Abstract
The associations of acute HIV infection (AHI) and other predictors with transmitted drug resistance (TDR) prevalence were assessed in a cohort of HIV-infected, antiretroviral-naïve patients. AHI was defined as being seronegative with detectable HIV RNA. Binomial regression was used to estimate prevalence ratios and 95% confidence intervals (CIs) for associations with TDR. Among 43 AHI patients, TDR prevalence was 20.9%, while prevalence was 8.6% among 677 chronically-infected patients. AHI was associated with 1.9 times the prevalence of TDR (95% CI: 1.0, 3.6) in multivariable analysis. AHI patients may represent a vanguard group that portends increasing TDR in the future.
Keywords: Transmitted drug resistance, HIV-1, Acute HIV Infection, Antiretroviral Resistance
Introduction
Transmitted drug resistance (TDR) may lead to a more rapid decline in CD4 cell counts prior to combination antiretroviral therapy (cART) initiation, and may increase the risk of virologic failure following cART initiation1–3. Therefore current HIV treatment guidelines recommend resistance testing at entry into HIV care and at cART initiation4.
TDR prevalence in the U.S. and internationally has been estimated to be between 8 and 15%5, 6; however, little is known about TDR in the Southeastern U.S., where the HIV epidemic continues to grow7. TDR prevalence may be higher among patients with acute HIV infection (AHI), than patients with chronic HIV infection (CHI)8, 9; but no direct comparisons have been made as AHI is typically either unidentified or crudely defined in large populations. Notwithstanding efforts at increasing HIV testing and early linkage to HIV care10, the vast majority of patients initiate HIV care with CD4 cell counts less than 500 cells/mL11. Therefore understanding the relationship between duration of HIV infection and TDR detection remains relevant. Knowledge of trends in TDR can guide clinical decisions about resistance testing and cART options, and may also inform community prevention efforts by identifying risk factors for acquiring TDR. In this study we used the University of North Carolina (UNC) Center for AIDS Research HIV Clinical Cohort (UCHCC) to characterize patients with TDR, evaluate trends over time, and contrast prevalence by HIV infection duration.
Methods
Patients at least 18 years of age participating in the UCHCC who initiated HIV care between January 1999 and September 2010 were eligible for this study. The UCHCC and its procedures have been described previously12. Briefly, information is collected on HIV diagnosis date, HIV transmission risk factors, antiretroviral (ARV) start and stop dates, and resistance reports through semi-annual standardized clinical record reviews. Demographic information (age, sex, and race), and laboratory information including CD4 cell counts and HIV viral loads are extracted from institutional electronic databases. For this study, laboratory results collected nearest to the genotype sample date were used to obtain values for CD4 cell counts and HIV viral loads. AHI was defined as either: a combination of non-reactive enzyme-linked immunosorbent assay (ELISA) or a negative or indeterminate Western blot (WB) paired with a positive HIV RNA or p24 antigen test, or a negative ELISA and WB less than 45 days before a documented positive ELISA or WB8, 13. All patients participating in the UCHCC provided informed consent. Ethical approval for this study was obtained from the UNC Institutional Review Board.
Patients were included who had at least one available genotype prior to cART initiation. Of the 720 eligible patients, 408 had genotypes available through routine clinical care and 312 patients had genotypes conducted on archived specimens. Population or “bulk” genotyping was conducted using commercially available assays, with over 90% of assays using HIV Genosure and HIV Genosure Plus (Laboratory Corporation of America, Research Triangle Park, North Carolina, USA). TDR was defined as the detection of any of the surveillance drug resistance mutations (SDRMs) listed by the World Health Organization14. We further characterized SDRMs into nucleoside reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI), or protease inhibitor (PI) mutations. Dual-class resistance was defined as the detection of at least one SDRM from two of the three drug classes considered: NRTI, NNRTI, and PI. Triple-class resistance was defined as the detection of at least one SDRM from all three drug classes. Integrase inhibitor resistance was not assessed.
Prevalence of TDR was calculated as the number of patients with detectable SDRMs divided by all patients with an available genotype; 95% confidence intervals (CI) were calculated using the exact binomial method. We assessed TDR prevalence over calendar time using the Cochran-Armitage trend test. Multivariable log-binomial regression was used to estimate adjusted prevalence ratios (PR) and 95% CIs of TDR across patient characteristics 15. Covariates were selected based on a priori knowledge and associations with AHI and TDR in the data. All analyses were conducted using SAS 9.2 statistical software (SAS Institute, Cary, North Carolina, USA).
Results
Of the 720 patients included in this study, 71% were male, 57% black, and 28% white; 43% of the patients were men who have sex with men (MSM), and 9% reported prior injection drug use (IDU). The median year of genotype sample date was 2005 (interquartile range, IQR: 2001–2007) and the average time from HIV diagnosis to genotype was 60 days (IQR: 22–280). The median CD4 cell count at genotype testing was 256 cells/mm3 (IQR: 62–454) and the median HIV RNA level was 4.8 log10 copies/mL (IQR: 4.2–5.3). Almost all patients were infected with HIV subtype B with <1% non-B subtypes. Forty-three patients were identified as AHI. AHI compared to CHI patients were more likely to be male (88 versus 70%, p=0.02), MSM (81 versus 41%, p<0.001), have higher CD4 cell counts (median 515 versus 236 cells/mm3, p<0.001) and HIV RNA levels (median 5.2 versus 4.8 log10 copies/mL, p=0.003). AHI patients had more recent genotypes (median year 2006 versus 2005, p<0.001) and shorter time from diagnosis to genotype (median 0.6 months versus 2.2 months, p<0.001).
The overall prevalence of TDR was 9.3% (95% CI: 7.3, 11.7): 1.5% with dual-class drug resistance and none with triple-class drug resistance. NNRTI resistance was most common (5.7% of all patients), while PI resistance was least common (1.5% of all patients). The most common SDRMs for the NRTI, NNRTI, and PI classes were D67N (1.1%), K103N (4.4%), and L90M (1.3%), respectively. Twenty-one percent (n=9) of AHI patients had evidence of TDR. Eight AHI patients had NNRTI mutations, six patients had K103N, and one each had Y188L and K103S. One AHI patient had a PI mutation (L90M) and none had dual class resistance. Nine percent (n=58) of CHI patients had TDR. Thirty-three CHI patients had NNRTI resistance; the most frequent were K103N (n=26), G190A (n=7), and K101E (n=3). The most common NRTI and PI mutations detected among CHI patients were D67N (n=8) and L90M (n=8), respectively. Eleven CHI patients had dual class resistance.
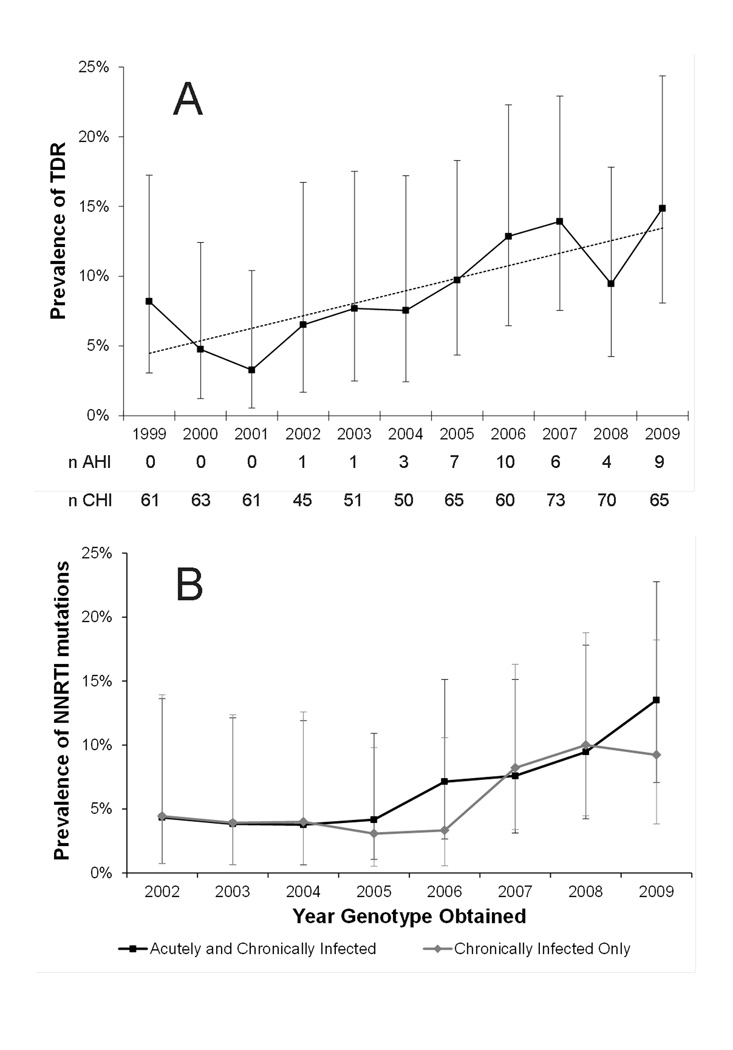
Patients with AHI had 2.4 times the prevalence of TDR than patients with CHI (95% CI: 1.3, 4.6; Table 1). The prevalence of TDR had a relative increase of 7% with each 100 CD4 cell count increase (95% CI: 0%, 14%), and was higher among MSM. Prevalence of TDR increased with calendar time (P=0.01 for trend; Figure 1A). This was primarily due to increases in NNRTI TDR with a relative increase in NNRTI TDR of 20% with each additional calendar year (95% CI: 10, 30; Figure 1B).
Table 1.
Demographic and clinical characteristics associated with Transmitted HIV Drug Resistance among antiretroviral naïve acutely and chronically HIV-infected patients, UCHCC 1999–2010
| TDR N (%) |
No TDR N (%) |
PR | 95% CI | P | |
|---|---|---|---|---|---|
| Total | 67 (9.3) | 653 (90.7) | |||
| Acute HIV Infection | |||||
| No | 58 (8.6) | 619 (91.4) | Ref | ||
| Yes | 9 (20.9) | 34 (79.1) | 2.44 | 1.30, 4.59 | 0.006 |
| Sex | |||||
| Male | 52 (10.1) | 462 (89.9) | Ref | ||
| Female | 15 (7.3) | 191 (92.7) | 0.72 | 0.41, 1.25 | 0.24 |
| Race | |||||
| Black | 39 (9.4) | 374 (90.6) | Ref | ||
| White | 19 (9.6) | 180 (90.5) | 1.01 | 0.60, 1.70 | 0.97 |
| Hispanic | 3 (4.4) | 65 (95.6) | 0.47 | 0.15, 1.47 | 0.19 |
| Other | 6 (15.0) | 34 (85.0) | 1.59 | 0.72, 3.52 | 0.25 |
| MSM | |||||
| No | 29 (7.1) | 382 (92.9) | Ref | ||
| Yes | 38 (12.3) | 271 (87.7) | 1.74 | 1.10, 2.76 | 0.02 |
| IDU | |||||
| No | 64 (9.7) | 595 (90.3) | Ref | ||
| Yes | 3 (4.9) | 58 (95.1) | 0.51 | 0.16, 1.56 | 0.24 |
| Year of Genotype | |||||
| Median (IQR) | 2006 (2003–2008) | 2005 (2001–2007) | 1.09† | 1.02, 1.18† | 0.02 |
|
Months Between HIV Diagnosis and Genotype |
|||||
| Median (IQR) | 2.4 (0.8–15.4) | 1.9 (0.7–8.5) | 1.00 | 0.99, 1.01 | 0.95 |
| Age at Diagnosis | |||||
| Mean (SD) | 34.1 (11.1) | 36.3 (10.8) | 0.84† | 0.67, 1.04† | 0.11 |
| CD4 cell Count (cells/mm3) | |||||
| Median (IQR) | 255 (60–446) | 290 (98–539) | 1.07† | 1.00, 1.14† | 0.06 |
| HIV RNA level (log10 copies/mL) | |||||
| Median (IQR) | 4.8 (4.2–5.4) | 4.7 (4.2–5.3) | 0.99† | 0.75, 1.29† | 0.92 |
Year of genotype estimates per 1 calendar year increase; Age estimates per 10 year increase in age; CD4 cell count estimates per 100 cells/ml increase; HIV RNA level estimates per 1 log10 copies/mL increase
UCHCC= University of North Carolina Center for AIDS Research HIV Clinical Cohort; TDR= Transmitted Drug Resistance; MSM= Men who have sex with men; IDU= Injection drug user
Figure 1.
A) Prevalence of Transmitted Drug Resistance across calendar time and B) Prevalence of Non-Nucleoside Reverse Transcriptase Inhibitor Transmitted Drug Resistance across calendar time
Error bars indicate 95% confidence intervals calculated using the binomial exact test. Gray dotted line in (A) indicates trend across all calendar years. TDR=Transmitted drug resistance, n AHI= number of patients with acute HIV infection who had a genotype obtained in each calendar year, n CHI=number of patients with chronic HIV infection who had a genotype obtained in each calendar year, NNRTI=Non-nucleoside reverse transcriptase inhibitor
In multivariable analyses, after adjusting for age, MSM, and calendar year of genotype test, AHI remained positively associated with TDR (PR: 1.9; 95% CI: 1.0, 3.6). Adjustment for additional variables including sex, race, CD4 cell count and HIV RNA level did not meaningfully change the PR estimate (PR: 1.8; 95% CI: 0.9, 3.7). In further multivariable analyses we did not identify other factors that were independently predictive of TDR among all patients.
Results were stratified by infection duration to assess whether demographic and clinical characteristics predicted TDR differently among AHI versus CHI patients. Consistent with our overall results, TDR prevalence was higher in more recent calendar years within both strata (AHI: 1999–2005=8%, 2006–2010=28%; CHI: 1999–2005=7%, 2006–2010=11%). NNRTI prevalence was also higher in more recent calendar years within both strata (AHI: 1999–2005=8%, 2006–2010=24%; CHI: 1999–2005=3%, 2006–2010=7%). Among AHIs, TDR prevalence was 1.3 times higher with each passing calendar year (95% CI: 0.9, 1.8, P=0.13 for trend) and prevalence was 1.1 times higher with each calendar year increase in CHIs (95% CI: 1.0, 1.2, P<0.01 for trend). MSM appeared predictive of TDR among CHI patients, but was not predictive of TDR among AHI patients. No other factors appeared predictive within either strata of infection duration, though power was not sufficient to conduct multivariable analyses.
Discussion
We observed a high prevalence of TDR in this Southeastern US cohort. Prevalence was highest for NNRTI mutations and lowest for PI mutations. As our study period began after the first case reports of TDR for NRTIs (1993), NNRTIs (1997), and PIs (1998), all of these mutations were expected to be present in our population from the beginning of the study period, at least at low levels16. TDR prevalence rose over calendar time in our population, with the latest estimates similar to prevalence estimates from recent US surveillance data6, 17. By contrast, studies in Europe have shown stabilizing and possibly decreasing trends in TDR prevalence in more recent years5.
Within our study, the rise in TDR across calendar time was mainly due to a rise in NNRTI mutations. This may be due in part to a rise in use of NNRTI-based fixed-dose combination regimens during this same time interval. This could also be evidence of the persistence of common NNRTI mutations such as K103N which are known to be long-lived even in the absence of ARV exposure18, 19. Persistence of TDR may be longer in the genital tract20, which can lead to reservoirs of resistance and transmission chains of TDR among antiretroviral naïve individuals21. As such, the observed increase in TDR prevalence may be due to onward transmission from individuals failing cART, individuals who are cART naive with TDR, or both.
Our most notable finding was the substantial difference in TDR prevalence by infection duration. A comparison of TDR by infection duration has not been made in larger studies because of the lack of a precise definition of AHI such as was available in our study. AHI patients had over twice the prevalence of TDR compared to CHI patients, largely driven by a higher frequency of NNRTI mutations. Several hypotheses may explain the association of infection duration with TDR. The lower prevalence of TDR in CHIs compared to AHIs may be a result of the reversion of mutations over time. We were unable to assess changes in detection of SDRMs over time within individual ARV naïve patients. Reversion of mutations to wild type has been observed, although certain SDRMs may persist despite the absence of ARV exposure18, 19. NNRTI mutations, specifically K103N, reduce replicative capacity only moderately and thus can persist for long periods of time18. Additionally, minority variants may persist and have a meaningful influence on treatment response22. Genotype testing shortly after AHI diagnosis may be informative for clinicians even if immediate ARV initiation is not expected, as detectable mutations in AHI may possibly persist as minority variants in CHI.
The association of infection duration with TDR could also be due to differences between AHI and CHI patients not accounted for in this analysis. Individuals with high risk behaviors not measured in this study may be more likely to be a part of sexual networks with TDR and may undergo more frequent HIV testing increasing the probability of being diagnosed with AHI23. Some CHI patients may have been infected before the widespread use of cART, while AHI patients with more recent infection dates may have been infected when there were higher levels of SDRMs circulating in the HIV population. Given the high prevalence of NNRTI mutations among AHI patients and the increasing trend in NNRTI mutation prevalence over time, patients with AHI may serve as a harbinger of future TDR trends in CHI individuals.
No covariates other than AHI appeared to independently predict TDR. Prior literature has not identified consistent individual-level risk factors for TDR5, 6, 8, 24, which may be a result of patients contracting TDR for heterogeneous reasons, as well as differing treatment practices and treatment histories by geographic region. The absence of strong predictors of TDR underscores the importance of genotype testing for all antiretroviral-naïve HIV patients regardless of demographic or behavioral characteristics.
TDR limits treatment options, increases the risk of poor treatment outcomes2, 3 and results in onward transmission of resistant virus. The rising prevalence of TDR and high prevalence among AHI patients suggest that ARV treatment options with higher genetic barriers to resistance may be indicated. Ongoing monitoring for TDR will remain important in considering appropriate clinical practices and anticipating future challenges as fewer new ARVs are developed. Monitoring resistance specifically within the AHI population may serve as an important tool in forecasting future patterns of drug resistance. Further investigation into the reasons for differences in TDR prevalence between AHI and CHI individuals, including in-depth comparisons of risk behaviors and community antiretroviral use, can optimize the interpretation of TDR monitoring data in both groups in the future.
Acknowledgements
This study was supported by the Center for AIDS Research at the University of North Carolina at Chapel Hill (P30 AI50410) and the University of North Carolina at Chapel Hill Training in Sexually Transmitted Diseases and HIV NIH Grant (5T32AI007001-35). Laboratory Corporation of America and Virco provided partial financial support for sample retrieval and performed drug resistance testing. In addition, we would like to thank Anna Barry for help with the Duke/UNC Acute Consortium database and all of the patients who consented to participate in the UCHCC.
Footnotes
Conflicts of Interest:
SN has received grant support from Pfizer, Bristol-Myers Squibb, and Merck. JJE has received consulting fees from Tibotec, Bristol-Myers Squibb, Merck, GlaxoSmithKline, ViiV and Pfizer, lecture fees from Roche, Bristol-Myers Squibb, Tibotec, and Merck, and grant support from GlaxoSmithKline, Merck, ViiV and Boehringer-Ingelheim. JS is an employee of Laboratory Corporation of America.
Parts of this work were previously presented at the International HIV and Hepatitis Resistance Workshop, June 8–10, 2011, and the International Conference on Pharmacoepidemiology, August 14–17, 2011.
References
- 1.CASCADE Virology Collaboration. The impact of transmitted drug resistance on the natural history of HIV infection and response to first-line therapy. AIDS. 2006;20:21–28. doi: 10.1097/01.aids.0000196172.35056.b7. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi T, Nurutdinova D, Grubb JR, et al. Transmitted Drug-Resistant HIV Type 1 Remains Prevalent and Impacts Virologic Outcomes Despite Genotype-Guided Antiretroviral Therapy. AIDS Research and Human Retroviruses. 2011;27 doi: 10.1089/aid.2011.0022. [DOI] [PubMed] [Google Scholar]
- 3.Wittkop L, Gunthard HF, de Wolf F, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis. 2011;11:363–371. doi: 10.1016/S1473-3099(11)70032-9. [DOI] [PubMed] [Google Scholar]
- 4.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescent. 2011 Jan 10; Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 5.Vercauteren J, Wensing AMJ, van de Vijver DAMC, et al. Transmission of Drug-Resistant HIV-1 Is Stabilizing in Europe. The Journal of Infectious Diseases. 2009;200:1503–1508. doi: 10.1086/644505. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler WH, Ziebell RA, Zabina H, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS. 2010;24:1203–1212. doi: 10.1097/QAD.0b013e3283388742. [DOI] [PubMed] [Google Scholar]
- 7.Peterman TA, Lindsey CA, Selik RM. This Place Is Killing Me: A Comparison of Counties Where the Incidence Rates of AIDS Increased the Most and the Least. The Journal of Infectious Diseases. 2005;191(Suppl 1):S123–S126. doi: 10.1086/425284. [DOI] [PubMed] [Google Scholar]
- 8.Hurt CB, McCoy SI, Kuruc J, et al. Transmitted Antiretroviral Drug Resistance among Acute and Recent HIV Infections in North Carolina, 1998 to 2007. Antivir Ther. 2009;14(5):673–678. [PMC free article] [PubMed] [Google Scholar]
- 9.Jain V, Liegler T, Vittinghoff E, et al. Transmitted Drug Resistance in Persons with Acute/Early HIV-1 in San Francisco, 2002–2009. PLoS ONE. 2010;5(12):e15510. doi: 10.1371/journal.pone.0015510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Divisions of HIV/AIDS Prevention. Expanded Testing Program Overview. 2011 Aug 22; http://www.cdc.gov/hiv/resources/factsheets/HIV-ETP.htm.
- 11.Althoff KN, Gange SJ, Klein MB, et al. Late Presentation for HIV Care in the United States and Canada. Clinical Infectious Diseases. 2010;50(11):1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napravnik S, Eron JJ, McKaig RG, et al. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care. 2006;18(Supplement 1):S45–S50. doi: 10.1080/09540120600838928. [DOI] [PubMed] [Google Scholar]
- 13.Pilcher CD, Fiscus SA, Nquyen TQ, et al. Detection of Acute Infections during HIV Testing in North Carolina. NEJM. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 14.Bennett DE, Camacho RJ, Otelea D, et al. Drug Resistance Mutations for Surveillance of Transmitted HIV-1 Drug-Resistance: 2009 Update. PLoS ONE [serial online] 2009;4(3):e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Medical Research Methodology. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurt CB. Transmitted resistance to HIV integrase strand-transfer inhibitors: right on schedule. Antivir Ther. 2011;16:137–140. doi: 10.3851/IMP1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon AFY, Aldous JL, Mathews WC, et al. Transmitted Drug Resistance in the CFAR Network of Integrated Clinical Systems Cohort: Prevalence and Effects on Pre-Therapy CD4 and Viral Load. PLoS ONE. 2011;6(6):e21189. doi: 10.1371/journal.pone.0021189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain V, Sucupira MC, Bacchetti P, et al. Differential Persistence of Transmitted HIV-1 Drug Resistance Mutation Classes. The Journal of Infectious Diseases. 2011;203:1174–1181. doi: 10.1093/infdis/jiq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little SJ, Frost SDW, Wong JK, et al. Persistence of Transmitted Drug Resistance among Subjects with Primary Human Immunodeficiency Virus Infection. Journal of Virology. 2008;82(11):5510–5518. doi: 10.1128/JVI.02579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DM, Wong JK, Shao H, et al. Long-Term Persistence of Transmitted HIV Drug Resistance in Male Genital Tract Secretions: Implications for Secondary Transmission. JID. 2007;196:356–360. doi: 10.1086/519164. [DOI] [PubMed] [Google Scholar]
- 21.Hue S, Gifford RJ, Dunn D, et al. Demonstration of Sustained Drug-Resistant Human Immunodeficiency Virus Type 1 Lineages Circulating among Treatment-Naive Individuals. Journal of Virology. 2009;83(6):2645–2654. doi: 10.1128/JVI.01556-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li JZ, Paredes R, Ribaudo HJ, et al. Low-Frequency HIV-1 Drug Resistance Mutations and Risk of NNRTI-Based Antiretroviral Treatment Failure. JAMA. 2011;305(13):1327–1335. doi: 10.1001/jama.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leaity S, Sherr L, Wells H, et al. Repeat HIV testing: high-risk bevahiour or risk reduction strategy? AIDS (London, England) 2000;14:547–552. doi: 10.1097/00002030-200003310-00010. [DOI] [PubMed] [Google Scholar]
- 24.Bartmeyer B, Kuecherer C, Houareau C, et al. Prevalence of Transmitted Drug Resistance and Impact of Transmitted Drug Resistance on Treatment Success in the German HIV-1 Seroconverter Cohort. PLoS ONE. 2010;5(10):e12718. doi: 10.1371/journal.pone.0012718. [DOI] [PMC free article] [PubMed] [Google Scholar]