Abstract
Background
Major racial and gender differences have been documented in the natural history and treatment responses of chronic hepatitis C virus (HCV) infection, however, potential mechanisms have remained enigmatic. We hypothesized that racial- and gender-related differences in NK cell populations may explain altered natural history and treatment responses.
Methods
Our study cohort consisted of 29 African-American (AA, 55% male) and 29 Caucasian-American (CA, 48% male) normal uninfected control subjects. Multi-parameter flow-cytometric analysis was used to characterize levels, phenotype with respect to 14 NK receptors, and lymphokine-activated killing (LAK) function. Gene expression was assessed by real-time RT-PCR after 6 hour in vitro stimulation with TLR ligands. The ability to control HCV infection was assessed in the Huh-7.5/JFH-1 co-culture system.
Results
NK expression of natural cytotoxicity receptor NKp46 was strongly associated with CA race and female gender and correlated positively with LAK activity (p=0.0054). NKp46high NKs were more efficient at controlling HCV than their NKp46low counterparts (p<0.001). Similarly, ligation of NKp46 on isolated NK cells resulted in a significant reduction in the HCV copy number detected in Huh-7.5/JFH-1 co-culture (MOI 0.01) at an effector:target ratio of 5:1 (p<0.005). After TLR stimulation, genes involved in cytotoxicity but not cytokine genes were significantly upregulated in NKp46high NKs. Cytokine stimulation (IL-12 and IL-15) demonstrated that NKp46high NK cells have significantly higher IFN-γ production than NKp46low cells. TLR stimulation significantly induced degranulation as well as TRAIL, Fas and TNF-α protein expression in NKp46high NKs. NKp46 ligand was induced on HCV-infected hepatocytes .
Conclusions
NKp46 expression may contribute to differential HCV responses. NKp46 expression correlates with anti-HCV activity in vitro and thus may prove to be a useful therapeutic target.
Keywords: Human, Natural Cytotoxicity Receptors, Cytotoxicity, IFN-γ
Natural killer (NK) cells constitute the first line of host defense against viral pathogens (1) (2);they eliminate virus-infected cells both directly via cytolytic mechanisms and indirectly by secreting cytokines such as IFN-y (3-4). NK cell activity is stringently controlled by inhibitory NK receptors (NKRs), which in steady-state conditions override signals provided by engagement of activating receptors. NKRs include the predominantly inhibitory killer immunoglobulin-like receptors (KIR), C-type lectin-like receptors of the CD94/NKG2 family comprising inhibitory (NKG2A) and activatory (NKG2C/D) isoforms, as well as the natural cytotoxicity receptors (NCRs) such as NKp30 (NCR3/CD337), NKp44 (NCR2/CD336) and NKp46 (NCR1/CD335) that deliver activatory signals (5) (6) (7).
The NKp46 receptor, expressed on both resting and activated NK cells, is considered the major human NCR involved in NK cell cytotoxicity (8) (9). Levels of expression of this NCR have been shown to differ significantly amongst donors and to correlate directly with natural cytotoxicity in these individuals (8). In addition to playing a significant role in anti-tumor immunity, NKp46 is clearly important for anti-viral immunity. Originally reported to interact with influenza hemaggglutinin (9), NKp46 is involved in the NK response against influenza-infected monocyte-derived dendritic cells (mDCs) (10). As further corroboration, a murine model demonstrates a critical function for NKp46 in the in vivo eradication of influenza virus (11). Although the cellular ligand has yet to be identified, recent work demonstrated the dominant contribution of this receptor to the activation of NK cells in response to human cytomegalovirus (HCMV)-infected mDCs (12). Down-regulation of NKp46 has been implicated in HPV-16 infection and cervical cancer progression (13). Down-regulation of NCRs including NKp46 has been implicated in attenuation of NK cell activity in HIV-infection (14). To date, there are limited data on NKp46 expression in HCV-infected individuals. A decrease in NKp46 has been demonstrated in acute and chronic HCV in some studies (15) (16); however, others have reported increased expression (17). A recent study suggests that up-regulation of NKp46 in response to IFN-α is predictive of SVR in chronic HCV infection (18).
Toll-like receptors (TLRs) constitute a family of conserved pattern recognition sensors that play a prominent role during the early anti-viral response via the induction of type I interferons and inflammatory cytokines (19). Several viruses have been shown to activate the TLR signaling cascade (20). NK cells express pattern-recognition receptors including several members of the TLR family, although not all are functional. Human NK cells express functional TLR2 (21), TLR3 (22),TLR7/8 (23) and TLR9 (24). Thus, in addition to classical NKRs, TLRs are likely to be important for effective NK anti-viral responses.
Considerable evidence indicates that the risk of viral persistence, natural history, and response to antiviral therapy in chronic HCV infection varies among racial groups (25) (26). In a large study of the natural history of HCV, patients with spontaneous viral clearance were more likely to be non-black and female (25). Symptomatic females have an eight-fold greater chance of clearing HCV infection in the acute infection setting (27). African Americans (AAs) with chronic hepatitis C genotype 1 infection have lower rates of virologic response to peginterferon and ribavirin than Caucasians (CAs) and these differences are not explained by disease characteristics, baseline viral levels, or amount of medication taken (28). Taken together, the above studies provide evidence that both gender and race influence the natural history of and treatment efficacy in some human viral infections. In this study, we show race and gender-related differences in the expression of the NCR NKp46 correlates with increased anti-viral cytolytic activity and gene transcription. Our data suggest that expression patterns of NKp46 on NK cells may explain some of the differences observed for HCV natural history and treatment responses. NKp46 expression correlates with anti-HCV activity in vitro and thus may prove to be a useful therapeutic target.
Materials and Methods
Study population
This study utilized 58 uninfected control subjects equally distributed by African American (AA, n=29) or Caucasian American (CA, n=29) race. The median age for AAs was 33.5 (range 21-62) and 55% were male. For CA subjects the median age was 34 (21-60) and 48% were male. This research was conducted in accordance with the Helsinki principles: all patients gave informed, written consent prior to their participation, and was approved by the University of Colorado Denver (UCD) Institutional Review Board.
Sample collection and storage
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by cellular preparation tubes (Becton-Dickinson, Franklin Lakes, New Jersey; anticoagulant sodium citrate). PBMCs were viably frozen in 80% FBS (BioWhittaker, Walkersville, MD), 10% DMSO, and 10% RPMI 1640 Media (Life Technologies, Grand Island, NY) and stored in liquid nitrogen for subsequent analyses.
Antibodies for detection and FACS analysis of antigen expression
Four-color multiparameter flow cytometry was performed using a BD FACSCanto II or BD FACScan instrument (BD Biosciences, San Jose, CA) compensated with single fluorochromes and analyzed using Diva™ or CellQuest™ software (BD Biosciences). Lymphocyte populations were identified by their characteristic forward scatter/side scatter (fsc:ssc) properties. Fluorochrome-labeled (PerCP/APC) monoclonal antibodies (MAb) specific for CD3 and CD56 (BD Biosciences) were used to identify NK (CD3-CD56+) and NT (CD3+CD56+) cells within the overall lymphocyte population. Anti-NK receptor (NKR) antibodies (FITC/PE) CD161, CD94, CD95, CD16, CD158a, CD158b, CD158e and NKG2D were obtained from BD Biosciences. Anti-NKG2C-PE and TRAIL-PE MAbs were purchased from R&D systems (Minneapolis, MN). Anti-NKG2A-PE, NKp30-PE, NKp44-PE, and NKp46-PE were obtained from Immunotech (Beckman Coulter, Fullerton, CA). Anti-FasL-Pe was purchased from eBioscience (San Diego, CA). Thawed PBMCs (1-2 × 106) were stained for cell surface antigen expression at 4°C in the dark for 30 minutes. Then, washed twice in 2 ml phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.01% sodium azide (Facs Wash) and subsequently fixed in 200ul of 1% paraformaldehyde (PFA, Sigma-Aldrich, St. Louis, MO). Isotype-matched control antibodies were used to determine background levels of staining.
Cytotoxicity assays
Thawed mononuclear cell suspensions were enriched for NKs using the NK Isolation Kit II from Miltenyi Biotec (Gladbach, Germany) according to the manufacturer’s instructions. Median purity of NKs was >90% in all cases. Following isolation, the NKs were cultured +/− IL-2 (25ng/ml, R&D) for 48 hours at 37°C and 5% CO2. Following culture, carboxy fluorescein succinimidyl ester (CFSE) labeled target cells (K562s) were added to the NKs at effector:target concentrations of 0:1 (negative control) and 10:1 (test) and incubated at 37°C for 4 hours. After incubation, cytotoxicity was measured using the flow-cytometry based Total Cytotoxicity & Apoptosis Detection Kit from Immunochemistry (Bloomington, MN). Immediately before acquisition, 7-aminoactinomycin D (7-AAD) was added to effector:target (E:T) populations and incubated for 15 minutes on ice. Cells treated with 0.1% Triton-X served as positive controls.
Hepatocyte cytotoxicity assay
NKs were enriched using magnetic beads and surface stained for CD3, CD56 and NKp46 as described above. NKs (CD3-CD56+) were FACS sorted on expression of NKp46 using a FACS Aria instrument (BD). Huh 7.5 cells (Apath LLC, St. Louis, MO) were seeded at a concentration of 1.25×105 cells/well in 24-well plates. After 24 hours NKp46high and NKp46low/neg fractions of NKs were added at a ratio of 5 NK to 1 Huh 7.5 cell (or 1:1). Cells were infected simultaneously with JFH-1 (National Institute of Infectious Diseases, Tokyo, Japan) at an MOI=0.01. Five days post infection; cells were harvested for RNA extraction (RNeasy mini Kit, Qiagen). RNA was transcribed to cDNA using the QuantiTect Reverse Transcription Kit (Qiagen) and HCV transcripts were detected using a 7300 Real Time PCR instrument (Applied Biosystems; Carlsbad, CA). A standard curve was created using JFH-1 plasmid stock (range 1×107 – 1×101). PCR Taqman Master Mix, primers and probes were purchased from Applied Biosystems. Primer and probe sequences were as follows; HCV-forward GCA CAC TCC GCC ATC AAT CAC T; HCV-reverse CAC TCG CAA GCG CCC TAT CA; HCV-probe 6FAM AGG CCT TTC GCA ACC CAA CGC TAC T TAMRA.
TLR response
NK cells were enriched from PBMCs (n=4) and stained for expression of CD56/CD3 and NKp46 as described above. FACS sorting (Aria, BD) was used to isolate NK cells based on the expression of NKp46. Sorted populations were cultured for 6 hours at a concentration of one million/ml in the presence or absence of a TLR-stimulation cocktail (100ug/ml poly-IC [TLR3], 5uM Loxoribine [TLR7] and 5um CpG [TLR9]). After culture, NK cells were washed and RNA extracted from cell pellets using the PicoPure™ RNA Isolation Kit (Arcturus, Applied Biosystems, Carlsbad, CA). cDNA was transcribed using 500ng of RNA in a 20ul reaction using the Quantitect RT Kit (Qiagen, Valencia, CA). For the detection of TLR responses at a protein level, PBMCs (n=5) were incubated with the TLR cocktail as above for 24 hours and cell surface expression of TRAIL, Fas and Fas-Ligand were assessed by flow cytometric analysis. Intracellular flow cytometic staining was used to measure TNF-α and IFN-γ responses after six hour stimulation. For some experiments cytokine stimulation (IL-12/IL-15) was used.
Real-time PCR
Gene expression was assessed using the Step One Plus Real time PCR system using the Fast SYBR Green Master Mix Protocol (Applied Biosystems). QuantiTect primer assays for use with Sybr Green detection were purchased from Qiagen/Superarray.
Immunofluorescent staining of hepatocytes
Huh 7.5 cells (Apath LLC, St. Louis, MO) were seeded onto coverslips (1.25 × 105 cells/well in 24-well plates) and incubated overnight. Cells were then infected with JFH-1 (National Institute of Infectious Diseases, Tokyo, Japan) at an MOI of 0.01. Five days post infection coverslips were removed, washed, fixed and stained for expression of NKp46 ligands using an fc fusion protein of the NKp46 receptor coupled to FITC. Images were acquired with a 20x objective lens at constant exposure. An isotype control was used to exclude background staining.
Statistics
Results are expressed as median (range). Non-parametric Mann Whitney U was used to compare differences between patient groups. Significance was defined as a p value of <0.05. The JMP 6.0 (SAS Institute, Inc, Cary NC) statistical software package was used.
Results
NKp46 expression is associated with female gender and Caucasian race:
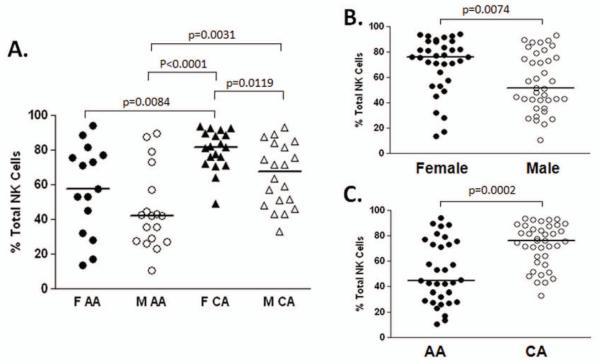
We hypothesized that innate racial- and gender-related difference in NK cell populations may explain altered natural history of chronic viral infection and disparate treatment responses to anti-viral therapy. To address this question we used a cohort of fifty eight age- and sex-matched uninfected control subjects (Table 1). Multi-parameter flow cytometric analysis was used to characterize NK cell levels and phenotype with respect to 14 different activating and inhibitory NK receptors (NKRs). Total NK cell levels did not differ according to race or gender (Table 1). The phenotype of NK cells was remarkably similar in our four test groups (Table 2). However, NK expression of the activatory natural cytotoxicity receptor (NCR) NKp46 was strongly associated with Caucasian American (CA) race and female gender. Female CAs had significantly higher expression than all other groups tested (Figure 1).
Table 1. Lymphocyte distribution.
| Population | F AA† | M AA | F CA | M CA |
|---|---|---|---|---|
| CD56+ Cells* | 17.5 (5.9-26.2)± | 20.35 (7.5-55.66) | 14.8 (6.3-33.95) | 16.32 (3.9-30.12) |
| NK Cells* | 12.2 (4.6-24.2) | 15.01 (6.3-38.64) | 8.8 (2.6-27.81) | 12.25 (3.2-20.6) |
| CD56+ NT Cells* | 2.9 (0.73-14.4) | 3.36 (1.55-17.02) | 3.7 (1.2-22.88) | 2.32 (0.4-17.8) |
| CD56bright NKs* | 6.61 (2.88-59.77) | 5.7 (0.4-15.15) | 7.56 (0.97-24.06) | 6 (0.93-19.26) |
F=Female; M=Male; AA=African American; CA=Caucasian American
% of total Lymphocytes
Median (range)
% of NK Cells
Table 2. Natural Killer cell phenotype.
| Receptor* | F AA† | M AA | F CA | M CA |
|---|---|---|---|---|
| NKp30 | 39.1 (12.4-79.67)‡ | 40.16 (2.4-92.92) | 44.4 (15.62-82.79) | 45.3 (16.03-79.1) |
| NKp44 | 1.5 (0.5-10.1) | 2.8 (1.0-29.79) | 2.35 (1.2-4.43) | 2.51 (0.65-7.3) |
| NKp46 | 57.6 (13.8-94.04) | 42.18 (10.5-89.48) | 81.64 (49.2-93.34) | 67.49 (33.3-92.88) |
| NKG2A | 51.3 (38.72-84) | 58.5 (20-79.05) | 49.73 (32.4-69.69) | 51.27 (26-66.93) |
| NKG2C | 9.8 (0.8-57.36) | 12.2 (0.5-51.1) | 6.22 (2.06-32.44) | 5.61 (1.62-31.02) |
| NKG2D | 88.2 (60.7-98.82) | 91.02 (48.5-98.4) | 93.5 (40.3-99.23) | 89.24 (55.4-98.11) |
| CD158a | 36.4 (8.4-61.93) | 36.84 (9.23-55.69) | 25.8 (5.12-60.23) | 26.59 (9-64.44) |
| CD158b | 25.8 (19.84-62.5) | 33 (8.93-50.66) | 32.02 (15.37-49.83) | 26.69 (21.9-49.99) |
| CD158e | 9.35 (3.9-21.65) | 13.2 (0-48.7) | 12.22 (0-47.2) | 11.35 (0-27.4) |
| TRAIL | 1.4 (0.6-22.2) | 5.53 (0.75-58.39) | 1.63 (0.68-8.7) | 4.09 (0.52-26.6) |
| Fas | 32.4 (4.1-82.08) | 73.68 (11.2-86.75) | 56.6 (15.5-82.95) | 52.45 (11-80.5) |
| Fas-L | 1.3 (0.5-7.9) | 1.9 (0.48-29.92) | 1.4 (0.59-8.42) | 3.11 (0.6-14.4) |
| CD16 | 79 (37-92.2) | 67.71 (20.1-88.27) | 83.35 (40.53-92.6) | 81.17 (29.8-95.15) |
| CD161 | 56.7 (18.53-95.44) | 59.84 (22.8-91.39) | 69.9 (12.28-97.34) | 68.22 (22-94.77) |
% of NK Cells
F=Female; M=Male; AA=African American; CA=Caucasian American
Median (range)
Figure 1. Higher NKp46 expression in females and Caucasians.
Multi-parameter flow cytometric analysis was used to assess the expression of a range of NK receptors on NK cells from 58 normal control subjects. Of the 14 receptors assayed only the natural cytotoxicity receptor NKp46 differed significantly amongst donors. The highest expression of NKp46 was detected in the female (F) Caucasian American (CA) group and the lowest in the male (M) African American (AA) group (A). Relatively increased levels of expression of NKp46 were associated with female gender (B) and Caucasian race (C).
LAK activity correlates with NKp46 expression
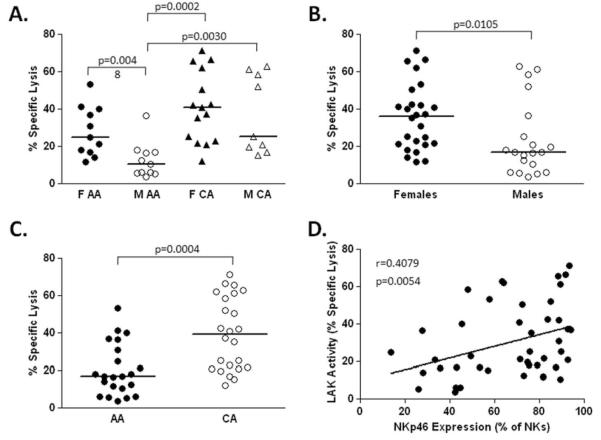
NK cells eliminate virus-infected cells directly via cytolytic mechanisms; therefore, we examined the ability of bead-purified NK cells to kill the NK-sensitive cell line K562 using a standard flow-based assay (29). LAK activity mirrored the pattern seen for NKp46 expression with female CAs demonstrating highest activity and male AAs being weakest. NK cells isolated from females had greater activity than males (35.93% vs 17%, p=0.0105). In addition, CA subjects had significantly higher lymphokine activated killing (LAK) activity than AA subjects (39.08% vs 16.92%, p=0.0004). NKp46 expression correlated positively with LAK activity (p=0.0054) suggesting that increased NKp46 expression contributes to more effective cytolytic activity of NK cells (Figure 2). We confirmed this by testing the ability of FACS-purified NKp46high/low subsets of NK cells to lyse NK cell sensitive targets (K562) in the presence (lymphokine activated killing, LAK) or absence (natural cytotoxicity) of IL-2. NK cells expressing high levels of NKp46 have increased LAK activity compared to NKp46low NK cells. No difference was seen in natural cytotoxicity (data not shown).
Figure 2. LAK activity correlates with NKp46 expression.
Interleukin 2-induced (25ng/ml) lymphokine activated killing (LAK) activity was measured against K562 target cells as described in materials and methods. The highest LAK activity was detected in the female (F) Caucasian American (CA) group and the lowest in the male (M) African American (AA) group (A). Relatively increased LAK activity was associated with female gender (B) and Caucasian race (C). LAK activity correlated directly with expression levels of NKp46 (D).
NKp46 expression is associated with increased anti-viral NK cell activity
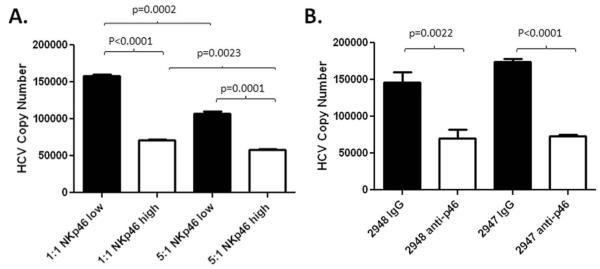
As NKp46 is important for cytotoxic function and expression on NK cells was significantly correlated with LAK activity, we next tested the functional significance of NKp46 expression in a more relevant viral model. We used the Huh 7.5 JFH-1 in vitro HCV infection system to compare the ability of FACS-sorted NKp46low/neg and NKp46high subsets of NK cells to attenuate infection of hepatocytes by HCV. NKp46high NKs were more efficient at controlling HCV copy number in this system than their NKp46low counterparts even at an effector to target ratio (E:T) of 1:1 (Figure 3A). Similarly, direct ligation of NKp46 using an agonist antibody on isolated NK cells resulted in a significant reduction in the HCV copy number detected in Huh-7.5/JFH-1 co-culture at an E:T of 5:1 (Figure 3B).
Figure 3. NKp46 expression is associated with increased anti-viral NK cell activity.
(A) The Huh 7.5 JFH-1 in vitro infection system was used to assess the efficacy FACS-sorted NKp46low/neg and NKp46high subsets of NK cells to attenuate infection of hepatocytes by HCV. Experiments were carried out in triplicate. NK cells expressing high levels of NKp46 (white bars) were more effective in preventing infection of Huh 7.5 cells than their NKp30low/neg counterparts (black bars) at effector:target ratios of 1:1 or 5:1. (B) The same system was used to compare the ability of bead-purified NK cells stimulated with an agonist antibody targeting NKp46 to attenuate infection. Infection of Huh 7.5 cells in the presence of control IgG at an MOI=0.01 results in robust infection after 5 days (black bars). NK cell activation through NKp46 in the absence of exogenous cytokine significantly reduces HCV copy number detected in hepatocytes.
NKp46 level is associated with increased NK cell expression of death receptor genes and proteins
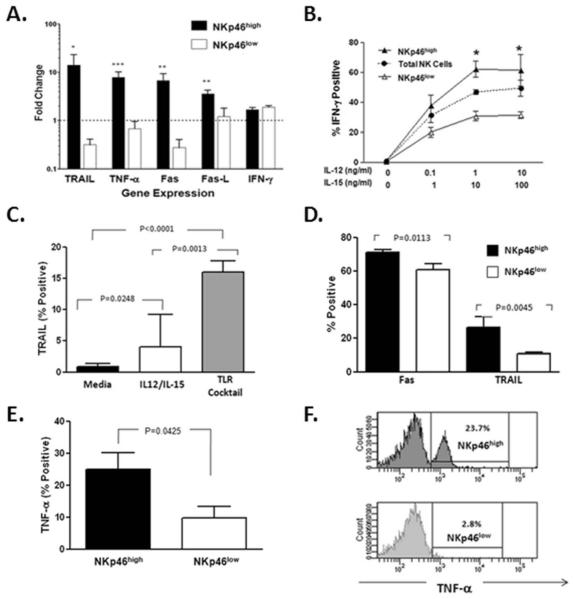
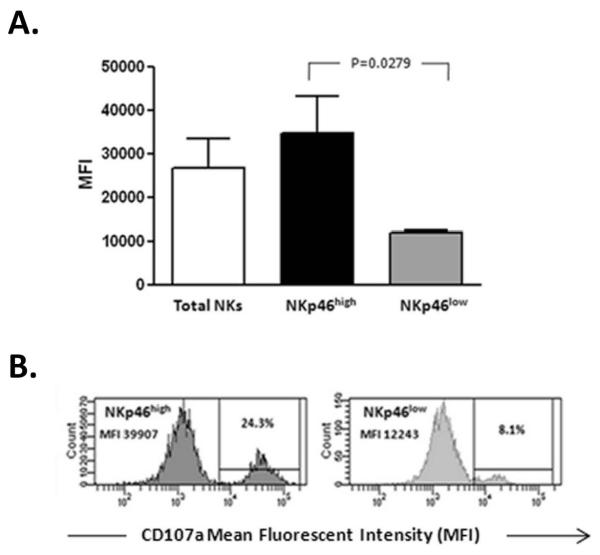
TLRs expressed by NK cells play a prominent role during the early anti-viral response via the induction of type I interferons and inflammatory cytokines (19); therefore, we tested the ability of FACS-purified NKp46high/low subsets of NK cells to respond to stimulation via TLR pathways. Gene expression in NK cell subsets was assessed by real-time RT-PCR after 6 hour in vitro stimulation with TLR ligands. As shown in Figure 4A, several genes involved in cytotoxicity are up-regulated in NK cells expressing high levels of NKp46 in contrast to the expression of the IFN-γ gene which was unchanged. No difference was observed in the expression of type I and type III interferon or SOCS-3 in the NK cell subsets. As production of IFN-γ represents a major function of NK cells in addition to cytotoxicity, we wanted to assess if NKp46 expression was related to the NK cell response to cytokines. IFN-γ expression in NK cell subsets based on the expression of NKp46 was assessed by flow cytometry after 6 hour in vitro stimulation with varying concentrations of IL-12 and IL-15. After stimulation, a higher percentage of NKp46high NKs expressed IFN-γ than NKs expressing low levels of this receptor (Figure 4B). As has been reported previously (30), we saw no enhancement of IFN-γ on ligation of NKp46 with an agonist antibody. These data suggest that the ability to produce IFN-γ is higher in NK cells expressing high levels of NKp46 but is not enhanced by activation of TLR pathways or NKp46 itself. As TLR stimulation resulted in upregulation of genes involved in NK cytolytic activity, we wanted to see if this upregulation was also evident at a protein level. After 24 hour stimulation, Fas protein was significantly upregulated on total NK cells with the TLR cocktail or cytokine stimulation. Upregulation of TRAIL was also observed but to a much greater extent with TLR stimulation compared to cytokine activation (Figure 4C). Of note, in these cultures the expression of both Fas and TRAIL was more prominent for the NKp46high NK cell subset compared to NK cells expressing low levels of NKp46 (Figure 4D). Short-term (6 hour) stimulation with the TLR cocktail resulted in increased TNF-α production predominantly in NK cells expressing high levels of NKp46 (Figure 4E and F). Fas-L expression was unchanged by TLR stimulation (data not shown). A functional correlate of this gene and protein upregulation is suggested by the significantly higher induction of degranulation (CD107a expression) by TLR on NKp46high NK cells in the same cultures (Figure 5).
Figure 4. NKp46 expression is associated with increased expression of killing genes and proteins.
(A) Six hour stimulation of FACS-sorted NKp46high/low NK cell subsets with TLR ligands results in relative up-regulation of genes encoding death receptor/ligands, TRAIL, TNF-α, Fas/Fas-L but not IFN-γ in the NK cell subset expressing high levels (NKp46high). (B) Intracellular IFN-γ production was assessed by flow cytometric analysis of total NK cells or NK subsets gated on the expression of high or low levels of NKp46 after short-term cytokine stimulation in the presence of varying concentrations of IL-12 and IL-15. More robust production of IFN-γ is evident in the NKp46 high NK cell subset (* p<0.05, **p<0.001, ***p<0.0001). (C) Stimulation of PBMCs (n=5) for 24 hours with TLR ligands or IL-12/IL-15 (1ng/ml and 10ng/ml respectively) results in upregulation of TRAIL to a greater extent on NK cells treated with TLR agonists. (D) NK cells sub-gated on the expression of NKp46 demonstrated that upregulation of Fas and TRAIL occurs predominantly in the NKp46high subset. (E) Six hour stimulation with TLR agonists results in upregulation of TNF-α in total NK cells but to a much greater extent in the NKp46high NK cell subset. (F) Representative histograms for TNF-α expression in NKp46high/low subsets are shown.
Figure 5. TLR stimulation induces NK cell degranulation.
(A) Flow cytometric analysis demonstrates that six hour stimulation of PBMCs (n=5) with TLR ligands results in increased degranulation (CD107a expression) of NK cells compared to control cultures. The mean fluorescent intensity (MFI) which correlates directly with the number of molecules on the cell surface is significantly higher for NK cells expressing high levels of NKp46. (B) Representative histograms for CD107a expression for NKp46high/low subsets are shown.
HCV infection upregulates expression of NKp46-Ligand on hepatocytes
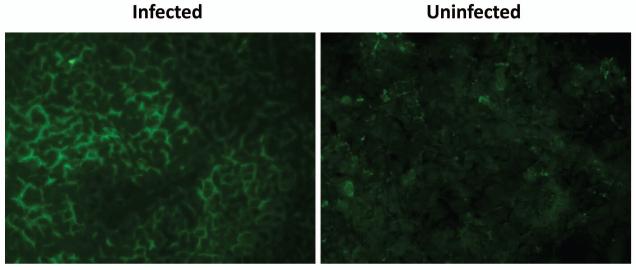
Next, taking advantage of the availability of soluble NCR-Fc fusion proteins, we determined whether replication within the Huh7.5 cell line induced upregulation of the NKp46 ligand. As shown in Figure 6, HCV-infected hepatocytes demonstrate a cell surface pattern of staining for NKp46 ligand. Although uninfected control hepatocytes also stain positive for the ligand for NKp46, the intensity of the staining is much lower. In contrast to the staining pattern seen for infected hepatocytes, NKp46 ligand staining is more diffuse, and, for the most part, localized to the cytoplasm. These data suggest that not only is NKp46 ligand up-regulated but is also transported to the cell surface upon HCV infection.
Figure 6. NKp46 ligand is expressed on hepatocytes.
HCV-infected hepatocytes demonstrate a cell surface pattern of staining for NKp46 ligand. Uninfected control hepatocytes also stain positively for the ligand for NKp46; however, the intensity of the staining is much lower. In contrast to the staining pattern seen for infected hepatocytes, NKp46 ligand staining is more diffuse and for the most part, localized to the cytoplasm. These data suggest that not only is NKp46 ligand upregulated but is also transported to the cell surface upon HCV infection. Images were acquired with a 20x objective lens at constant exposure and are representative of three independent experiments. No FITC signal was detected for the isotype control.
Discussion
NK cells comprise a central component of the innate immune response, representing the first line of defense against a variety of microbrial pathogens, including viruses, bacterial, fungi, as well as tumors (31). The surface density of natural cytotoxicity receptors (NCRs) on NK cells is associated with the magnitude of cytolytic activity against NK-susceptible target cells. Our comprehensive analysis of NCRs (Table 2) identified race- and gender-related differences. Although the ligands recognized by NCRs remain incompletely defined, it is known that soluble NKp46-Ig fusion protein binds to both the hemagglutinin of influenza virus and the hemagglutinin-neuraminidase of parainfluenza virus (9). For the first time, we demonstrate that HCV infection in the Huh7.5 replicon cell line induces expression of the NKp46 ligand on the surface of hepatocytes. Whether this is due to a specific viral component or a stress response to the infection is the focus of ongoing work. Regardless, the findings that NKp46 is associated with increased expression of killing molecules following TLR stimulation, increased IFN-γ production, LAK, degranulation and in vitro control of HCV replication corroborate the strong epidemiological data that both race and gender are associated with spontaneous recovery from HCV infection (25). The fact the outcome of other viral infections do not consistently track with race and gender as in HCV infection may be related to differences in viral tropism or expression of the NKp46 ligand(s) in target tissues. Our collective findings suggest that for HCV infection, NKp46 may represent a useful therapeutic target.
Acknowledgements
Grant Information: supported by U19 AI 1066328, K24AI083742, VA Merit Review grant.
We would like to thank Dr. Takaji Wakita (National Institute of Infectious Diseases, Tokyo, Japan) for kindly providing the JFH-1 plasmid. We thank the Colorado Center for AIDS Research (CFAR) Laboratory Core for access to FACS sorting.
Abbreviations
- AA
African American
- CA
Caucasian American
- LAK
Lymphokine Activated Killing
- TLR
Toll Like Receptor
- NKR
Natural Killer Receptor
- NCR
Natural Cytotoxicity Receptor
Footnotes
Author contributions: LGM, planned, performed experiments, wrote the paper; AS and LC, performed experiments; KMB, analyzed data; HRR, designed study and wrote the paper.
The authors declare no conflict of interest.
References
- 1.Biron CA. Initial and innate responses to viral infections--pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 5.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 7.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228–234. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 8.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, Moretta L, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29:1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 10.Draghi M, Pashine A, Sanjanwala B, Gendzekhadze K, Cantoni C, Cosman D, Moretta A, et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178:2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 11.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 12.Magri G, Muntasell A, Romo N, Saez-Borderias A, Pende D, Geraghty DE, Hengel H, et al. NKp46 and DNAM-1 NK-cell receptors drive the response to human cytomegalovirus-infected myeloid dendritic cells overcoming viral immune evasion strategies. Blood. 2011;117:848–856. doi: 10.1182/blood-2010-08-301374. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Duenas MG, Balderas-Pena LM, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. doi: 10.1186/1471-2407-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 15.Alter G, Jost S, Rihn S, Reyor LL, Nolan BE, Ghebremichael M, Bosch R, et al. Reduced frequencies of NKp30+NKp46+, CD161+, and NKG2D+ NK cells in acute HCV infection may predict viral clearance. J Hepatol. 2011;55:278–288. doi: 10.1016/j.jhep.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 18.Bozzano F, Picciotto A, Costa P, Marras F, Fazio V, Hirsch I, Olive D, et al. Activating NK cell receptor expression/function (NKp30,NKp46,DNAM-1) during chronic viraemic HCV infection is associated with the outcome of combined treatment. Eur J Immunol. 2011 doi: 10.1002/eji.201041361. [DOI] [PubMed] [Google Scholar]
- 19.Carty M, Bowie AG. Recent insights into the role of Toll-like receptors in viral infection. Clin Exp Immunol. 2010;161:397–406. doi: 10.1111/j.1365-2249.2010.04196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 21.Becker I, Salaiza N, Aguirre M, Delgado J, Carrillo-Carrasco N, Kobeh LG, Ruiz A, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003;130:65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 22.Pisegna S, Pirozzi G, Piccoli M, Frati L, Santoni A, Palmieri G. p38 MAPK activation controls the TLR3-mediated up-regulation of cytotoxicity and cytokine production in human NK cells. Blood. 2004;104:4157–4164. doi: 10.1182/blood-2004-05-1860. [DOI] [PubMed] [Google Scholar]
- 23.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 24.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 26.Rosen HR, Weston SJ, Im K, Yang H, Burton JR, Jr., Erlich H, Klarquist J, et al. Selective decrease in hepatitis C virus-specific immunity among African Americans and outcome of antiviral therapy. Hepatology. 2007;46:350–358. doi: 10.1002/hep.21714. [DOI] [PubMed] [Google Scholar]
- 27.Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, Shaw-Stiffel T, et al. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474–1482. doi: 10.1086/522608. [DOI] [PubMed] [Google Scholar]
- 28.Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, Brown RS, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Golden-Mason L, Castelblanco N, O’Farrelly C, Rosen HR. Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection. J Virol. 2007;81:9292–9298. doi: 10.1128/JVI.00834-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Hubeshy ZB, Coleman A, Nelson M, Goodier MR. A rapid method for assessment of natural killer cell function after multiple receptor crosslinking. J Immunol Methods. 2011;366:52–59. doi: 10.1016/j.jim.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Marras F, Bozzano F, De Maria A. Involvement of activating NK cell receptors and their modulation in pathogen immunity. J Biomed Biotechnol. 2011;2011:152430. doi: 10.1155/2011/152430. [DOI] [PMC free article] [PubMed] [Google Scholar]