Abstract
Background and Purpose
Hypertension is the most important risk factor associated with intracerebral hemorrhage (ICH). We explored racial differences in blood pressure (BP) control after ICH and assessed predictors of BP control at presentation, 30 days, and 1 year in a prospective cohort study.
Methods
Subjects with spontaneous ICH were identified from the DiffErenCes in the Imaging of Primary Hemorrhage based on Ethnicity or Race (DECIPHER) Project. Blood pressure was compared by race at each time point. Multivariable linear regression was used to determine predictors of presenting mean arterial pressure (MAP), and longitudinal linear regression was used to assess predictors of MAP at follow-up.
Results
A total of 162 patients were included (mean age 59, 53% male, 77% black). MAP at presentation was 9.6 mmHg higher in blacks than whites despite adjustment for confounders (p=0.065). Fewer than 20% of patients had normal blood pressure (<120/80 mmHg) at 30 days or 1 year. While there was no difference at 30 days (p=0.331), blacks were more likely than whites to have Stage I/II hypertension at one year (p=0.036). Factors associated with lower MAP at follow-up in multivariable analysis were being married at baseline (p=0.032) and living in a facility (versus personal residence) at the time of BP measurement (p=0.023).
Conclusions
Long-term blood pressure control is inadequate in patients following ICH, particularly in blacks. Further studies are needed to understand the role of social support and barriers to control to identify optimal approaches to improve blood pressure in this high-risk population.
Keywords: Intracerebral hemorrhage, hypertension, secondary prevention, racial differences
Hypertension is a critical risk factor for primary intracerebral hemorrhage (ICH) and is the most important modifiable risk factor for prevention of recurrent ICH.1 Minority populations, including blacks, are at increased risk of both hypertension and ICH.2, 3 Preliminary clinical trial evidence suggests that blood pressure (BP) lowering immediately after ICH may be an important treatment target to prevent hematoma expansion, though it has not been proven to be beneficial.4 In the chronic phase, poor BP control has been associated with increased risk of recurrent stroke and cardiovascular events.5
Prior studies based on the National Health and Nutrition Examination Survey (NHANES) have identified that control of BP in stroke survivors is often suboptimal.6, 7 These studies used patient self-report to identify stroke; therefore they did not have information on stroke type, clinical presentation, or time from stroke to BP assessment in these studies. Since the burden of ICH is highest in black and Hispanic/Latino populations compared with other race-ethnic groups,3 long-term BP control after ICH is even more important in these populations.
We performed a longitudinal cohort study of ICH survivors in a multiracial population from the Washington, DC metro area. The goals of this study were to 1) explore racial differences in BP after ICH; 2) investigate predictors of higher BP at the time of ICH presentation; and 3) identify factors associated with higher BP in the first year after ICH.
Methods
Study setting, case identification, and eligibility
This study was a part of the DiffErenCes in the Imaging of Primary Hemorrhage based on Ethnicity or Race (DECIPHER) Project.8 DECIPHER is a longitudinal observational cohort study of individuals with ICH designed primarily to investigate racial differences in ICH with a focus on MRI findings including cerebral microbleeds. Patients with ICH from the Washington, DC metro area were identified at one of the following hospitals: Washington Hospital Center, Georgetown University Hospital, Suburban Hospital (Bethesda, Maryland), National Rehabilitation Hospital, and Howard University Hospital. Patients were identified through active surveillance of all patients admitted to a study hospital with brain hemorrhage. Eligibility criteria for DECIPHER included a primary (non-traumatic) ICH within 30 days prior to enrollment, age 18 or older, no contraindication to brain magnetic resonance imaging (MRI), and informed consent signed by the patient or a legally authorized representative. Exclusion criteria were pregnancy, coagulopathy (international normalized ratio >3 at the time of ICH), central nervous system (CNS) tumor, need for craniotomy or craniectomy, active CNS infection or inflammatory process, CNS arteriovenous malformation or aneurysm, or CNS trauma within 2 weeks. The current study did not focus on MRI findings, though MRI is required for the primary aims of DECIPHER and therefore all patients in the current study had to be able to undergo MRI.
Baseline data collection
Pre-stroke medical co-morbidities, education, occupation, health insurance status, martial status, medication use, drug or alcohol use (> 2 drinks per day), and smoking were collected from the medical record and via interviews with patients or a proxy (if patient was unable). Race was determined by self-report; individuals of non-black or non-white race ethnic groups were excluded from the current analysis due to low numbers. Marital status at baseline was dichotomized as married versus not married for analysis. BP medications were reviewed and classified into one of the following categories: beta blocker, alpha blocker, calcium channel blocker, diuretic, angiotensin converting enzyme inhibitor, angiotensin receptor blocker, nitrates, and other.
Initial Glasgow Coma Scale (GCS) and baseline body mass index (BMI) were determined based on the medical chart. Admission National Institutes of Health Stroke Scale was abstracted from the first documented examination using a previously validated method.9 The first BP listed in the emergency department record was recorded as the baseline BP. Education and occupation were combined to generate the Hollingshead two factor index of social position (ISP) as a measure of socioeconomic status10 which was dichotomized as middle to upper (ISP ≤ 47) and lower to lower-middle (referent, ISP ≥ 48) for analysis.
ICH volume was calculated based on initial head CT scan in DICOM format by volumetric analysis employing a semi-automated approach using Multi-Image Analysis GUI (MANGO) software.11 Five cases had only a baseline MRI rather than CT and volume data was converted using a validated method.12
Data collection at 30 days and 1 year
Enrolled subjects underwent an in-person evaluation at approximately 30 days and 1 year from the time of initial presentation. The subjects’ location was recorded and dichotomized as living in a personal residence versus living in a facility for analysis. The interview consisted of questions about medication use as well as a BP measurement. Trained study staff measured BP with an automated monitor (LifeSource UA-767 Plus, cuff size varied to fit arm circumference) according to a protocol recommended by the American Heart Association.13 In the initial stages of the project, a single BP measurement was taken, though the study procedures were later changed to include two measurements taken at least two minutes apart, with the average used for analysis. Medication management was left up to the patients’ primary care doctors and there was no direct physician intervention or interview regarding BP control. The current study consisted of DECIPHER patients enrolled between December 7, 2007 and November 3, 2011 who had BP data available at either 30 days or 1 year.
Statistical analysis
Baseline characteristics were summarized for categorical variables as frequencies and percents and for continuous variables as means and standard deviations or medians and interquartile ranges (IQR). BP at follow-up (30 days and 1 year) was reported by race continuously and categorized as normal, pre-hypertension, Stage I Hypertension, or Stage II Hypertension based on the Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII).14 Baseline characteristics and BP were compared by race using t-tests, chi square tests, or non-parametric rank sum tests as appropriate.
Multivariable linear regression was used to investigate predictors of higher MAP at presentation and longitudinal linear regression models were developed (excluding four patients discharged to hospice) to investigate BP control at 30-days and 1 year after ICH. Candidate variables for inclusion in the models were pre-selected based on biological plausibility or expected association with BP. Age (continuous, centered at mean value), race, gender, were used to generate the base models. Covariates were individually added to the base model with a plan to retain variables if they either improved the model fit based on R2 (baseline model) or Akaike information criterion (AIC) (longitudinal model) or if they altered the association of other variables with the outcome. Time was treated as a dummy variable for year 1 with day 30 as the reference.
Statistical analysis was done in SAS version 9.2 (SAS Institute, Cary, N.C.) This study was approved by the Institutional Review Boards of Georgetown University and all participating institutions. Patients or their legally authorized representatives gave their written informed consent for participation.
RESULTS
The study population consisted of 162 patients who had BP data available at either 30 days (140 patients) or 1 year (103 patients). A total of 806 patients with spontaneous ICH were screened for DECIPHER during the study period, with198 of these enrolled (reasons for non-enrollment included died or discharged prior to consent: 218; refused: 164; MRI contraindication: 40; other exclusion: 186). Four patients were ineligible for the current study due to non-black or non-white race, and 32 were excluded due to missing blood pressure data at both 30 days and 1 year. There was no significant difference in age, race, gender, or initial National Institutes of Health Stroke Scale comparing those included to those excluded for lack of BP data.
Mean age was 58.9 years, 53% of the cohort was male, 77% were black, and 85% had a history of hypertension. Baseline demographics, medical history, and clinical characteristics are shown in Table 1 overall and by race. Seven of the included 162 patients died within the first year after ICH, while four had recurrent ICH and two had a subsequent ischemic stroke. Black participants were younger than whites (p=<0.001), more likely to be smokers (p=0.014), and less likely to be married at baseline (p=0.005). Blacks were also more likely than whites to have a baseline diagnosis of hypertension (p=0.001) and had higher BP at presentation (p=0.002 for MAP).
Table 1.
Description of the population
| Characteristic | Overall (N=162) |
White (N=37) |
Black (N=125) |
p |
|---|---|---|---|---|
| Age | 58.9 (12.3) | 65.3 (12.3) | 57.1 (11.7) | <0.001 |
| Male | 85 (52.5%) | 20 (54.1%) | 65 (52.0%) | 0.826 |
| Black Race | 125 (77.2%) | - | - | - |
| Body mass index (kg/m2) | 28.9 (24.8, 34.7) |
26.4 (22.9, 33.3) |
30.0 (25.7, 34.9) |
0.05* |
| Married | 68 (42.0%) | 23 (62.2%) | 45 (36.0%) | 0.005 |
| Private Health Insurance | 92 (56.8%) | 23 (62.2%) | 69 (55.2%) | 0.453 |
| Socioeconomic status middle class or greater (4 missing) |
95 (60.1%) | 28 (77.8%) | 67 (54.9%) | 0.014 |
| Current Smoker | 43 (26.5%) | 4 (10.8%) | 39 (31.2%) | 0.014 |
| Cocaine Use (2 missing) | 25 (15.6%) | 2 (5.6%) | 23 (18.6%) | 0.059† |
| Hypertension (3 missing) | 135 (84.9%) | 24 (66.7%) | 111 (90.2%) | 0.001 |
| Systolic blood pressure | 179.9 (34.8) | 160.9 (28.6) | 185.5 (34.6) | <0.001 |
| Diastolic blood pressure | 99.8 (23.6) | 92.4 (20.6) | 102.0 (24.0) | 0.029 |
| Mean arterial pressure | 126.5 (25.8) | 115.2 (21.6) | 129.8 (26.0) | 0.002 |
| National Institutes of Health Stroke Scale |
6 (3, 13) | 6 (3, 13) | 6 (3, 12) | 0.590* |
| ICH volume in cc (5 missing) | 7.5 (3.2, 20.2) |
11.4 (3.6, 29.5) |
7.2 (3.1, 16.7) |
0.212* |
| On any antihypertensive medication | 88 (55.0%) | 14 (37.8%) | 74 (60.2%) | 0.017 |
| Number of classes of blood pressure medication |
1.2 (1.4) | 0.8 (1.1) | 1.3 (1.4) | 0.066 |
Wilcoxon
Fisher’s exact
Continuous variables presented as mean (standard deviation) or median (inter-quartile range)
The racial difference in MAP at presentation persisted when adjusting for age and gender in a multivariable linear regression model as shown in Table 2. Younger age was also associated with higher MAP at presentation in this model, and age did confound the association between race and baseline MAP (>10% change in parameter estimate, data not shown). Adding the other pre-selected covariates did not result in a substantial improvement in model fit based on the R2 and did not alter the magnitude of the association between race and MAP (e.g. less than 10% change in parameter estimates). Therefore, none of the other covariates met our pre-specified criteria for inclusion in the model, though both the demographics-only model and a model with all pre-specified covariates are shown in Table 2 to demonstrate the minimal change in the race and age effects when adjusting for other factors. Note that the model beta estimates can be interpreted as the average difference in MAP (in mmHg) for a one-unit change in the predictor variable, adjusted for all other model covariates. In other words, black participants had a MAP that was on average about 9.6 mmHg higher than whites, adjusted for other model covariates.
Table 2.
Predictors of Mean Arterial Blood Pressure at Presentation
| Demographics only | Fully adjusted model* | |||
|---|---|---|---|---|
| Parameter | β (standard error) |
p | β (standard error) |
p |
| Black Race (reference: white) |
9.60 (4.72) | 0.044 | 9.62 (5.18) | 0.065 |
| Age (centered at 59) | −0.61 (0.16) | <0.001 | −0.59 (0.20) | 0.003 |
| Male | 1.80 (3.82) | 0.639 | 3.90 (4.36) | 0.373 |
| Intercept | 118.10 (4.64) | <0.001 | 112.86 (11.87) | <0.001 |
Additionally adjusted for body mass index, private health insurance (reference: all others), hemorrhage volume, current smoking, marital status, cocaine use, and socioeconomic status.
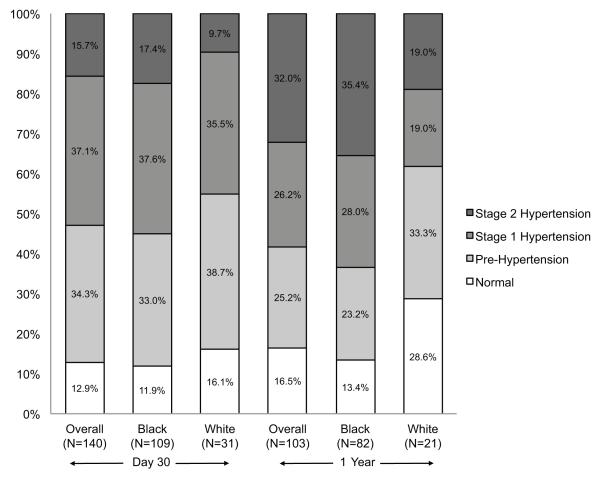
Average BP at 30 days and 1 year after ICH are shown in Table 3 by race. Blacks had higher BP than whites at each time point, though only the diastolic BP at 1 year was significantly higher (p=0.029). BP categories by JNC VII criteria at 30 days and 1 year after ICH are shown in the Figure. At each time point, fewer than 20% of the overall population had a BP in the normal range. Although there was no significant racial difference at 30 days (p=0.331), blacks were more likely than whites to have BP measurements consistent with Stage I/II hypertension at one year (p=0.036). At 30 days, blacks had a higher mean number of classes of anti-hypertensive medications than whites at 30 days (2.6 vs. 1.6; p=0.001). Although this trend continued at 1 year, it was not statistically significant (2.3 vs. 1.7, p=0.072).
Table 3.
Unadjusted blood pressure at follow-up by race
| Day 30 | Year 1 | |||||
|---|---|---|---|---|---|---|
| Black (N=109) | White (N=31) | p | Black (N=82) | White (N=21) | p | |
| SBP | 137.7 (19.1) | 132.7 (18.1) | 0.196 | 142.5 (27.1) | 132.5 (24.4) | 0.126 |
| DBP | 81.7 (14.8) | 78.3 (13.1) | 0.246 | 87.2 (18.3) | 79.7 (12.0) | 0.029 |
| MAP | 100.4 (14.2) | 96.4 (12.1) | 0.162 | 105.6 (19.9) | 97.3 (14.5) | 0.076 |
SBP indicates systolic blood pressure, DBP indicates diastolic blood pressure, and MAP indicates mean arterial pressure.
Figure. Blood Pressure Category at Follow-up by Race at 30 days and 1 year after ICH.
Blacks were more likely than whites to have blood pressure measurements consistent with Stage I/II hypertension at one year (p=0.036) but not 30 days (p=0.331).
The longitudinal model predicting MAP at follow-up is shown in Table 4. In contrast to the analysis of MAP at baseline, there was no association of race with MAP at follow-up (β=1.35, p=0.62). The only covariates associated with MAP at follow-up were married status at baseline and living in a personal residence at the time of the follow-up BP measurement. Married individuals had a MAP that was about 5mmHg lower than non-married individuals (β=-5.17, p=0.03). Individuals living in a personal residence at the time of the BP measurement had a MAP that was about 5mmHg higher than those living in a facility (β=5.47, p=0.02).
Table 4.
Longitudinal regression model predicting mean arterial pressure at 30 days and 1 year
| Parameter | β (standard error) | p |
|---|---|---|
| Black Race (reference: white) | 1.35 (2.70) | 0.618 |
| Age (centered at 59) | −0.15 (0.09) | 0.111 |
| Male | 1.62 (2.22) | 0.467 |
| Socioeconomic status*† | −1.67 (2.29) | 0.465 |
| Private Health Insurance (reference: all others)† |
2.06 (2.22) | 0.353 |
| Married (reference: not married)† | −5.17 (2.38) | 0.032 |
| Current smoking† | −2.89 (2.48) | 0.246 |
| Cocaine Use† | 4.15 (3.06) | 0.177 |
| National Institutes of Health Stroke Scale† | 0.15 (0.13) | 0.268 |
| Living in a personal residence (reference: living in a facility) |
5.47 (2.38) | 0.023 |
| Year 1 (reference: day 30) | 2.17 (2.12) | 0.308 |
| Intercept | 102.92 (7.72) | <0.001 |
Hollingshead index, dichotomized as middle class or higher vs. lower or lower-middle
at baseline
DISCUSSION
We found that BP control was extremely poor at 30 days and 1 year after ICH, particularly in black patients, in this longitudinal cohort study of ICH survivors. Fewer than 20% of the study population had normal BP at follow-up measurements and more than half had BP consistent with Stage I or Stage II hypertension. Poor control of BP in stroke survivors has been described previously in studies based on NHANES.6, 7, 15 However, these studies had limitations in that they identified stroke based on self-report, had BP measurements at varying times from their stroke, and were unable to adjust for baseline stroke characteristics such as severity. Though there is some evidence that BP control in stroke survivors may be improving over the last several years,6 our results suggest that efforts to improve BP control after ICH remain critically important in both blacks and whites.
We found that blacks had a higher BP at the time of initial ICH presentation, with a MAP that was on average 15 mmHg higher than whites on unadjusted analysis. Our fully adjusted linear regression model suggested that even when accounting for other factors, blacks had a MAP that was still on average 9.6mm higher than whites, though this finding was of borderline significance. Elevated BP in the acute phase after ICH is a well-known phenomenon that has been associated with poor outcome16 and hematoma expansion.17 Few studies have specifically investigated racial differences in presenting BP in the acute phase after ICH. One study used 24 hour ambulatory BP monitoring acutely after ischemic and hemorrhagic stroke and identified that blacks had higher ambulatory mean BP than whites.18 The treatment implications of a racial difference in presenting BP are unclear in light of recent evidence suggesting that BP lowering after ICH may be associated with increased risk of ischemic lesions on MRI8 and considering that trials of acute BP lowering in ICH are still ongoing. Future studies will hopefully clarify whether the potential benefits of BP lowering on hematoma expansion outweigh the potential detrimental effects of ischemic lesions on outcome.
When examining BP at follow-up, we found that blacks were somewhat more likely than whites to have elevated BP at 1 year (combined Stage I/II hypertension and higher DBP). However, there was no racial difference seen in BP at 30 days, and race was not associated with MAP at follow-up in our final multivariable model. Due to the relatively small number of white participants at 1 year, we did not have sufficient power to systematically investigate which specific factors may have explained the racial difference in BP seen on unadjusted analysis. However, a post-hoc exploratory analysis identified that both age and marital status acted as confounders of the relationship between race and MAP (more than a 10% reduction in the parameter estimate for race when adding age or marital status to the model). Future studies with larger samples should be performed to allow a more detailed understanding of what factors explain the racial difference in BP post ICH and to verify whether this racial difference becomes larger over time. In particular, it would be important to understand whether inadequate control is caused by poor medication adherence, poor access to healthcare, more treatment resistant hypertension in blacks, failure to prescribe or adjust medications by health care providers, or a combination of the above.
Our results suggest that future efforts to improve BP control after ICH may wish to target social and environmental factors in addition to traditional medical factors. Marital status at baseline may be a surrogate marker for social support, which we did not directly measure in this study. It is possible that individuals with stroke related physical, emotional, or cognitive problems may need the assistance of a dedicated social support network to assist with attending regular physician appointments, medication adherence, and maintaining lifestyle modifications which can help BP control.19, 20 Further, being married may represent a set of specific social support dimensions not able to be performed by other networks.21 Our finding of higher BP among those living in a personal residence at the time of follow-up could be due to individuals in an institution receiving more frequent BP monitoring or more consistent medication administration than those living in a personal residence. However, other unmeasured factors could account for these observed differences as well, and these factors should be assessed in larger studies of BP control after stroke to confirm these findings and better understand reasons for these associations.
Few studies have investigated predictors of long-term BP specifically in the ICH population. Studies of BP control in the general population based on NHANES data have had conflicting results on the association with marital status, with some,22 but not all studies suggesting an association.2 Health insurance status and disability have been associated with BP control,2 though we did not find insurance or disability (as measured by initial stroke severity) to be associated with MAP in our models. Some of the differences between our study and NHANES data that may account for these different findings include the study population (ICH survivors vs. general population sample) and the precise definition of the outcome variables (continuous vs. categorical analysis of BP).
This study has limitations. The requirement that participants in DECIPHER be able to undergo MRI and complete study assessments may have skewed our population toward milder ICH cases. However, optimal BP control after ICH is important for all ICH survivors regardless of severity, and therefore this limitation would not diminish the importance of our findings. Furthermore, if there is a healthy participant bias in this study population, we might expect that BP control in non-participants would be even worse. We did not have data on some potentially important covariates such as medication adherence, regular access to a primary care physician, or detailed assessment of social support and environmental factors.
Summary.
In conclusion, we identified suboptimal BP control at 30 days and 1 year after ICH in this predominantly black population. Black participants had higher BP than whites acutely and were more likely to have Stage I/II hypertension than whites at 1 year. Urgent efforts are needed to better understand the factors leading to poor long-term BP control and identify optimal approaches to improve BP management after ICH in both blacks and whites.
Acknowledgements
None
Sources Of Funding This work was supported by Award Number U54NS057405 from the National Institute of Neurological Disorders And Stroke (NINDS) and National Institute on Minority Health and Health Disparities (NIMHD) (U54NS057405). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the NIMHD, or the NIH. Dr. Zahuranec is supported by grant K23AG038731 from the National Institute on Aging.
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr., et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keenan NL, Rosendorf KA. Prevalence of hypertension and controlled hypertension - United States, 2005-2008. MMWR Surveill Summ. 2011;60(Suppl):94–97. [PubMed] [Google Scholar]
- 3.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65:518–522. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 5.Lakhan SE, Sapko MT. Blood pressure lowering treatment for preventing stroke recurrence: a systematic review and meta-analysis. Int Arch Med. 2009;2:30. doi: 10.1186/1755-7682-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muntner P, DeSalvo KB, Wildman RP, Raggi P, He J, Whelton PK. Trends in the prevalence, awareness, treatment, and control of cardiovascular disease risk factors among noninstitutionalized patients with a history of myocardial infarction and stroke. Am J Epidemiol. 2006;163:913–920. doi: 10.1093/aje/kwj124. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AI, Suri MF, Guterman LR, Hopkins LN. Ineffective secondary prevention in survivors of cardiovascular events in the US population: report from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2001;161:1621–1628. doi: 10.1001/archinte.161.13.1621. [DOI] [PubMed] [Google Scholar]
- 8.Menon RS, Burgess RE, Wing JJ, Gibbons MC, Shara NM, Fernandez S, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71:199–205. doi: 10.1002/ana.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31:858–862. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 10.Hollingshead AB. Two factor index of social position. Conn.; New Haven: 1957. [Google Scholar]
- 11.Research Imaging Center: UTHSCSA [Accessed on 2/22/2012];Multi-image Analysis GUI (MANGO) Available from: http://ric.uthscsa.edu/mango.
- 12.Burgess RE, Warach S, Schaewe TJ, Copenhaver BR, Alger JR, Vespa P, et al. Development and validation of a simple conversion model for comparison of intracerebral hemorrhage volumes measured on CT and gradient recalled echo MRI. Stroke. 2008;39:2017–2020. doi: 10.1161/STROKEAHA.107.505719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. Epub 2003 Dec 1201. [DOI] [PubMed] [Google Scholar]
- 15.Kesarwani M, Perez A, Lopez VA, Wong ND, Franklin SS. Cardiovascular comorbidities and blood pressure control in stroke survivors. J Hypertens. 2009;27:1056–1063. doi: 10.1097/hjh.0b013e32832935ce. [DOI] [PubMed] [Google Scholar]
- 16.Fogelholm R, Avikainen S, Murros K. Prognostic value and determinants of first-day mean arterial pressure in spontaneous supratentorial intracerebral hemorrhage. Stroke. 1997;28:1396–1400. doi: 10.1161/01.str.28.7.1396. [DOI] [PubMed] [Google Scholar]
- 17.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–2375. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 18.Lip GY, Zarifis J, Farooqi IS, Page A, Sagar G, Beevers DG. Ambulatory blood pressure monitoring in acute stroke. The West Birmingham Stroke Project. Stroke. 1997;28:31–35. doi: 10.1161/01.str.28.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005;64:1888–1892. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 20.Willey JZ, Paik MC, Sacco R, Elkind MSV, Boden-Albala B. Social Determinants of Physical Inactivity in the Northern Manhattan Study (NOMAS) Journal of Community Health. 2010;35:602–608. doi: 10.1007/s10900-010-9249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boden-Albala B, Tehranifar P, Stillman J, Paik MC. Social Network Types and Acute Stroke Preparedness Behavior. Cerebrovascular Diseases Extra. 2011;1:75–83. doi: 10.1159/000328726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J, Muntner P, Chen J, Roccella EJ, Streiffer RH, Whelton PK. Factors associated with hypertension control in the general population of the United States. Arch Intern Med. 2002;162:1051–1058. doi: 10.1001/archinte.162.9.1051. [DOI] [PubMed] [Google Scholar]