Summary
Premotor circuits help generate complex behaviors, including those learned by imitation. Premotor circuits also can be activated during observation of another animal’s behavior, leading to speculation that they also participate in sensory learning important to imitation. Here we tested this idea by focally manipulating the brain activity of juvenile zebra finches, which learn to sing by memorizing and vocally copying the song of an adult tutor. Tutor song-contingent optogenetic or electrical disruption of neural activity in the pupil’s song premotor nucleus HVC prevented song copying, indicating that a premotor structure important to the temporal control of birdsong also helps encode the tutor song. In vivo multiphoton imaging and neural manipulations delineated a pathway and candidate synaptic mechanism through which tutor song information is encoded by premotor circuits. These findings provide evidence that premotor circuits help to encode sensory information about the behavioral model prior to shaping and executing imitative behaviors.
The cultural transmission of behavior involves observation of a behavioral model followed by imitation of the observed behavior. How the brain encodes the formative sensory experience provided by the behavioral model remains poorly understood. Although sensory structures are undoubtedly activated during observation of the model, premotor structures that play a role in generating imitative behaviors also can be activated during observation of another animal’s behavior1–5. This has led to speculation that premotor circuits may also help to encode sensory information about the model that is important to subsequent behavioral imitation6–9. Birdsong is a culturally transmitted vocal behavior with strong parallels to human speech learning, including obligatory auditory experience of a vocal model during a juvenile sensitive period followed by a phase of vocal copying10–13. Juvenile male zebra finches first listen to and memorize the song of an adult male tutor during a sensory learning phase (Fig. 1a; ~30–60 days post-hatching (dph)), then engage in vocal practice to emulate this memorized song model during a partially overlapping and more prolonged phase of sensorimotor learning (~45–90 dph)11. Moreover, the male zebra finch’s brain contains well described auditory and song motor pathways that are thought to be critical to these two phases of learning (Fig. 1b)13,14. Nonetheless, how experience of the tutor song is initially encoded in the juvenile’s brain and how this information interacts with song motor circuits to guide song development remain unclear.
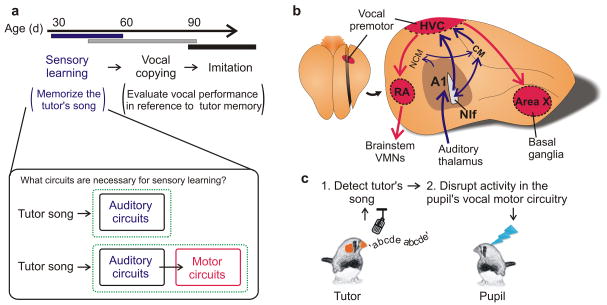
Figure 1. Testing the role of premotor circuits in sensory learning in songbirds.
a, Song learning (upper panel) in juvenile male zebra finches comprises a sensory learning phase, during which the pupil memorizes the song of a tutor, and a longer sensorimotor learning phase, during which the pupil uses auditory feedback to match its song to the memorized model. The brain regions important to sensory learning could be restricted to auditory circuits or also require the participation of motor circuits. b, Dorsal view of the zebra finch brain (left panel) and a parasagittal view through the medial forebrain (right panel) showing song premotor circuitry (red), including HVC, and auditory circuitry (blue). A1, primary auditory regions (Field L); CM, caudal mesopallium; A2–3, secondary and tertiary auditory regions; Area X, striatal component of the song system; HVC (used here as a proper name); NIf, nucleus interface of the nidopallium; RA, robust nucleus of the arcopallium; VMNs, vocal motor neurons. c, Schematic of tutor song-contingent disruption of neural activity in the pupil’s brain.
One possibility is that the auditory memory of the tutor song is encoded in forebrain structures analogous to secondary and tertiary auditory cortices of mammals (Fig. 1b). In support of this idea, vocal imitation is impaired after pharmacological manipulations of the secondary auditory regions of juvenile zebra finches during tutoring15, and immediate early gene and electrophysiological studies in adult zebra finches suggest that neurons in these regions could encode a long-lasting representation of the tutor song16–19. However, these findings do not address whether encoding of the tutor song also requires activity in downstream structures, including motor structures that directly control singing (Fig. 1b). Indeed, secondary auditory regions provide direct and indirect input to the telencephalic nucleus HVC20,21, a premotor structure that is essential for song generation22, and where neurons encode precise timing information for song patterning and respond to auditory presentation of tutor song23–25. Moreover, exposing a juvenile zebra finch to tutor song can trigger rapid structural and functional changes to synapses in its HVC that correlate with the quality of subsequent song imitation26. The finding that tutor song exposure can rapidly alter the HVC network suggests a possible role for HVC in the encoding of tutor song experience.
One challenge to testing this idea is that juvenile zebra finches often interleave periods of singing and other forms of vocal activity with periods of listening to a tutor song. Consequently, while pharmacological manipulations either upstream or downstream of HVC can affect the quality of song copying15,27, it is unclear whether these effects are due to interference with vocal premotor activity, auditory activity evoked by the tutor song, or auditory feedback activity evoked by the pupil’s own singing. To examine whether HVC plays a critical role in encoding experience of the tutor song, we sought a method that would allow us to disrupt HVC activity only when the pupil listened to his tutor’s song, but not at other times, including periods of vocal rehearsal (Fig. 1c). The transgenic expression of light-activated cation channels (i.e., channelrhodopsins) provides a means for precise spatiotemporal control of neural activity, without the potential confound of activating fibers of passage that can accompany electrical stimulation or the typically prolonged (i.e., minutes to hours) modulation of neural activity observed with pharmacological methods 28–31.
Premotor circuits are essential to sensory learning
We found that viral-mediated expression of humanized channelrhodopsin-2 (hChR2 expressed using scAAV2.9-hChR2-YFP or HSV1-hChR2\Wcm) could be used in combination with light pulses to robustly alter HVC neuronal activity (Fig. 2a–c). Extracellular recordings made in anesthetized juvenile male zebra finches expressing hChR2 revealed that brief (50–500ms) pulses of laser light (473 nm) applied through a fiber optic cable could alter activity across the mediolateral and rostrocaudal extent of HVC (Fig. 1c; n = 13 birds, 26 hemispheres). Importantly, light-evoked responses were only detected in the dorsal aspect of HVC. Extracellular recordings in anesthetized birds confirmed that optogenetic activation of HVC did not evoke antidromic activity in auditory regions presynaptic to HVC (i.e., NIf or CM, see Figure 1B; 0/18 sites in n = 3 birds), and similarly did not activate neurons more ventrally in the HVC “shelf,” a distinct region that may play a role in auditory processing32,33 (in 25/26 hemispheres, light-evoked responses were only detected in the first ~250μm from the surface of the brain; in the 26th hemisphere, responses were detected up to a depth of ~350μm). Illuminating the dorsal surface of HVC strongly excited neurons at some recording sites, suppressed spontaneous activity at other sites, or elicited more prolonged and complex responses consisting of both suppression and excitation (Supplementary Fig. 1). These findings suggest that viral-mediated expression of ChR2 coupled with laser illumination can modulate the activity of both excitatory and inhibitory neurons that populate the HVC microcircuit34,35, an idea that we confirmed using intracellular recordings from physiologically-identified HVC neurons in brain slices prepared from male zebra finches previously injected in HVC with AAV2.9-hChR2-YFP (Supplementary Fig. 2). Therefore, a virally mediated optogenetic approach is well suited to disrupt HVC network dynamics in juvenile birds learning to sing.
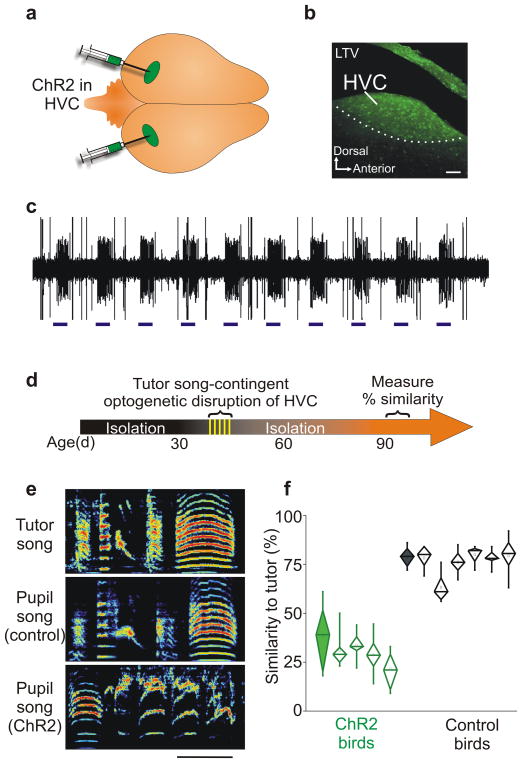
Figure 2. Optogenetic disruption of neural activity in the pupil’s HVC during tutoring impairs copying.
a, Dorsal view of the finch brain showing bilateral viral delivery of hChR2-YFP to the song nucleus HVC. b, Parasagittal section through HVC showing neuronal expression of hChR2-YFP immunoreacted with anti-GFP, 11 days after scAAV-hChR2-YFP injection into the same region; scale bar = 100μm; LTV, lateral telencephalic ventricle. c, In vivo extracellular recording of light-evoked action potentials (473nm, 500ms, 10 trials) in the HVC of a juvenile zebra finch injected with HSV-hChR2. d, Sketch of the experimental timeline in which activity in the pupil’s HVC is optogenetically disrupted while the tutor is singing, but not at other times. e, Sonograms of a tutor’s song and the adult songs of two of his pupils, including a control and one that received optogenetic activation of HVC during tutoring (ChR2); scale bar = 200ms; ordinate = 0 – 9 kHz. f, Optogenetic disruption of a juvenile finch’s HVC only when its tutor is singing disrupts subsequent copying of the tutor’s song (green-experimental; black-controls; p = 1.7 × 10−6; green filled diamond–average for birds raised in isolation from a tutor (n = 3 birds); black filled diamond-average for birds raised with free access to the same tutor used for optogenetic experiments (n = 3 birds); diamond plot whiskers denote 10–90% range of similarity scores for each bird; learning outcomes measured in adulthood).
To selectively disrupt HVC activity during tutoring, software36 was used to detect recognizable features of the tutor’s song and directly trigger optogenetic stimulation of neurons in the pupil’s HVC (Supplementary Movie 1). Functional expression of hChR2 in the left and right HVC of tutor-naïve juvenile zebra finches was assessed with in vivo extracellular recordings and illumination over the recording site prior to implanting optical fibers (200μm diameter) immediately dorsal to each HVC. The morning following implantation, the optical fibers were connected via an optical commutator to a 473 nm laser, and an adult male tutor was introduced to the holding cage with the implanted juvenile. Beginning 43–53dph, juveniles were exposed to their tutors for 2h per day for five consecutive days, and then raised in isolation to adulthood (Fig. 2d; adulthood > 90 dph). During tutoring, 200 ms (n = 2 birds) or 500 ms (n = 2 birds) long laser pulses were triggered on features of the tutor song (hit rate > 80%). Juveniles subjected to such tutor song-contingent optogenetic disruption of HVC activity developed adult songs that bore little resemblance to the song of their tutor (Fig. 2e–f; n = 4 birds, all exposed to the same tutor). In fact, the adult songs of ChR2-birds and untutored birds were equally dissimilar to the tutor song (mean similarity to the tutor song: ChR2 = 28.7%, isolate = 37.6% (n = 3 birds); two sample t (5) = 1.0, p = 0.35). In contrast, birds in four different control conditions all copied significantly more from their tutors (n = 6; two sample t (8) = 2.3, p = 1.7 × 10−6, power (1-β) = 1). These control conditions included: (i) juveniles subjected to the same temporal pattern of optogenetic stimulation in HVC, but immediately after the removal of the tutor (see Methods); (ii) juveniles subjected to tutor song-contingent optical stimulation of HVC following injection with AAV virus expressing eGFP into HVC (Fig. 2e–f; n=2); (iii) a juvenile expressing HSV-ChR2 in HVC, and subjected to optical stimulation in the primary auditory forebrain; (iv) a juvenile expressing HSV-ChR2 in HVC without optical activation. Indeed, these control birds and juvenile birds raised with unlimited access to the same tutor displayed similar copying (mean similarity to tutor song: control birds = 75.8%, n = 6; unlimited access birds = 77.7.%, n = 3). Post hoc analysis of similarity scores indicated that the adult songs of birds subjected to tutor song-contingent optogenetic stimulation of HVC and the birds from the four control groups fell into highly non-overlapping distributions (two sample t (8) = 2.3, p = 1.7 × 10−6, power (1-β) = 1). These observations indicate that the pattern of neural activity in the pupil’s HVC during exposure to the tutor’s song is necessary for accurate copying of that song.
HVC helps encode tutor experience with temporal precision
Adult zebra finches sing a highly stereotyped “motif” comprising a fixed sequence of several spectrally distinct syllables whose temporal features are controlled with millisecond precision11,24. Various studies in singing birds suggest that HVC precisely encodes the temporal features of song23,24,37,38, raising the possibility that the HVC network also helps to encode tutor song experience in a temporally precise fashion. Testing this idea requires a method of altering HVC activity during tutoring on the time scale of individual syllables (~100 msec). Optogenetic modulation of HVC activity can lag behind light onset and persist up to several hundred milliseconds following light offset and hence lacks the required temporal specificity (Supplementary Fig. 1, see rasters in middle and bottom panels, e.g.).
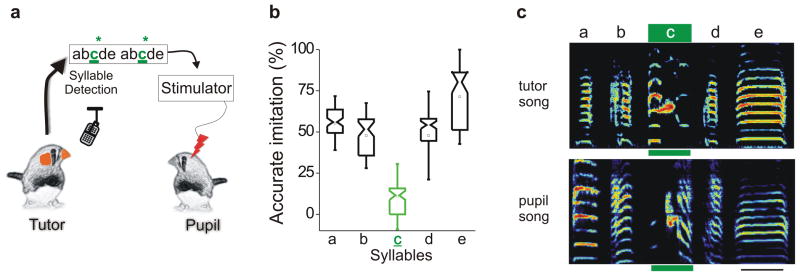
To attempt to increase the temporal specificity of our perturbation, we implanted monopolar platinum stimulating electrodes (0.1M ) bilaterally in the HVC of tutor-naïve juveniles, and used software36 to target electrical stimulation of HVC during the utterance of a specific syllable in the tutor’s song motif (Fig. 3a). Beginning at 43–53 dph, juveniles were subjected to tutor song-contingent microstimulation (20uA per HVC, biphasic pulses at 170HZ for 200ms) for 4h per day for five consecutive days and then raised in isolation to adulthood (> 90 dph). These birds produced poor copies of the tutor song syllable paired with microstimulation, even though they accurately copied syllables that preceded and followed the syllable targeted with microstimulation (Fig. 3b, c; Supplementary Fig. 3; ANOVA: F(4,14) = 7.508, p = 0.001; n = 4 birds, all of which were exposed to the same tutor and received microstimulation paired with the tutor’s syllable ‘c’). Together with our optogenetic manipulations, these results suggest that the premotor structure important to the precise temporal control of birdsong also plays an observational role during sensory learning, helping to encode auditory experience of the tutor song in a temporally specific manner.
Figure 3. Tutor song syllable-triggered microstimulation of HVC disrupts copying of the targeted syllable.
a, Sketch of the experimental design in which the pupil’s HVC is microstimulated (20μA per HVC, biphasic pulses, 300 μs each phase at 170Hz for 200ms) while the tutor is singing syllable ‘c’. b, Pupils fail to imitate the syllable paired with HVC microstimulation (syllable c; F(4,14) = 7.508, P = 0.001; n = 4 birds; notched box plot whiskers = 1.5 standard deviations). c, Sonograms of the tutor’s song and the adult song of one his pupils that was microstimulated in HVC when the tutor sang syllable ‘c’. Green bar under syllable ‘c’ and scale bar at lower right = 130ms; ordinate = 0 – 9 kHz.
A candidate mechanism for encoding tutor experience
A remaining question is how the HVC network helps to encode tutor song experience. Recent in vivo multiphoton imaging experiments performed in juvenile zebra finches show that tutoring can trigger the rapid enlargement of previously stable dendritic spines in HVC26, a structural correlate of synaptic strengthening that in other systems has been shown to depend on the activation of postsynaptic N-methyl D-aspartate (NMDA) receptors39,40. To test whether the enlargement of HVC dendritic spines seen following tutoring is NMDA receptor-dependent, we combined in vivo multiphoton imaging of HVC dendritic spines with acute pharmacological blockade of NMDA receptors on HVC neurons (Fig. 4a). A lentivirus expressing enhanced green fluorescent protein (eGFP) was used to label HVC neurons and their dendritic spines, cranial windowing provided optical access to HVC under a multiphoton microscope, and retrograde tracing from HVC’s efferent targets helped to visualize the borders of HVC.
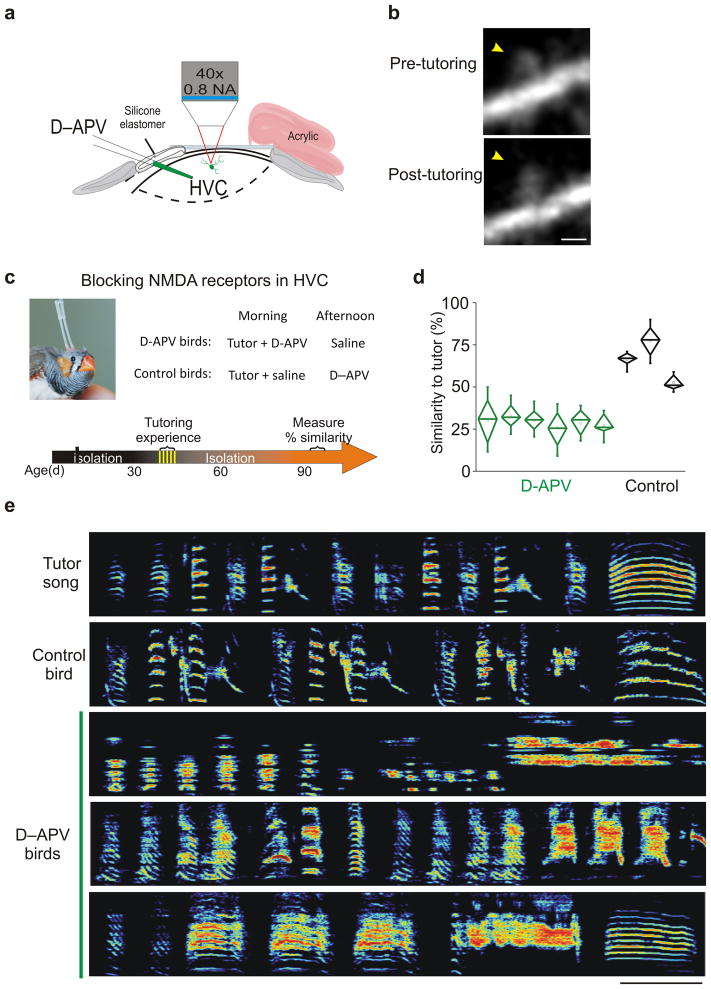
Figure 4. Blocking NMDA receptors in HVC during tutoring prevents spine enlargement and disrupts copying of the tutor song.
a, Schematic of in vivo multiphoton imaging of dendritic spines in HVC and the pharmacological blockade of NMDA receptors achieved by injecting D-APV (25mM) into HVC immediately prior to tutoring. b, Example of a stable spine (yellow arrowheads) imaged in HVC before and after tutoring + D-APV; scale bar = 1μm. Spine size did not change when tutoring was preceded by infusion of D-APV (tutoring + D-APV: P = 0.30, 74 dendritic spines from 4 birds; tutoring alone: P = 0.001, 47 dendritic spines from 5 birds, tutoring alone data are provided from Roberts et al. (2010) 26). c, Schematic for reversibly blocking NMDA receptors in HVC during tutoring. Upper left: a zebra finch with reverse microdialysis probes bilaterally implanted in HVC. Upper right: treatment groups and tutoring schedule used in these experiments. Lower panel: the timeline of the experiments. d, Infusion of D-APV in HVC during tutoring sessions (green diamond plots), but not during periods of vocal practice (black diamond plots), prevents subsequent copying of the tutor song (P = 0.0001, diamond plot whiskers denote 10–90% range of similarity scores for each bird). d, Sonograms of a tutor’s song and the adult songs of 4 of his pupils in which APV was infused in HVC during (D-APV birds) or immediately after each of five morning tutoring sessions (control bird); scale bar = 200ms; ordinate = 0 – 9 kHz.
To establish a baseline measurement of spine size, dendritic spines on GFP-expressing HVC neurons were imaged in tutor-naïve juvenile male zebra finches during their subjective nighttime (the first imaging session occurred between 43–53 dph). The following morning, the NMDA receptor antagonist D-APV ((2R)-amino-5-phosphonopentanoate (100nl, 25mM)) was pressure injected into HVC immediately prior to a single, brief (~1.5h) tutoring session. Neurons in these recently tutored birds were then re-imaged the following night to assess changes in the size of the HVC dendritic spines that persisted between the two nightly imaging sessions (i.e., stable spines). Spine size did not change when tutoring was preceded by infusion of D-APV (Fig. 4b; paired, two-sample t-test comparing relative spine brightness before and after tutoring, t (72) = 1.0, p = 0.3 (n=4 birds, 73 dendritic spines). This finding indicates that the tutoring-induced enlargement of dendritic spines in HVC depends on an NMDA receptor-dependent mechanism.
If spine enlargement in HVC helps to encode tutor song experience, then blocking NMDA receptors in HVC during tutoring should prevent accurate imitation of the tutor song. To test this prediction, we implanted reverse microdialysis probes bilaterally in HVC and infused D-APV during five consecutive 4h (9am–1pm) tutoring sessions, allowing us to reversibly block NMDA receptors in the HVC of juvenile zebra finches (Fig. 4c; n = 6 male juveniles, 43–53 dph, all of which were tutor-naive prior to the first tutoring session; tutor + APV; 25mM APV). Following the end of the morning tutoring session, the probes were flushed with saline, and the bird was isolated in a sound-attenuating chamber until the next morning. Following the last tutoring session (i.e., the afternoon of the 5th day of tutoring), these APV-treated animals were isolated from other birds and raised to adulthood (> 90 dph), when their songs were recorded and compared to their tutors’ songs. Juveniles treated with D-APV during tutoring sessions developed adult songs that bore little resemblance to those of their tutors, based on quantitative measurements of song similarity and other comparisons of their songs’ spectral and temporal features (Fig. 4d, e; Supplementary Fig. 4; mean similarity to the tutor songs = 29.1%, n = 6 birds). In contrast, juveniles that received saline in HVC during morning tutoring sessions and D-APV (25mM) in the afternoon (4h; 1–5 pm), when they were housed in isolation, copied significantly more of their tutors’ songs (Fig. 4c–f; mean similarity to the tutor songs = 65.3%, n = 3 birds; two sample t (7) = 7.4, p = 0.0001, power (1-β) = 0.999). Together, these findings promote an NMDA receptor-dependent process at HVC synapses as a candidate mechanism for encoding the tutor song experience.
A pathway that conveys tutor experience to HVC
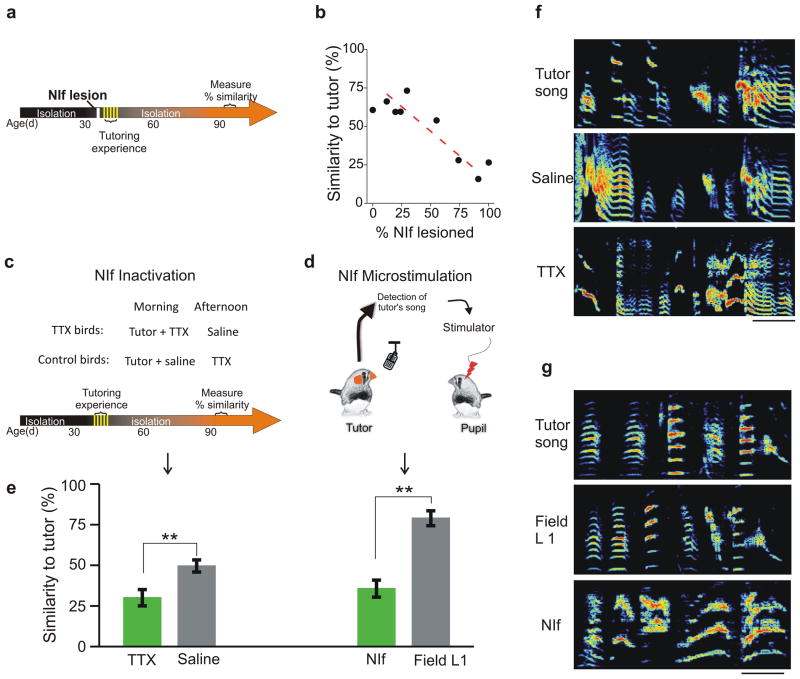
A remaining question is how tutor song-related information is conveyed to the premotor network in HVC. Although HVC receives input from several sources20,21,33,41, the telencephalic nucleus interface (NIf) has been identified as a major source of auditory input to HVC and is also the putative source of spontaneous bursting activity that is augmented in HVC immediately following tutoring26,42–45. Permanent lesions of NIf in adult zebra finches do not exert persistent effects on singing behavior42, nor do they interfere with auditory feedback-dependent vocal plasticity45. To test whether NIf is required for sensory learning, we made permanent bilateral lesions of NIf in tutor-naïve juvenile zebra finches one day prior to their initial exposure to a tutor (Fig. 5a; tutoring started between 36–47 dph). Juveniles were housed with their tutors for five consecutive days, and then raised in isolation to adulthood (> 90 dph). Lesions to NIf severely impaired tutor song imitation, and the lesion size was strongly correlated with the degree of impairment (Fig. 5b; Supplementary Fig. 5; n= 9 birds, R2 = 0.79). To test whether the effects of NIf lesions on imitation were indeed due to the disruption of input to HVC during tutoring, rather than possible secondary effects of NIf lesions, we reversibly inactivated NIf during tutoring in a separate set of birds. Reversibly inactivating NIf just prior to each of five consecutive daily tutoring sessions also severely disrupted subsequent tutor song imitation (Fig. 5c, e, f; Supplementary Fig. 6; n = 7 previously tutor-naïve juvenile birds received 14 nl TTX (50 μm) injected bilaterally into NIf prior to a 1.5 hr morning tutoring session, beginning 40–45 dph, followed by an afternoon injection of 14 nl saline). In contrast, juveniles subjected to a reversed TTX and saline treatment schedule ultimately produced better copies (Fig. 5c, e, f; two-sample t (10) = 2.9, p = 0.016, n = 5 control birds). These findings suggest that NIf plays a critical role in sensory learning by interacting with HVC during tutoring.
Figure 5. Tutor experience is conveyed to HVC from nucleus NIf.
a, Timeline for the NIf lesion experiments. b, Lesioning NIf prior to tutoring severely disrupts subsequent imitation of the tutor song (R2 = 0.79; n= 9 birds). Birds with >50% of NIf lesioned (n=4 birds) showed severe disruption in tutor song imitation compared with birds with <30% of NIf lesioned (n=5 birds; P = 0.001). See Supplementary Fig. 4 for sonograms of NIf lesioned birds. c, Schematic of NIf inactivation experiments. Upper panel shows the treatment groups and tutoring schedule used in these experiments. Lower panel shows the timeline for the NIf inactivation experiments. d, Sketch of the experimental design in which the pupil’s NIf or Field L1 was microstimulated (20uA per side at 76–170HZ for 200–400ms) while the tutor was singing.. e, Reversible inactivation of NIf (left columns, green bar; 14 nl of 50μM TTX) during but not immediately after tutoring sessions (left columns, gray bar) impairs subsequent copying (P = 0.016; tutor + TTX, n = 7 birds; tutor + saline, n = 5 birds; error bars = s.e.m). Tutor song-triggered microstimulation of NIf (right columns, green bar), but not Field L1 (right columns, gray bar), disrupts subsequent imitation of the tutor song (P = 0.0067, NIf = 3 birds, Field L1 = 4 birds; error bars = s.e.m). f, Sonograms of a tutor’s song and the adult songs of two of his pupils in which NIf was inactivated with TTX either during (TTX) or after (saline) morning tutoring sessions; scale bar = 100ms; ordinate = 0 – 9 kHz. g, Sonograms of a tutor’s song and the adult songs of two of his pupils in which tutor-triggered microstimulation was applied to either Field L1 or NIf during tutoring sessions; scale bar = 100ms; ordinate = 0 – 9 kHz.
To better delineate the timescale of this interaction, we applied tutor-song triggered electrical microstimulation methods to NIf or to an adjacent auditory region (i.e., Field L146) in juvenile zebra finches (Fig. 5d). Previously tutor-naïve juveniles (n = 6; 43–53dph on the first day of tutoring) were exposed to a live tutor for 4h per day for five consecutive days then raised in isolation to adulthood (> 90 dph). As adults, all of the NIf-stimulated birds produced poor copies of their tutors’ songs, whereas birds stimulated in the adjacent auditory region learned the song of their tutor (Fig. 5e, g; Supplementary Fig. 7; mean similarity of NIf stimulated birds to the tutor song = 35.6%; for L1-stimulated birds = 78.9%; two-sample t(5) = 2.5, p = 0.001; n = 3 NIf-stimulated birds, n = 4 Field L1-stimulated birds). Together, these findings point to NIf as a critical conduit for conveying auditory information to HVC when the tutor is singing.
Discussion
Here we used a novel combination of song-triggered optogenetic and focal electrical stimulation methods to manipulate the activity of vocal premotor neurons in juvenile zebra finches as they listened to the song of a tutor. These manipulations impaired the quality of song imitation, indicating that the pattern of neural activity in the vocal premotor circuitry during this formative auditory experience is critical to subsequent vocal motor learning. Furthermore, blocking NMDA receptors in HVC during tutoring blocked spine enlargement and also impaired vocal imitation of the tutor song, suggesting that an NMDA receptor-dependent strengthening of synapses in HVC is important to encoding tutor song experience underlying imitative learning. Along with the recent observation that tutoring rapidly stabilizes and strengthens synapses in HVC26, the current findings support the idea that synapses in HVC are sites where experience of the tutor song is encoded in the brain, and that this encoding depends on NIf, which supplies auditory input to HVC42,43. Although these findings do not exclude the involvement of other regions downstream of HVC in this sensory encoding process27, they do rule out a prevailing model in which auditory experience of the tutor song is first encoded in auditory regions upstream of HVC and only later used to guide changes in the vocal motor network during sensorimotor learning15,18,47.
By using optogenetic and electrical stimulation methods to disrupt activity in HVC only during the juvenile’s auditory experience of the tutor song, the current study delineates a role for the song motor system in sensory learning. An earlier study showed that blocking NMDA receptor in a song system nucleus downstream of HVC during tutoring also impaired the quality of song copying27, raising the possibility that the encoding of tutor song experience involves distributed activity in the song system, an idea that can be tested in the future with the tutor song contingent stimulation methods developed here. Furthermore, although a prior study demonstrated that pharmacological blockade of extracellular signal-regulated kinase activation in the secondary auditory telencephalic region NCM during tutoring disrupted song copying15, we found that tutor song contingent electrical stimulation in Field L1, an auditory region presynaptic to NCM, did not disrupt song learning. Although tutor song contingent stimulation methods will be necessary to better define the role that NCM plays in song learning, these contrasting findings may constrain the locus of tutor song encoding to levels of the auditory system above Field L and to the song motor system. Moreover, because HVC also provides input to secondary regions of the auditory telencephalon20, HVC may also transmit information to the auditory system20, such as a sensorimotor registration signal, that is important to song learning. Even in these distributed models, the current findings emphasize that HVC is a critical node for encoding information about the tutor song.
The current study also reveals that auditory experience of the tutor song interacts with the premotor network in a temporally precise fashion because microstimulation targeted to a single syllable in the tutor’s song disrupted copying of the targeted syllable but not adjacent syllables. Prior studies in singing birds show that HVC premotor neurons fire precise bursts of action potentials that are tightly linked to the temporal organization of song23,24, raising the possibility that the same neural machinery that controls the song’s temporal organization in adults also is employed to encode the temporal features of the song model early in juvenile life. In this view, auditory experience of the tutor song influences the functional organization of synaptic connections in the HVC premotor network and this functional synaptic organization ultimately shapes the temporal structure of the pupil’s song. In primates, sensory-evoked activity in premotor structures is speculated to facilitate imitation, including speech learning1,3,7–9,48,49. Our results extend this view, providing evidence that premotor circuits initially function in an observational mode to help store information about the behavioral model. Later in development, this information could help to instruct these same circuits when they operate to shape and execute the motor programs underlying behavioral imitation.
Materials & Methods
Juvenile male zebra finches, obtained from the Duke University or the Harvard University breeding facility, were isolated from adult male song tutors between 7–12 days post hatching (dph) and then exposed to a song tutor for five consecutive days starting between 40–53 dph. Prior to tutoring, juvenile male zebra finches were housed in nesting groups in sound attenuation chambers and cared for by 1–3 adult female zebra finches. Juvenile males were removed from the nesting groups and separately housed in sound attenuation chambers starting by 34–40 dph. Following five days of tutoring, the juvenile zebra finches were raised to adulthood (> 90 dph) in visual and acoustic isolation from other birds. Songs were recorded with microphones (Shure SM 93) pre-amplified and saved to a computer using Sound Analysis Pro (http://ofer.sci.ccny.cuny.edu/sound_analysis_pro) or with custom written software (Labview, National Instruments). Adult song of each bird was then compared to the song of its tutor to measure song imitation. We quantified the amount that juvenile birds copied from their tutor using the percentage similarity score for whole motif comparisons or the percentage local similarity score (% accuracy imitation) for syllable-level comparisons using Sound Analysis Pro (α = 0.05). Standard parametric and non-parametric statistical methods were used to calculate significant differences (α = 0.01) and retrospective power analysis was used to determine inferential power of our analyses (1-β > 0.95; see Table 1 for list of experimental manipulations and outcomes). Experimental procedures were conducted in accordance with the National Institutes of Health guidelines and were reviewed by the Duke University Medical Center Animal Care and Use Committee (IACUC) or the Harvard University IACUC.
Viral, tracer and ibotenic acid injections
Male zebra finches were anaesthetized using isoflurane inhalation (2%) and placed in a stereotaxic apparatus. Target sites in the brain were located using stereotaxic coordinates and multi-unit neural recordings. A glass pipette attached to a pressure injection unit (Drummond Nanoject II; Drummond Scientific, Bro Broomall, Pennsylvania, United States) was used to deliver virus or neural tracer to target brain regions. For behavioral optogenetic experiments in HVC we used a self-complementary AAV expressing hChR2 under the control of CMV promoter (scAAV2.9.CMV.hChR2.YFP, UNC Vector Core, custom prep) or a HSV1 expressing hChR2 (HSV1.hChR2\Wcm, BioVex). scAAV2.9.CMV.hChR2.YFP (600–700 nl) and HSV1.hChR2\Wcm (400 nl) injections were made into HVC 5–6 days before in vivo electrophysiological recordings and fiber optic cable implantation. For in vitro optogenetic experiments in HVC we used AAV2.9.CAG.hChR2-Venus or mCherry (Penn Vector Core) injected 40–60 days prior to cutting brain slices to drive expression of hChR2. For in vivo multiphoton imaging of dendritic spines in HVC we used a lentivirus (FRGW) expressing enhanced GFP under the control of the Rous sarcoma virus long terminal repeat50. Lentiviral injections (1 ul) were made 15–20 d before imaging and retrograde tracer injections were made into the two targets of HVC 5–7 d before imaging (Fast Blue (Polysciences Inc.) to Area X and Alexa Fluor 594 conjugated dextran amines (Invitrogen) to RA). For neurotoxic lesion of NIf, the location of NIf was verified electrophysiologically by recording antidromic responses to stimulation in nucleus HVC (bipolar stimulation electrodes, 200 μs pulses of ~500 uAmp at 1 Hz). 23 nl of 1% Ibotenic acid (#Asc-041, Ascent Scientific, Princeton, New Jersey, United States) dissolved in 0.1M NaOH was then injected bilaterally into NIf using a Nanoject II injector.
Tutor song-triggered optogenetics
One to two days prior to tutoring, isolate juvenile male zebra finches (41–51 dph) previously injected with a virus expressing hChR2 in HVC were anesthetized with isoflurane (2%) and multi-unit neural recordings were used to assess light-evoked optogenetic responses (473nm light; Ikecool, IKE-473-200-OP) in HVC. In a subset of these birds, multi-unit recordings from HVCs auditory afferents, NIf and CM were also combined with optical stimulation of HVC to examine if optogenetic stimulation of HVC was capable of antidromically exciting NIf and CM. Only birds exhibiting light evoked responses in at least three different recordings sites in each HVC were implanted with fiber optic cables (200μm diameter core, 0.37 NA; Thor Labs (BFL37)) and used as pupils in subsequent tutor song-triggered behavioral experiments. Fiber optic cable guide cannulae (PlasticsOne, C315GS-4-SP guide 26GA cut 2mm below pedestal) were implanted immediately dorsal to HVC. Fiber optic were connected to a diode-pumped solid-state 473nm laser (Ikecool, IKE-473-200-OP) via 1×2 fiber optic commutator (Doric Lenses, FRJ_1×2i_FC-2FC). Custom software36 was used to detect components of the tutor’s song and trigger optical stimulation (200–500ms, 5–8mW/mm2 per hemisphere) of HVC. Pupils were tutored for 2hr/day for five consecutive days then raised to adulthood in isolation.
A separate group of birds were used as optogenetic controls and subjected to one of the four following conditions. 1) Juvenile birds were tutored 2hr/day for five consecutive days. Immediately following each tutoring session, and out of earshot of the juvenile, an audio recording of the tutor’s singing behavior from that day’s tutoring session was played back to voice recognition software to trigger optogenetic stimulation of the pupil’s HVC. This approach ensured that the juveniles received a pattern and amount of optogenetic stimulation in HVC highly similar to the tutor song contingent stimulation group, except that the stimulation was not coincident with tutor song experience. 2) Juveniles were subjected to tutor song-contingent optical stimulation of HVC following injection with AAV virus expressing eGFP into HVC. 3) A juvenile was subjected to tutor song-contingent optical stimulation in the primary auditory forebrain following injection with HSV expressing hChR2 in HVC. 4) A juvenile was tutored following injection with HSV expressing hChR2 in HVC without optical activation.
In a blind post hoc analysis of learning outcomes, data from all experimental and control birds were pooled and were found to be bimodally distributed, constituting two non-overlapping groups. One group (n = 4) showed very low similarity to the tutor and the other group (n = 6) showed very high similarity to the tutor. All of the birds that received tutor song-contingent optogenetic stimulation of HVC fell into the population with very low similarity to the tutor song, whereas all of the birds that received any of our 4 control manipulations fell into a second population with very high similarity to the tutor song. A two-sample t-test was used to examine statistical differences between these two groups.
Tutor song-triggered microstimulation
One to two days prior to tutoring, isolate juvenile male zebra finches (41–51 dph) were anesthetized with isoflurane (2%), placed in a stereotaxic holder and multi-unit neural recordings used to identify target structures in the brain (HVC, NIf or Field L1; HVC was identified by its characteristic bursting activity, NIf was identified by antidromic stimulation from HVC, and Field L1 implants were placed anterior to NIf). Platinum monopolar electrodes (0.1M; MPI) were bilaterally implanted in HVC, NIf or Field L1 and secured in place with dental acrylic. A small grounding screw was then implanted over the cerebellum. Stimulating electrodes and the grounding screw were then wired to a custom built adapter and secured with additional dental acrylic. Custom software36 was used to detect specific acoustic features associated with a given syllable in the tutor’s song and to trigger electrical bilateral stimulation of HVC, NIf or Field L1 (200–400ms, 20μA/hemisphere, 73–170Hz biphasic pulses; A–M Systems isolated pulse stimulator model 2100). Pupils were tutored for 4hr/day for five consecutive days starting at 43–53 dph then raised to adulthood in isolation.
In vivo multiphoton imaging
In vivo multiphoton imaging of dendritic spines measurement of changes in dendritic spines on HVC neurons was conducted as previously described26, with additional modifications described below. Cranial windows were bilaterally implanted over the HVC of isolate juvenile male zebra finches (43–53 dph), previously injected with a lentivirus expressing GFP in HVC and retrograde tracers in the targets of HVC, RA and Area X. A 200μm gap between the custom cut glass coverslip covering HVC and skull at the caudal border of HVC was covered with Kwik-Sil (MPI) to allow targeted infusion of D-APV under the coverslip with a glass pipette. Juvenile zebra finches used for these experiments were maintained in a reversed day-night cycle, and images of HVC neurons and their dendritic spines were obtained during the animal’s subjective nighttime with a multiphoton microscope (Zeiss LSM 510). Immediately before the beginning of the bird’s subjective daytime, the subject was briefly anesthetized with isoflurane (2%) and a D-APV (25mM) filled glass pipette attached to a pressure injection unit (Drummond, Nanoject II) was advanced at 45° to the pial surface to the center of HVC. 100 nl of D-APV was injected in the center of HVC bilaterally. Immediately following recovery from anesthesia (~5min) a tutor was placed with the isolate bird for 1.5 hrs. The following evening the same neurons, stretches of dendrite and dendritic spines were re-imaged under the multiphoton microscope.
Reverse microdialysis
Custom designed reverse microdialysis probes (K. Hamaguchi) with a 200μm diameter dialysis membrane of which 200μm was exposed (Spectra/Por; 13kD mw cutoff) were bilaterally implanted into HVC and secured to the skull with dental acrylic one to two days prior to tutoring. Pupils received morning tutoring sessions, 4hr/day for five consecutive, days starting at 43–53 dph. D-APV (25mM) was dialyzed into HVC during morning tutoring sessions and saline was dialyzed into HVC in the afternoon when the birds were not with their tutor. Control birds received the opposite treatment: saline was dialyzed into HVC during morning tutoring sessions and D-APV was dialyzed into HVC in the afternoon when the birds were not with their tutor. Following five days of tutor exposure, birds were raised to adulthood in isolation from other finches in sound attenuating chambers. Post mortem histological analysis was used to confirm placement of the probe.
Transient Inactivation
Three to four days prior to the tutoring experiments (age range: 38–42 dph), birds were anesthetized, and small holes made in the skull above NIf bilaterally. A head-holder was implanted on the anterior part of the skull as described previously51. The craniotomies were covered with Kwik-Kast (World Precision Instruments, Sarasota, Florida, United States).
In the morning and afternoon of experimental days, birds were placed in a foam restraint and the head holder attached to the stereotaxic apparatus for approximately 10 minutes. Kwik-Kast was removed from the craniotomies, and TTX (14 nl, 50 μM, # T5651, Sigma, St. Louis, Missouri, United States) or PBS was injected bilaterally into Nif using a Nanoject II. Based on previous studies51, we estimate the inactivation radius resulting from the TTX injections to be <200 μm. Visual inspection of the fluid level in the injection pipette confirmed successful drug injection. Dye-conjugated dextrans (#D-22912 or #D-22910, Molecular Probes, Eugene, Oregon, United States) were co-injected with TTX for post-hoc verification of the injection site.
In vitro intracellular recordings from HVC neurons
40–60days post virus-ChR2 injection, birds were anaesthetized with isoflurane (5%) and decapitated. The brain was quickly removed and moved into a solution of ice cold artificial CSF (aCSF). 400μm sagittal brain slices including HVC were cut using a vibratome (Leica, VT 1000s). Borosilicate glass electrodes (80–200MΩ) filled with 2M potassium acetate and 5% Neurobiotin were used to obtain sharp intracellular recordings. Membrane potential recordings were amplified with an Axoclamp 2B amplifier (Axon Instruments) in bridge mode, low-pass filtered at 1–3 kHz, and digitized at 10 kHz. Data were collected using a data acquisition board (National Instruments) controlled by custom Labview software. The different HVC cell types (HVCX, HVCRA, and HVCINT) were identified by their response to families of current pulses52 (−600 to +1000 pA, 500 msec duration). Short collimated light pulses (3–100ms duration) at 473nm (3–5mW/mm2;) were delivered to HVC by a 200μm diameter fiber optic coupled to a diode-pumped solid-state laser (model BL473T3-150 Shanghai Laser and Optics). Electrophysiological data were analyzed offline using custom written MATLAB software (K. Hamaguchi and M. Murugan).
Histology
Birds were anesthetized with 0.08 ml Natriumpentobarbital (Nembutal, i.m. injection) and subsequently perfused with phosphate-buffered saline (PBS), followed by fixation with 4% paraformaldehyde in PBS (PFA). Brains were dissected out and post-fixed in 4% PFA at 4° C overnight. Parasagittal sections (50 μm) were cut on a Vibratome (Leica). Tissue sections were mounted and stained with cresyl violet to reconstruct the location of implanted dialysis probes, stimulating electrodes or fiber optic cables. Injection sites for the TTX inactivation experiments were verified in alternate brain slices by fluorescence microscopy (Fig. S 5). The remaining slices were stained with cresyl violet and the location of NIf confirmed based on nucleus shape and size, and its orientation between the anatomical landmarks Lamina Mesopallialis (LaM) and Lamina pallio-subpallialis (LPS)53 (Fig. S 5a). Photomicrographs of fluorescent injection sites were superimposed on their alternate cresyl violet sections using Adobe Photoshop to determine the location of the injection (Fig. S 5b–e). We measured the distance between the center of the injection and the center of NIf with ImageJ (NIH) software (Left hemisphere: 194 μm ± 38 (SEM), right hemisphere: 189 μm ± 36 (SEM); see Fig. S 5e). Outlier analysis using z-scores confirmed that the centers of the injections relative to the center of NIf were not significantly different from the group mean in any of the birds for both hemispheres, and thus all birds were included for further statistical analysis. For lesions, location and size were determined by outlining the area of visually damaged tissue (based on loss of neurons and gliosis6) on photomicrographs of cresyl violet stained sections with Spot Basic image capture software. Lesion size was expressed as percentage of intact NIf size53. All analyses were performed blind as to experimental treatment.
Supplementary Material
Supplementary Movie 1. Tutor song-triggered optogenetic disruption of neural activity in the pupils’ HVC. This video displays a juvenile zebra finch being tutored for the first time, using tutor song-contingent optogenetic methods to disrupt neural activity in HVC. The song of the tutor (bird on the right in this video) is detected by software36 and used to drive brief pulses of blue light delivered to the HVC of the pupil (bird on left in this video) via chronically implanted fiber optic cables.
Supplementary Table 1. Summary of experimental groups, sample sizes and results. Table lists all the behavioral experiments conducted in this research, the sample size for each group and the average result of this manipulation (% similarity score to tutor song).
Supplementary Figure 1. In vivo extracellular recordings in the HVC of anesthetized finches reveal that optogenetic stimulation can evoke a variety of neural responses.Raster plots depict action potentials in HVC in response to 10 consecutive trials of light illumination applied to the dorsal surface of HVC. These recordings were obtained from three different juvenile zebra finches that had been injected with scAAV-ChR2 five to ten days prior to the recording session.
Supplementary Figure 2. Intracellular recordings in brain slices further characterize viral mediated optogenetic responses in HVC neurons. a, Confocal Images of an hChR2-expressing HVCRA neuron recorded in vitro. The green channel (left) shows hChR2-EYFP immunoreacted with an anti-GFP antibody; the red channel (middle) shows intracellular staining of the impaled cell with neurobiotin, which was visualized with streptavidin-conjugated alexafluor 594. The right panel is the merged view showing that the impaled cell is ChR2-positive. Scale bar = 20 um. In vitro brain slices were prepared from a bird injected with AAV2/9-hChR2-EYFP. b, left column: Putative direct excitatory responses to 473 nm fiber optic illumination could be detected in neurons expressing hChR2. HVC neurons that projected to either the song motor nucleus RA or the striatal Area X (HVCRA or HVCX cells) were identified by their membrane potential responses to positive and negative current pulses injected through the recording electrode (data not shown). The HVCRA cell in the top panel is the cell visualized in panel a). Right column: Illumination could also evoke excitatory and inhibitory synaptic responses in physiologically identified HVC projection neurons, presumably reflecting the direct activation of other PNs, which are a source of local excitatory input to HVC PNs, and the direct and indirect activation of local interneurons, which are a source of inhibitory input onto HVC PNs35 (middle and right columns; total of responsive cells recorded: HVCRA = 9 neurons, HVCX = 17 neurons, HVCINT = 2 cells (not shown), obtained from 7 hemispheres prepared from 4 birds). Panel insets show timing differences between putative direct and synaptic responses evoked by HVC illumination.
Supplementary Figure 3. Tutor song syllable-triggered microstimulation of HVC disrupts copying of the targeted syllable without changing the overall length of the learned motif. Sonograms of the tutor’s song and the adult song of four of his pupils that were microstimulated in HVC when the tutor sang syllable ‘c’. Green bar marks syllable ‘c’ in all sonograms. The adult motifs of the pupils were approximately the same length as their tutor’s motif. Scale bar at upper right = 130ms; ordinate = 0 – 9 kHz.
Supplementary Figure 4. Infusing D-APV in HVC during tutoring results in adult birds with syllables that are noisier, longer, and higher frequency than syllables in tutor songs. Cumulative probability plots showing the distribution of spectral and temporal features of song syllables from six D-APV treated birds (green) and from three of the tutors used in this study (black). a, Syllables in the adult songs of birds treated with D-APV during tutoring are noisier (lower entropy) than the syllables of their tutors (Kolmogorov-Smirnov test ( KS test), p = 1.21 × 10−6; D-APV birds = 3,084 syllables; tutors = 1,034 syllables). b, Syllables in the adult songs of birds treated with D-APV during tutoring are longer than the syllables of their tutors (KS test, p = 1.01 × 1017; D-APV birds = 2,024 syllables; tutors = 1,285 syllables). c, Syllables in the adult songs of birds treated with D-APV during tutoring are higher in frequency than the syllables of their tutors (KS test, p = 4.13 × 10−75; D-APV birds = 3,084 syllables; tutors = 1,034 syllables).
Supplementary Figure 5. Juvenile finches with larger NIf lesions develop poorer copies of their tutor’s song. Sonograms of a tutor’s song and the adult songs of two of his pupils in which 12% or 91% of NIf was lesioned prior to tutoring; scale bar = 100ms; ordinate = 0 – 9 kHz.
Supplementary Figure 6. Histology confirming the injection sites for the NIf inactivation experiments. a, Photomicrograph of a cresyl violet-stained parasagittal section with anatomical boundaries of NIf indicated with arrow heads. b, Fluorescent photomicrograph superimposed on the alternate section shown in a (NIf indicated with arrow heads). c, d, Higher magnification photomicrographs of alternate cresyl-violet and fluorescent sections centered on NIf. e, Composite of cresyl-violet stained section and alternate fluorescence section. The origin of the first, smaller circle is in the center of NIf, and the diameter of the circle spans the maximum width of the nucleus along its anterior-posterior axis. The larger circle indicates the estimated spread of TTX, assuming an inactivation radius of ~200 μm. Scale bar indicates 200 μm. f, g. Combined data for all birds. Location of NIf and estimated spread of TTX was determined for all birds and hemispheres (left hemisphere: f, right hemisphere: g) based on superimposed fluorescence and cresyl violet-stained photomicrographs as shown in e. Dashed ellipse: boundaries of NIf. Solid circles: estimated spread of TTX; different colors indicate different animals.
Supplementary Figure 7. Location of stimulating electrodes in an experiment where tutor song-contingent microstimulation was applied in Field L1. Photomicropgraphs of brain sections showing NIf (red outline) and Field L1 (white arrows) from a juvenile finch subjected to tutor song-contingent microstimulation in which the stimulating electrode tips (blue arrowheads) were implanted in a region of Field L1 adjacent to NIf; scale bar = 250μm.
Acknowledgments
The authors thank H. Greenside, S. Lisberger, D. Purves, K. Tschida, and F. Wang for reading and commenting on the manuscript, K. Hamaguchi for data analysis software support, T. Otchy, K. Bohon, A. Bae and S. Lotfi for technical assistance and assistance with data processing and J. Baltzegar for animal husbandry and laboratory support. This research was supported by grants from the National Science Foundation (R.M.), the National Institutes of Health (R.M. (R01 DC02524) and B.P.O. (R01 NS066408)), by a Rubicon fellowship from the Netherlands Organization for Scientific Research (NWO) to S.M.H.G., and grants from the Klingenstein, Sloan and McKnight Foundations (B.P.O.).
Footnotes
Author Contributions This manuscript combines the synergistic results of two independently conceived and executed research studies arriving at similar conclusions. T.F.R. and R.M. designed all experiments involving tutor song-contingent optogenetic or electrical manipulations of HVC activity, in vivo multiphoton imaging of HVC dendritic spines, pharmacological manipulations of NMDA receptors in HVC during tutoring, and tutor song-contingent microstimulation of NIf and Field L. T.F.R. conducted all of the aforementioned experiments and analyzed resultant data with input from R.M. S.M.H.G. and B.P.Ö. designed, executed and analyzed the results of experiments involving permanent or reversible NIf lesions. M. M. adapted optogenetic methods for use in the zebra finch, and characterized the effects of ChR2 activation on HVC neurons in brain slices. T.F.R. and R.M. wrote the manuscript, with extensive input from B.P.Ö. and S.M.H.G. All authors read and commented on the manuscript.
The authors declare no competing financial interests.
References
- 1.Dehaene-Lambertz G, et al. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci U S A. 2006;103:14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur J Neurosci. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- 3.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119 ( Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 4.Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- 5.Prather JF, Peters S, Nowicki S, Mooney R. Persistent representation of juvenile experience in the adult songbird brain. J Neurosci. 2010;30:10586–10598. doi: 10.1523/JNEUROSCI.6042-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbib MA, Liebal K, Pika S. Primate vocalization, gesture, and the evolution of human language. Curr Anthropol. 2008;49:1053–1063. doi: 10.1086/593015. discussion 1063–1076. [DOI] [PubMed] [Google Scholar]
- 7.Iacoboni M, et al. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 8.Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- 9.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 10.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 11.Immelman K. In: Bird Vocalizations. Hinde RA, editor. Cambridge University Press; 1969. pp. 61–74. [Google Scholar]
- 12.Marler P, Tamura M. Culturally Transmitted Patterns of Vocal Behavior in Sparrows. Science. 1964;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- 13.Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16:655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- 14.Hahnloser RH, Kotowicz A. Auditory representations and memory in birdsong learning. Curr Opin Neurobiol. 2010;20:332–339. doi: 10.1016/j.conb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 15.London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolhuis JJ, Hetebrij E, Den Boer-Visser AM, De Groot JH, Zijlstra GG. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur J Neurosci. 2001;13:2165–2170. doi: 10.1046/j.0953-816x.2001.01588.x. [DOI] [PubMed] [Google Scholar]
- 17.Gobes SM, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Curr Biol. 2007;17:789–793. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 18.Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. An analysis of the neural representation of birdsong memory. J Neurosci. 2004;24:4971–4977. doi: 10.1523/JNEUROSCI.0570-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akutagawa E, Konishi M. New brain pathways found in the vocal control system of a songbird. J Comp Neurol. 2010;518:3086–3100. doi: 10.1002/cne.22383. [DOI] [PubMed] [Google Scholar]
- 21.Bauer EE, et al. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci. 2008;28:1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 23.Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- 24.Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol. 2005;62:231–242. doi: 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- 26.Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basham ME, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata) Neurobiol Learn Mem. 1996;66:295–304. doi: 10.1006/nlme.1996.0071. [DOI] [PubMed] [Google Scholar]
- 28.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 29.Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 32.Kelley DB, Nottebohm F. Projections of a telencephalic auditory nucleus-field L-in the canary. J Comp Neurol. 1979;183:455–469. doi: 10.1002/cne.901830302. [DOI] [PubMed] [Google Scholar]
- 33.Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Wild JM, Williams MN, Howie GJ, Mooney R. Calcium-binding proteins define interneurons in HVC of the zebra finch (Taeniopygia guttata) J Comp Neurol. 2005;483:76–90. doi: 10.1002/cne.20403. [DOI] [PubMed] [Google Scholar]
- 35.Mooney R, Prather JF. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci. 2005;25:1952–1964. doi: 10.1523/JNEUROSCI.3726-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature. 2007;450:1240–1244. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- 37.Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- 39.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fortune ES, Margoliash D. Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata) J Comp Neurol. 1995;360:413–441. doi: 10.1002/cne.903600305. [DOI] [PubMed] [Google Scholar]
- 42.Cardin JA, Raksin JN, Schmidt MF. Sensorimotor nucleus NIf is necessary for auditory processing but not vocal motor output in the avian song system. J Neurophysiol. 2005;93:2157–2166. doi: 10.1152/jn.01001.2004. [DOI] [PubMed] [Google Scholar]
- 43.Coleman MJ, Mooney R. Synaptic transformations underlying highly selective auditory representations of learned birdsong. J Neurosci. 2004;24:7251–7265. doi: 10.1523/JNEUROSCI.0947-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahnloser RH, Fee MS. Sleep-related spike bursts in HVC are driven by the nucleus interface of the nidopallium. J Neurophysiol. 2007;97:423–435. doi: 10.1152/jn.00547.2006. [DOI] [PubMed] [Google Scholar]
- 45.Roy A, Mooney R. Song decrystallization in adult zebra finches does not require the song nucleus NIf. J Neurophysiol. 2009;102:979–991. doi: 10.1152/jn.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells of the field L complex in male zebra finches (Taenopygia guttata) J Comp Neurol. 1992;325:388–404. doi: 10.1002/cne.903250306. [DOI] [PubMed] [Google Scholar]
- 47.Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- 48.Arbib MA. From grasp to language: embodied concepts and the challenge of abstraction. J Physiol Paris. 2008;102:4–20. doi: 10.1016/j.jphysparis.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Fabbri-Destro M, Rizzolatti G. Mirror neurons and mirror systems in monkeys and humans. Physiology (Bethesda) 2008;23:171–179. doi: 10.1152/physiol.00004.2008. [DOI] [PubMed] [Google Scholar]
- 50.Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brainstem and forebrain premotor networks necessary for song. J Neurosci. 2008;28(13):3479–3489. doi: 10.1523/JNEUROSCI.0177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ölveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biology. 2005;5(30):902–909. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dutar P, Vu HM, Perkel DJ. Multiple cell types distinguished by physiological, pharmacological and anatomic properties in nucleus HVC of the adult zebra finch. J Neurophys. 1998;80:1828–1838. doi: 10.1152/jn.1998.80.4.1828. [DOI] [PubMed] [Google Scholar]
- 53.Cardin JA, Raksin JN, Schmidt MF. Sensorimotor nucleus NIf is necessary for auditory processing but not vocal motor output in the avian song system. J Neurophysiol. 2005;93:2157–2166. doi: 10.1152/jn.01001.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1. Tutor song-triggered optogenetic disruption of neural activity in the pupils’ HVC. This video displays a juvenile zebra finch being tutored for the first time, using tutor song-contingent optogenetic methods to disrupt neural activity in HVC. The song of the tutor (bird on the right in this video) is detected by software36 and used to drive brief pulses of blue light delivered to the HVC of the pupil (bird on left in this video) via chronically implanted fiber optic cables.
Supplementary Table 1. Summary of experimental groups, sample sizes and results. Table lists all the behavioral experiments conducted in this research, the sample size for each group and the average result of this manipulation (% similarity score to tutor song).
Supplementary Figure 1. In vivo extracellular recordings in the HVC of anesthetized finches reveal that optogenetic stimulation can evoke a variety of neural responses.Raster plots depict action potentials in HVC in response to 10 consecutive trials of light illumination applied to the dorsal surface of HVC. These recordings were obtained from three different juvenile zebra finches that had been injected with scAAV-ChR2 five to ten days prior to the recording session.
Supplementary Figure 2. Intracellular recordings in brain slices further characterize viral mediated optogenetic responses in HVC neurons. a, Confocal Images of an hChR2-expressing HVCRA neuron recorded in vitro. The green channel (left) shows hChR2-EYFP immunoreacted with an anti-GFP antibody; the red channel (middle) shows intracellular staining of the impaled cell with neurobiotin, which was visualized with streptavidin-conjugated alexafluor 594. The right panel is the merged view showing that the impaled cell is ChR2-positive. Scale bar = 20 um. In vitro brain slices were prepared from a bird injected with AAV2/9-hChR2-EYFP. b, left column: Putative direct excitatory responses to 473 nm fiber optic illumination could be detected in neurons expressing hChR2. HVC neurons that projected to either the song motor nucleus RA or the striatal Area X (HVCRA or HVCX cells) were identified by their membrane potential responses to positive and negative current pulses injected through the recording electrode (data not shown). The HVCRA cell in the top panel is the cell visualized in panel a). Right column: Illumination could also evoke excitatory and inhibitory synaptic responses in physiologically identified HVC projection neurons, presumably reflecting the direct activation of other PNs, which are a source of local excitatory input to HVC PNs, and the direct and indirect activation of local interneurons, which are a source of inhibitory input onto HVC PNs35 (middle and right columns; total of responsive cells recorded: HVCRA = 9 neurons, HVCX = 17 neurons, HVCINT = 2 cells (not shown), obtained from 7 hemispheres prepared from 4 birds). Panel insets show timing differences between putative direct and synaptic responses evoked by HVC illumination.
Supplementary Figure 3. Tutor song syllable-triggered microstimulation of HVC disrupts copying of the targeted syllable without changing the overall length of the learned motif. Sonograms of the tutor’s song and the adult song of four of his pupils that were microstimulated in HVC when the tutor sang syllable ‘c’. Green bar marks syllable ‘c’ in all sonograms. The adult motifs of the pupils were approximately the same length as their tutor’s motif. Scale bar at upper right = 130ms; ordinate = 0 – 9 kHz.
Supplementary Figure 4. Infusing D-APV in HVC during tutoring results in adult birds with syllables that are noisier, longer, and higher frequency than syllables in tutor songs. Cumulative probability plots showing the distribution of spectral and temporal features of song syllables from six D-APV treated birds (green) and from three of the tutors used in this study (black). a, Syllables in the adult songs of birds treated with D-APV during tutoring are noisier (lower entropy) than the syllables of their tutors (Kolmogorov-Smirnov test ( KS test), p = 1.21 × 10−6; D-APV birds = 3,084 syllables; tutors = 1,034 syllables). b, Syllables in the adult songs of birds treated with D-APV during tutoring are longer than the syllables of their tutors (KS test, p = 1.01 × 1017; D-APV birds = 2,024 syllables; tutors = 1,285 syllables). c, Syllables in the adult songs of birds treated with D-APV during tutoring are higher in frequency than the syllables of their tutors (KS test, p = 4.13 × 10−75; D-APV birds = 3,084 syllables; tutors = 1,034 syllables).
Supplementary Figure 5. Juvenile finches with larger NIf lesions develop poorer copies of their tutor’s song. Sonograms of a tutor’s song and the adult songs of two of his pupils in which 12% or 91% of NIf was lesioned prior to tutoring; scale bar = 100ms; ordinate = 0 – 9 kHz.
Supplementary Figure 6. Histology confirming the injection sites for the NIf inactivation experiments. a, Photomicrograph of a cresyl violet-stained parasagittal section with anatomical boundaries of NIf indicated with arrow heads. b, Fluorescent photomicrograph superimposed on the alternate section shown in a (NIf indicated with arrow heads). c, d, Higher magnification photomicrographs of alternate cresyl-violet and fluorescent sections centered on NIf. e, Composite of cresyl-violet stained section and alternate fluorescence section. The origin of the first, smaller circle is in the center of NIf, and the diameter of the circle spans the maximum width of the nucleus along its anterior-posterior axis. The larger circle indicates the estimated spread of TTX, assuming an inactivation radius of ~200 μm. Scale bar indicates 200 μm. f, g. Combined data for all birds. Location of NIf and estimated spread of TTX was determined for all birds and hemispheres (left hemisphere: f, right hemisphere: g) based on superimposed fluorescence and cresyl violet-stained photomicrographs as shown in e. Dashed ellipse: boundaries of NIf. Solid circles: estimated spread of TTX; different colors indicate different animals.
Supplementary Figure 7. Location of stimulating electrodes in an experiment where tutor song-contingent microstimulation was applied in Field L1. Photomicropgraphs of brain sections showing NIf (red outline) and Field L1 (white arrows) from a juvenile finch subjected to tutor song-contingent microstimulation in which the stimulating electrode tips (blue arrowheads) were implanted in a region of Field L1 adjacent to NIf; scale bar = 250μm.