Abstract
Background and Purpose
The pattern of antenatal brain injury varies with gestational age at the time of insult. Deep brain nuclei are often injured at older gestational ages. Having previously shown postnatal hypertonia following preterm fetal rabbit hypoxia-ischemia (H-I), the objective of this study was to investigate the causal relationship between the dynamic regional pattern of brain injury on MRI and the evolution of muscle tone in the near-term rabbit fetus.
Methods
Serial MRI was performed on New Zealand White rabbit fetuses to determine equipotency of fetal H-I during uterine ischemia comparing 29 days gestation (E29, 92% gestation) with E22 and E25. E29 postnatal kits at 4, 24, 72 hours after H-I underwent T2- and diffusion weighted imaging. Quantitative assessments of tone were made serially using a torque apparatus in addition to clinical assessments.
Results
Based on the brain apparent diffusion coefficient (ADC), 32 min of uterine ischemia was selected for E29 fetuses. At E30, 58% of the survivors manifested hind limb hypotonia. By E32, 71% of the hypotonic kits developed dystonic hypertonia. Marked and persistent ADC reduction in basal ganglia, thalamus and brainstem was predictive of these motor deficits.
Conclusions
MRI observation of deep brain injury 6–24 hours following near-term H-I predicts dystonic hypertonia postnatally. Torque-displacement measurements indicate that motor deficits in rabbits progressed from initial hypotonia to hypertonia, similar to human CP, but in a compressed time-frame. The presence of deep brain injury and quantitative shift from hypo- to hypertonia may identify patients at risk for developing CP.
Keywords: hypoxic-ischemic encephalopathy, hypertonia, diffusion-weighted imaging
INTRODUCTION
Cerebral palsy is a clinical syndrome commonly designating a group of conditions characterized by chronic motor impairment due to early occurrence of a non-progressive lesion to the developing brain 1–2. The type and site of lesions depend on the stage of brain maturation during which pathogenic events occurred 3–5.
Newborns with hypoxic-ischemic encephalopathy are initially hypotonic and subsequently develop hypertonia after some months 6. The pathophysiology and evolution of dysfunctional motor control in CP patients are largely unknown.
In a clinically relevant animal model after fetal H-I at 70–79% (E22 and E25) gestation in rabbits, newborn kits at E32 manifest hypertonia, resembling motor deficits of human CP7–10. MRI markers on diffusion weighting imaging (DWI) during the insult in utero at E25 are predictive of hypertonia 11. Compared to humans, rabbit motor development is compressed in time. Given the 7–10 day period in utero after H-I in the rabbit model, it was possible that the hypotonic period occurred in utero and evolved into hypertonia before birth.
Muscle tone is typically assessed clinically by ordinal measures, with distinction into spasticity, rigidity or dystonia for hypertonia 12. Laboratory measurements objectively quantify the different types of hypertonia, by determining contributions of velocity dependent (viscous) and independent (elastic) components in human 13–14 and animal studies 15–16.
The hypothesis of this study was that muscle tone in the rabbit would evolve from hypotonia to hypertonia after an H-I insult and that apparent diffusion coefficient (ADC) changes on MRI could be used to predict the eventual motor phenotype. We could not test this hypothesis previously as the preterm fetuses could not be observed ex utero. Sufficient near-term rabbit fetuses, survive delivery immediately after H-I, to allow immediate neurological examination. The secondary hypothesis was that the pattern of injury in near-term H-I would differ from preterm H-I (E22 and E25), with greater involvement of deep brain structures. We utilized dynamic MRI imaging for evaluation of regional brain injury and torque apparatus measurement of passive joint resistance to stretch for longitudinal and quantitative evaluation of muscle tone changes. The study was designed to investigate short-term outcomes at E32 for comparison with previous studies of earlier gestational ages.
METHODS
The Institutional Animal Care and Use Committee of NorthShore University HealthSystem approved all animal experiments.
Prenatal hypoxia-ischemia model and neurobehavioral assessment
New Zealand White pregnant rabbits (Myrtle’s Rabbits, Thompson Station, Tennessee) at 29 days gestation (90% term) underwent uterine ischemia induced by inflation of intra-aortic balloon catheter, which caused in vivo global fetal hypoxia–ischemia 10. Newborn kits were delivered 4 hours after H-I by hysterotomy 10. Neurobehavioral measurements, tone assessment by a torque-displacement apparatus, and serial MRI were performed at 6, 18, 24 and 72 hours following H-I.
Muscle tone assessment was based on a modified Ashworth scale, 10 which takes into account hypotonia and hypertonia, as well as differences in forelimb and hind limb tone. Final assessment of motor deficits was at 72 h following H-I. Kits were labeled as “with” or “without” motor deficits. The former category included kits having abnormal muscle tone (hypertonia or hypotonia), abnormal posture or locomotion.
Objective measurements of muscle tone
We built a torque-displacement apparatus that measured passive resistance and stretch angle during sinusoidal joint stretch (supplementary methods and Figure S1, http://stroke.ahajournals.org). Joint stiffness (slope of torque-displacement curve), derived from the laboratory muscle tone was assessed and correlated with manual muscle tone assessment (Figure S1D).
Fetal MR imaging
A subset of dams was studied in a clinical 3T magnet. T2-weighted and diffusion weighted imaging (DWI) of fetal brains (b=0, 0.8 ms/µm2) were acquired as previously described 11. Uterine position of each fetus was first identified and ADC of fetal brain was measured 11. Whole brain ADC could be obtained reliably but regional changes could not be consistently quantified because of fetal motion.
Postnatal MR imaging
Newborn kits after delivery were studied in an animal 4.7T magnet and T2-weighted and diffusion tensor images (6 diffusion directions, b= 0, 0.8 ms/µm2) were acquired as previously described 8. Injury in the region of interest was determined by quantifying the volume of voxels on ADC maps below a predefined threshold value of 0.7 µm2/ms.
Statistical analysis
Data were analyzed using SPSS 14.0 software and expressed as means±SEM. Differences between groups were tested using one-way ANOVA with Tukey post-hoc group comparisons. Longitudinal data series were tested with repeated measures ANOVA. The cut-off threshold of fetal brain ADC nadir during H-I was determined by linear discriminant analysis, separating hypertonia from non-hypertonia outcomes.
RESULTS
Perinatal death increases with gestation age for the same duration of H-I
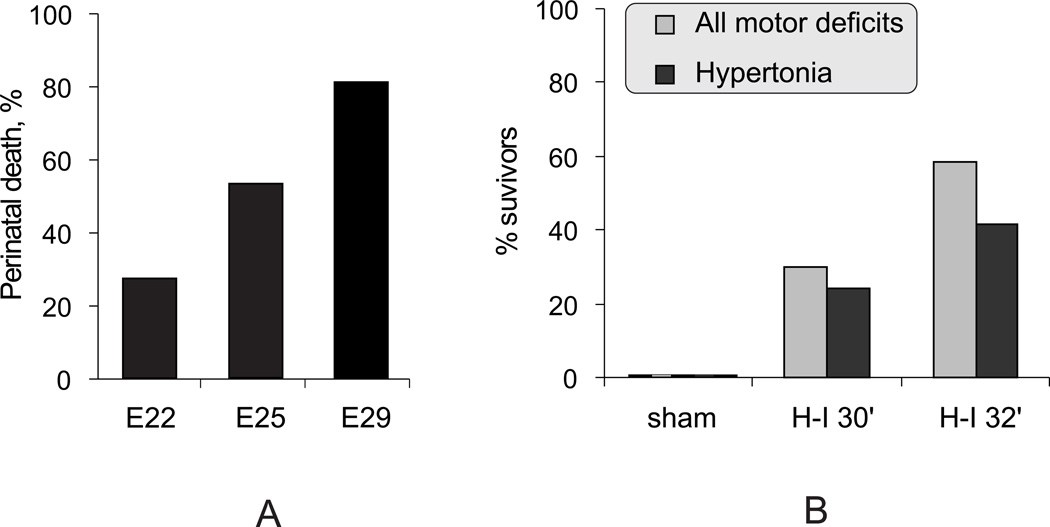
We first compared the severity of 40-min fetal H-I in near-term E29 fetuses, to preterm E22 and E25 in terms of mortality and morbidity (E22/E25 data is partly from previously published data7.) The term (E32) death rate increased with increasing age, suggesting that fetal vulnerability to H-I increases with age (Figure 1A).
Figure 1.
A. Perinatal death increased with age following 40 min global fetal H-I at E22 (n=369 fetuses), E25 (186 fetuses) and E29 (89 fetuses) (p <0.001, Chi-square).
B. Increase of postnatal neurobehavioral deficits with increasing durations of H-I at E29 (p <0.05, Chi-square for both motor deficits and hypertonia). Sham controls had no deficits. n=10, 67, 60 for sham, 30 min H-I, and 32 min H-I correspondingly.
Establishing equipotent insult
Previously, we showed that 30 min H-I at E22 resulted in all normal appearing kits 10. The E29 H-I duration was decreased from 40 min to 30 or 32 min (Figure 1B) resulting in a similar proportion of live born kits at 72 hr (40.0 % and 55.2% respectively) compared to 59% live kits after 40 min H-I at E22 7. 30 min H-I at E29 resulted in a lower incidence of hypertonia than following 40 min H-I at E22 7, whereas 32 min H-I had a similar incidence and was chosen for subsequent studies.
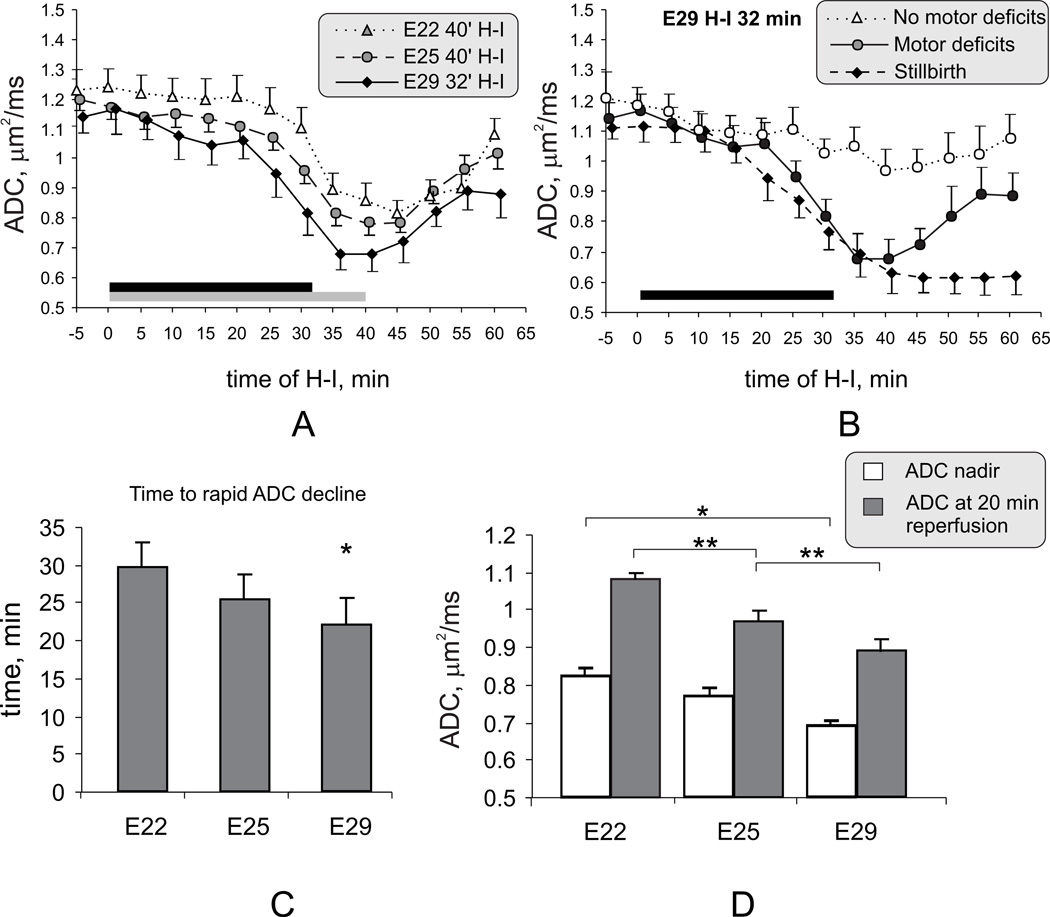
ADC decline occurs faster and more pronounced during H-I at E29 fetuses
Another parameter to compare brain vulnerability to H-I insult between preterm and near term fetuses was ADC time course during H-I, since ADC decrease below a threshold level of in E25 fetal brains was predictive of postnatal hypertonia 11. The pattern of the ADC response to H-I with increasing fetal age for E22 and E29 fetuses was compared with our previous study of E25 fetuses11. The fall in ADC at E29 started earlier, was deeper and recovered more slowly (Figure 2A,C,D). The ADC took a median of 28.4 min (interquartiles 25.4–31.3) to fall below the threshold of 0.83 µm2/ms for E25 fetuses which distinguished postnatal hypertonia from non-hypertonia. The time of ADC to reach the corresponding mean value at end of 40 minutes H-I in E25 (0.78 µm2/ms) was 29.6 min (median 28.4, interquartiles 27.5–32.9) in E29 fetuses.
Figure 2.
A. Dynamic ADC changes during H-I and reperfusion-reoxygenation in E22, E25, E29 fetuses that were born alive with motor deficits. Duration of H-I was 40 min for E22 and E25 fetuses (gray bar) and 32 min for E29 fetuses (black bar). With increasing age there is an earlier and deeper fall in ADC during H-I.
B. Fetuses that developed motor deficits later showed a pattern of ADC different from sham controls (not shown), non-hypertonic and stillbirths with 32 min H-I at E29.
C. The onset of rapid phase of ADC decline during H-I occurred significantly earlier at E29 than at E22. * - p<0.05 in E29 group compared with E22
D. The lowest value of ADC during time course (nadir) was lower in E29 compared to E22 (* p<0.05). The ADC value at 20 min reperfusion progressively decreased with increasing gestational age. ** - p <0.05 in E25 group compared with E22 and E29 groups. ANOVA with Tukey post-hoc group comparisons.
For 32 min H-I in E29 fetuses, the predictive threshold of whole brain ADC for distinguishing hypertonia from non-hypertonia was 0.75 µm2/ms, determined by discriminant analysis. Also, as shown previously, if ADC did not recover in the late reperfusion-reoxygenation period, those fetuses were more likely to die (Figure 2B).
Muscle tone of after near-term fetal H-I evolves from hypotonia to hypertonia
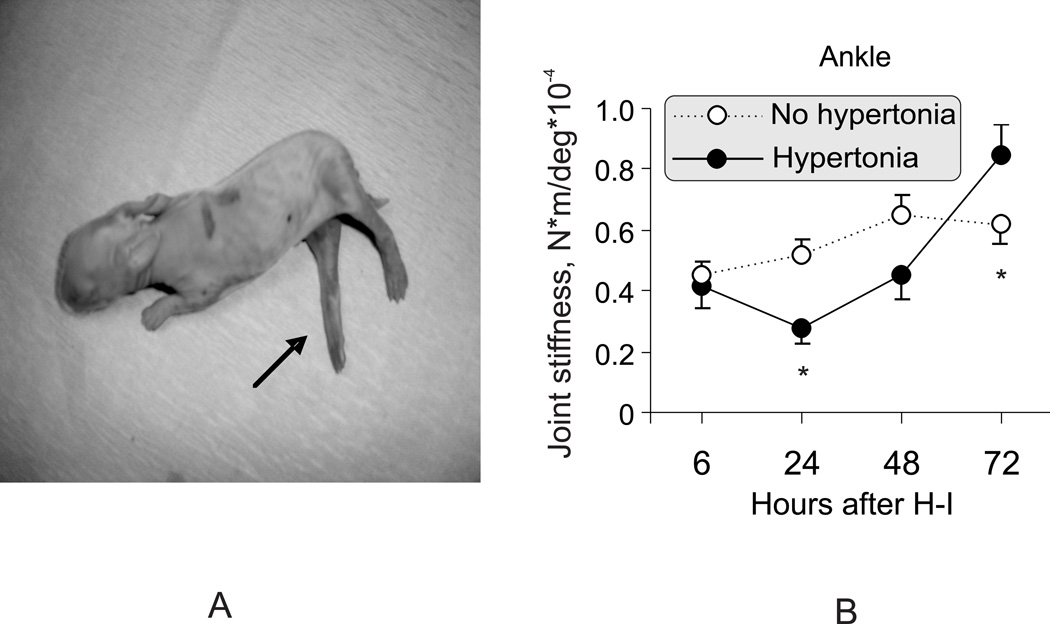
The incidence and pattern of motor deficits after 32 min H-I at E29 was compare with previously published data at E22 and E25. The incidence of hypertonia following H-I at E22 was 75%, using a clinical score based on modification of the Ashworth scale 7. In E29 animals, 24 hours after the H-I, 14/24 surviving kits (58%) had hypotonia of the hind limbs. Of these hypotonic kits, 10 (71%) subsequently developed hypertonia at 72 hours, 7 in hind limbs (Figure 3A) and 3 in all limbs. The other 4 kits continued to have hind limb hypotonia at 72 hours. In addition to manual evaluation7, the qualitative assessment of tone was validated and subsequently quantified by measures of muscle resistance to passive stretch using the torque-displacement apparatus. Kits that developed hypertonia by 72 hour after H-I, had initial resistance to stretch that was lower than corresponding age matched control kits at 24 hour after H-I (Figure 3B). Limb resistance to passive stretch did not change with stretch velocity, indicative of dystonic hypertonia and not spastic hypertonia as assessed by human criteria 12. The dystonic hypertonia following H-I at E29 kits was similar to that found following H-I at E22 10. The quantitative data confirms that hypertonia is preceded by a period of hypotonia in rabbit kits. The short duration of hypotonia also confirms the rapid development of rabbit motor function, similar to other non-human mammals and distinct from the slower human development.
Figure 3.
A. Illustrative case of hind limb hypertonia at 72 hours after E29 fetal H-I. Increased tone in knee and ankle extensors (arrow).
B. Biphasic change of muscle tone in surviving kits after E29 fetal H-I. The tone measure, joint stiffness, was calculated as a slope between joint resistance to passive stretch and joint displacement angle, obtained with torque-displacement apparatus (supplementary Figure S1). The hypertonic group (at 72 hours) had initially lower hind limb tone at 24 hours, replaced by elevated tone by 72 hours after H-I. * - p <0.05 in tone difference between groups, repeated measures ANOVA
Deep brain nuclei pattern of injury after E29 H-I
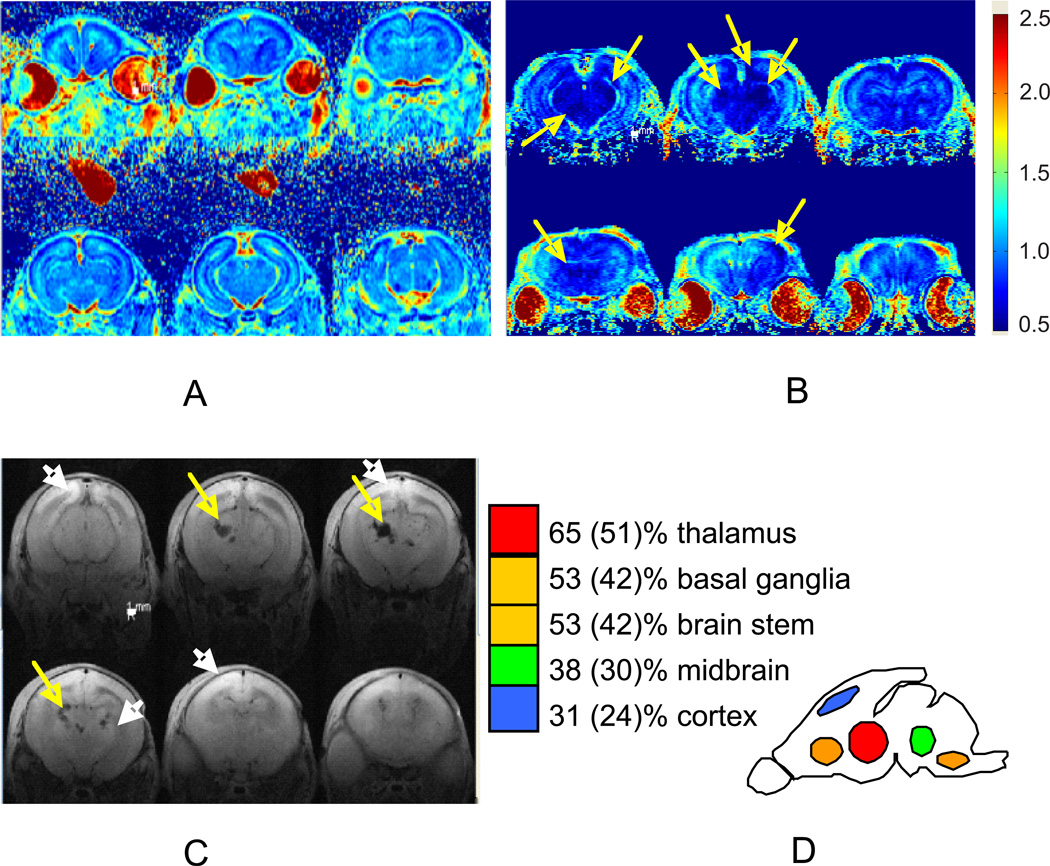
Regional injury could be demarcated by abnormally low ADC at 24 hours after H-I (Figure 4A, B). We also found a spectrum of abnormalities on T2 weighted MRI 72 hours after H-I (Figure 4C), including hyper-intensive areas (white arrows), indicative of high water content and also hypo-intensive lesions (yellow arrows) in some severely affected kits, suggestive of hemorrhages. The prevalence and locations of brain injury are depicted in Figure 4D. Thalamic injury was most common (65%) and often seen in combination with other regions. In addition there was frequent injury to the other deep brain structures such as basal ganglia and brain stem, including midbrain, pons and medulla. Cortical injury, predominantly parasagittal, was observed less frequently and mostly in severe cases with pronounced hypertonia of all limbs. There were also cases with predominantly basal ganglia injury without thalamic involvement (31% of cases with any injury on MRI and 24% of all studied E29 kits after H-I).
Figure 4.
Diffusion weighted images 24 hours after H-I in E29 brain on showing no injury in A and severe injury in B, in areas of cortex, basal ganglia, thalamus, midbrain, brain stem (arrows indicates areas with abnormally low ADC less 0.7 µm2/ms). Color map bar units are µm2/ms.
C. T2-weighted images 72 hours after H-I in E29 brain (n=19 kits) show ischemic regions (white arrows, bright image) and hemorrhagic lesions (yellow arrows, black image) in thalamus and cortex.
D. The frequency of injury is depicted as a proportion of all kits with any injury on MRI and a proportion of all studied kits after H-I in parenthesis.
Persistent regional reduction of ADC within 24 hours after H-I is indicative of injury
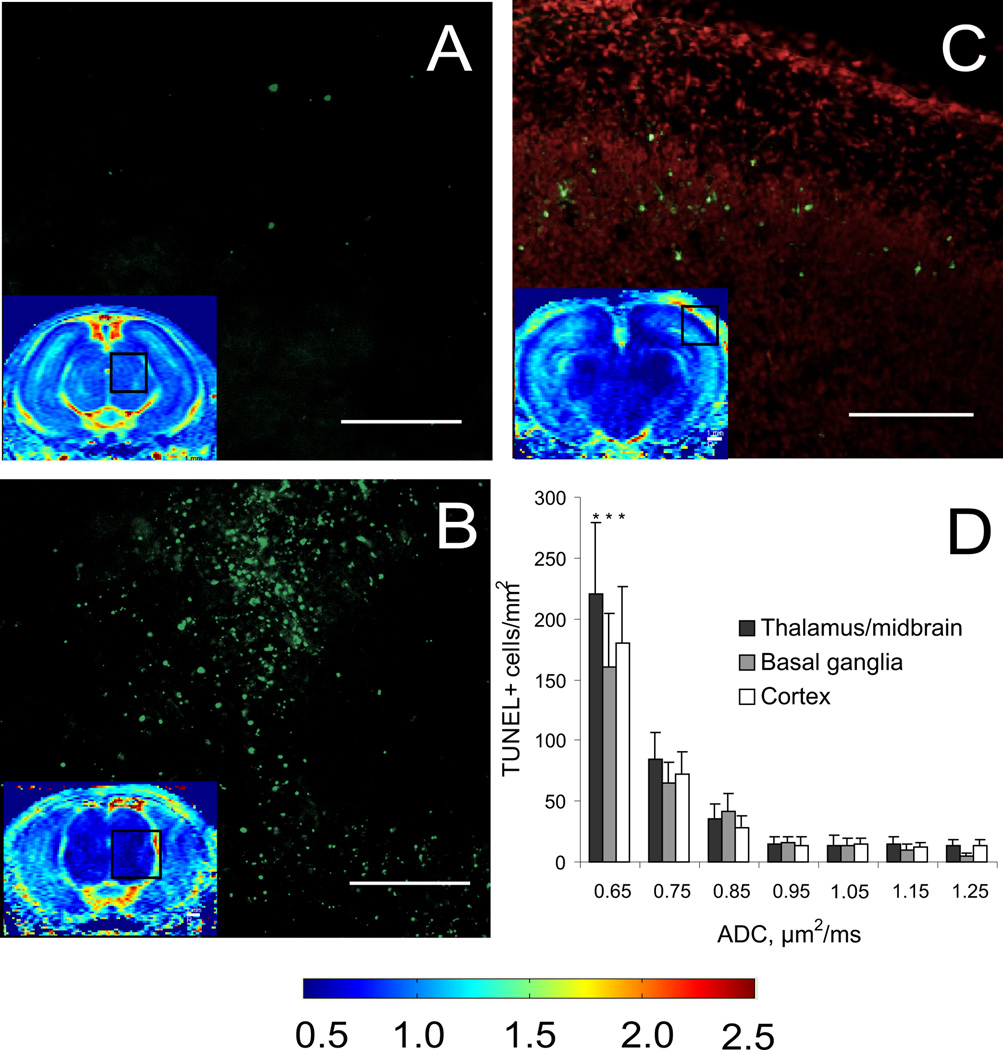
The regions with abnormally low ADC (Figure 4) corresponded to areas with abundant TUNEL labeling, indicative of cell injury (Figure 5A–C). The number of TUNEL positive cells were significantly higher in regions with ADC below 0.7 µm2/ms (Figure 5D)
Figure 5.
Regions of injury defined by ADC decrease below threshold 0.7 µm2/ms, shown on ADC maps from the same animals (color inserts) on sections at midbrain (A,B) and thalamus (C) levels in controls (A) and hypertonic (B,C) kits, had numerous injured cells with positive TUNEL staining 24 hours after H-I injury at E29. Overlay TUNEL (green) + propidium iodide (red) (C) demonstrate that cortical injury was mostly in layer 4. The number of TUNEL positive cells was significantly higher in regions with ADC below 0.7 µm2/ms (D) in all counted gray matter regions (p<0.05, ANOVA). Color map bar units are µm2/ms. Scale bar 500 µm. Number of samples n=4/group in H-I and control groups.
The kits that developed hypertonia had a marked and persistent reduction of ADC during acute H-I in basal ganglia, thalamus and midbrain compared to non-hypertonic kits (supplementary Figure S2) and the decrease persisted for 24 hours. At 72 hours after H-I, abnormally high ADC, tissue loss or hyper-intensities on T2-weighted images were observed in the areas of initially decreased ADC (supplementary FigureS2), similar to the evolution of perinatal brain H-I injury in other animal models and humans 17.
Deep brain nuclear injury, diagnosed by ADC decrease, is a biomarker of motor deficits
In kits that developed motor deficits, ADC in thalamus declined at the end of H-I and remained low at 6, 18 and 24 hours (Figure 6A) and this decline was greater than in those kits without motor deficits.). A similar pattern of ADC change was observed in the other deep gray matter regions and parietal cortex. ADC changes in frontal and motor cortex were not significantly different from sham controls. The decrease of ADC values after H-I at 6 and 18 hours was significantly lower than corresponding values in control kits (p<0.05, repeated measures ANOVA), even on the background of significant maturational changes of ADC in the perinatal period 18.
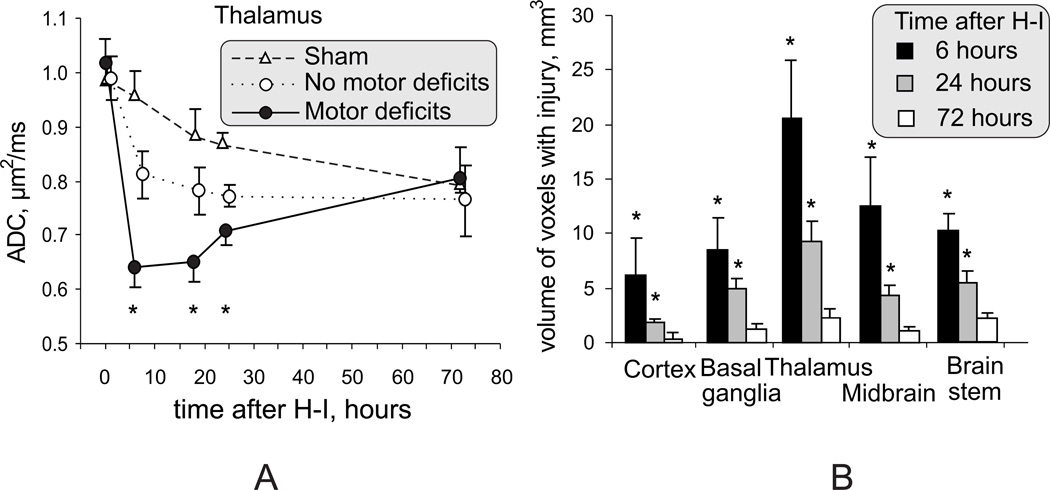
Figure 6.
A. Dynamic changes of ADC in the region of thalamus at the level of third ventricle. Significant ADC decrease persisted in thalamus 6–24 hours after H-I and is an early marker of acute hypoxic thalamic injury. * - p<0.05 comparing motor deficits with group without motor deficits, repeated measures ANOVA.
B. Volume of voxels with ADC below 0.7 µm2/ms in the brains of kits with motor deficits, as an indicator of injury severity, declined from 6 hours to 24 hours after H-I, but remained significant at the 24-hour time point. * - p<0.05, t-test and Bonferroni correction
The severity of H-I injury was delineated by the volume of voxels with an ADC value lower than 0.7 µm2/ms. We chose this empirical threshold since gray and white matter ADC in control animals between E29 and E32 were always above this value and since increased cell injury was observed in regions below this threshold (Figure 5). The volume of voxels with ADC below the cut-off value in injured animals decreased from 6 to 24 h after H-I (Figure 6B) but was still significantly higher at the latter time point in all examined regions (except cerebral cortex). The ADC normalized to control values beyond 24 hours after H-I, in deep gray matter and cortex (Figure 6A). Therefore, a persistent ADC decrease between 6 and 24 hours in deep gray matter regions can be used as an early marker of acute hypoxic injury in deep gray matter and is predictive of subsequent motor deficits. Interestingly, T2-weighted images at 72 hours, but not at 24 hours showed changes that suggest possible hemorrhages in thalamus/midbrains of 2 kits (6%), in areas that had abnormally low ADC at 24 hours after H-I. However, these T2 changes did not possess independent predictive value of hypertonia and seems to be secondary to the severe injury observed with ADC. To test the association between pattern of injury observed in kits 24 hours and presence of neurological abnormalities at 72 hours after H-I, a regression analysis was applied. The best combination of predictors for any motor deficits was injury in basal ganglia and in brain stem and for hypertonia - in thalamus and brain stem (supplementary table S3).
DISCUSSION
1. Biphasic muscle tone changes after antenatal H-I
For the first time in a perinatal H-I model evolution of motor abnormalities was quantitatively assessed by measuring resistance to passive joint stretch (Figure S1). The present study shows the rapid evolution (a few days) from hypotonia to hypertonia in the developing rabbit. Previously only hypertonia was observed after delivery at E32 or P1 following H-I at E22 and E25 7, 9, 11. This evolution of hypotonia to hypertonia following E29 H-I is similar to that of humans with H-I injury. Patients with neonatal encephalopathy have mostly hypotonia as an initial finding and hypertonia may develop months or years later with the diagnosis of spasticity or dystonia 19. This time span make causal etiological linkages difficult in human clinical studies. The rapid transition in perinatal rabbits makes the model a convenient platform to study the etiology of motor deficits of CP, after H-I. Furthermore, we show structural lesions on MRI are predictive of motor deficits postnatally and there is a regional predilection to brain injury.
2. Sensitivity of deep brain nuclei to H-I injury and importance of the injury in pathogenesis of CP
Human term newborns often have “cerebral cortical – deep nuclear” or “deep nuclear – brain stem” types of brain injury 4–6, 19. The basal ganglia/thalamus pattern in term and premature infants5 is associated with the most impaired motor and cognitive outcome at 30 months 4–5, 20. Thalamic motor nuclei are especially injured 21. Similarly, the near-term rabbit studies at E29 confirm the predictive value of basal ganglia-thalamus-brain stem injury to postnatal motor deficits (Table S4).
The injury also involved cortex and periventricular white matter, albeit to a lesser extent. In human infants after perinatal asphyxia, decreased brain MRI ADC values of the brain stem and basal ganglia correlated with abnormal or adverse outcome 22. The implication of neuronal loss and gliosis being more common in the thalamus in human PVL 21, 23 is either due to a primary injury or a secondary anterograde or retrograde injury. The secondary consequences of a primary neuronal injury could involve white matter axons, with subsequent hypomyelination and impaired development of cerebral cortex and thalamus/basal ganglia 24. This study, however, was designed only to study short term outcome at 72 hours because we wanted to compare with E32 findings of previous studies of earlier gestational ages. Longer term white matter and cortical/subcortical abnormalities and involvement in motor and cognitive impairment in rabbits require further investigation.
3. MRI as a biomarker
A decrease of whole brain ADC below 0.83 µm2/ms in E25 11 and below 0.75 µm2/ms in E29 rabbits during and immediately after H-I (Figure 2) was predictive of motor deficits. We have extended the time window to 6–24 hour postnatally after H-I and demonstrated a threshold of ADC decrease below 0.7 µm2/ms to be a reliable diagnostic tool for injury to the deep brain nuclei and was predictive of hypertonia and other motor deficits. The implication is also that MRI indicates a level of regional injury that is significant clinically to cause a motor phenotype. The absence of the specific ADC fall also may be useful prognostically.
4. Comparison of timing
Our study underlines the importance of timing in diagnosing perinatal brain injury by DWI. In contrast to rodent25 and piglet26 neonatal stroke models, we did not observe a secondary decline of ADC 24 hr after H-I. In rabbits, whole brain ADC only partially recovered after 20 min of reperfusion-reoxygenation and was consistently low at 6–24 hours in deep brain nuclei and cortex. This discrepancy can be attributed to species differences and global nature of H-I and are more analogous to the human situation. In a prospective human longitudinal study with known time of insult, ADC declined and reached a nadir in injured regions of 35% of the control values between 2–3 days17. ADC returned to normal levels by 7 days. In the present study, the corresponding nadir of ADC occurred around 6 hours and returned to normal levels around 3 days, suggesting the evolution of H-I injury follows the human pattern s but occurs 4–6 times faster. This may correspond to the relatively short time frame of neurobehavioral evolution from hypotonia to hypertonia in rabbits.
SUMMARY
The near-term rabbit model may be a useful platform to test mechanisms for evolution of muscle tone changes. We have shown that quantification of lesions is important both for prediction and studying evolution of muscle tone. Abnormally low ADC values suggestive of deep brain injury 6–24 hours following near-term H-I predicts dystonic hypertonia postnatally. The first torque-displacement studies to be done in perinatal models indicate that motor deficits in rabbits progressed from initial hypotonia to hypertonia, similar to human CP, but in a compressed time-frame. The presence of deep brain injury and quantitative shift from hypo- to hypertonia may identify patients at risk for developing CP.
Supplementary Material
Acknowledgement
Xinhai Ji, Aiping Liu.
Source of funding: ENH pilot award, NIH NS062367, UCP/Hearst foundation (AD), NIH NS 43285, NS051402 (ST)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest/ Disclosures: none
REFERENCES
- 1.Laura LD, Deborah G-S. Assessment and treatment of movement disorders in children with cerebral palsy. The Orthopedic clinics of North America. 2010;41:507–517. doi: 10.1016/j.ocl.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: The definition and classification of cerebral palsy april 2006. Dev. Med. Child Neurol. Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 3.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr. Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford M, Biarge M, Allsop J, Counsell S, Cowan F. Mri of perinatal brain injury. Pediatr. Radiol. 2010;40:819–833. doi: 10.1007/s00247-010-1620-z. [DOI] [PubMed] [Google Scholar]
- 5.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J. Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Volpe JJ. Neurology of the newborn. Philadelphia: W.B. Saunders Company; 2001. [Google Scholar]
- 7.Tan S, Drobyshevsky A, Jilling T, Ji X, Ullman LM, Englof I, et al. Model of cerebral palsy in the perinatal rabbit. J. Child Neurol. 2005;20:972–979. doi: 10.1177/08830738050200120801. [DOI] [PubMed] [Google Scholar]
- 8.Drobyshevsky A, Derrick M, Wyrwicz AM, Ji X, Englof I, Ullman LM, et al. White matter injury correlates with hypertonia in an animal model of cerebral palsy. J. Cereb. Blood Flow Metab. 2007;27:270–281. doi: 10.1038/sj.jcbfm.9600333. [DOI] [PubMed] [Google Scholar]
- 9.Derrick M, Drobyshevsky A, Ji X, Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- 10.Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, et al. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: A model for human cerebral palsy? J. Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drobyshevsky A, Derrick M, Prasad PV, Ji X, Englof I, Tan S. Fetal brain magnetic resonance imaging response acutely to hypoxia-ischemia predicts postnatal outcome. Ann. Neurol. 2007;61:307–314. doi: 10.1002/ana.21095. [DOI] [PubMed] [Google Scholar]
- 12.Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:e89–e97. doi: 10.1542/peds.111.1.e89. [DOI] [PubMed] [Google Scholar]
- 13.Chen JJ, Wu YN, Huang SC, Lee HM, Wang YL. The use of a portable muscle tone measurement device to measure the effects of botulinum toxin type a on elbow flexor spasticity. Arch. Phys. Med. Rehabil. 2005;86:1655–1660. doi: 10.1016/j.apmr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Lee HM, Chen JJ, Ju MS, Lin CC, Poon PP. Validation of portable muscle tone measurement device for quantifying velocity-dependent properties in elbow spasticity. J. Electromyogr. Kinesiol. 2004;14:577–589. doi: 10.1016/j.jelekin.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Kakinohana O, Hefferan MP, Nakamura S, Kakinohana M, Galik J, Tomori Z, et al. Development of gaba-sensitive spasticity and rigidity in rats after transient spinal cord ischemia: A qualitative and quantitative electrophysiological and histopathological study. Neuroscience. 2006;141:1569–1583. doi: 10.1016/j.neuroscience.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 16.Wu YN, Hyland BI, Chen JJ. Biomechanical and electromyogram characterization of neuroleptic-induced rigidity in the rat. Neuroscience. 2007;147:183–196. doi: 10.1016/j.neuroscience.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 17.McKinstry RC, Miller JH, Snyder AZ, Mathur A, Schefft GL, Almli CR, et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology. 2002;59:824–833. doi: 10.1212/wnl.59.6.824. [see comment]. [DOI] [PubMed] [Google Scholar]
- 18.Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J. Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpe JJ. Neurology of the newborn. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 20.Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics. 2008;121:906–914. doi: 10.1542/peds.2007-0770. [DOI] [PubMed] [Google Scholar]
- 21.Ligam P, Haynes RL, Folkerth RD, Liu L, Yang M, Volpe JJ, et al. Thalamic damage in periventricular leukomalacia: Novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr. Res. 2009;65:524–529. doi: 10.1203/PDR.0b013e3181998baf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liauw L, van Wezel-Meijler G, Veen S, van Buchem MA, van der Grond J. Do apparent diffusion coefficient measurements predict outcome in children with neonatal hypoxic-ischemic encephalopathy? AJNR. Am. J. Neuroradiol. 2009;30:264–270. doi: 10.3174/ajnr.A1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpe JJ. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedelcu J, Klein MA, Aguzzi A, Boesiger P, Martin E. Biphasic edema after hypoxic-ischemic brain injury in neonatal rats reflects early neuronal and late glial damage. Pediatr. Res. 1999;46:297–304. doi: 10.1203/00006450-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Thornton JS, Ordidge RJ, Penrice J, Cady EB, Amess PN, Punwani S, et al. Temporal and anatomical variations of brain water apparent diffusion coefficient in perinatal cerebral hypoxic-ischemic injury: Relationships to cerebral energy metabolism. Magn. Reson. Med. 1998;39:920–927. doi: 10.1002/mrm.1910390609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.