These increased levels of depression among children of HIV-positive parents may contribute to increased engagement in HIV risk behaviors.
PTSRC in Families with HIV-Positive Parents
In general, researchers have found that children of HIV-positive parents report more frequent discussions about HIV and express greater comfort with the topic compared to children of uninfected parents [39]. However, to date, only two studies (four resultant publications) have examined parent–child communication about sex in HIV-infected families [28, 39–41]. Of these four publications, two specifically evaluated the influence of maternal HIV sero-status on adolescents’ engagement in HIV-related risk behaviors [28, 41]. Mothers living with HIV were more likely to report discussing, and their children were more likely to report greater comfort discussing, sex-related topics. However, Corona et al. [40] found that some children of HIV-positive parents reported feeling uncomfortable talking with their parent about HIV for fear of upsetting or reminding them of their illness.
Limitations of the Existing Literature
Although it is well established that PTSRC is associated with reduction in HIV risk behaviors in youth, little is known about this type of communication in HIV-affected families. Among the studies that have begun to explore this topic, a clear definition about the meaning of “discussing HIV-related topics” has not been established. The dialogue could include disclosing status and talking about HIV as a disease or providing risk reduction interventions and strategies to youth. Mere disclosure and basic education alone may not facilitate the intended results—that is, reducing HIV risk engagement by adolescents. To effect changes in adolescent beliefs (including increased condom use self-efficacy) and resultant changes in intention and behaviors, specific PTSRC is necessary. Thus, this work sought to explore PTSRC among HIV-infected women and how maternal HIV status influenced adolescent girls’ beliefs and intentions related to abstinence and condom use.
Theoretical Framework
The theory of planned behavior provided the overarching structure for this investigation; the theory posits that behavioral intentions (viewed as the immediate antecedents to behavior) are a function of beliefs about the likelihood that performing the behavior will lead to a specific outcome [42]. Within the theory of planned behavior, an important determinant of an individual’s behavior is said to be his/her behavioral intention. Intentions result from a combination of one’s behavioral beliefs (attitude) towards performing the behavior, his/her subjective norms (the perception that those important to him/her approve/disapprove of the behavior), and his/her confidence in the ability to perform the behavior (control beliefs) [43]. Three conditions impact the strength of the relationship between intention and behaviors: (a) the degree to which the measure of intention and behavioral criteria correspond; (b) the stability of intentions between time of measurement and performance of behavior; and (c) the degree to which carrying out the behavior is under the individual’s volitional control [44].
The family expansion of the theory of planned behavior [45] builds on this framework, allowing the researcher to conceptualize how external influences may impact parenting behaviors (e.g., PTSRC) and how these parenting behaviors may, in turn, act as external influences on adolescents’ HIV-related sexual risk beliefs, intentions, and behaviors. Including parental influences is consistent with the ecological view (parents being one of the proximal influences on adolescent behaviors [45]). This model accommodates the influences on and behaviors of both mothers and daughters, helping to elucidate the interactions between the two. Most important for practice, the model highlights the multiple influences of HIV-related behaviors that exist in an adolescent’s proximal and distal environments [45]. The model’s constructs (see Fig. 1), starting from the left side of the model and moving towards the right, are described below. The model described mirrors the constructs used in this investigation.
Fig. 1.
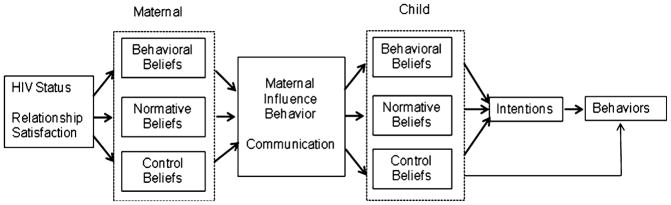
A family expansion of the theory of planned behavior (modified from Hutchinson and Wood [45])
Maternal Qualities
External factors may impact a mother’s parenting influence behaviors (communication); in this study, HIV status was included as the external factor as we hypothesized that a positive diagnosis would positively influence PTSRC. Based on literature, we also included relationship satisfaction, and mental and physical health as external factors influencing PTSRC.
Maternal Beliefs (Behavioral, Normative, and Control)
Maternal beliefs included attitudes (behavioral belief) towards PTSRC, perception of whether others approve or disapprove of them engaging in PTSRC (normative belief), and ability to perform PTSRC (control belief).
Maternal Influence Behavior
In this study, maternal influence behavior was operationalized as PTSRC. PTSRC is the dialogue between mother and daughter that can include any or all of the following subjects: sexuality, abstinence, substance use, sexual intercourse, familial beliefs and values, partner negotiation, condom skills, and other contraception. Communication acts as external influence factors on the adolescent side of the model. These influences are hypothesized to be indirect and mediated through the daughter’s beliefs [45].
Child Beliefs (Behavioral, Normative, and Control)
Adolescent daughters’ beliefs included attitudes towards abstinence and condom use (behavioral beliefs), perception of whether others approve or disapprove of them abstaining from sex or using condoms (normative beliefs), and ability to either abstain from having sex or use condoms during sexual intercourse (control belief).
Intentions and Behaviors
In this model, intention is theorized to be influenced by behavioral, normative, and control belief and is the hypothesized prerequisite step to performing the proposed behavior (abstaining from sex or using condoms).
Summary of Proposed Model
This family expansion of the theory of planned behavior is an important step in moving beyond the individual-level investigation of behavior. The model illustrates the theoretical rationale for undertaking research that seeks to understand how mothers’ parenting beliefs and behaviors influence their daughters’ sexual risk-related beliefs and behaviors [45]. Extending an individual-level approach like the theory of planned behavior helps to elucidate the factors that influence adolescent engagement in sexual risk behaviors. The expansion provides a more advanced understanding of the multiple influences of HIV-related behaviors that exist within an adolescent’s proximal and distal environments [45].
Based on this theoretical model, the study hypotheses were: (1) HIV-positive mothers will report higher rates of PTSRC than HIV-negative women; (2) mother–daughter dyads with greater relationship satisfaction will report higher rates of PTSRC; (3) adolescent girls who report higher rates of PTSRC will endorse abstinence and condom use; and (4) adolescent girls who report higher levels of relationship satisfaction will endorse abstinence and condom use.
Methods
This cross-sectional study was conducted with 176 African American and Latina mother–daughter dyads (N = 352 individuals) recruited from three cities in the northeastern United States. Data were collected between May 2008 and July 2009 via self-administered (paper and pencil) surveys in participants’ homes or at community-based service organizations. Mothers and daughters completed surveys separately but simultaneously. Help was provided (in the form of reading the survey aloud) to those who needed/ wanted this type of assistance. The surveys were developed, in part, from elicitation work undertaken through focus groups [26]. All procedures for the protection of human subjects were reviewed and approved by the University of Pennsylvania.
Sample and Sampling Procedures
Convenience samples of HIV-positive and HIV-negative mothers with adolescent daughters (14–18 years) were recruited from clinics and service organizations in Philadelphia, PA, Newark, NJ, and New York, NY. A list of relevant agencies was compiled utilizing a database of service providers. A total of 11 agencies assisted in the recruitment of participants for the study. Recruitment was accomplished through flyers and provider and participant referrals. Inclusion criteria for adults were: (1) female; (2) a diagnosis of symptomatic HIV, AIDS, or HIV-negative; (3) live at least 50 % of the time with an uninfected daughter between the ages of 14 and 18 who is aware of HIV diagnosis, and (4) English speaking. When the adult reported more than one daughter within the inclusion age range, the daughter closest in age to 16 (measured in months) was selected to participate (this reduced any potential maternal bias; e.g. choosing the daughter they felt would give “better” answers). Adult participants were asked to provide written consent for themselves and their daughters; minor participants provided written assent. Each mother and daughter received $20 and $15, respectively, for their participation.
The majority of mothers self-identified as African American (86.3 %; see Table 1). Thirty-seven percent (n = 65) of the dyads included HIV-positive mothers. The mean age of adolescent girls in the sample was 15.8 years; 87.5 % of daughters self-identified their race as African American (see Table 2). The majority of adolescent participants (91 %) were currently in school. Forty-two percent of the adolescent girls reported having had sex.
Table 1.
Demographics—mothers
| Variable | Total sample (N = 176) | HIV+ (N = 65) | HIV− (N = 111) | t (df) |
|---|---|---|---|---|
| Mean/ % | Mean/ % | |||
| Age | 40.89 [27–70] | 41.77 [30–70] | 40.31 [27–64] | −1.260 (171) |
| Race—African American | 86.3 % (145) | 76.7 % (46) | 91.6 % (98)* | 2.451 (87.384) |
| Latina | 17.2 % (28) | 22.2 % (14) | 14.1 % (14) | .061 (161) |
| Ever married, yes | 30.5 % (51) | 40 % (24) | 25.5 % (27)* | −2.000 (165) |
| Level of education* | 1.672 (165) | |||
| Less than HS | 35.9 % (60) | 46.9 % (30) | 29.4 % (30) | |
| HS Graduate | 42.5 % (71) | 32.8 % (21) | 48 % (49) | |
| Voc Ed/College | 21.6 % (36) | 20.3 % (13) | 22.5 % (23) | |
| Employed—yes | 47.4 % (83) | 36.9 % (24) | 54.1 % (59)* | −2.156 (173) |
| Other parent involved | 65.9 % (110) | 54.7 % (35) | 72.5 % (74)* | −2.430 (165) |
| Are you HIV+ | 37.1 % (65) | – | – | |
| Other family member HIV+ | 34.3 % (60) | 85.9 % (55) | 4.5 % (5)*** | −19.295 (173) |
| Family friend HIV+ | 45.7 % (80) | 73.8 % (48) | 29.4 % (32)*** | −6.338 (173) |
| Religion—Christian | 43.3 % (74) | 50 % (32) | 39.6 % (42) | −1.372 (169) |
| Age at first sex | 16.05 (11–22) | 15.21 (11–21) | 16.57 (12–22) | – |
| Ever + for STD—yes | 49.4 % (83) | 63.9 % (39) | 41.5 % (44)** | −2.898 (166) |
| Last sex, condom—yes | 53.4 % (93) | 59.4 % (38) | 49.5 % (54) | −1.194 (172) |
| Last time sex, high—yes | 16.6 % (29) | 14.1 % (9) | 18.2 % (20) | .675 (173) |
| Ever smoked cigarettes—yes | 70.5 % (122) | 75.4 % (49) | 68.2 % (73) | −1.086 (171) |
| Ever used marijuana—yes | 45.8 % (70) | 59.7 % (37) | 36.7 % (33)** | −2.914 (151) |
| Ever used alcohol—yes | 80.1 % (141) | 83.1 % (54) | 79.1 % (87) | −.596 (174) |
| Ever used cocaine—yes | 28 % (45) | 49.2 % (31) | 14.4 % (14)** | −5.210 (151) |
| Ever used heroin—yes | 8.6 % (15) | 14.1 % (9) | 5.5 % (6)* | −1.981 (173) |
p<.05,
p<.01,
p<.001
Table 2.
Demographics—adolescents
| Variable | Total sample (N = 176) | HIV+ Mom (N = 65) | HIV− Moms (N = 111) | t (df) |
|---|---|---|---|---|
| Mean/ % | Mean/ % | |||
| Age | 15.8 [14–19] | 15.88 [14–19] | 15.78 [14–19] | – |
| Grade in school | .640 (158) | |||
| 8th grade | 17.5 % (28) | 17.9 % (10) | 16.5 % (18) | |
| 9th grade | 23.1 % (37) | 23.2 % (13) | 23.3 % (24) | |
| 10th grade | 25.6 % (41) | 21.4 % (12) | 28.2 % (29) | |
| 11th grade | 15 % (24) | 14.3 % (8) | 15.5 % (16) | |
| 12th grade | 14.4 % (23) | 19.6 % (11) | 11.7 % (12) | |
| More than HS | 4.4 % (7) | 3.6 % (2) | 4.9 % (5) | |
| Race—African American | 87.5 % (147) | 77.6 % (45) | 92.7 % (101) | −.507 (174) |
| Father HIV-positive—yes | 16.1 % (28) | 31.7 % (20) | 7.3 % (8)*** | −4.266 (172) |
| Other family member HIV+ | 17.8 % (31) | 31.3 % (21) | 10.1 % (38)*** | −3.909 (172) |
| Family friend HIV-positive | 25 % (44) | 50.8 % (33) | 10 % (11)*** | −6.747 (174) |
| Ever had sex—yes | 42.6 % (75) | 56.9 % (37) | 34.5 % (38)** | −2.995 (174) |
| Age at first sex | 14.27 [11–18] | 14.36 [11–17] | 14.20 [12–18] | – |
| First sex forced—yes | 10.7 % (8) | 5.6 % (2) | 15.4 % (6) | −1.884 (172) |
| First sex condom use—yes | 71.4 % (55) | 70.3 % (26) | 72.5 % (29)* | −2.450 (173) |
| Ever test for STD—yesa | 41 % (71) | 46.2 % (30) | 38 % (41) | −1.420 (171) |
| Ever tested for HIV—yesb | 39.9 % (67) | 58.1 % (36) | 29.5 % (31)** | −3.816 (166) |
| Last sex, condom used—yesa | 63.6 % (49) | 67.6 % (25) | 60 % (24)** | −2.769 (173) |
| Ever smoked cigarettes—yes | 32.2 % (56) | 37.5 % (24) | 29.4 % (32) | −1.143 (172) |
| Ever used marijuana—yes | 22.2 % (39) | 35.4 % (23) | 14.5 % (16)** | −3.314 (174) |
| Ever used alcohol—yes | 39.2 % (69) | 43.1 % (28) | 37.3 % (41) | −.802 (174) |
p<.05,
p<.01,
p<.001
One missing participant
Two missing participants
Instrumentation
The survey instrument was comprised of scales that have all been previously validated with urban adults and adolescents. To reduce problems with recall, respondents were asked to remember specific behaviors during a relatively brief period (3 months) [9]. Adolescents and mothers were assured that their responses would not be shared with each other, a technique known to increase adolescents’ willingness to disclose sensitive information.
Parent-Adolescent Sexual Risk Communication
Daughters completed a measure of sexual communication, the 8-item PTSRC Scale (PTSRC-III) [6], which as an internal reliability of .92. Participants were asked how much information their mother shared about: (a) birth control, (b) sexually transmitted infections (STIs), (c) HIV, (d) how to prevent STIs and HIV, (e) condoms, (f) pressure from peers and partners to have sex, (g) how to resist pressure to have sex, and (h) waiting to have sex. Daughters’ reports were used in the analyses [46]. Scale reliability was α = .940 in this study; this is consistent with previously reported reliability scores [6].
Relationship Satisfaction
Mother–daughter relationship satisfaction was measured using an 11-item scale [47]. Participants were asked about satisfaction with various aspects of their mother–daughter relationship (e.g., communication, emotional support, conflict resolution, and fun). In Model 1, mothers’ reports of satisfaction were used; in Model 2, daughters’ reports were used, as their intention to abstain from sex or use condoms was the outcome of interest. Internal reliability of the scale in this study was α = .939 for mothers and α = .958 for daughters.
Behavioral, Normative, and Control Beliefs
Maternal behavioral, normative, and control beliefs about PTSRC were assessed with multi-item scales (see Table 3 for questions). Behavioral beliefs were measured with four items; normative beliefs were assessed with three items asking about approval of PTSRC by partners, family, and friends. Control beliefs were assessed with four items asking how difficult it was to communicate with daughters about sexual risk topics. Internal reliability alphas for the three constructs were α = .906, α = .833, and α = .943, respectively. Maternal intention was measured by two single-item measures: “Do you intend to talk to your daughter about sex and abstinence in the next three months?” and “How likely is it that you will talk to your daughter about HIV/AIDS?” Daughters’ behavioral, normative, and control beliefs regarding abstinence and condom use were also assessed. Behavioral beliefs about abstinence included three items (α = .644); beliefs about condoms included three items (α = .901). Normative beliefs about sex was a single item; normative beliefs about condoms included five items (α = .866). Single items were used to assess control beliefs and intentions related to abstinence and condom use including: “How easy or hard would it be for you to not have sex in the next 3 months?”; “How easy or hard would it be to use condoms when you have sex?”; “I plan to have sex in the next three months”; and “I plan to use condoms in the next three months.”
Table 3.
Standardized factor loadings for latent constructs used in hypothetical models
| Standardized factor loading | |
|---|---|
| Depression | |
| Depressive affect | .903 |
| Somatic retardation | .915 |
| Relationship satisfactiona | |
| Parcel 1 | .915–.925 |
| Parcel 2 | .818–.882 |
| Parcel 3 | .829–.944 |
| PTSRC-behavioral belief | |
| I know enough information to talk to my daughter about how to prevent HIV/AIDS | .951 |
| I know enough information to talk to my daughter about how to use a condom. | .948 |
| I know enough information to talk to my daughter about different kinds of birth control | .872 |
| I know how to tell my daughter good ways to put off sex until she is older | .897 |
| PTSRC-normative belief | |
| How important is it to you that your family approves of what you do with your daughter? | .872 |
| How important is it to you that your partner/husband approves of what you do with your daughter? | .642 |
| How important is it to you that your friends approve of what you do with your daughter? | .792 |
| PTSRC-control belief | |
| How easy/hard is it for you to talk to your daughter about sex and abstinence | .928 |
| How easy/hard is it for you to talk to your daughter about dating | .838 |
| How easy/hard is it for you to talk to your daughter about resist | .852 |
| How easy/hard is it for you to talk to your daughter about STDs and HIV | .787 |
| Communication intention | |
| How likely is it that you will talk to your daughter about sex and abstinence | .795 |
| How likely is it that you will talk to your daughter about HIV/AIDS | .797 |
| PTSRCa | |
| Parcel 1 | .882–.914 |
| Parcel 2 | .869–.900 |
| Parcel 3 | .711–.719 |
| Abstinence-behavioral belief | |
| If I do not have sex, people will call me names | .817 |
| If I do not have sex, boys will not want to go out with me | .885 |
| If I do not have sex with my boyfriend, then he will break up with me | .859 |
| Condom use-behavioral belief | |
| Condoms help prevent pregnancy | .849 |
| Condoms help prevent STDs | .985 |
| Condoms help prevent HIV | .783 |
| Condom use-normative belief | |
| Would most people who are important to you approve or disapprove of you using a condom if you have sex in the next 3 months? | .731 |
| Would your sexual partner approve or disapprove of you using a condom if the two of you have sex in the next 3 months? | .741 |
| Would your mother approve or disapprove of you using a condom if you have sex in the next 3 months? | .918 |
| Would your father approve or disapprove of you using a condom if you have sex in the next 3 months? | .858 |
| Would your friends approve or disapprove of you using a condom if you have sex in the next 3 months? | .567 |
All factor loadings are significant at p < .001
Standardized factor loadings from hypothetical model 1 and model 2
HIV Risk Behaviors
A sexual behavior questionnaire was used to assess risk intention to be abstinent or use condoms in the next 3 months. Actual behavior was also assessed; the items have been widely used [9]. Items assessed whether the adolescent had ever engaged in each type of behavior, and how frequently they had done so in the previous 3 months.
Demographic and Other Variables
Both mothers and daughters reported on age, educational attainment, race/ethnicity, religion, and living arrangements. Mothers were also asked for HIV serostatus, marital status, and employment status. Mothers and daughters were asked two questions related to “knowing someone with HIV” (within family and among friends). Depression was measured using the Center for Epidemiologic Studies Depression Scale (CES-D 10) [48]. Reliability in the current study was α = .818. Mothers’ physical symptoms were measured with an adapted version of the Physical Symptoms Inventory [49] created by Armistead and colleagues [25]. The 23-item inventory highlights the physical symptoms most often seen in HIV-positive women. Possible scores range from 0 to 108, with higher scores indicating more symptoms (α = .91) [25]. The inventory was completed by both HIV-positive and negative mothers, and the reliability was α = .936.
Data Analysis
Preliminary analyses were conducted in SPSS v.17 and included cleaning and identifying outliers in the data. Scales were constructed and descriptive statistics calculated for all items and scales. Internal reliability alpha coefficients were calculated for all multi-item scales. Structural equation modeling (SEM) was used to examine the impact of maternal HIV status on PTSRC and the influence of PTSRC on daughters’ beliefs and intentions. All SEM analyses were performed in mPLUS 6.1.
PTSRC, maternal beliefs about PTSRC, PTSRC intentions, depression, and mother–daughter relationship satisfaction were specified as latent factors measured by indicators identified through exploratory factor analysis and the published literature. For latent factors measured by more than five items, three parcels were constructed by assigning each item to one of the parcel groupings for that latent factor. The assignment process was guided by an exploratory factor analysis algorithm that used an iterative estimator with an oblique rotation [50]. Before developing the structural model, we conducted a confirmatory factor analysis (CFA) to assess how well the observed measures or random parcels reflected the hypothesized latent constructs. This analysis also allowed us to examine the correlations among the unobserved latent factors. The same factor structure or measurement portion of the CFA model was used as the basis for the initial structure model. In order to investigate the measurement invariance and population heterogeneity, we also conducted the multiple group analysis to test the structural equality across HIV-positive and -negative participants.
Missing data were handled using the optimal full information maximum likelihood (FIML) estimation method. In the context of SEM, FIML is frequently recommended as the best approach to handling missing data [51]. Model fit indexes, including χ2, the comparative fit index (CFI) [52], and the root-mean-square error of approximation (RMSEA), were the criteria used for estimating model fit. Bentler’s recommendation, to not reject a model when the ratio of the model’s χ2 to the degrees of freedom is<2.00, was followed [53]. The CFI, a sample-size adjusted analogue to the normed fit index [37], indicated the amount of covariation in the data accounted for by the hypothesized model. A CFI > .90 is generally accepted as representative of good model fit [52]. The RMSEA, a noncentrality measure, indicates a good model with a value of .05 or less.
Results
Preliminary Results: Mothers and Daughters
Table 1 provides an overview of the demographic characteristics of the adult participants (mothers) overall and by HIV serostatus. The mean age for mothers was 40.89 years. Approximately 65 % of the mothers were high school graduates; 47 % were currently employed. Among the adults, 34.3 % reported having another family member who was HIV-positive and 45.7 % reported having a family friend who was HIV-positive. Women who reported being HIV-positive had significantly lower levels of education (t = 1.672; p < .034), were less likely to be employed (t = 2.156; p < .032), and were less likely to be married (t = −2.000; p < .047). They were more likely to have a history of using marijuana (t = −2.914; p < .004), cocaine (t = −5.210; p < .000), and heroin (t = −1.981; p < .049).
Table 2 provides an overview of the demographic characteristics of the adolescent participants. Among adolescent girls who reported being sexually experienced (n = 75), the mean age at their first sexual encounter was 14.27 years (SD = 2.26; range = 11–18); 71.4 % reported condom use during their first sexual encounter and 63.6 % reported using a condom the last time they had sex. Daughters of HIV-positive mothers were more likely than other girls to report having a father who was HIV-positive (t = −4.266; p < .000), ever having had sex (t = −2.595; p < .003), having used marijuana (t = −3.314; p < .001), and having been tested for HIV (t = −3.816; p < .000).
Main Analyses
Two SEM models were constructed to explore the associations between HIV status, beliefs, communication, and behavioral intentions.
Measurement Model
All factor loadings for the measured variables on the latent factors were sizable and significant (see Table 3). PTSRC was reflected by indicators (parcels) with standardized factor loadings ranging from .711 to .914. Exploratory factor analyses using both principal axis and common factoring methods provided empirical support for a single reliable factor that characterizes the sexual-risk communication between mother and daughter. The latent factor for depression (CES-D 10) was represented by indicators that have been previously examined [54].
Structural Model
To capture the patterns in the data, we developed the initial structural model by testing for specific or nonstandard paths guided by the Lagrangian multiplier tests [55]. Although adding other correlated residuals may have improved the model fit, we avoided including them due to the potential for unreliable effects. Results from the multiple-group analysis by HIV status indicated there was no significant difference in the structural relationships between the HIV-positive and -negative group. Therefore, HIV status was controlled in the model as a covariate.
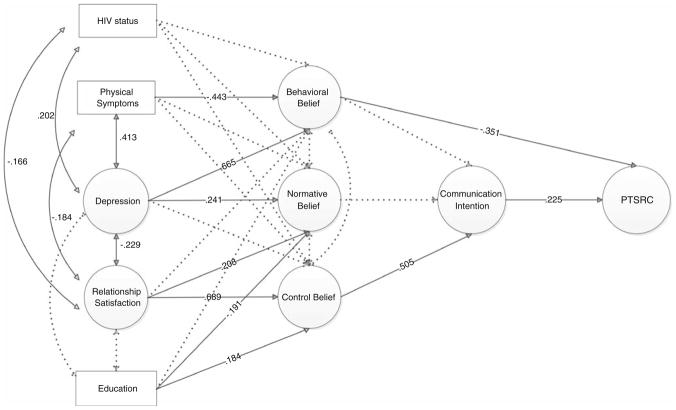
Model 1 examined the influence of maternal characteristics (depression, HIV status, relationship satisfaction with daughters, and physical symptoms) on the PTSRC beliefs and behaviors of mothers. The final model demonstrated adequate fit (χ2 = 340.957; df = 222; ratio of χ2 and df = 1.54; CFI = .95; RMSEA = .058). Figure 2 depicts the significant paths (solid lines) between factors in the final model. HIV status was not significantly related to behavioral, normative, and control beliefs about PTSRC. HIV status was positively associated with depression and negatively associated with relationship satisfaction. Mothers who were more depressed had greater behavioral and normative beliefs about PTSRC but no significant links were found between depression and control beliefs; relationship satisfaction was positively associated with normative and control beliefs. As predicted by the theory of planned behavior, PTSRC communication intentions were associated with PTSRC. For clarification, believing PTSRC was important was associated with a lower sum score on the behavioral beliefs measure. Those who felt strong control beliefs related to PTSRC were more likely to report sexual risk communication with their daughters.
Fig. 2.
Model 1 influences on maternal reports of parent-teen sexual risk communication. Note All responses, except PTSRC, are from mothers. Model fit indexes: χ2 = 340.957, df = 222, CFI = .95, RMSEA = .058. N = 160; 16 subjects are excluded from this model due to missing data on independent observed variables. Solid lines indicate parameter estimates than are statistically significant at p < .05
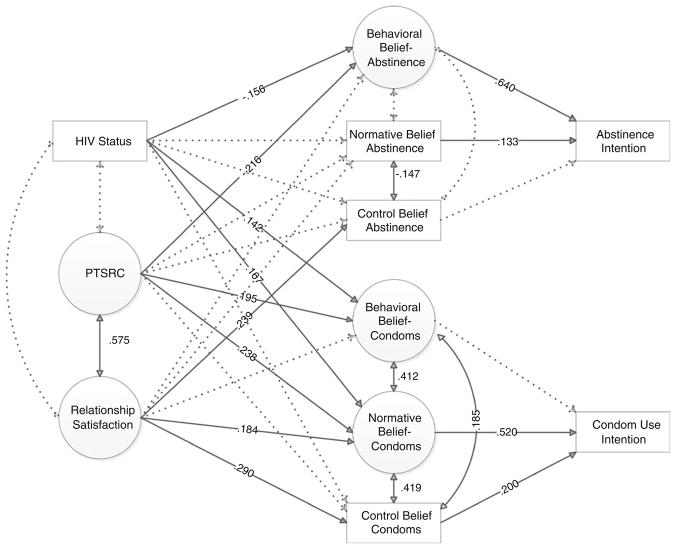
In Model 2, we examined how maternal communication, relationship satisfaction (from the perspective of the daughter), and HIV status influenced the beliefs and intentions of daughters in relation to abstinence and condom use. Again, the final model demonstrated adequate fit (χ2 = 374.734; df = 221; ratio of χ2 and df = 1.70; CFI = .935; RMSEA = .063). Figure 3 depicts the significant paths in this final structural model. In Model 2, maternal HIV status was associated with more permissive behavioral beliefs about sex but stronger behavioral and normative beliefs about condoms among daughters. PTSRC was associated with behavioral beliefs about abstinence and condoms as well as normative beliefs about condoms. Relationship satisfaction was associated with control beliefs about both abstinence and condoms. Whereas behavioral and normative beliefs about condoms were predictive of abstinence intentions, normative and control beliefs regarding condoms were predictive of condom use intentions. These paths are consistent with the theory of planned behavior.
Fig. 3.
Influences of daughter’s abstinence and condom use intentions. Note All responses, except HIV status, are from daughters. Model fit indexes: χ2 = 374.734, df = 221, CFI = .935, RMSEA = .063. N = 175; one subject is excluded from this model due to missing data on HIV status. Solid lines indicate parameter estimates that are statistically significant at p < .05
Discussion
Children of HIV-positive women have been shown in several studies to be at increased risk for sexually acquired HIV infection [3, 27, 28]. This study is one of a few to explore the influence of maternal HIV status on adolescent risk intentions [2, 28], and the first to use a family expansion of the theory of planned behavior to better elucidate which maternal characteristics are associated with parent–child sexual risk communication. Although others have examined how knowing someone with HIV or who has died of AIDS impacts sex behaviors [3, 56, 57], the findings here highlight the complexity of the influence of maternal HIV, including the influence of emotional and physical health and mother–daughter relationship quality on mother–daughter sexual risk communication, and daughters’ beliefs and intentions regarding abstinence and condom use.
Compared to HIV-negative mothers, HIV-positive mothers in this study generally represented a more vulnerable group of women. HIV-positive mothers were more likely to have histories of marijuana, cocaine, and heroin use, were less likely to be employed, and were less likely to report that their daughter’s father remained involved in her life, compared to HIV-negative mothers. This may have increased the likelihood that daughters of HIV-positive mothers were living in environments similar to those in which their mothers were exposed to HIV [24]. Yet we saw no significant differences in PTSRC between HIV-positive and HIV-negative mother-daughter dyads. We believe this null finding is important to share. This finding may be attributable to the fact that both adolescents of HIV-positive and HIV-negative mothers came from similar vulnerable inner-city neighborhoods with high rates of blight, violence, and drug use. The finding may imply that communication about HIV behaviors may be triggered by residence in high-risk, HIV-dense neighborhoods, and not by individual HIV status itself. If this is the case, then the important target group may be those mothers who reside in high-poverty environments (who are potentially more limited in their knowledge, comfort, and/or ability to communicate with their daughters about sex).
Because mean scores on PTSRC did not vary by maternal HIV status, alternative maternal qualities were explored. The most salient quality related to PTSRC among these mother–daughter dyads was relationship satisfaction. Although the findings here are consistent with the findings of previous studies that link parent–child relationship satisfaction with positive outcomes for adolescents [18, 20, 21, 58], few have examined relationship satisfaction and parent–child communication specifically [21, 46, 59]. Women who scored higher on the depression inventory had higher behavioral beliefs about PTSRC; they were also more likely to be influenced by their normative groups, which could lead to or deter them from PTSRC. This finding highlights the importance of exploring normative beliefs related to PTSRC among mothers, but also identifies the potential need for changing community norms, helping to create communities where it is normative to talk to preadolescents and adolescents about both abstinence and safer sex. When community norms are shifted, positive HIV risk reduction messages provided by parents may be repeated and reinforced outside the home.
Maternal HIV-status was associated with less relationship satisfaction between mothers and daughters. This finding differs some from one earlier study that found that although HIV-affected families have poorer family functioning, they have stronger parent–child bonds [60]. The finding that HIV-positive women report poorer relationship satisfaction with their daughters provides us with a second potential explanation for the null findings between HIV-positive serostatus and PTSRC. Perhaps there are two ways in which HIV influences mothers; there are those who are more proactive because of their status, and those who have limitations because of individual and structural barriers, including physical symptoms associated with HIV. It is possible that factors like physical symptoms are relevant predictors of ability to engage with daughters in the ways measured by the relationship satisfaction scale [61]. Given the significant relationship between control beliefs and PTSRC intention, women may be less likely to have the needed energy and self-efficacy to communicate with their daughters about reducing HIV risk behaviors.
Important in these analyses was the relationship between mother–daughter relationship satisfaction and daughters’ control beliefs about both abstinence and condoms. The concept of control beliefs emerges from Bandura’s [62] work on self-efficacy and can determine how a person thinks, motivates, and behaves. Control beliefs encompass confidence in one’s ability to carry out a specific behavior, with the path from control beliefs to behavior thought emerging when there is relative agreement between an individual’s perception of control and their actual control over a behavior [44]. The data suggest that in stronger mother–daughter relationships, daughters have greater control beliefs about their ability to abstain from sex or use condoms when engaging in sexual intercourse. This belief is critical to the adolescent’s ability to carry out these protective behaviors. These findings about parent–child relationship point to the importance of increasing mother–daughter relationship satisfaction, as this relationship increases the likelihood of self-efficacy related to reduction in risk engagement. We know that among participants in the sample, reports of greater parent–child relationship satisfaction mitigated that risk of negative peer pressure regarding sex [63]. As such, relationship satisfaction may also serve to buffer the distal influences within the community.
Consistent with the work of others [3, 23, 26], daughters of HIV-positive mothers were more likely to report ever having had sex and had more permissive behavioral beliefs about sex, reflecting the potential for riskier behavior. Although more likely to engage in sex, daughters of HIV-positive mothers also had stronger behavioral and normative beliefs about condoms, suggesting that although they were not abstinent, these adolescents had the necessary attitudinal antecedents to increase the likelihood of future condom use. This is further reflected in their increased likelihood of reporting condom use during their initial and most recent sexual encounters. Daughters of HIV-positive mothers were also more likely to report having been tested for HIV. The Centers for Disease Control and Prevention [64] recommends universal HIV testing for all people over the age of 13, targeting those at highest risk. A recent Institute of Medicine report [65] echoes these recommendations, suggesting annual screening for STIs and HIV among women who are sexually active. Based on these findings, it appears that maternal HIV status, if disclosed, may serve as a trigger for both condom use and testing uptake among adolescent girls.
Limitations of the Study
This study contributed to the literature by examining the influence of maternal HIV status on adolescent daughters’ intentions to engage in HIV risk behaviors using a family expansion of the theory of planned behavior [45]. Few others have examined these processes in HIV-positive families. However, there were a number of limitations to the study. First, this study included convenience samples of HIV-positive and HIV-negatives families. The sampling does not ensure true representation of the population; participants were limited to those who self-selected to participate. This is particularly important given the focus of the study. It may be that mothers who did not have positive relationships with their daughters self-selected out because they would have been unable to get their daughter to agree to complete the survey or did not want to share the weaknesses within their mother–daughter relationship. The homogeneity among study participants is another limitation; there were few group differences among those who were HIV-positive compared to HIV-negative. Larger groups or representative sampling techniques to increase the heterogeneity of the samples may have shown more meaningful differences. Given the smaller sample size, there may have been insufficient power in this study to detect differences between the two groups. Lastly, these data are cross-sectional, which limits our ability to examine relationships over time or control for issues of temporal order. To reduce this limitation, we chose to explore intentions instead of actual performed behaviors when examining adolescent risk activities.
Conclusion
Findings from this study provide useful directions for working with families infected and affected by HIV/AIDS, or those families who may be at higher risk for HIV infection. Mothers need to be supported in disclosing their HIV status and building strong and open relationships with daughters in order to increase opportunities to communicate with their daughters, teach negotiation skills, and create an environment that maximizes the child’s opportunities to feel safe communicating with her mother about HIV risk reduction issues. Interventions will need to allow participants to discuss how their families have been fractured. We know that adolescents who receive messages more frequently report better relationships with their parents, are better able to communicate with their parents in general and about sex specifically, and perceive greater openness in discussions with their parents. Based on our findings, the elements necessary to reach the proposed goal of reducing sexual risk engagement by HIV-negative youth must (1) include parents, (2) emphasize relationship building between parents and adolescents, and (3) include skills-building activities for both parents and adolescents to increase openness, comfort, and communication about abstinence and/or condom use. Given the recommendation of universal HIV testing, reducing stigma and barriers to testing can further increase opportunities for linking risk assessment with health screening.
Acknowledgments
This research was supported by the National Institute of Mental Health (F31 MH076697-01A1; PI: Cederbaum) and a secondary grant from the Center for AIDS Research at the University of Pennsylvania (CFAR; P30-AI-045008-10, PI: Hoxie). Many thanks to the agencies that support the data collection efforts: CFAR at the University of Pennsylvania, Children’s Hospital of Philadelphia, St. Christopher’s Hospital, the New Jersey Women and AIDS Network (NJWAN), El Club del Barrio, Action AIDS, Congresso de Latinos, Women in Transition, and the Consortium.
Contributor Information
Julie A. Cederbaum, Email: jcederba@usc.edu, School of Social Work, University of Southern California, Montgomery Ross Fisher Building, Room 214, Los Angeles, CA 90089, USA
M. Katherine Hutchinson, College of Nursing, New York University, New York, NY, USA.
Lei Duan, School of Social Work, University of Southern California, Montgomery Ross Fisher Building, Room 214, Los Angeles, CA 90089, USA.
Loretta S. Jemmott, School of Nursing, University of Pennsylvania, Philadelphia, PA, USA
References
- 1.Centers for Disease Control and Prevention. [Accessed 22 June 2011];Pregnancy and childbirth. 2007 www.cdc.gov/hiv/topics/perinatal.
- 2.Brackis-Cott E, Mellins CA, Block M. Current life concerns of early adolescents and their mothers: influence of maternal HIV. J Early Adolesc. 2003;23(1):51–77. [Google Scholar]
- 3.Chabon B, Futterman D, Hoffman ND. HIV infection in parents of youths with behaviorally acquired HIV. Am J Public Health. 2001;91(4):649–50. doi: 10.2105/ajph.91.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiIorio C, Pluhar E, Belcher L. Parent–child communication about sexuality: a review of the literature from 1980–2002. J HIV/AIDS Prev Educ Adolesc Child. 2003;5(3–4):7–32. [Google Scholar]
- 5.Guilamo-Ramos V, Jaccard J, Dittus P, Bouris AM. Parental expertise, trustworthiness, and accessibility: parent–adolescent communication and adolescent risk behavior. J Marriage Fam. 2006;68(5):1229–46. [Google Scholar]
- 6.Hutchinson MK. The parent-teen sexual risk communication scale (PTSRC-III): instrument development and psychometrics. Nurs Res. 2007;56(1):1–8. doi: 10.1097/00006199-200701000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson MK, Jemmott JB, III, Jemmott LS, Braverman P, Fong GT. The role of mother–daughter sexual risk communication in reducing sexual risk behaviors among urban adolescent females: a prospective study. J Adolesc Health. 2003;33(2):98–107. doi: 10.1016/s1054-139x(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 8.Markham CM, Lormand D, Gloppen KM, et al. Connectedness as a predictor of sexual and reproductive health outcomes for youth. J Adolesc Health. 2010;46(3):S23–41. doi: 10.1016/j.jadohealth.2009.11.214. [DOI] [PubMed] [Google Scholar]
- 9.Jemmott JB, III, Jemmott LS, Braverman PK, Fong GT. HIV/STD risk reduction interventions for African American and Latino adolescent girls at an adolescent medicine clinic. Arch Pediatr Adolesc Med. 2005;159(5):440–9. doi: 10.1001/archpedi.159.5.440. [DOI] [PubMed] [Google Scholar]
- 10.Aspy CB, Vesely SK, Oman RF, Rodine S, Marshall L, McLeroy K. Parental communication and youth sexual behaviour. J Adolesc. 2007;30(3):449–66. doi: 10.1016/j.adolescence.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Wilson KM, Klein JD. Just say no? The impact of messages from parents and sex education classes. J Adolesc Health. 2002;30(2):105. [Google Scholar]
- 12.Guzmán BL, Schlehofer-Sutton MM, Villanueva CM, Dello Stritto ME, Casad BJ, Feria A. Let’s talk about sex: how comfortable discussions about sex impact teen sexual behavior. J Health Commun. 2003;8(6):583–98. doi: 10.1080/716100416. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson MK. The influence of sexual risk communication between parents and daughters on sexual risk behaviors. Fam Relat. 2002;51(3):238–47. [Google Scholar]
- 14.Kapungu CT, Baptiste D, Holmbeck G, et al. Beyond the “birds and the bees”: gender differences in sex-related communication among urban African–American adolescents. Fam Process. 2010;49(2):251–64. doi: 10.1111/j.1545-5300.2010.01321.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin KA, Luke K. Gender differences in the ABC’s of the birds and the bees: what mothers teach young children about sexuality and reproduction. Sex Roles. 2010;62(3–4):278–91. [Google Scholar]
- 16.Pluhar EI, DiIorio CK, McCarty F. Correlates of sexuality communication among mothers and 6–12-year-old children. Child Care Health Dev. 2008;34(3):283–90. doi: 10.1111/j.1365-2214.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 17.Wyckoff SC, Miller KS, Forehand R, et al. Patterns of sexuality communication between preadolescents and their mothers and fathers. J Child Fam Stud. 2008;17(5):649–62. [Google Scholar]
- 18.Ackard DM, Neumark-Sztainer D, Story M, Perry C. Parent–child connectedness and behavioral and emotional health among adolescents. Am J Prev Med. 2006;30(1):59–66. doi: 10.1016/j.amepre.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Bulanda RE, Majumdar D. Perceived parent–child relations and adolescent self-esteem. J Child Fam Stud. 2009;18(2):203–12. [Google Scholar]
- 20.Boutelle K, Eisenberg ME, Gregory ML, Neumark-Sztainer D. The reciprocal relationship between parent–child connectedness and adolescent emotional functioning over 5 years. J Psychosom Res. 2009;66(4):309–16. doi: 10.1016/j.jpsychores.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Henrich CC, Brookmeyer KA, Shrier LA, Shahar G. Supportive relationships and sexual risk behavior in adolescence: an ecological–transactional approach. J Pediatr Psychol. 2006;31(3):286–97. doi: 10.1093/jpepsy/jsj024. [DOI] [PubMed] [Google Scholar]
- 22.Ream GL, Savin-Williams RC. Reciprocal associations between adolescent sexual activity and quality of youth–parent interactions. J Fam Psychol. 2005;19(2):171–9. doi: 10.1037/0893-3200.19.2.171. [DOI] [PubMed] [Google Scholar]
- 23.Jones DJ, Foster SE, Zalot AA, Chester C, King A. Knowledge of maternal HIV/AIDS and child adjustment: the moderating role of children’s relationships with their mothers. AIDS Behav. 2007;11(3):409–20. doi: 10.1007/s10461-006-9188-1. [DOI] [PubMed] [Google Scholar]
- 24.Brackis-Cott E, Mellins CA, Dolezal C, Spiegel D. The mental health risk of mothers and children: the role of maternal HIV infection. J Early Adolesc. 2007;27(1):67–89. [Google Scholar]
- 25.Armistead L, Tannenbaum L, Forehand R, Morse E, Morse P. Disclosing HIV status: are mothers telling their children? J Pediatr Psychol. 2001;26(1):11–20. doi: 10.1093/jpepsy/26.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Cederbaum JA. The experience of sexual risk communication in African American families living with HIV. J Adolesc Res. 2011 doi: 10.1177/0743558411417864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MB, Lester P, Rotheram-Borus MJ. The relationship between adjustment of mothers with HIV and their adolescent daughters. Clin Child Psychol Psychiatry. 2002;7(1):71–84. [Google Scholar]
- 28.Mellins CA, Brackis-Cott E, Dolezal C, Meyer-Bahlburg HFL. Behavioral risk in early adolescents with HIV+ mothers. J Adolesc Health. 2005;36(4):342–51. doi: 10.1016/j.jadohealth.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Gurung RAR, Taylor SE, Kemeny M, Myers H. “HIV is not my biggest problem”: the impact of HIV and chronic burden on depression in women at risk for AIDS. J Soc Clin Psychol. 2004;23(4):490–511. [Google Scholar]
- 30.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–30. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 31.Morrison MF, Petitto JM, Ten Have T, et al. Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry. 2002;159(5):789–96. doi: 10.1176/appi.ajp.159.5.789. [DOI] [PubMed] [Google Scholar]
- 32.Richardson J, Barkan S, Cohen M, et al. Experience and covariates of depressive symptoms among a cohort of HIV infected women. Soc Work Health Care. 2001;32(4):93–111. doi: 10.1300/J010v32n04_05. [DOI] [PubMed] [Google Scholar]
- 33.Remien RH, Exner T, Kertzner RM, et al. Depressive symptomatology among HIV-positive women in the era of HAART: a stress and coping model. Am J Community Psychol. 2006;38(3–4):275–85. doi: 10.1007/s10464-006-9083-y. [DOI] [PubMed] [Google Scholar]
- 34.Yi MS, Mrus JM, Wade TJ, et al. Religion, spirituality, and depressive symptoms in patients with HIV/AIDS. J Gen Intern Med. 2006;21(5):S21–7. doi: 10.1111/j.1525-1497.2006.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy DA, Marelich WD, Dello Stritto ME, Swendeman D, Witkin A. Mothers living with HIV/AIDS: mental, physical, and family functioning. Aids Care. 2002;14(5):633–44. doi: 10.1080/0954012021000005461. [DOI] [PubMed] [Google Scholar]
- 36.Forehand R, Jones DJ, Kotchick BA, et al. Noninfected children of HIV-infected mothers: a 4-year longitudinal study of child psychosocial adjustment and parenting. Behav Ther. 2002;33(4):579–600. [Google Scholar]
- 37.Lester P, Rotheram-Borus MJ, Lee SJ, et al. Rates and predictors of anxiety and depressive disorders in adolescents of parents with HIV. Vulnerable Child Youth Stud. 2006;1(1):81–101. [Google Scholar]
- 38.Pilowsky DJ, Zybert PA, Hsieh PW, Vlahov D, Susser E. Children of HIV-positive drug-using parents. J Am Acad Child Adolesc Psychiatry. 2003;42(8):950–6. doi: 10.1097/01.CHI.0000046888.27264.17. [DOI] [PubMed] [Google Scholar]
- 39.O’Sullivan LF, Dolezal C, Brackis-Cott E, Traeger L, Mellins CA. Communication about HIV and risk behaviors among mothers living with HIV and their early adolescent children. J Early Adolesc. 2005;25(2):148–67. [Google Scholar]
- 40.Corona R, Cowgill BO, Bogart LM, et al. Brief report: a qualitative analysis of discussions about HIV in families of parents with HIV. J Pediatr Psychol. 2009;34(6):677–80. doi: 10.1093/jpepsy/jsn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marhefka SL, Mellins CA, Brackis-Cott E, Dolezal C, Ehrhardt AA. Perceptions of adolescents’ sexual behavior among mothers living with and without HIV: does dyadic sex communication matter? Arch Sex Behav. 2009;38(5):788–801. doi: 10.1007/s10508-007-9284-y. [DOI] [PubMed] [Google Scholar]
- 42.Ajzen I. Perceived behavioral control, self-efficacy, locus of control, and the theory of planned behavior. J Appl Soc Psychol. 2002;32(4):665–83. [Google Scholar]
- 43.Ajzen I. From intentions to actions: a theory of planned behavior. In: Kuhl J, Beckmann J, editors. Action control: from cognition to behavior. Berlin: Springer; 1985. pp. 11–39. [Google Scholar]
- 44.Madden TJ, Ellen PS, Ajzen I. A comparison of the theory of planned behavior and the theory of reasoned action. Pers Soc Psychol Bull. 1992;18(1):3–9. [Google Scholar]
- 45.Hutchinson MK, Wood EB. Reconceptualizing adolescent sexual risk in a parent-based expansion of the theory of planned behavior. J Nurs Scholarsh. 2007;39(2):141–6. doi: 10.1111/j.1547-5069.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- 46.Jaccard J, Dittus PJ, Gordon VV. Parent-teen communication about premarital sex: factors associated with the extent of communication. J Adolesc Res. 2000;15(2):187–208. [Google Scholar]
- 47.Jaccard J, Dittus PJ, Gordon VV. Parent–adolescent congruency in reports of adolescent sexual behavior and in communications about sexual behavior. Child Dev. 1998;69(1):247–61. [PubMed] [Google Scholar]
- 48.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–62. [PubMed] [Google Scholar]
- 49.Wahler HJ. The physical symptoms inventory: measuring levels of somatic complaining behavior. J Clin Psychol. 1968;24(2):207–11. doi: 10.1002/1097-4679(196804)24:2<207::aid-jclp2270240223>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 50.Little TD, Cunningham WA, Shahar G, Widaman KF. To parcel or not to parcel: exploring the question, weighing the merits. Struct Equ Model. 2002;9(2):151–73. [Google Scholar]
- 51.Cheung MWL. Comparison of methods of handling missing time-invariant covariates in latent growth models under the assumption of missing completely at random. Organ Res Methods. 2007;10(4):609–34. [Google Scholar]
- 52.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–46. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 53.Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88(3):588–606. [Google Scholar]
- 54.Bradley KL, Bagnell AL, Brannen CL. Factorial validity of the center for epidemiological studies depression 10 in adolescents. Issues Ment Health Nurs. 2010;31(6):408–12. doi: 10.3109/01612840903484105. [DOI] [PubMed] [Google Scholar]
- 55.Chou CP, Bentler PM. Model modification in covariance structure modeling: a comparison among likelihood ratio, lagrange multiplier, and Wald tests. Multivar Behav Res. 1990;25(1):115–36. doi: 10.1207/s15327906mbr2501_13. [DOI] [PubMed] [Google Scholar]
- 56.Cederbaum JA, Marcus SC, Hutchinson MK. The influence of knowing someone with AIDS on youth HIV sexual risk behaviors. J HIV AIDS Prev Child Youth. 2008;8(2):31–44. [Google Scholar]
- 57.Palekar R, Pettifor A, Behets F, MacPhail C. Association between knowing someone who died of AIDS and behavior change among South African youth. AIDS Behav. 2008;12(6):903–12. doi: 10.1007/s10461-007-9325-5. [DOI] [PubMed] [Google Scholar]
- 58.Deptula DP, Henry DB, Shoeny ME, Slavick JT. Adolescent sexual behavior and attitudes: a costs and benefits approach. J Adolesc Health. 2006;38(1):35–43. doi: 10.1016/j.jadohealth.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 59.Markham CM, Tortolero SR, Escobar-Chaves SL, Parcel GS, Harrist R, Addy RC. Family connectedness and sexual risk-taking among urban youth attending alternative high schools. Perspect Sex Reprod Health. 2003;35(4):174–9. doi: 10.1363/psrh.35.174.03. [DOI] [PubMed] [Google Scholar]
- 60.Lester P, Peterson K, Reeves J, et al. The long war and parental combat deployment: effects on military children and at-home spouses. J Am Acad Child Adolesc Psychiatry. 2010;49(4):310–20. [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy DA, Marelich WD, Herbeck DM, Payne DL. Family routines and parental monitoring as protective factors among early and middle adolescents affected by maternal HIV/AIDS. Child Dev. 2009;80(6):1676–91. doi: 10.1111/j.1467-8624.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice-Hall; 1986. [Google Scholar]
- 63.Adhikari AB, Cederbaum JA. Maternal and peer influences on sexual intention among urban African American and Hispanic female adolescents. Under review and unpublished data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55(RR14):1–17. [PubMed] [Google Scholar]
- 65.Institute of Medicine. [Accessed 15 July 2011];Clinical preventive services for women: closing the gaps. http://www.iom.edu/preventiveserviceswomen.