Abstract
Pilocarpine-treated mice are an increasingly used model of temporal lobe epilepsy. However, outcomes of treatment can be disappointing, because many mice die or fail to develop status epilepticus. To improve animal welfare and outcomes of future experiments we analyzed results of previous pilocarpine treatments to identify factors that correlate with development of status epilepticus and survival. All treatments were performed by one investigator with mice of the FVB background strain. Results from 2413 mice were evaluated for effects of sex, age, body weight, and latency between administration of atropine methyl bromide and pilocarpine. All parameters correlated with effects on outcomes. Best results were obtained from male mice, 6–7 weeks old, and 21–25 g, with pilocarpine administered 18–30 min after atropine methyl bromide. In that group only 23% failed to develop status epilepticus, and 64% developed status epilepticus and survived. Those results are substantially better than that of the total sample in which 31% failed to develop status epilepticus and only 34% developed status epilepticus and survived.
Keywords: pilocarpine, mice, status epilepticus, sex, age, body weight
Introduction
Rodents that survive status epilepticus after systemic treatment with kainic acid or pilocarpine are among the most widely used models in epilepsy research (Cavalheiro et al., 2006). Mice are increasingly popular because of the abundant availability of transgenic and knockout animals. Kainate treatment in mice can be problematic, however, because some commonly used strains are resistant to kainate’s normally excitotoxic (and epileptogenic) effects (Schauwecker and Steward, 1997), which is not the case for pilocarpine (Shibley and Smith, 2002; Schauwecker, 2012). Consequently, pilocarpine-treated mice have become an important model of temporal lobe epilepsy. In its most common application the model involves intraperitoneally administering a peripherally acting muscarinic acetylcholine receptor antagonist, such as methyl scopolamine or atropine methyl bromide, minutes before a single high-dose of pilocarpine (Turski et al., 1984). Pilocarpine’s actions include activation of M1 receptors, which evokes status epilepticus (Maslanksi et al., 1994; Hamilton et al., 1997). After surviving status epilepticus, mice begin displaying spontaneous seizures days later, and their epileptic condition is permanent (Turski et al., 1989; Cavalheiro et al., 1996).
Treating mice with pilocarpine is difficult, however, because many animals fail to develop status epilepticus and many of those that do die acutely. Mortality rates for mice treated with pilocarpine range from 25–100% depending in part on dose (Turski et al., 1984; Maslanksi et al., 1994; Cavalheiro et al., 1996; Winawer et al., 2007b). Respiratory failure following convulsions is a common cause of acute death after pilocarpine treatment (Boyd and Fulford, 1961). Lower pilocarpine doses are associated with lower mortality rates, but fewer mice develop status epilepticus (Shibley and Smith, 2002; Borges et al., 2003). If status epilepticus does not occur or is too short, epilepsy will not develop (Lemos and Cavalheiro, 1995).
To improve animal welfare and outcomes of future experiments we analyzed results of previous pilocarpine treatments to identify factors that correlate with development of status epilepticus and survival. We asked whether sex, age, body weight, and timing of administration of a peripherally acting muscarinic acetylcholine receptor antagonist relative to pilocarpine affected outcome of treatment.
Methods
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by a Stanford University Institutional Animal Care and Use Committee. All pilocarpine treatments were performed by the same investigator using GIN mice (FVB-Tg(GadGFP)4570Swn/J, The Jackson Laboratory, Bar Harbor, Maine) (Oliva et al., 2000). A colony of GIN mice was maintained by our laboratory for use in other experiments (Zhang et al., 2009; Halabisky et al., 2010; Buckmaster and Lew, 2011; Buckmaster and Wen, 2011; Lew and Buckmaster, 2011). The present study is a retrospective analysis of pilocarpine treatments performed for those experiments. Mice were bred in-house, lived in potentially mixed-sex sibling groups of up to 5 per cage, and had food and water available ad libitum. On the day of treatment, mice were weighed, sexed, and individually identified by tail bands made by marker pen. Female mice that appeared to be pregnant because of obvious abdominal distension were noted. Pilocarpine and atropine methyl bromide (Sigma-Aldrich, St. Louis, Missouri) were dissolved in bacteriostatic 0.9% sodium chloride solution to 50 and 1 mg/ml, respectively. Pilocarpine (300 mg/kg, i.p.) was administered 18 min or longer after atropine methyl bromide (5 mg/kg, i.p.), which began at 9:40 am ± 1 h (mean ± standard deviation, range 6:50 am till noon). Mice were behaviorally monitored continuously. Status epilepticus was recognized as persistent head nodding following stage 3 or greater seizures on the Racine (1972) scale. Some mice that failed to develop status epilepticus experienced one or more convulsive seizures but did not display continuous head nodding. Diazepam (10 mg/kg, i.p.) was administered 2 h after the onset of stage 3 or greater seizures and was repeated 1–6 h later if necessary to suppress convulsions. During recovery, mice received lactated ringers with 5% dextrose subcutaneously, and body temperature was maintained by placing cages on a heating pad. In the present study, survival/death refers only to the first 8 h following pilocarpine treatment. Results are reported as mean ± standard deviation. Statistics were performed using Sigma Stat (Systat, Chicago, Illinois) with p<0.05 considered significant.
Results
Results were obtained from 2413 mice that were treated in 32 batches (75 ± 23 mice/batch, range 14–129). For the entire sample, 744 (30.8%) failed to develop status epilepticus, 860 (35.6%) developed status epilepticus and died, and 809 (33.5%) developed status epilepticus and survived. Therefore, a total of 1669/2413 (69.2%) developed status epilepticus. The percentage of mice that developed status epilepticus and survived ranged widely from 11–72% per batch. All parameters (sex, age, body weight, and time between atropine and pilocarpine) correlated with differences in outcome (p<0.05, Kruskal-Wallis one way ANOVA on ranks with Dunn’s method). Therefore, each parameter was evaluated in detail.
Gender
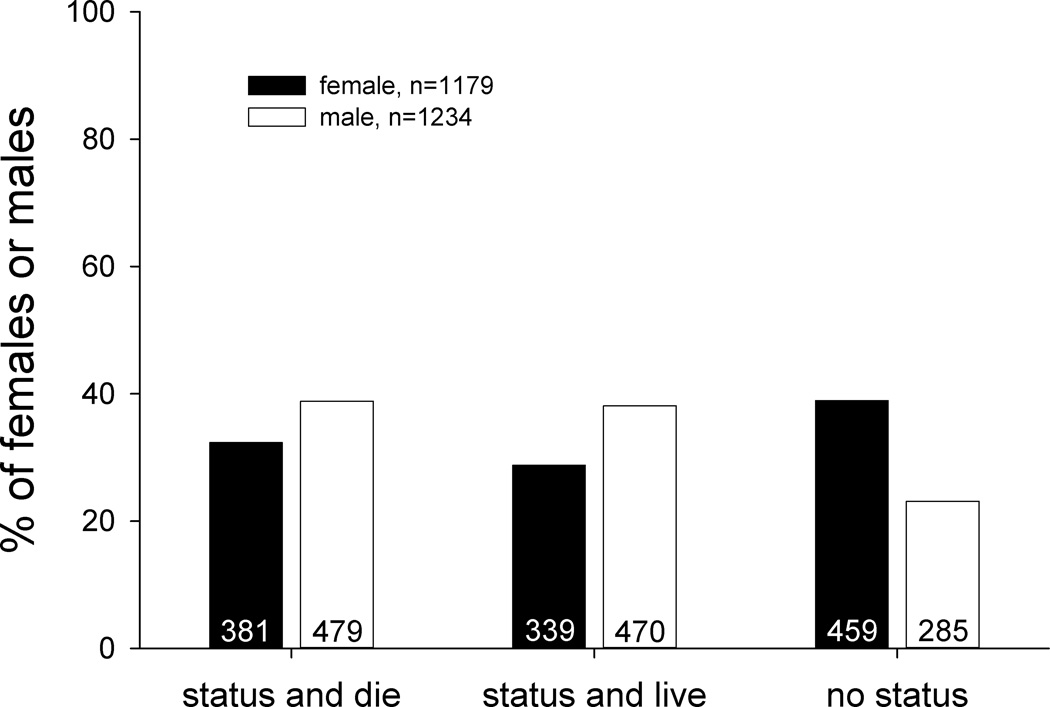
Of 2413 mice, 1179 (49%) were female. Male mice were 1.3-times more likely to develop status epilepticus than females (Figure 1). Of male mice, 77% (949/1234) developed status epilepticus, compared to only 61% (720/1179) of females (p<0.001, Chi-square test). However, males and females that developed status epilepticus had similar probabilities of surviving (50%, 470/949 and 47%, 339/720, respectively). Consequently, male mice were 1.3-times more likely to develop status epilepticus and survive than females (38%, 470/1234 versus 29%, 339/1179, respectively, p<0.001, Chi-square test).
Figure 1.
Percentage of male and female mice that developed status epilepticus and died or survived or failed to develop status epilepticus after pilocarpine treatment. Numbers from which percentages were calculated are indicated in bars. Male and female mice were significantly different (p<0.001, Chi-square test).
Of 1179 female mice, 105 displayed obvious abdominal distension and therefore appeared to be pregnant. Average body weight of these mice (33 ± 5 g) was 1.6-times that of other females (21 ± 3 g; p<0.0001, t test). Outcomes of pilocarpine treatment were similar in both groups. For example, 59% (62/105) of obviously pregnant mice developed status epilepticus compared to 61% (658/1073) of other females, and 30% (31/105) of obviously pregnant mice developed status epilepticus and survived compared to 29% (308/1073) of other females.
Age
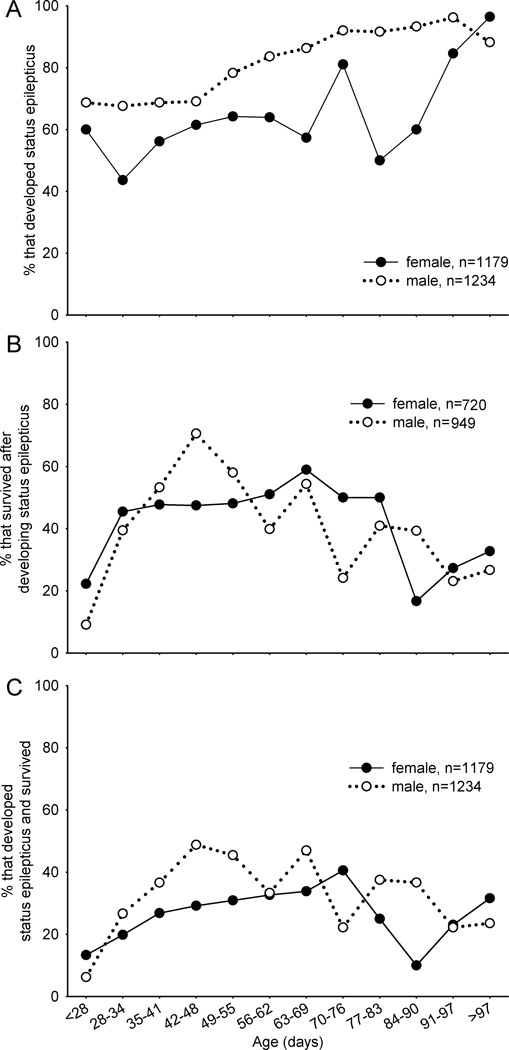
For the entire sample, median and average age at treatment was 46 d and 53 ± 24 d, respectively (range 21–384 d). Effects of age on outcomes of pilocarpine treatment were sex specific. To evaluate effects of age, female and male mice were divided into 12 groups ranging from <28 d old through 7 d increments to >97 d old. For each age group there were 96 ± 91 (range 8–276) and 101 ± 83 (range 16–246) female and male mice, respectively. The probability of developing status epilepticus increased with age in both genders but at different time points (Figure 2A). In male mice up to 48 d old the percentage that developed status epilepticus remained close to 70% before gradually increasing to a new constant level of ~90% at 70 d old and beyond. The percentage of female mice that developed status epilepticus was lower and more variable, averaging ~60% in mice <90 d old and increasing to ~90% in older animals. Younger (<28 d old) and older (>84 d old) mice of both sexes that developed status epilepticus were less likely to survive than animals of intermediate ages (Figure 2B). Survival of status epilepticus peaked at 71% (120/170) in male mice at 42–48 d old, which was 1.5-times that of age-matched females (47%, 66/139) (p<0.001, Chi-squared test). The percentage of female mice that survived status epilepticus was relatively constant (~50%) from 28–83 d old. In summary, compared to females, male mice were more likely to develop status epilepticus (Figure 2A) and to survive if they developed status epilepticus (Figure 2B). Consequently, male mice were more likely to develop status epilepticus and survive especially at 35–55 d old (Figure 2C). For example, 49% (120/246) of male mice at 42–48 d old developed status epilepticus and survived, which was 1.7-times higher than that of age-matched female mice (29%, 66/226) (p<0.001, Chi-squared test). Female mice were most likely to develop status epilepticus and survive when they were slightly older, up to 76 d old.
Figure 2.
Outcomes of pilocarpine treatment as a function of sex and age. A Percentage of mice that developed status epilepticus. B Of mice that developed status epilepticus, percentage that survived. C Of all mice treated, percentage that developed status epilepticus and survived. See text for statistical analyses.
Body weight
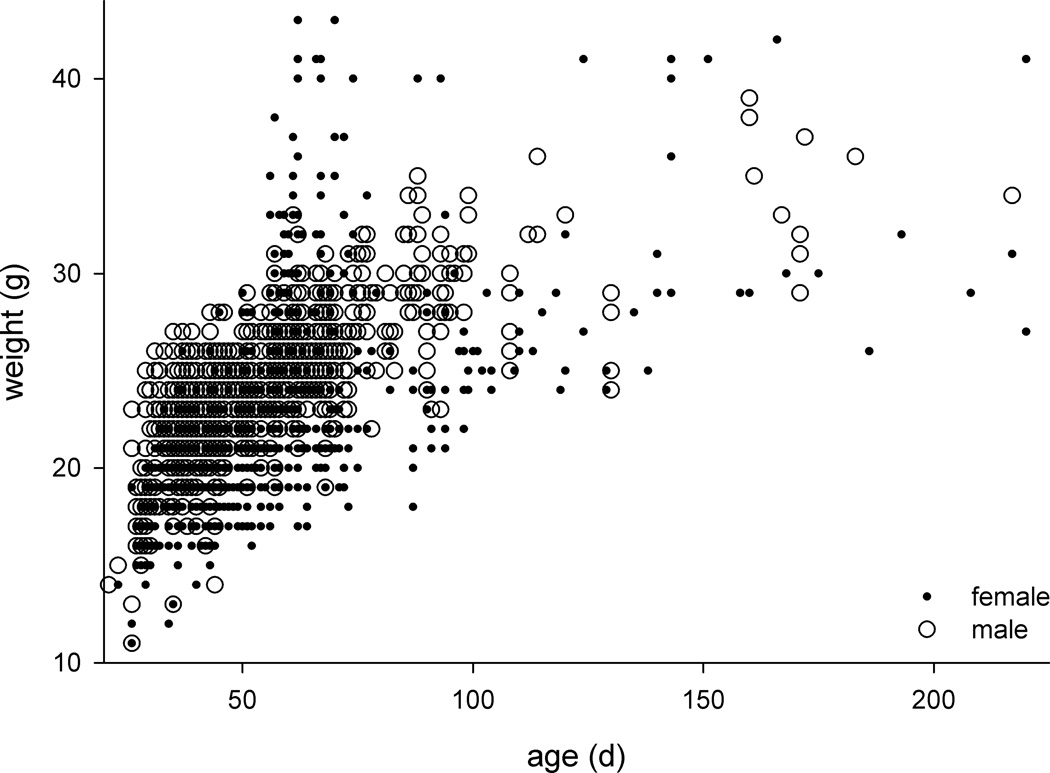
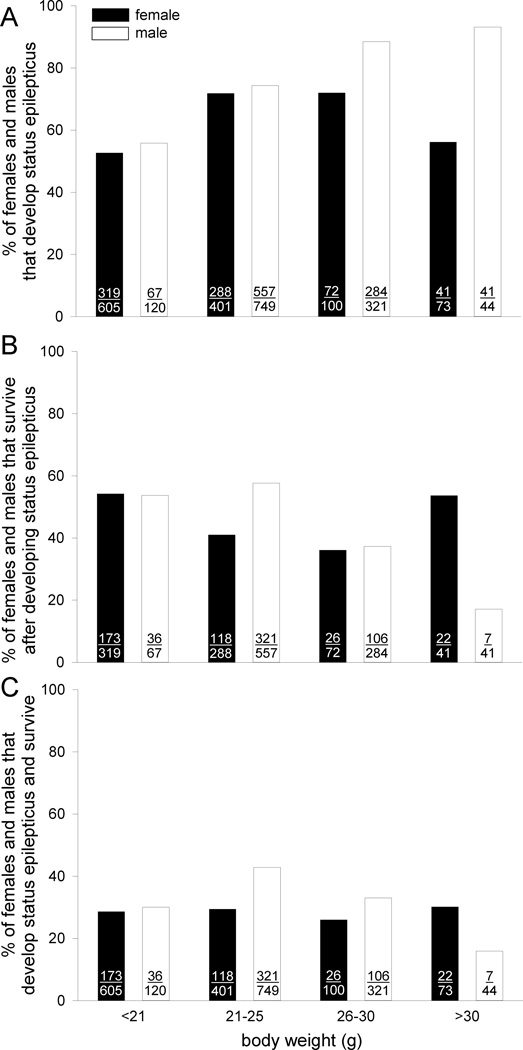
For the entire sample, median and average body weight was 23 g and 23 ± 4 g, respectively (range 11–57g). As expected, body weight increased with age (Figure 3). Median and average body weight of males (24 g and 24 ± 3 g, range 11–39 g) was 1.2-times higher than that of females (20 g and 22 ± 5 g, range 11–57 g; p<0.001, t test), although there was a group of pregnant female mice (mostly ~70 d old) with high body weights. To evaluate effects of body weight, female and male mice were divided into 4 groups: <21 g, 21–25 g, 26–30 g, and >30 g. Weight groups consisted of 369 ± 254 (range 73–605) and 309 ± 316 (range 44–749) female and male mice, respectively. Female mice at intermediate weights (21–30 g) were more likely to develop status epilepticus (Figure 4A) but less likely to survive status epilepticus (Figure 4B) (p<0.001, Chi-squared tests). The opposing effects canceled out, and the percentage of female mice that developed status epilepticus and survived remained constant at ~28% across all body weights (Figure 4C) (p=0.883, Chi-squared test). In contrast, male mice were increasingly likely to develop status epilepticus as body weight increased (Figure 4A) (p<0.001, Chi-squared test). However, survival of status epilepticus in males dropped progressively from 58% (321/557) at 21–25 g to only 17% (7/41) at >30 g (Figure 4B) (p<0.001, Chi-squared test). Consequently, male mice at 21–25 g were most likely to develop status epilepticus and survive, and those >30 g were least likely (Figure 4C) (p<0.001, Chi-squared test).
Figure 3.
In general, body weight increased with age, and males were larger than females, with the exception of obviously pregnant females (high weight females mostly ~70 d old). To improve visualization of results, mice that weighed >44 g (n=2) or were >225 d old (n=2) were excluded from the plot.
Figure 4.
Outcomes of pilocarpine treatment as a function of gender and body weight. A Of all mice treated (indicated in bars), percentage that developed status epilepticus. Numbers of animals are indicated in bars. B Of mice that developed status epilepticus, percentage that survived. C Of all mice treated, percentage that develop status epilepticus and survive. See text for statistical analyses.
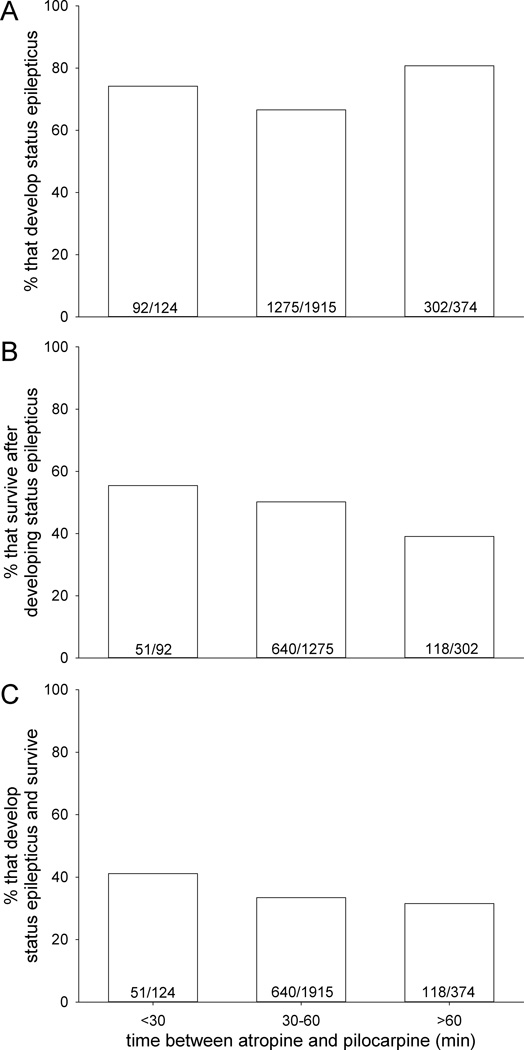
Latency between atropine and pilocarpine treatment
For the entire sample, median and average time between administration of atropine methyl bromide and pilocarpine was 46 min and 49 ± 17 min (range 18 min to 2 h and 19 min). Male and female mice responded similarly to changes in latency between atropine and pilocarpine, so data from both sexes were combined. To test for correlations with outcomes of pilocarpine treatment, mice were divided into groups based on time between atropine and pilocarpine: <30 min (n=124), 30–60 min (n=1915), and >60 min (n=374). Mice were less likely to develop status epilepticus when atropine was administered 30–60 min before pilocarpine compared to <30 min or >60 min (p<0.001, Chi-squared test) (Figure 5A). Mice were 1.4-times more likely to survive after developing status epilepticus if the timing between atropine and pilocarpine was <30 min (55%, 51/92) versus >60 min (39%, 118/302) with intermediate results at 30–60 min (50%, 640/1275) (Figure 5B) (p<0.001, Chi-squared test). Ultimately, mice were 1.2-times more likely to develop status epilepticus and survive if they were treated with pilocarpine <30 min after atropine (41%, 51/124) versus longer (33%, 758/2325) (Figure 5C) (p<0.001, Chi-squared test).
Figure 5.
Outcomes of pilocarpine treatment as a function of time between administration of atropine methyl bromide and pilocarpine. A Of all mice treated, percentage that developed status epilepticus. Numbers of animals are indicated in bars. B Of mice that develop status epilepticus, percentage that survived. C Of all mice treated, percentage that developed status epilepticus and survived. Time between treatment with atropine methyl bromide and pilocarpine correlated with each parameter (p<0.001, Chi-squared test).
Optimal group
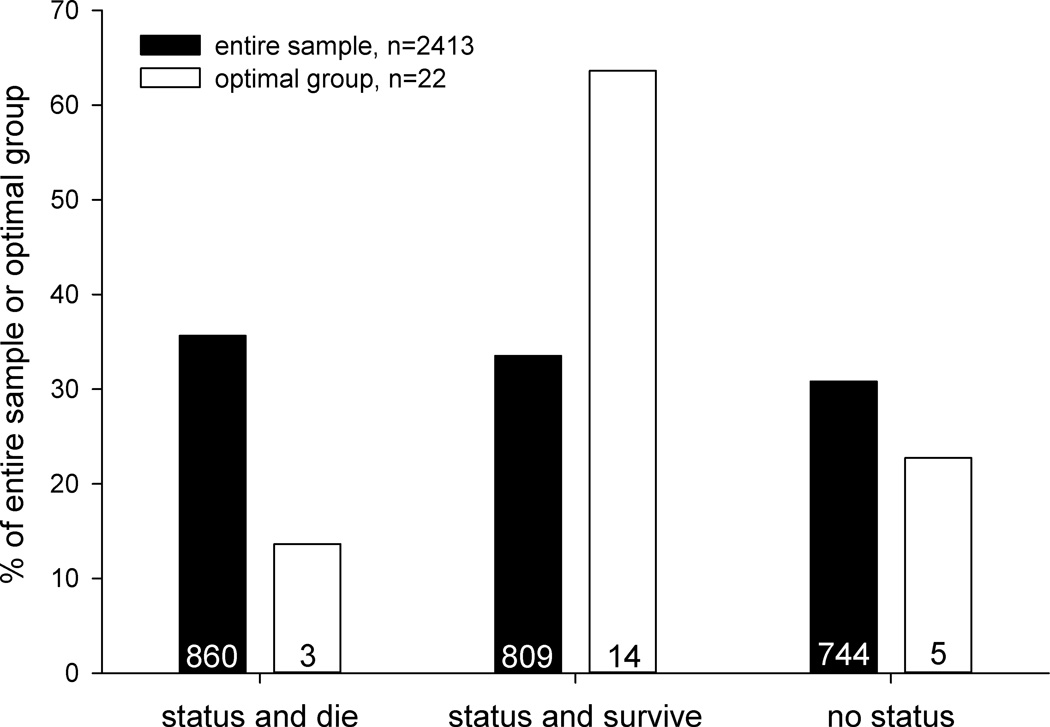
Based on results above, the optimal group for developing status epilepticus and surviving would be males, 6–7 weeks old, and 21–25 g, with pilocarpine administered 18–30 min after atropine methyl bromide. Our sample included 22 mice that matched these parameters. Compared to the entire sample, that group was 1.1-times more likely to develop status epilepticus (77%, 17/22 versus 69%, 1669/2413), 1.7-times more likely to survive if they developed status epilepticus (82%, 14/17 versus 48%, 809/1669), and 1.9-times more likely to develop status epilepticus and survive (64%, 14/22 versus 34%, 809/2413) (Figure 6).
Figure 6.
Outcomes of pilocarpine treatment for the entire sample of mice and the optimal group based on analysis of sex (male), age (6–7 weeks old), body weight (21–25 g), and latency between atropine methyl bromide and pilocarpine (18–30 min).
Discussion
The principal findings of the current study on pilocarpine treatment in FVB mice are the following. Males were more likely to develop status epilepticus than females, but sexes were equally likely to survive status epilepticus. Pregnancy did not affect the outcome of pilocarpine treatment. The probability of developing status epilepticus increased at ~70 d old in males and at ~90 d old in females. Younger (<28 d) and older (>84 d) mice were less likely to survive status epilepticus than animals at intermediate ages. Male mice weighing 21–25 g were most likely to develop status epilepticus and survive, whereas body weight had no net effect on this outcome parameter in females. Finally, mice were most likely to develop status epilepticus and survive when atropine methyl bromide was administered ~25 min before pilocarpine. These findings suggest that to maximize the percentage of mice that develops status epilepticus and survives it is best to use males, ~23 g, and ~50 d old, and administer pilocarpine 20–30 min after atropine methyl bromide.
The present study evaluated mice on the FVB background strain. FVB mice are more likely to display seizures after pilocarpine treatment than are C57BL/6 mice (Mahajeri et al., 2004), are vulnerable to kainate-induced excitotoxic neuron loss, unlike some other strains (Schauwecker and Steward, 1997), and are more sensitive to electroshock-induced seizures than several other strains (Frankel et al., 2001). Some female FVB mice over 16 weeks of age, which is older than almost all mice included in the present study, develop spontaneous seizures that can be fatal (Mahler et al., 1996; Goelz et al., 1998). The underlying epileptogenic mechanisms in older female FVB mice are unclear. They might be related to reduced survival after status epilepticus of ~6 week old female versus male mice in the present study. However, that possibility is difficult to reconcile with the finding that compared to males, which have little if any predisposition to develop spontaneous seizures, females were less likely to develop status epilepticus. The FVB mice used in the present study are transgenic, which raises the possibility that observed phenotypes might be attributable to positional effects or copy numbers of the GAD-GFP transgene (Crusio et al., 2009).
Factors affecting outcomes of pilocarpine treatment might vary depending on species and strain, because different species and strains vary with respect to seizure sensitivity and seizure-induced effects. For example, mice are more sensitive to the convulsant effects of pilocarpine than are rats (Zablocka and Esplin, 1963). Many studies have shown differences in seizure susceptibility and seizure-induced effects between different mouse strains (Engstrom and Woodbury, 1988; McKhann et al., 2003; Mohajeri et al., 2004; Winawer et al., 2007a,b; Schauwecker, 2012) and even between mice of the same strain that have been bred separately (Borges et al., 2003; Müller et al., 2009c). Nevertheless, results of the present study are similar to those of previous studies on rats. For example, male FVB mice in the present study were more likely to develop status epilepticus than female mice. Similarly, male rats are more likely to develop status epilepticus after pilocarpine treatment, an effect that has been attributed at least in part to testosterone (Mejías-Aponte et al., 2002). In the present study, mortality after developing status epilepticus was highest in mice <28 d old. Similarly, lethal toxicity of pilocarpine (Cavalheiro et al., 1987; Priel et al., 1996) and kainate (Albala et al., 1984) is highest in rats during the third and fourth weeks of life. And in the present study older mice (>90 d) were more likely to develop status epilepticus, similar to pilocarpine treated rats (Patel et al., 1988). Therefore, findings of the present study on outcomes of pilocarpine treatment in FVB mice might be generally applicable to rats and other mouse strains. It would be helpful to test this possibility by performing additional retrospective analyses on experiments that have utilized C57BL/6 mice, because many transgenic mice have been backcrossed on that strain.
The present study identified the optimal sex, age, and body weight and latency between administration of atropine methyl bromide and pilocarpine for pilocarpine treatment. However, even under the best conditions, only 64% of treated mice developed status epilepticus and survived. Some epilepsy research experiments (for example, many anti-epileptogenesis studies) require only animals that have experienced status epilepticus. In those cases, mice that do not develop status epilepticus and survive are not useful. How might outcomes be further improved? To increase the proportion of animals that develops status epilepticus, the convulsant effects of pilocarpine can be enhanced by pretreatment with lithium in rats (Honchar et al., 1983). However, lithium fails to have the same effect in mice (Turski et al., 1989; Müller et al., 2009a). To increase the percentage of animals that develops status epilepticus and reduce mortality associated with single high-dose administration, repeated low-dose protocols were developed for kainate (Hellier et al., 1998) and pilocarpine treatment in rats (Glien et al., 2001). A similar approach was developed for pilocarpine treatment in mice (Gröticke et al., 2007; Müller et al., 2007a,b,c). However, mortality rates after repeated low-dose pilocarpine treatment in mice remain high (~50%). An anecdotal report suggests addition of a central muscle relaxant (xylazine) might improve survivability in mice after pilocarpine treatment (Curia et al., 2008). Intrahippocampal injection of pilocarpine in rats does not evoke status epilepticus in all animals, but survival of those that develop status epilepticus is high (Furtado et al., 2002). A similar intrahippocampal pilocarpine protocol might work in mice, but the necessity of surgical cannulation imposes additional work for investigators and additional stress for animals.
Another concern is that the present study measured mortality only for the first 8 h after pilocarpine treatment, because data were not available for longer periods. Most rodents that die after chemoconvulsant-induced status epilepticus do so within the time span evaluated in the present study, but additional mortality occurs later (Boyd and Fulford, 1961), and it can be substantial in some mouse strains (Winawer et al., 2007b). Supportive care for up to a week after pilocarpine treatment, including maintenance of hydration and blood glucose with fluid therapy and maintenance of body temperature, reduces delayed mortality after status epilepticus.
In summary, findings of the present study identified characteristics of sex, age, body weight, and relative timing of administration of a peripherally acting muscarinic acetylcholine receptor antagonist that correlated with better outcomes of pilocarpine treatment. However, much room for improvement remains, and more work is needed to optimize treatment outcomes and animal welfare.
Acknowledgements
Supported by NINDS and NCRR of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albala BJ, Moshé SL, Okada R. Kainic-acid-induced seizures: a developmental study. Dev Brain Res. 1984;13:139–148. doi: 10.1016/0165-3806(84)90085-3. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Boyd EM, Fulford RA. Pilocarpine-induced convulsions and delayed psychotic-like reaction. Can J Biochem Physiol. 1961;39:1287–1294. [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wen X. Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy. Epilepsia. 2011;52:2057–2064. doi: 10.1111/j.1528-1167.2011.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Naffah-Mazzacoratti MG, Mello LE, Leite JP. The pilocarpine model of seizures. In: Pitkänen A, Schwartzkroin PA, Moshé SL, editors. Models of Seizures and Epilepsy. New York: Elsevier; 2006. pp. 433–448. [Google Scholar]
- Cavalheiro EA, Santos NF, Priel MR. The pilocarpine model of epilepsy in mice. Epilepsia. 1996;37:1015–1019. doi: 10.1111/j.1528-1157.1996.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Silva DF, Turski WA, Calderazzo-Filho LS, Bortolotto ZA, Turski L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Dev Brain Res. 1987;37:43–58. doi: 10.1016/0165-3806(87)90227-6. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RSG, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Meth. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom FL, Woodbury DM. Seizure susceptibility in DBA and C57 mice: the effects of various convulsants. Epilepsia. 1988;29:389–395. doi: 10.1111/j.1528-1157.1988.tb03736.x. [DOI] [PubMed] [Google Scholar]
- Frankel WN, Taylor L, Beyer B, Tempel BL, White HS. Electroconvulsive thresholds of inbred mouse strains. Genomics. 2001;74:306–312. doi: 10.1006/geno.2001.6564. [DOI] [PubMed] [Google Scholar]
- Furtado MA, Braga GK, Oliveira JAC, Del Vecchio F, Garcia-Cairasco N. Behavioral, morphologic, and electroencephalographic evaluation of seizures induced by intrahippocampal microinjection of pilocarpine. Epilepsia. 2002;43(suppl 5):37–39. doi: 10.1046/j.1528-1157.43.s.5.41.x. [DOI] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Voigt H, Ebert U, Löscher W. Repeated low-dose treatment of rats with pilocarpine: low mortality but high proportion of rats developing epilepsy. Epilepsy Res. 2001;46:111–119. doi: 10.1016/s0920-1211(01)00272-8. [DOI] [PubMed] [Google Scholar]
- Goelz MF, Mahler J, Harry J, Myers P, Clark J, Thigpen JE, Forsythe DB. Neuropathologic findings associated with seizures in FVB mice. Lab Anim Sci. 1998;48:34–37. [PubMed] [Google Scholar]
- Halabisky B, Parada I, Buckmaster PS, Prince DA. Excitatory input onto hilar somatostatin interneurons is increased in a chronic model of epilepsy. J Neurophysiol. 2010;104:2214–2223. doi: 10.1152/jn.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Loose MD, Qi M, Levey A, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci USA. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Honchar MP, Olney JW, Sherman WR. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983;220:323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- Lemos T, Cavalheiro EA. Suppression of pilocarpine-induced status epilepticus and the late development of epilepsy in rats. Exp Brain Res. 1995;102:423–428. doi: 10.1007/BF00230647. [DOI] [PubMed] [Google Scholar]
- Lew FH, Buckmaster PS. Is there a critical period for mossy fiber sprouting in a mouse model of temporal lobe epilepsy? Epilepsia. 2011;52:2326–2332. doi: 10.1111/j.1528-1167.2011.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler JF, Stokes W, Mann PC, Takaoka M, Maronpot RR. Spontaneous lesions in aging FVB/N mice. Toxicol Path. 1996;24:710–716. doi: 10.1177/019262339602400606. [DOI] [PubMed] [Google Scholar]
- Maslanski JA, Powelt R, Deirmengiant C, Patelt J. Assessment of the muscarinic receptor subtypes involved in pilocarpine-induced seizures in mice. Neurosci Lett. 1994;168:225–228. doi: 10.1016/0304-3940(94)90456-1. [DOI] [PubMed] [Google Scholar]
- McKhann GM, II, Wenzel HJ, Robbins CA, Sosunov AA, Schwartzkroin PA. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122:551–561. doi: 10.1016/s0306-4522(03)00562-1. [DOI] [PubMed] [Google Scholar]
- Mejías-Aponte CA, Jiménez-Rivera CA, Segarra AC. Sex differences in models of temporal lobe epilepsy: role of testosterone. Brain Res. 2002;944:210–218. doi: 10.1016/s0006-8993(02)02691-4. [DOI] [PubMed] [Google Scholar]
- Mohajeri MH, Madani R, Saini K, Lipp H-P, Nitsch RM, Wolfer DP. The impact of genetic background on neurodegeneration and behavior in seizured mice. Gene Brain Behav. 2004;3:228–239. doi: 10.1111/j.1601-1848.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- Müller CJ, Bankstahl M, Gröticke I, Löscher W. Pilocarpine vs. lithium-pilocarpine for induction of status epilepticus in mice: development of spontaneous seizures, behavioral alterations and neuronal damage. Eur J Pharmacol. 2009a;619:15–24. doi: 10.1016/j.ejphar.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Müller CJ, Gröticke I, Bankstahl M, Löscher W. Behavioral and cognitive alterations, spontaneous seizures and neuropathology developing after a pilocarpine-induced status epilepticus in C57BL/6 mice. Exp Neurol. 2009b;219:284–297. doi: 10.1016/j.expneurol.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Müller CJ, Gröticke I, Hoffmann K, Schughart K, Löscher W. Differences in sensitivity to the convulsants pilocarpine in substrains and sublines of C57BL/6 mice. Genes Brain Behav. 2009c;8:481–492. doi: 10.1111/j.1601-183X.2009.00490.x. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Meldrum BS, Fine A. Susceptibility to pilocarpine-induced seizures in rats increases with age. Behav Brain Res. 1988;31:165–167. doi: 10.1016/0166-4328(88)90019-8. [DOI] [PubMed] [Google Scholar]
- Priel MR, Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996;26:115–121. doi: 10.1016/s0920-1211(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE. Strain differences in seizure-induced cell death following pilocarpine-induced status epilepticus. Neurobiol Dis. 2012;45:297–304. doi: 10.1016/j.nbd.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibley H, Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002;49:109–120. doi: 10.1016/s0920-1211(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321:237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Calalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Winawer MR, Kuperman R, Niethammer M, Sherman S, Rabinowitz D, Guell IP, Ponder CA, Palmer AA. Use of chromosome substitution strains to identify seizure susceptibility loci in mice. Mamm Genome. 2007a;18:23–31. doi: 10.1007/s00335-006-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer MR, Makarenko N, McCloskey DP, Hintz TM, Nair N, Palmer AA, Scharfman HE. Acute and chronic responses to the convulsants pilocarpine in DBA/2J and A/J mice. Neuroscience. 2007b;149:465–475. doi: 10.1016/j.neuroscience.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablocka B, Esplin DW. Central excitatory and depressant effects of pilocarpine in rats and mice. J Pharmacol Exp Ther. 1963;140:162–169. [PubMed] [Google Scholar]
- Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]