Abstract
Background
Attention deficit/hyperactivity disorder and depression have been found to be comorbid with smoking behaviors, and all three behavioral syndromes have been shown to be familially transmitted. The present paper reports on the results of analyses testing whether child attention deficit/hyperactivity disorder and depression symptoms were mediators in the intergenerational transmission of cigarette smoking.
Method
Path analyses using bootstrapped mediation procedures were conducted on data from a community sample of 764 families (one or both parents and one adolescent offspring) from the Indiana University Smoking Survey. Parents reported on their smoking behaviors, ADHD, and depression and their child’s ADHD, while offspring reported on their smoking behaviors and depression.
Results
Although fathers’ and mothers’ smoking status, depression, and ADHD were not significantly correlated with boys’ smoking initiation, there was a significant mediated (indirect) pathway from mothers’ depression to boys’ smoking initiation through boys’ depression. Several parental variables were significantly correlated with smoking initiation in girls, and the pathways from mothers’ smoking status, mothers’ ADHD, and fathers’ smoking status to girls’ smoking initiation were significantly mediated by girls’ ADHD.
Conclusions
For adolescent girls, the intergenerational transmission of ADHD appears to be important in understanding the intergenerational transmission of cigarette smoking. Sex differences in the intergenerational transmission of psychopathology as it leads to smoking initiation were also discussed.
Keywords: smoking initiation, attention deficit/hyperactivity disorder, depression, familial transmission
1. Introduction
Every year over 438,000 people die from smoking related illness in the United States (Centers for Disease Control and Prevention, 2005), making cigarette smoking the leading preventable cause of death and disability. The public health significance of smoking prevention highlights the importance of understanding how smoking is first initiated.
Although influences on smoking are numerous and complex, studies have shown that parents have a significant influence on adolescent smoking (Chassin et al., 1998, 2008; Monteaux et al., 2008; Sherman et al., 2009; Melchior et al., 2010), that is, smoking behavior is intergenerationally transmitted. For example, Chassin et al. (1998) showed that parent smoking was related to offspring smoking in two generations, such that grandparent smoking influenced parent smoking, and parent smoking then influenced adolescent smoking. Melchior et al. (2010) found that children of long-term smokers were at a higher risk of initiating smoking than children of parents who were not long-term smokers, and Chassin et al. (2008) found that parent-smoking characteristics (age at initiation, frequency, longevity) were predictive of the child’s smoking initiation.
There are multiple explanations for the intergenerational transmission of smoking. First, there are likely to be genetically mediated pathways (True et al., 1997; Boomsma et al., 1994; Heath et al., 1993; Heath and Martin, 1993). For example, heritable individual differences in nicotine metabolism or nicotinic effects are likely to underlie the intergenerational transmission of smoking (e.g., Tobacco and Genetics Consortium, 2010). Environmental pathways may also influence the intergenerational transmission of smoking (Mulienburg et al., 2009; True et al., 1997; Boomsma et al., 1994; Kim et al., 2010). Parents who smoke provide models of smoking, access to cigarettes, and have more permissive rules about children’s smoking (Otten et al., 2008). The current study focuses on two additional possible mechanisms that mediate the intergenerational transmission of smoking, namely, whether smoking parents transmit symptoms of depression and/or Attention Deficit/Hyperactivity Disorder (ADHD), which then influence children’s smoking.
Multiple studies have found a relation between smoking and depression (Chang et al., 2005; Audrain-McGovern et al., 2009; Steuber and Danner, 2006; Brown et al., 1996; Fucito and Juliano, 2009; Fergusson et al., 2003; Upadhyaya et al., 2002; Lerman et al., 1998; Morrell et al., 2010; Vogel et al., 2003). Longitudinal studies of adolescents by Fergusson et al. (2003) and Brown et al. (1996) found significant relations between major depression and smoking, with the latter study finding evidence of a bi-directional relationship. Cigarette smoking may represent one way that adolescents cope with depressive symptoms.
ADHD has also been shown to have a strong relation with smoking (McClernon and Kollins, 2008; Chang et al., 2005; Lynskey and Fergusson, 1995; Upadhyaya et al., 2002; Barkley et al., 1990; Monteaux et al., 2008; Burke et al., 2001). Barkely et al. (1990) found that hyperactive children were more likely to have smoked cigarettes than were controls, and Burke et al. (2001) found that the inattentive aspect of ADHD was linked with tobacco use. Adolescents with ADHD may smoke to obtain the beneficial effects of nicotine on attention. Several studies have shown that nicotine can improve attention in both people with and without ADHD (Potter and Newhouse, 2008; Rezvani and Levin, 2001; McClernon et al., 2008; Levin et al., 2006).
Not only is smoking associated with both depression and ADHD, but both ADHD and depression may be intergenerationally transmitted. ADHD has been shown to be transmitted from parent to child (Biederman et al., 2008; Faraone and Biederman, 1998; Monteaux et al., 2008; Lange et al., 2005; Cunningham and Boyle, 2002; Minde et al., 2003). In the case of ADHD, this transmission appears to reflect strong genetic influence (Faraone and Biederman, 1998; Smalley et al., 2000; Levy et al., 1997; Biederman, 2005; Wallis et al., 2008).
Depression can also be transmitted through families (Jones et al., 2001; Silberg et al., 2010; Loeber et al., 2009; Pettit et al., 2008; Beardslee et al., 1996). In the case of depression, however, this intergenerational transmission may reflect environmental influence. For example, Silberg et al. (2010) confirmed in a study of children of twins that depression is transmitted from one generation to the next, but also that the transmission was due entirely to family environmental factors. Similarly, Tully et al.’s (2008) study of parental depression in adoptive and biological families found that parental depression significantly predicted adolescent depression in both biological and adoptive families.
Given that ADHD, depression, and smoking are all intergenerationally transmitted, and given that both ADHD and depression are predictive of smoking, the current study hypothesizes that a possible mechanism for the intergenerational transmission of smoking is the co-transmission of ADHD and/or depression. In particular, the present study tests whether ADHD and depression act as mediators of the intergenerational transmission of smoking. Although Monteaux et al. (2008) have shown that ADHD and smoking are co-transmitted intergenerationally, to our knowledge, no studies have simultaneously examined the intergenerational co-transmission of smoking, depression, and ADHD in a community sample.
Separate models for both parent and child gender were considered in this study of intergenerational co-transmission of smoking, depression, and ADHD because previous literature demonstrates gender differences in depression and in ADHD as well as in genetic and environmental influences on smoking. For example, depression is more prevalent in female adolescents and adults than it is in males (Elliot et al., 2001; Jenkins et al., 2002), with about twice as many females reaching clinical levels of depression compared to males. However, studies have also shown that this gender difference does not appear until adolescence (Angold and Worthman, 1993; Doménech-Llaberia et al., 2009; Hankin et al., 1998)—around the age of 15. In other words, there is no gender difference in depression prevalence in children. In contrast, ADHD is more prevalent in males than females (Biederman et al., 1994; DuPaul et al., 2006), with boys having higher rates of ADHD than girls by at least a ratio of 2:1. Finally, studies have shown that environmental and genetic influences on smoking differ between genders (Heath et al., 1993; Boomsma et al., 1994; Hamilton et al., 2006), with genetic influences being found to be stronger in smoking initiation for males, whereas environmental influences are stronger in females.
In addition to child gender being important, parent gender is also an important factor in analysis. Mothers and fathers can contribute to ADHD vulnerability through both environment and genetics. One study by Nomura et al. (2010) found that mothers’ smoking during pregnancy was a significant influence on ADHD outcome; children of women who smoked had significantly higher inattention, hyperactivity and ADHD scores than did children of women who did not smoke. This was true regardless of whether or not fathers smoked. Knopik et al. (2006) and D’Onofrio et al. (2008) found similar results. However, fathers have been reported to be more important than mothers in terms of heritable serotonergic and dopaminergic influences on the development of ADHD (Hawi et al., 2002; Kent et al., 2005; Hawi et al., 2005; Quist et al., 2003), although a similar study by Anney et al. (2008) failed to find any significant parent gender effects in different genes that may influence susceptibility to ADHD. Differing from ADHD, mothers primarily influence on depression. Studies by Brennan et al. (2002), Marmorstein et al. (2004) and Tully et al. (2008) all found that maternal, but not paternal, depression was a significant predictor of offspring depression. There are inconsistent findings concerning the impact of parent gender on the intergenerational transmission of smoking. Whereas some studies have found that parental influence can depend on adolescent gender, such that mothers have stronger influence on daughters or that fathers have stronger influence on sons, (Wickrama et al., 1999; Kandel and Wu, 1995; Gilman et al., 2009), one study found that paternal smoking had no effect on child smoking initiation (Rohde et al., 2003), and still another found that gender had no effect on the influence that parents have on smoking initiation (Peterson et al., 2006). Given the previous findings, albeit inconsistent, that mothers and fathers may influence sons and daughters differently, the current study tested mothers’ and fathers’ effects in separate models.
In the current study, the independent variables were parent smoking status, ADHD symptoms, and depression symptoms, the mediators were child ADHD symptoms and depressive symptoms, and the dependent variable was child smoking initiation. The following hypotheses were tested: 1) Parents who smoke will have higher levels of ADHD symptoms than parents who do not smoke; 2) Parents with higher levels of ADHD symptoms will have children who have higher levels of ADHD symptoms than will parents with lower rates of ADHD symptoms; 3) Children with higher levels of ADHD symptoms will be more likely to begin to smoke than will children with lower rates of ADHD symptoms; 4) Parents who smoke will have higher levels of depression symptoms than parents who do not smoke; 5) Parents with higher levels of depression symptoms will have children with higher levels of depression symptoms than will parents with lower rates of depression symptoms; 6) Children with higher levels of depression symptoms will be more likely to begin to smoke than children with lower rates of depression symptoms. We also tested the indirect effects of parental smoking and parents’ ADHD symptoms on child’s smoking through the child’s ADHD symptoms and the indirect effects of parental smoking and parents’ depression symptoms on child’s smoking through the child’s depression symptoms. Finally, we tested possible child gender moderation of all of these direct and indirect effects.
2. Methods
2.1. Participants
Participants were adolescents, ages 11–16, and their parents who were part of an 18-month longitudinal study on families and smoking. Participants were recruited through the Indiana University (IU) Smoking Survey (Chassin et al., 1990; Sherman et al., 2009; Chassin et al., 2000), an ongoing cohort-sequential study of the natural history of cigarette smoking. The larger study has been ongoing since 1980. In-school assessments took place from 1980–1983 with 6th–12th graders in a Midwestern county school system. Mail follow-ups were conducted in 1987, 1993, 1999, and 2005. At each wave there was a 70% or more retention rate of the original sample (total N assessed at least once = 8,487). The sample is representative of the community: well-educated and predominantly white (96% non-Hispanic Caucasian). For each follow-up, biases have been small, but dropouts were more likely to be smokers and to have parents and friends who were more likely to smoke.
In 2005, IU Smoking Survey participants (ages 32–42) who had adolescent children (ages 10–18) were recruited, with one of their children and their spouses/partners, into an 18-month longitudinal study (N = 1335 families, i.e., one adolescent child + one or both parents). Participants were paid $25 for their participation. For the current analysis, we selected all adolescents who reported not smoking at Time 1, in order to study smoking initiation (N = 921 families). Only participants with a Time 2 report of child smoking status were selected (N = 785 families). At the 18 month follow up, 85.2% of these adolescents were retained. To reduce age heterogeneity, only child participants between the ages of 11 and 16 were selected (final N = 764 families; 394 boys, 370 girls), with 91.6% of participants being White non Hispanic, 51.6% being male, and participants having a median age of 13.27 years at Time 1. It should be noted that child age correlated less than .09 with child depression and ADHD and only .23 with initiation of smoking for the sample used in the present analyses.
Data on participant smoking, ADHD symptoms, and depression symptoms were collected by paper and pencil survey via mail and by web survey.
2.2. Measures
All variables used in the analyses are based on Time 1 reports, with the exception of child smoking status at Time 2.
2.2.1. Parent and Adolescent Smoking
Parents and adolescents self-reported their smoking status using an item from the IU Smoking Survey (Chassin et al., 1990). The scale assessed participant smoking status asking “Which of the following best describes your cigarette smoking?”: “Never smoked, not even a single puff,” “Smoked once or twice ‘just to try’ but not in the last month,” “Do not smoke, but in the past I was a regular smoker,” “Smoke regularly, but not more than once a month,” “Smoke regularly, but not more than once a week,” “Smoke regularly, but not more than once a day,” and “Smoke more than once a day.” Parental smoking was recoded on a four-point scale ranging from 1) never smoked, 2) experimented with smoking, 3) used to smoke, to 4) currently smoking. At Time 1, 12.5% of mothers reported being regular smokers, and 15.0% of fathers reported being regular smokers. The logic of this coding was that, since some lifetime smoking is extremely common among adults, parents who are current smokers may have a greater genetic liability for smoking and/or have had a longer time to model smoking behaviors for their offspring. Although including frequency of smoking for current smokers in the variable might have added additional information in terms of possible genetic load or environmental modeling of smoking, including this information would create other issues of the distributional properties and construct validity of the resulting variable.
Adolescents were selected to be those who never smoked at Time 1, and their smoking status at Time 2 was dichotomized as either continuing not to smoke or any increase in smoking. By Time 2, 16.8% of adolescents had initiated smoking.
According to the National Survey on Drug Use and Health (2009), in 2005, 10.8% of adolescents reported cigarette use, which is somewhat lower than the rate found in the current study. The NSDUH survey, however, found higher rates of smoking in Whites than non-Whites, which may account for the discrepancy in the present mostly White sample. The lower rate of parent smoking compared to the NSDUH may be explained by the fact that all participants are parents, which is associated with greater likelihood of quitting smoking (Chassin et al., 1996), and is magnified by the fact that they were parents of nonsmoking adolescents, as well as the generally higher educational attainment of the present parent sample.
2.2.2. Attention Deficit/Hyperactivity Disorder Symptoms
ADHD symptoms were assessed using 18 items based on DSM IV criteria (see Barkley et al., 2002). Items on the scale asked participants to rate their behavior in the past six months (e.g.,“Talked excessively,” “Was easily distracted,” and “Shifted from one uncompleted activity to another.”) Responses were given on a four-point Likert Scale (Never/Rarely = 1 and Very Often = 4), with participant scores calculated by taking the mean score of the eighteen items in the scale. Self-reports of ADHD symptom suffer from optimistic bias (Barkeley et al., 2002). Thus, although we had to rely on self-report of parents’ ADHD symptoms, child ADHD symptoms were assessed by parent report. Child self-reports of ADHD symptoms were not obtained. Child scores were computed by taking the mean of the scale scores reported by each parent (if there were two participating parents or a single parent score if there was only one participating parent). Although scale items can be scored to distinguish between hyperactive/impulsive and inattention symptoms, these subscales were highly correlated (rs > .70). Mother and father reports of child were correlated .641. Cronbach’s alpha for mothers’ self report was .883, for fathers’ self report .893, for mothers’ child report .931 and fathers’ child report .983.
2.2.3. Depression Symptoms
Parent and adolescent depression symptoms were assessed using a shortened version (Andresen et al., 1994) of the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977). Participants self reported their depression symptoms by responding to the ten items (such as, “I was bothered by things that don’t usually bother me,” “I felt that everything I did was an effort,” and “I felt lonely”) using a four-point Likert scale (1 = Never/Rarely to 4 = Very Often). Participants were asked to report the frequency of their symptoms over the past month; the original shortened CES-D used a past week time frame. Individual scores were computed by averaging the responses across the ten questions. Cronbach’s alpha for fathers was .857, for mothers .871, and for children .711.
2.3. Modeling Analyses
In order to examine mediation of ADHD symptoms and depression symptoms in the intergenerational transmission of smoking, path analyses (Figure 1, Figure 2, Figure 3, and Figure 4) were conducted that modeled parent smoking, parent ADHD symptoms, and parent depression symptoms as correlated exogenous variables, with each predicting adolescent depression symptoms and adolescent ADHD (modeled as correlated variables) that, in turn, were tested as predictors of child smoking initiation—the dichotomous outcome variable. Separate models were estimated for father variables and mother variables. Combined and separate analyses were also run for sex of the offspring, with difference chi-squares calculated for the combined analyses to test for the equivalence of the estimated paths for the male versus female offspring matrices. Mediation effects were tested using the bias corrected bootstrapping of confidence intervals option in Mplus version 5.21 (Muthén and Muthén, 1998–2007) with maximum likelihood estimation using the covariance matrix. Indirect paths are significant if the resulting confidence interval does not include zero. Missing data were handled using full information maximum likelihood. Total N for the female models was 370; N for males was 394.
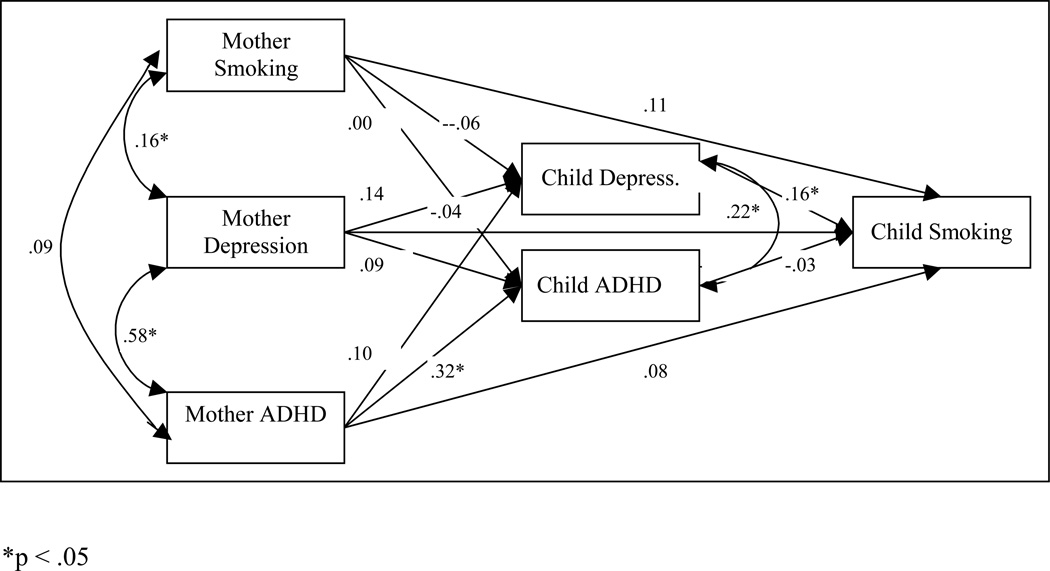
Figure 1.
The path model of depression and ADHD as mediators in the intergenerational co-transmission of smoking, depression and ADHD between mothers and sons.
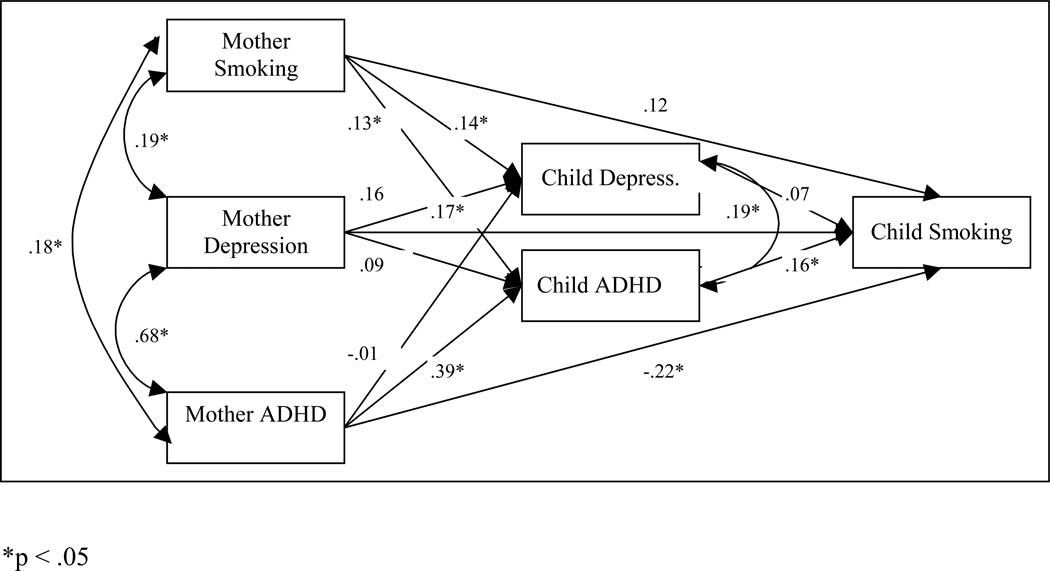
Figure 2.
The path model of depression and ADHD as mediators in the intergenerational co-transmission of smoking, depression and ADHD between mothers and daughters.
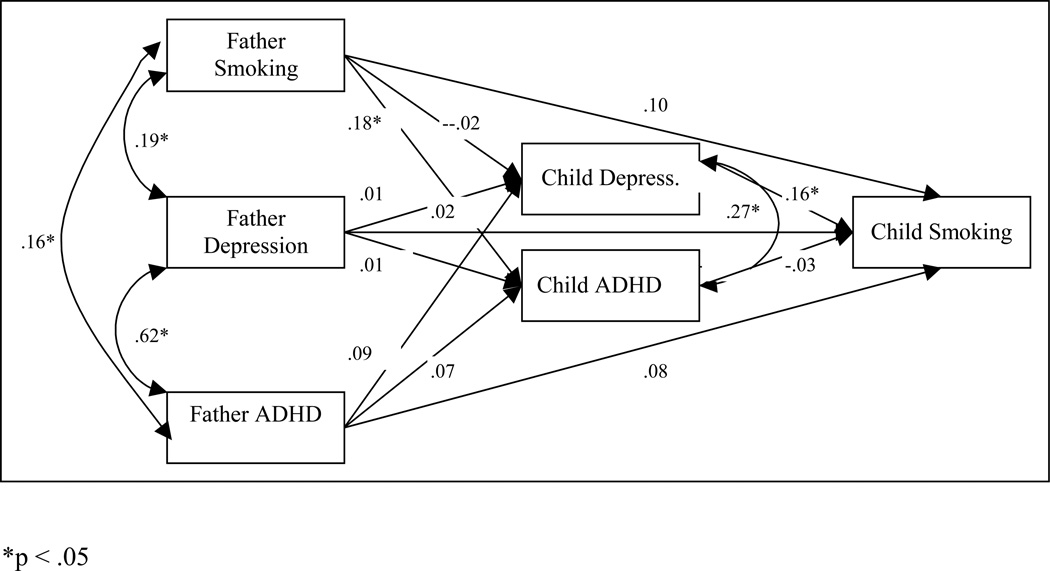
Figure 3.
The path model of depression and ADHD as mediators in the intergenerational co-transmission of smoking, depression and ADHD between fathers and sons.
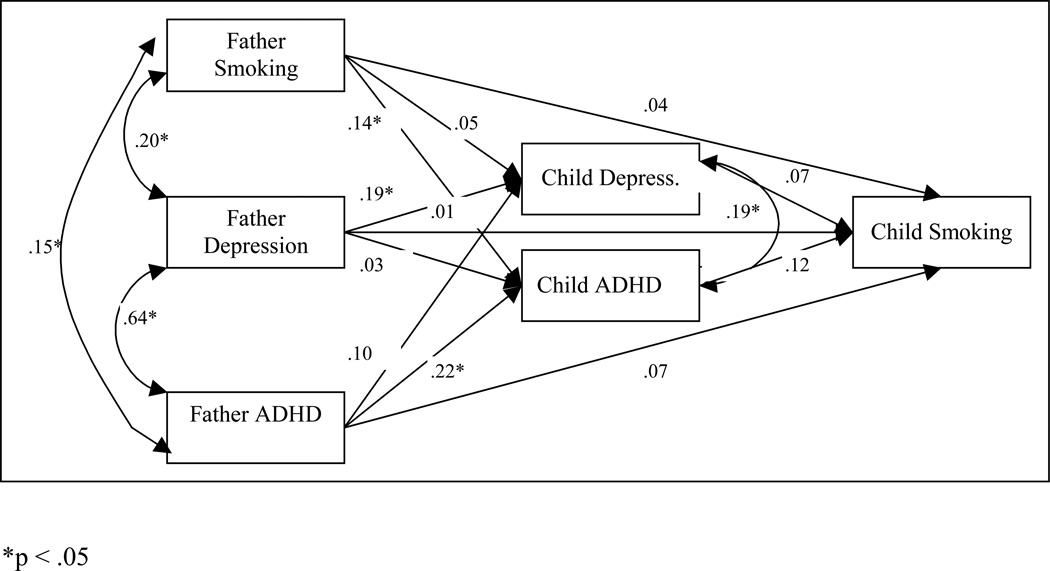
Figure 4.
The path model of depression and ADHD as mediators in the intergenerational co-transmission of smoking, depression and ADHD between fathers and daughters.
3. Results
Table 1 presents the means and standard deviations for child, mother, and father smoking status, depression symptoms, and ADHD symptoms separately by sex. As expected, boys scored higher than girls on ADHD symptoms, but there were no significant sex differences in child depression symptoms. Parent smoking status, depression symptoms, and ADHD symptoms did not differ by parent sex.
Table 1.
Means and Standard Deviations by Child Sex
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | t | |
| Child | |||||||
| Smoking Status | 394 | 0.17a | 370 | 0.17 | |||
| Depression | 388 | 1.61 | 0.40 | 361 | 1.63 | 0.47 | −0.38 |
| ADHD (mother) | 356 | 1.64 | 0.53 | 323 | 1.45 | 0.44 | 5.04** |
| ADHD (father) | 314 | 1.69 | 0.53 | 280 | 1.48 | 0.44 | 5.13** |
| Parent | |||||||
| Smoking Status | 481 | 2.19 | 1.01 | 597 | 2.15 | 0.97 | 0.66 |
| Depression | 597 | 1.64 | 0.53 | 684 | 1.67 | 0.56 | −0.98 |
| ADHD | 594 | 1.40 | 0.36 | 680 | 1.38 | 0.35 | 1.00 |
Child smoking status is a dichotomous variable with 0 indicating no smoking initiation and 1 indicating initiation.
p < .05
p < .01
Tables 2 and 3 present the correlations among child, mother, and father smoking status, depression symptoms, and ADHD symptoms separately by child sex. With the exception of mothers’ and girls’ smoking status, parental smoking status was not correlated with child smoking status, but parental depression and ADHD symptoms were correlated with child depression and ADHD symptoms. Child depression and ADHD symptoms, mothers’ depression, and fathers’ ADHD were correlated with child smoking status only for girls.
Table 2.
Correlations Among Child and Parent Smoking, ADHD, and Depression for Boys
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child Smoking Status | -- | |||||||||
| 2. Child Depression | .15** | -- | ||||||||
| 3. Child ADHD (mother) | .07 | .28** | -- | |||||||
| 4. Child ADHD (father) | .02 | .25** | .66** | -- | ||||||
| 5. Mother Smoking Status | .08 | −.04 | .05 | .01 | -- | |||||
| 6. Mother Depression | .05 | .19** | .28** | .17** | .14* | -- | ||||
| 7. Mother ADHD | .09 | .18** | .38** | .21** | .09 | .58** | -- | |||
| 8. Father Smoking Status | .09 | .00 | .13* | .10 | .33** | .03 | .05 | -- | ||
| 9. Father Depression | .08 | .06 | .08 | .26** | .15* | .15* | −.03 | .18** | -- | |
| 10. Father ADHD | .11 | .09 | .08 | .34** | .01 | .12 | .12* | .15* | .62** | -- |
p < .05
p < .01
Table 3.
Correlations Among Child and Parent Smoking, ADHD, and Depression for Girls
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child Smoking Status | -- | |||||||||
| 2. Child Depression | .13* | -- | ||||||||
| 3. Child ADHD (mother) | .17** | .24** | -- | |||||||
| 4. Child ADHD (father) | .21** | .26** | .58** | -- | ||||||
| 5. Mother Smoking Status | .16** | .17** | .22** | .06 | -- | |||||
| 6. Mother Depression | .11* | .18** | .39** | .34** | .19** | -- | ||||
| 7. Mother ADHD | .01 | .13* | .48** | .31** | .18** | .68** | -- | |||
| 8. Father Smoking Status | .10 | .11 | .19** | .09 | .42** | .26** | .19** | -- | ||
| 9. Father Depression | .11 | .27** | .23* | .36** | .18** | .30** | .34** | .20** | -- | |
| 10. Father ADHD | .13* | .23** | .28** | .42** | .08 | .25** | .29** | .16** | .64** | -- |
p < .05
p < .01
3.1. Path Models for Mothers’ Influence
The mother models for boys and girls were significantly different (difference χ2 (15) = 25.207, p = .047). Accordingly, models for boys and girls were estimated separately. In the mother-boy model (Figure 1), boys’ depression symptoms predicted boys’ smoking initiation, and mother ADHD predicted boys’ ADHD symptoms. There was a significant indirect path from mothers’ depression symptoms to boys’ smoking status through boys’ depression symptoms (95% confidence interval: .001, .046), such that mothers with higher levels of depression had sons with higher levels of depression, which was predictive of boys’ smoking initiation.
For the mother-girl model (Figure 2), girls’ ADHD predicted girls’ smoking initiation, and mothers’ smoking status predicted girls’ depression and ADHD symptoms, mothers’ depression predicted girls’ depression symptoms, and mothers’ ADHD predicted girls’ ADHD symptoms. There was a significant indirect path from mothers’ ADHD symptoms to girls’ smoking status through girls’ ADHD symptoms (95% C.I.: .013, .146), such that mothers with higher levels of ADHD had daughters with higher levels of ADHD symptoms, which was predictive of girls’ smoking initiation. There was also a marginally significant indirect path from mothers’ smoking status to daughters’ smoking status through daughters’ ADHD symptoms (95% C.I.: .000, .026), such that mothers with higher levels of smoking had daughters with higher levels of ADHD symptoms, which was predictive of girls’ smoking initiation.
3.2. Path Models for Fathers’ Influence
The father models for boys and girls did not statistically differ (difference χ2 (15) = 17.814, p = .273). However, given the differences in the mother models, boys and girls models were analyzed separately to facilitate comparison with the mothers’ models. As with the mother-boy model, in the father-boy model (Figure 3), boys’ depression predicted boys’ smoking initiation, but in this case boys’ depression was not predicted by any of the parent level variables. Fathers’ smoking status did predict boys’ ADHD symptoms, but there were no significant indirect pathways from any of the father variables to boys’ smoking initiation.
For the father-girl model (Figure 4), neither girls’ depression nor ADHD symptoms predicted girls’ smoking initiation. Father smoking status and father ADHD predicted girls’ ADHD symptoms, and father depression predicted girls’ depression symptoms. As was the case for the mother-girls’ model, there was a marginally significant indirect path from fathers’ smoking status to daughters’ smoking status through daughters’ ADHD symptoms (95% C.I.: .000, .020), where fathers with higher levels of smoking had daughters with higher levels of ADHD symptoms, which was predictive of girls’ smoking initiation.
3.3 Psychopathology Covariance and One- vs. Two-Parent Families
Given the high covariance between depression and ADHD symptoms, particularly for parents, the effects of each of these psychopathologies in the intergenerational transmission of smoking may have been attenuated. Additional path analyses were thus conducted separately for smoking with depression and smoking with ADHD. These analyses yielded all of the significant indirect path effects described above. For mothers and daughters, additional marginally significant indirect path effects emerged for mothers’ depression to daughters’ smoking through daughters’ depression (95% C.I.: .000, .028) and for mothers’ smoking to daughters’ smoking through daughters’ depression (95% C.I.: .000, .016).
Given the possibly greater risk for psychopathology in one- (N = 216) as opposed to two-parent (N = 548) families (in the present sample, in fact, mean differences in child smoking status, depression, and ADHD symptoms did not significantly differ across one- vs. two-parent families) and the possibly greater effects of parents in one-parent families, additional path analyses tested for differences in the path coefficients between these two kinds of families for mother-son, mother-daughter, father-son, and father-daughter pairs. There was no difference for mother-son (difference χ2 (15) = 13.975, p = .527) nor father-daughter (difference χ2 (15) = 21.954, p = .109), but the one- vs. two-parent difference was significant for mother-daughter (difference χ2 (15) = 28.772, p = .017) and near-significant for father-son (difference χ2 (15) = 23.232, p = .079) pairs. Father-son pairs did not yield any significant mediated effects in the full sample analyses nor when the sample was broken down by one- vs. two-parent status, and the most notable difference was the much larger relationship of father smoking to sons’ depression and ADHD in one-parent families, while the relationship between sons’ depression and ADHD was much stronger in two-parent families. Interestingly, similar differences were found for mother-daughter pairs, with mothers’ smoking being more highly correlated with daughters’ depression in one-parent-families, while the relationship between daughters’ depression and ADHD was much stronger in two-parent families. In terms of indirect effects, the mediated paths from mothers’ smoking to daughters’ smoking through daughters’ depression and from mothers’ ADHD to daughters’ smoking through daughters’ ADHD were only significant for two-parent families (95% C.I.: .004, .047; .038, .211, respectively).
4. Discussion
The present research confirms previous studies of the intergenerational transmission of cigarette smoking (for example, Chassin et al., 1998), depression (for example, Silberg et al., 2010), and ADHD (for example, Minde et al., 2003), at least for girls. The important new finding here is the demonstration that the intergenerational transmission of smoking behavior may be mediated by the intergenerational transmission of depression and ADHD. The sex differences in this mediated intergenerational transmission of smoking are also notable.
The finding that ADHD was a significant intergenerational mediator of smoking initiation for girls is similar to the results found by Monteaux et al. (2008), who found evidence that ADHD and smoking are cosegregated (are transmitted familially together) in a sample of girls. They proposed that there is a subtype of ADHD that is particularly vulnerable to smoking, with a common genetic factor associated with ADHD and nicotine vulnerability. A recent study by Miles and Weden (in press) also found that problem behaviors partially mediated the intergenerational transmission of smoking from mother to child. In terms of why this pathway occurs for adolescent girls but not boys, studies by DuPaul et al. (2006) and Smalley (2000) have found that the inattentive subtype of ADHD is more prevalent in girls than in boys, and a study by Burke et al. (2001) found that the inattentive dimension of ADHD was significantly linked to cigarette use. Since the variable examined here was smoking initiation, self-medication of attention deficits with tobacco use is an unlikely explanation. However, other findings suggest a different interpretation. Lee and Hinshaw (2006) found that it was the hyperactivity/impulsivity dimension of ADHD in girls that was related to later substance use, and they theorized that hyperactivity/impulsivity in girls is equivalent to conduct disorder in boys. The study, in fact, found that hyperactivity/impulsivity symptoms were significant predictors of antisocial behavior. So it may be antisocial behavior in girls with higher rates of ADHD that is driving smoking initiation. However, in the current data these subscales of ADHD symptoms were highly correlated and analyses with each subscale separately produced the same findings as the total scale.
In contrast, for boys the intergenerational transmission of smoking from mothers was mediated by depression. One explanation for this sex difference may involve the different coping strategies typically engaged in by adolescent boys compared to girls. Several studies show that boys, compared to girls, tend to use more negative coping strategies, including avoidance of problems through substance use (Eschenbeck et al., 2007; Compas et al., 1993; Seiffge-Krenke, 1993). Girls, in contrast, tend to use social support more often than do boys. If so, then boys may be more likely than girls to cope with depressive symptoms by smoking cigarettes. The current results might seem to be in conflict with previous studies by Heath et al. (1993), Boomsma et al. (1994), and Hamilton et al. (2006), which found that genetic influences were stronger for males and environmental influences were stronger for females. Of course, the influences on smoking initiation are numerous and complex, and each influence should be treated as an important part of the overall process.
The lack of indirect father effects through depression was not surprising. Previous studies (for example, Brennan et al., 2002) have found that maternal, but not paternal, depression influences adolescent depression. The lack of indirect effects through ADHD is unexpected. It could be for a number of reasons. First, it could have simply been an issue of power, given the smaller sample for fathers than for mothers. Second, mothers are generally the primary caretaker, and therefore may be more involved in children’s lives. Mothers can also influence their children prenatally, which might be particularly relevant if mothers smoked or used alcohol or other substances during pregnancy. For example, prenatal nicotine exposure can affect children in a number of ways (for a review, see Ernst et al., 2001).
The current study has several limitations that should be noted. The relatively homogeneous sample, in terms of racial/ethnic diversity and education, limits the generalizability of the findings. Use of depression and ADHD symptoms rather than clinical disorder diagnoses, and the use of a non-clinical sample, may underestimate the magnitudes of effects, if the relations of depression and ADHD symptoms to smoking are most apparent at clinical levels of the disorders. Given the above discussion, the relationship of ADHD to antisocial/externalizing behaviors needs to be considered in understanding the intergenerational transmission of smoking and ADHD. The six-month timeframe for the assessments of depression and ADHD may have attenuated findings by missing participants in remission for these syndromes. Finally, the study lasted only long enough to measure how depression and ADHD symptoms relate to smoking initiation. Studies have shown that the factors that influence smoking initiation and smoking persistence are different. For example, True et al. (1997) found that genetic influences accounted for more variability in smoking persistence than in smoking initiation. Despite these limitations, this study contributes to the literature by demonstrating that the intergenerational transmission of depressive and ADHD symptoms may contribute to the intergenerational transmission of cigarette smoking. The sex differences found here suggest that future research needs to consider differences in gender socialization and gender-based coping strategies that may act as protective or risk factors in the intergenerational transmission of problem behaviors, including substance use.
Acknowledgments
Support for this project was provided by the National Institute of Drug Abuse grant DA13555 to Drs. Chassin and Presson. We thank Dr. Jon Macy for coordination of the data collection and our participants who have given so generously of their time.
Role of Funding Source:
National Institute of Drug Abuse grant DA13555 to Drs. Chassin and Presson provided the financial support for the collection of the data the present analyses are based on. The sponsor had no role in the study design; collection, analysis, and interpretation of data; writing of the report; nor the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Contributors:
Alex Zoloto managed the literature searches and summaries of previous related work, as well as wrote the bulk of the manuscript. Craig Nagoshi undertook the statistical analyses and wrote those sections of the manuscript concerned with these analyses. Clark Presson and Laurie Chassin designed the study, wrote the study protocol, and contributed to the writing of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest:
All authors declare they have no conflicts of interest.
References
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short-form of the CES-D. Am. J. Prev. Med. 1994;4:77–84. [PubMed] [Google Scholar]
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J. Affect. Disord. 1993;29:145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Anney R, Hawi Z, Sheehan K, Mulligan A, Pinto C, Brookes K, Xu X, Zhou K, Franke B, Buitelaar J, Vermeulen S, Banaschewski T, Sonuga-Barke E, Ebstein R, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rommelse N, Rothenberger A, Sergeant J, Steinhausen HC, Taylor E, Thompson M, Asherson P, Faraone S, Gill M. Parent of origin effects in attention/deficit hyperactivity disorder (ADHD): analysis of data from the international multicenter ADHD genetics (IMAGE) program. Am. J. Med. Genet. Part B. 2008;147B:1495–1500. doi: 10.1002/ajmg.b.30659. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Kassel JD. Adolescent smoking and depression: evidence for self medication and peer smoking mediation. Addiction. 2009;104:1743–1756. doi: 10.1111/j.1360-0443.2009.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Social Learning Theory. Oxford, England: Prentice Hall; 1977. [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. an 8-year prospective follow-up study. J. Am. Acad. Child Adolesc. Psychiat. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J. Abnorm. Psychol. 2002;111:279–289. [PubMed] [Google Scholar]
- Beardslee WR, Keller MB, Seifer R, Lavori PW, Staley J, Podorefsky D, Shera D. Prediction of adolescent affective disorder: effects of prior parental affective disorders and child psychopathology. J. Am. Acad. Child Adolesc. Psychiat. 1996;35:279–288. doi: 10.1097/00004583-199603000-00008. [DOI] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol. Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Spencer T, Wilens T, Mick E, Lapey KA. Gender differences in a sample of adults with attention deficit hyperactivity disorder. Psychiatry Res. 1994;53:13–29. doi: 10.1016/0165-1781(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Wilens TE, Fraire MG, Purcell CA, Mick E, Monteaux MC, Faraone SC. Familial risk analysis of attention deficit hyperactivity disorder and substance use disorders. Am. J. Psychiatry. 2008;165:107–115. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Koopmans JR, Van Dooren LJP, Orlebeke JF. Genetic and social influences on starting to smoke: a study of Dutch adolescent twins and their parents. Addiction. 1994;89:219–226. doi: 10.1111/j.1360-0443.1994.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Hammen C, Katz AR, Brocque RML. Maternal depression, paternal psychopathology, and adolescent diagnostic outcomes. J. Counsel. Clin. Psychol. 2002;70:1075–1085. doi: 10.1037//0022-006x.70.5.1075. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J. Am. Acad. Child Adolesc. Psychiat. 1996;35:1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? J. Child. Psychol. Psychiat. 2001;4:493–502. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Annual smoking – attributable mortality, years of potential life lost, and productivity losses --- United States, 1997 – 2001. MMWR. 2005;54:625–628. [PubMed] [Google Scholar]
- Chang G, Sherritt L, Knight JR. Adolescent cigarette smoking and mental health symptoms. J. Adolesc. Health. 2005;36:517–522. doi: 10.1016/j.jadohealth.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a Midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychol. 2000;19:223–231. [PubMed] [Google Scholar]
- Chassin L, Presson C, Seo DC, Sherman SJ, Macy J, Wirth RJ, Curran P. Multiple trajectories of cigarette smoking and the intergenerational transmission of smoking: a multigenerational, longitudinal study of a Midwestern community sample. Health Psychol. 2008;27:819–828. doi: 10.1037/0278-6133.27.6.819. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol. 1990;9:701–716. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Todd M, Rose JS, Sherman SJ. Maternal socialization of adolescent smoking: the intergenerational transmission of parenting and smoking. Dev. Psychol. 1998;34:1189–1201. doi: 10.1037//0012-1649.34.6.1189. [DOI] [PubMed] [Google Scholar]
- Compas BE, Oroson PG, Grant KE. Adolescent stress and coping: implications for psychopathology during adolescence. J. Adolesc. 1993;16:331–349. doi: 10.1006/jado.1993.1028. [DOI] [PubMed] [Google Scholar]
- Cunningham CE, Boyle MH. Preschoolers at risk for attention-deficit hyperactivity disorder: family, parenting, and behavioral correlates. J. Abnorm. Child Psychol. 2002;30:555–569. doi: 10.1023/a:1020855429085. [DOI] [PubMed] [Google Scholar]
- Doménech-Llaberia E, Viñas F, Pla E, Jané MC, Mitjavila M, Corbella T, Canals J. Prevalence of major depression in preschool children. Eur. Child Adolesc. Psychiatry. 2009;18:597–604. doi: 10.1007/s00787-009-0019-6. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev. Psychopathol. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Jitendra AK, Tresco KE, Vile Junod RE, Volpe RJ, Lutz JG. Children with attention deficit hyperactivity disorder: are there gender differences in school functioning? School Psychol. Rev. 2006;35:292–308. [Google Scholar]
- Elliot M. Gender differences in causes of depression. Women Health. 2001;33:183–198. [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Eschenbeck H, Kohlmann CW, Lohaus A. Gender differences in coping strategies in children and adolescents. J. Indiv. Diff. 2007;28:18–26. [Google Scholar]
- Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biol. Psychiatry. 1998;44:951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol. Med. 2003;33:1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Juliano LM. Depression moderates smoking in response to a sad mood induction. Psychol. Addict. Behav. 2009;23:546–551. doi: 10.1037/a0016529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Rende R, Boergers J, Abrams DB, Buka SL, Clark MA, Colby SM, Hitsman B, Kazura AN, Lipsitt LP, Lloyd-Richardson EE, Rogers ME, Stanton CA, Stroud LA, Niaura RS. Parental smoking and adolescent smoking initiation: an intergenerational perspective on tobacco control. Pediatrics. 2009;123:274–281. doi: 10.1542/peds.2008-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AS, Lessov-Schlaggar CN, Cockburn MG, Unger JB, Cozen W, Mack TM. Gender differences in determinants of smoking initiation and persistence in California twins. Cancer Epidem. Biomark. Prev. 2006;15:1189–1197. doi: 10.1158/1055-9965.EPI-05-0675. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year study. J. Abnorm. Psychol. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hawi Z, Dring M, Kirley A, Foley D, Kent L, Craddock N, Asherson P, Curran S, Gould A, Richards S, Lawson D, Pay H, Turic D, Langley K, Owen M, O’Donovan M, Thapar A, Fitzgerald M, Gill M. Serotonergic system and attention deficit disorder (ADHD): a potential susceptibility locus at the 5-HT1B receptor gene in 273 nuclear families from a multi-centre sample. Molec. Psychiatry. 2002;7:718–725. doi: 10.1038/sj.mp.4001048. [DOI] [PubMed] [Google Scholar]
- Hawi Z, Segurado R, Conroy J, Sheehan K, Lowe N, Kirley A, Shields D, Fitzgerald M, Gallagher L, Gill M. Preferential transmission of paternal alleles at risk genes in attention/deficit hyperactivity disorder. Am. J. Hum. Genet. 2005;77:958–965. doi: 10.1086/498174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Genetic models for the natural history of smoking: evidence for a genetic influence on smoking persistence. Addict. Behav. 1993;18:19–34. doi: 10.1016/0306-4603(93)90005-t. [DOI] [PubMed] [Google Scholar]
- Heath AC, Cates R, Martin NG, Meyer J, Hewitt JK, Neale MC, Eaves LJ. Genetic contribution to risk of smoking initiation: comparisons across birth cohorts and across cultures. J. Subst. Abuse. 1993;5:221–246. doi: 10.1016/0899-3289(93)90065-j. [DOI] [PubMed] [Google Scholar]
- Jenkins SR, Goodness K, Buhrmester D. Gender differences in early adolescents? relationship qualities, self-efficacy, and depression symptoms. J. Early Adolesc. 2002;22:277–309. [Google Scholar]
- Jones DJ, Forehand R, Neary EM. Family transmission of depressive symptoms: replication across Caucasian and African American mother-child dyads. Behav. Thera. 2001;32:123–138. [Google Scholar]
- Kandel DB, Wu P. The contributions of mothers and fathers to the intergenerational transmission of cigarette smoking in adolescence. J. Res. Adolesc. 1995;5:225–252. [Google Scholar]
- Kent L, Green E, Hawi Z, Kirley A, Dudbridge F, Lowe N, Raybould R, Langley K, Bray N, Fitzgerald M, Owen MJ, O’Donovan MC, Gill M, Thapar A, Craddock N. Association of the paternally transmitted copy of common valine allele of the Val66Met polymorphism of the brain-derived neurotropic factor (BDNF) gene with susceptibility to ADHD. Molec. Psychiatry. 2005;10:939–943. doi: 10.1038/sj.mp.4001696. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Fleming CB, Catalano RF. Individual and social influences on progression to daily smoking during adolescence. Pediatrics. 2010;124:895–902. doi: 10.1542/peds.2008-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Waldron M, Martin NG. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children of twins design. Psychol. Med. 2006;36:1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Lange G, Sheerin D, Carr A, Dooley B, Barton V, Marshall D, Mulligan A, Lawlor M, Belton M, Doyle M. Family factors associated with attention deficit hyperactivity disorder and emotional disorders in children. J. Fam. Thera. 2005;27:76–96. [Google Scholar]
- Lee SS, Hinshaw SP. Predictors of adolescent functioning in girls with attention deficit hyperactivity disorder (ADHD): the role of childhood ADHD, conduct problems, and peer status. J. Clin. Child Adolesc. Psychol. 2006;35:356–368. doi: 10.1207/s15374424jccp3503_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvain AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacol. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a contiuum? Genetic analysis of a large-scale twin study. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Loeber R, Hipwell A, Battista D, Sembower M, Stouthamer-Loeber M. Intergenerational transmission of multiple problem behaviors: prospective relationships between mothers and daughters. J. Abnorm. Child Psychol. 2009;37:1035–1048. doi: 10.1007/s10802-009-9337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM. Childhood conduct problems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. J. Abnorm. Child Psychol. 1995;23:281–302. doi: 10.1007/BF01447558. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, Malone SM, Iacono WG. Psychiatric disorders among offspring of depressed mothers: associations of paternal psychopathology. Am. J. Psychiatry. 2004;161:1588–1594. doi: 10.1176/appi.ajp.161.9.1588. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DP, Redman C, Rose JE. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacol. 2008;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- Melchior M, Chastang J, MacKinnon D, Galera C, Fombonne E. The intergenerational transmission of tobacco smoking—the role of parents’ long-term smoking trajectories. Drug Alcohol Depend. 2010;107:257–260. doi: 10.1016/j.drugalcdep.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Miles JN, Weden MM. Is the intergenerational transmission of smoking from mother to child mediated by children’s behavior problems? Nicot. Tob. Res. doi: 10.1093/ntr/ntr328. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minde K, Eakin L, Hechtman L, Ochs E, Bouffard R, Greenfield B, Looper K. The psychosocial functioning of children and spouses of adults with ADHD. J. Child Psychol. Psychiatry. 2003;44:637–646. doi: 10.1111/1469-7610.00150. [DOI] [PubMed] [Google Scholar]
- Monteaux MC, Faraone SV, Hammerness P, Wilens TE, Fraire M, Biederman J. The familial association between cigarette smoking and ADHD: a study of clinically referred girls with and without ADHD, and their families. Nicotine Tob. Res. 2008;10:1549–1558. doi: 10.1080/14622200802326137. [DOI] [PubMed] [Google Scholar]
- Morrell HER, Cohen LM, McChargue DE. Depression vulnerability predicts cigarette smoking among college students: gender and negative reinforcement expectancies as contributing factors. Addict. Behav. 2010;35:607–611. doi: 10.1016/j.addbeh.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulienburg JL, Latham T, Annang L, Johnson WD, Burdell AC, West SJ, Clayton DL. The home smoking environment: influence on behaviors and attitudes in a racially diverse adolescent population. HealthEduc. Behav. 2009;36:777–793. doi: 10.1177/1090198109339461. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus Software Program. Fifth Ed. Los Angeles, CA: Muthén & Muthén; 1998–2007. [Google Scholar]
- Nomura Y, Marks DJ, Halperin JM. Prenatal exposure to maternal and paternal smoking on attention deficit hyperactivity disorders symptoms and diagnosis in offspring. J. Nerv. Ment. Dis. 2010;198:672–678. doi: 10.1097/NMD.0b013e3181ef3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten R, Engels R, Van den Eijnden R. General parenting, anti-smoking socialization and smoking onset. Health Educ. Res. 2008;23:859–869. doi: 10.1093/her/cym073. [DOI] [PubMed] [Google Scholar]
- Peterson AV, Leroux BG, Bricker J, Kealey KA, Marek PM, Sarason IG, Andersen MR. Nine-year prediction of adolescent smoking by number of smoking parents. Addict. Behav. 2006;31:788–801. doi: 10.1016/j.addbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Pettit JW, Olino TM, Roberts RE, Seeley JR, Lewinsohn PM. Intergenerational transmission of internalizing problems: effects of parental and grandparental major depressive disorder on child behavior. J. Clin. Child Adolesc. Psychol. 2008;37:640–650. doi: 10.1080/15374410802148129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Gilpin E. How long will today’s new adolescent smoker be addicted to cigarettes? Am. J. Pub. Health. 1996;86:253–256. doi: 10.2105/ajph.86.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piko B. Gender differences and similarities in adolescents’ ways of coping. Psychol. Record. 2001;51:223–235. [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol. Biochem. Behav. 2008;88:407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Quist JF, Barr CL, Schachar R, Roberts W, Malone M, Tannock R, Basile VS, Beitchman J, Kennedy JL. The serotonin 5-HT1B receptor gene and attention deficit hyperactivity disorder. Molec. Psychiatry. 2003;8:98–102. doi: 10.1038/sj.mp.4001244. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol. Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Brown RA, Gau JM, Kahler CW. Psychiatric disorders, familial factors, and cigarette smoking: I. associations with smoking initiation. Nicotine Tob. Res. 2003;5:85–98. doi: 10.1080/1462220031000070507. [DOI] [PubMed] [Google Scholar]
- Seiffge-Krenke I. Coping Behavior in normal and clinical samples: more similarities than differences? J. Adolesc. 1993;16:285–303. doi: 10.1006/jado.1993.1026. [DOI] [PubMed] [Google Scholar]
- Sherman SJ, Chassin L, Presson C, Seo D, Macy JT. The intergenerational transmission of implicit and explicit attitudes toward smoking: predicting adolescent smoking initiation. J. Exp. Soc. Psychol. 2009;45:313–319. doi: 10.1016/j.jesp.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg JL, Maes H, Eaves LJ. Genetic and environmental influences on the transmission of parental depression children’s depression and conduct disturbance: an extended children of twins study. J. Child Psychol. Psychiatry. 2010;51:734–744. doi: 10.1111/j.1469-7610.2010.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley SL, McGough JJ, Del Homme M, NewDelman J, Gordon E, Kim T, Liu A, McCraken JT. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1135–1143. doi: 10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Steuber T, Danner F. Adolescent smoking and depression: which comes first? Addict. Behav. 2005;31:133–136. doi: 10.1016/j.addbeh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Nong L, Eisen SA, Lyons MJ, Tsuang MT. Genetic and environmental contributions to smoking. Addiction. 1997;92:1277–1287. [PubMed] [Google Scholar]
- Tully EC, Iacono WG, McGue M. An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. Am. J. Psychiatry. 2008;165:1148–1154. doi: 10.1176/appi.ajp.2008.07091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya HP, Deas D, Brandy KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:1294–1305. doi: 10.1097/00004583-200211000-00010. [DOI] [PubMed] [Google Scholar]
- Vogel JS, Hurford DP, Smith JV, Cole A. The relationship between depression and smoking in adolescents. Adolescence. 2003;38:57–74. [PubMed] [Google Scholar]
- Wallis D, Russell HF, Muenke M. Review: genetics of attention deficit/hyperactivity disorder. J. Pediatr. Psychol. 2008;33:1085–1099. doi: 10.1093/jpepsy/jsn049. [DOI] [PubMed] [Google Scholar]
- Wickrama KAS, Conger RD, Wallace LE, Elder GH. The intergenerational transmission of health risk behaviors: adolescent lifestyles and gender moderating effects. J. Health Soc. Behav. 1999;40:258–272. [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance use? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]