Abstract
Spontaneous preterm birth is a heterogeneous phenotype. A multitude of pathophysiologic pathways culminate in the final common denominator of cervical softening, shortening and dilation that leads to preterm birth. A precise description of specific microstructural changes to the cervix is imperative if we are to identify the causative upstream molecular processes and resultant biomechanical events associated with each unique pathway. Currently, however, we have no reliable clinical tools for quantitative and objective evaluation, which likely contributes to the reason the singleton spontaneous preterm birth rate has not appreciably changed in more than 100 years. Fortunately, promising techniques to evaluate tissue hydration, collagen structure and/or tissue elasticity are emerging. These will add to the body of knowledge about the cervix and facilitate coordination of molecular studies, ultimately leading to novel approaches to preterm birth prediction and, finally, prevention.
Keywords: Cervical microstructural assessment, quantitative ultrasound, preterm birth, short cervix
PRETERM BIRTH IS A HETEROGENEOUS PHENOTYPE
Spontaneous preterm birth (sPTB), the leading global cause of neonatal death, affects 13 million babies annually.1 Premature babies who survive are at risk for serious complications including cerebral palsy, respiratory morbidity, mental retardation, blindness, deafness, cardiovascular disease and cancer.2 This problem has been the object of considerable research effort for decades, yet the singleton spontaneous preterm birth (sPTB) rate remains unacceptably high, both in the US and throughout the world. A prevailing theory is that this is because the approach to research and treatment has been simplistic, while the pathways to sPTB are extremely complex.3,4
sPTB is the final common denominator of the interaction of a multitude of factors such as social stress, infection/inflammation, poor nutrition, genetics, and others.3–7 Studies of biomarkers, genetics, and “-omics”, while adding essential information to the body of knowledge about sPTB, have thus far been disappointing with regard to its prevention. Of more than 100 biomarkers evaluated in a recent meta-analysis, none emerged as reliably predictive, assessing biomarkers for early intervention has not decreased the incidence of sPTB,8 associations between sPTB risks and polymorphisms in candidate genes have been modest,5 and “-omics” studies (genomics, proteomics, transcriptomics, metabolomics) have provided no concrete associations with sPTB.5,9 This is not unexpected, however, given the multiple and varied pathways to sPTB.
Fortunately for study, the pathways to sPTB dovetail into final unifying processes such as cervical remodeling.3,4,9 As recently stated by Global Alliance to Prevent Prematurity and Stillbirth (GAPPS), “The finding that heterogeneous origins result in common downstream biological pathways and outcomes … provides the opportunity to develop rational treatment strategies that target the upstream initiators … ”3 In other words, identification of cervical microstructural changes during specific stages of remodeling should facilitate study of isolated molecular events and interactions at critical time points.
THE ROLE OF THE CERVIX IN PRETERM BIRTH
Currently, a short cervix in the second trimester is our best predictor of sPTB. But treatment for the short cervix remains controversial despite more than 600 publications in the past 2 decades on the relationship between sPTB, the short cervix, and proposed interventions. Cerclage for a cervix ≤25mm in an unselected population demonstrated no benefit in a meta-analysis, but cerclage does reduce the risk of sPTB by 30% if there is also a history of sPTB.10 Two recent randomized controlled trials of progesterone for a short cervix in an unselected population had opposite results: intramuscular progesterone for a cervix <30mm demonstrated no benefit,11 but vaginal progesterone gel for a cervix 10–20mm was associated with a 45% reduction in sPTB.12 In February 2012, however, the FDA found the latter study insufficient to support approval of this gel.
This is unsurprising given the conflicting data about the effect of progestins on cervical length; some studies suggest it attenuates shortening13,14 while others suggest it does not.15,16 Further, the variety of doses, administration routes and formulations makes it difficult to reach definitive conclusions. And so, although measuring cervical length has become a fundamental part of clinical practice, the cervix remains mysterious: most women with a short cervix (but no history of sPTB) deliver at term without intervention, the risk reduction for those who have any intervention is usually modest, and the vast majority of preterm births in low risk women occur in those with a normal midtrimester cervical length.17
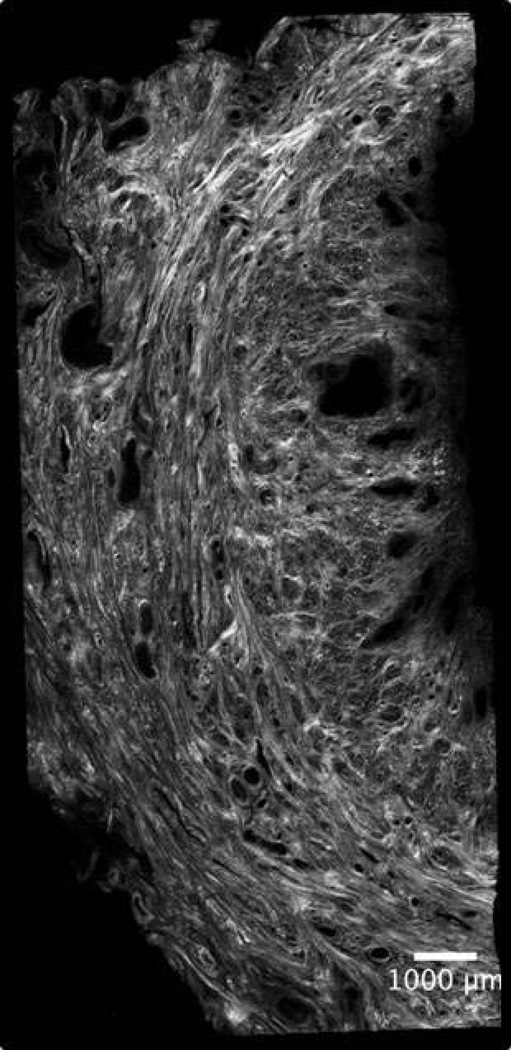
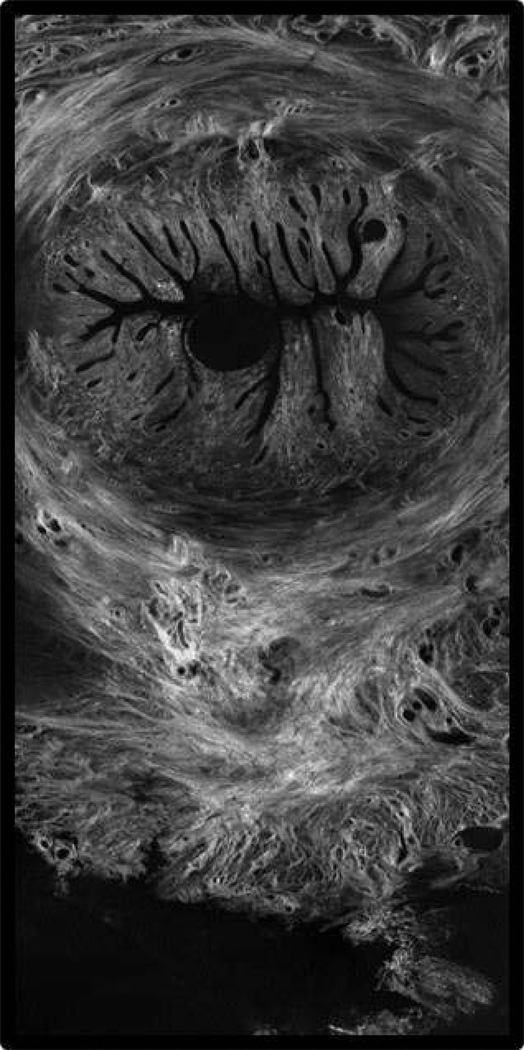
Perhaps our confusion can be blamed on the complexity of the cervix itself. This remarkable structure, with its diametrically opposed functions, is comprised of interwoven layers of collagen that remodel independently and progressively throughout gestation via, presumably, different molecular processes.18,19 (Figure 1) Shortening at term is associated with differential expression of 687 genes20 and preterm shortening appears even more complicated. Further, by the time shortening is recognizable, marked microstructural change has already occurred.21,22 Much less is understood about cervical softening than shortening, although the latter is arguably more critical. Softening starts soon after conception and occurs progressively throughout pregnancy.21.22 Testimony to its importance is that uterine contractions do not effect delivery if the cervix is firm,23 but a soft cervix is associated with preterm delivery even without contractions.24,25 Yet clinical assessment is entirely subjective; the cervix is labeled “soft, medium or firm” based solely on digital examination.
Figure 1. a and b. Complex Cervical Microstructure.
Shown are cross-sectional second harmonic generation (SHG) images of the extremely complex cervical microstructure: a nonpregnant human cervix (a, the cervical canal is on the right) and nonhuman primate (b). Note the dominant central circumferential band of collagen in both images; studies in several animal models suggest that this layer undergoes the most dramatic change during pregnancy.
Current data suggest that alterations to collagen processing, assembly and structure are primarily responsible for cervical remodeling.18 There are 4 stages, each associated with specific collagen alterations: the 1st is softening, the 2nd shortening and marked softening (“ripening”), the 3rd active dilation during labor and the 4th recovery after delivery.18,21,22 The inability to objectively describe microstructural change within each of these stages limits our ability to target the associated molecular processes. Ultimately, this makes it nearly impossible to conceive of novel approaches to prediction and treatment. This review discusses emerging methods for objective assessment of the pregnant cervix.
APPROACHES TO ASSESSSMENT OF CERVICAL MICROSTRUCTURE
Tissue hydration, collagen structure, and tissue elasticity all change progressively with cervical remodeling: hydration increases, collagen disorganizes, and elasticity (softness) increases. Current approaches to microstructural assessment attempt to assess hydration, describe various aspects of collagen structure, and/or directly measure some aspect of tissue elasticity. Methods are grouped below by approach.
Tissue Hydration
Cervical surface area
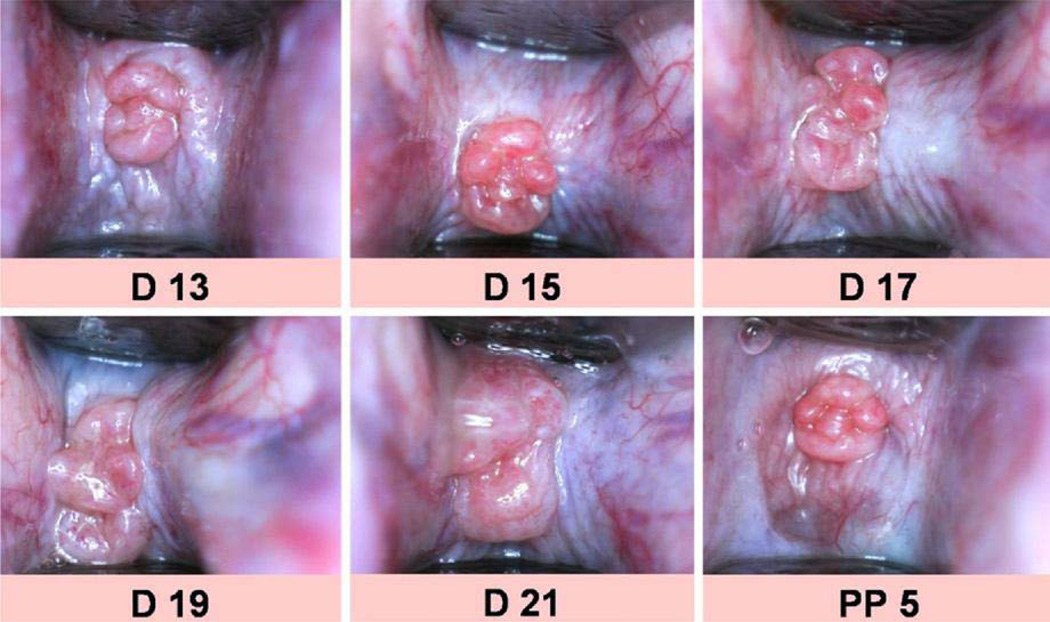
Cervical tissue hydration increases as pregnancy progresses. Measuring cervical surface area indirectly evaluates that property.26 In a rat model, digital images of the cervix were captured from which cervical surface area was calculated. (Figure 2) The surface area of the cervix increased throughout gestation, leading the authors to hypothesize this may be useful for evaluation of human cervical remodeling.
Figure 2. Cervical Surface Area.
Digital images of the rat cervix through gestation and postpartum. Surface area is calculated from these images. (Reprinted with permission from Kuon et al, A novel optical method to assess cervical changes during pregnancy and use to evaluate the effects on progestins on term and preterm labor. Am J Obstet Gynecol 2011; 205:82.e15–20.)
The advantage of this technique is its ease of use. The main disadvantage is noted by the authors: all the “folds and furrows” of the rat cervix cannot be measured, making the calculation imprecise. This also makes it unclear how well it would translate to the human cervix.
Electrical impedance
Flow of electrical current is affected by tissue hydration. Impedance to flow can be measured by placing electrodes on the cervix. Good correlation between impedance and cervical consistency was demonstrated in hysterectomy specimens,27 and a significant difference was found between pregnant and nonpregnant tissue.28 However, a recent study demonstrated low predictive values, leading the authors to conclude that the method “offers no immediate clinical utility.”29
Acoustic Attenuation
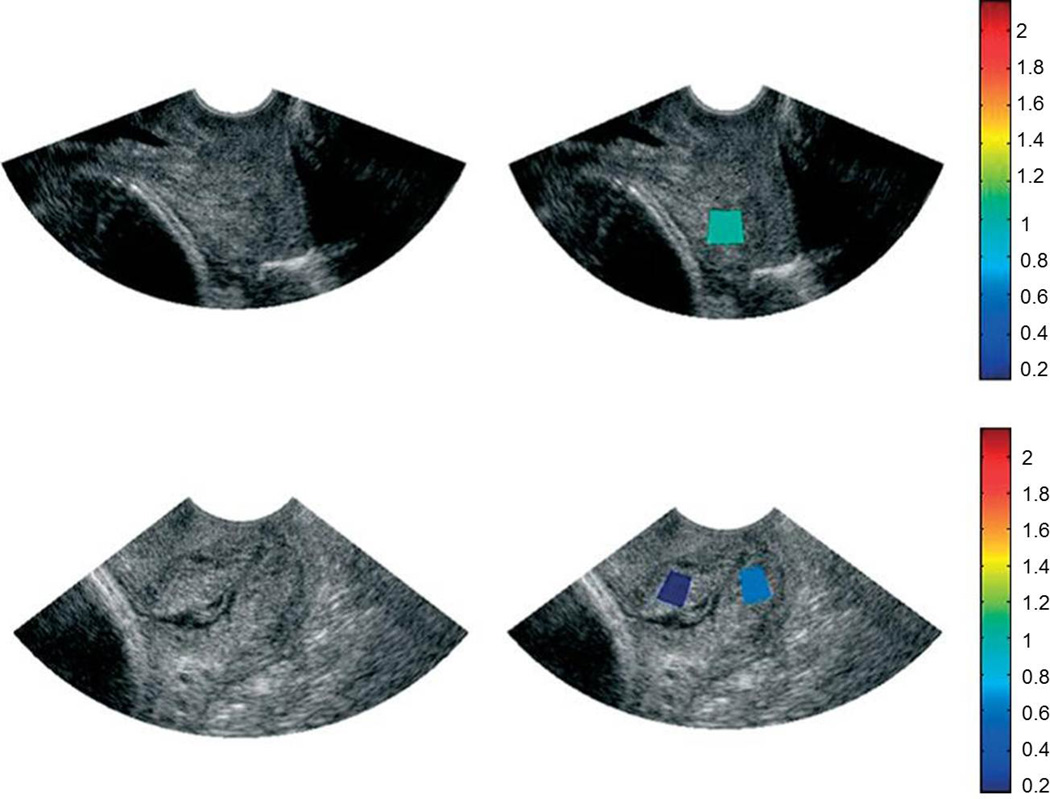
Attenuation is the loss of ultrasound signal amplitude with depth as a function of ultrasound frequency. Attenuation should decrease with hydration. Forty-one women (10 – 41 weeks gestation) underwent transvaginal ultrasound.30 Images were converted to raw radiofrequency (RF) echo signals. Attenuation estimates were calculated within regions of interest (ROIs) manually chosen in areas that “appeared to be homogeneous” because tissue inhomogeneity violates the assumptions of the attenuation algorithm. (Figure 3)
Figure 3. Acoustic Attenuation.
Ultrasound B-mode images (a,c) and B-mode images superimposed with maps of mean attenuation estimates (b,d) from 2 women at 14 weeks (a/b) and 38 weeks (c/d) of gestation. The colors of the regions of interest represent a mean attenuation (dB/cm-MHz) consistent with the corresponding scale bar (Reprinted with permission from McFarlin et al, Ultrasonic attenuation estimation of the pregnant cervix: a preliminary report. Ultrasound Obstet Gynecol 2010; 36:218–225.)
Attenuation was a predictor of interval from ultrasound exam to delivery, but not of gestational age or cervical length. The study was not powered to look at attenuation as a predictor of sPTB, but its potential was suggested by 2 cases of women with a short cervix but moderate to high attenuation values at 18 or 29 weeks, respectively, both of whom delivered at term. The authors suggested that attenuation may be a better predictor of sPTB than cervical length.
Advantages of this method are that it is quantitative and relatively easy. Disadvantages noted by the authors are considerable between-subject variability and inability to standardize comparisons between subjects (because ROIs are selected manually). A seemingly more significant issue is discussed by Parra-Saavreda et al: tissue inhomogeneity violates the assumptions of their attenuation algorithm, but the cervix is definitely not homogeneous.31
Cervical stromal differentiation
On T2-weighted MRI images, the signal intensity difference between the inner (near the canal) and outer cervix diminishes with remodeling as tissue hydration increases. A prospective cohort study of 100 women admitted for preterm labor at 18–34 weeks determined “stromal differentiation” as high, intermediate, or low based on visual inspection of signal intensity difference between the inner and outer cervix.32 Low differentiation was associated with shorter cervical length, and low or intermediate differentiation with a higher risk of preterm delivery. This method is relatively easy, but its subjectivity, low sensitivity and negative predictive values, and the fact that cervical length via ultrasound was as predictive led the authors to conclude the “clinical usefulness of this test is limited by practical and cost issues”.32
Collagen Structure
Cervical gland area (CGA)
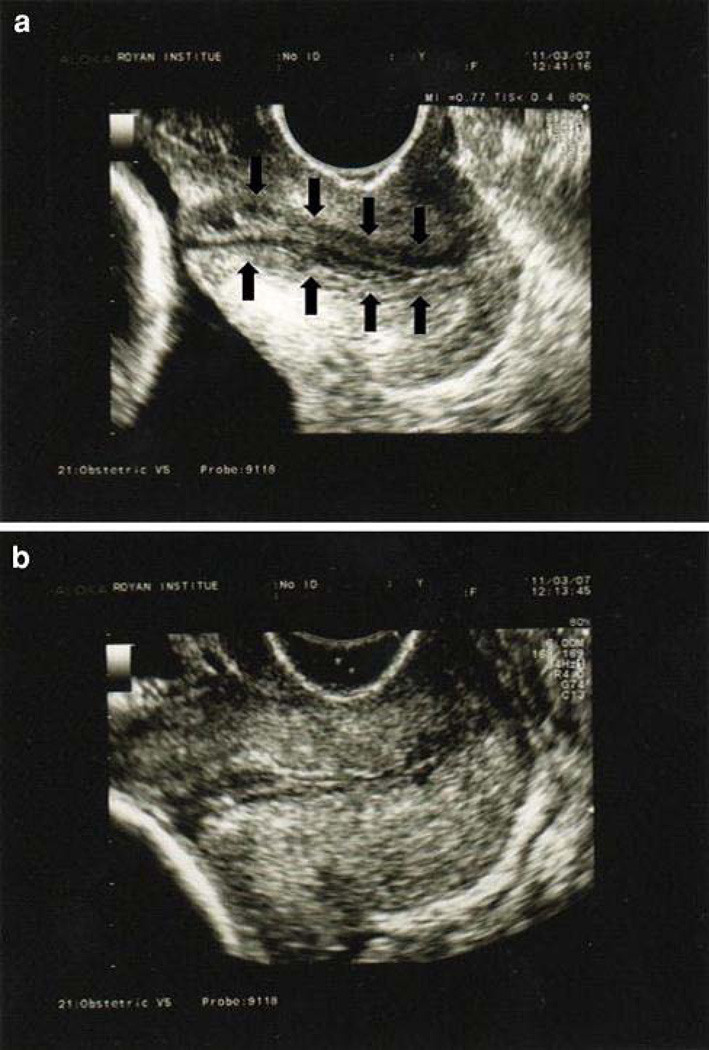
Absence of normal mucosal glands (cervical gland area), demonstrated by a “hyperechoeic or hypoechoeic segment around the cervical mucosa” has been proposed as a predictor of sPTB.33 600 pregnant women were scanned at 16–19 weeks of gestation. CGA was detected in 77% of women who delivered at term versus 55% of those who did not. (Figure 4)
Figure 4. Cervical Gland Area.
CGA is characterized as a hypoechoic band around the cervical canal (arrows in image (a)). The top image shows a normal CGA, and in the bottom image, the CGA is absent. (Reprinted with permission from Afzali N et al, Cervical gland area: a new sonographic marker in predicting preterm delivery. Archives Gynecology Obstetrics 2012; 285 (1):255–258.)
The advantage of this method is it uses standard imaging techniques. The disadvantages are the relative subjectivity of ascertaining the presence of mucus glands (limits of detection depend on the system, transducer and scanning technique). The other disadvantage is low performance; CGA was detected in most of the women in their study, irrespective of delivery timing.
Light-induced fluorescence (LIF)
LIF measures the natural fluorescence of non-soluble (cross-linked) collagen in the cervix. As the cervix remodels, the amount of cross-linked collagen decreases, and a “collascope” can measure this.34–37 The instrument is a steel probe attached by a fiber optic cable to a large main unit, which is attached to a computer. The probe, placed against the anterior lip of the cervix, delivers excitation light onto the cervix and carries the fluorescent light back to the instrument, and the computer displays the spectrum of fluorescence.
The collascope was used weekly in 21 pregnant patients beginning at 24–40 weeks, as well as 29 patients in labor.34 LIF was (weakly) inversely correlated with gestational age, and (weakly) correlated with time to delivery. Another study enrolled 41 patients presenting for induction of labor.35 LIF and Bishop score before and 4 hours after prostaglandin application were recorded. There was no correlation between initial LIF and Bishop score, but there was an inverse relationship between final LIF and Bishop score. Eleven of 22 patients with initial low LIF showed no change after ripening, in contrast to 16/19 with a high pre-ripening LIF. The authors suggested that the technique may help distinguish women who would benefit from pre-induction ripening.
The advantage of this technique is its ability to objectively assess cervical crosslinking, a measure of collagen organization. A disadvantage is that it requires a specialized, fairly cumbersome, apparatus.
Second harmonic generation (SHG)
SHG is a nonlinear microscopic method for imaging collagen. In ex vivo mouse cervices, SHG identified changes in signal intensity and collagen fiber size that accompanied normal remodeling changes.19 (Figure 5) The advantage of this method is that it directly visualizes collagen microstructure. Its disadvantage is that it would require development of an endoscopic microscope for in vivo use.
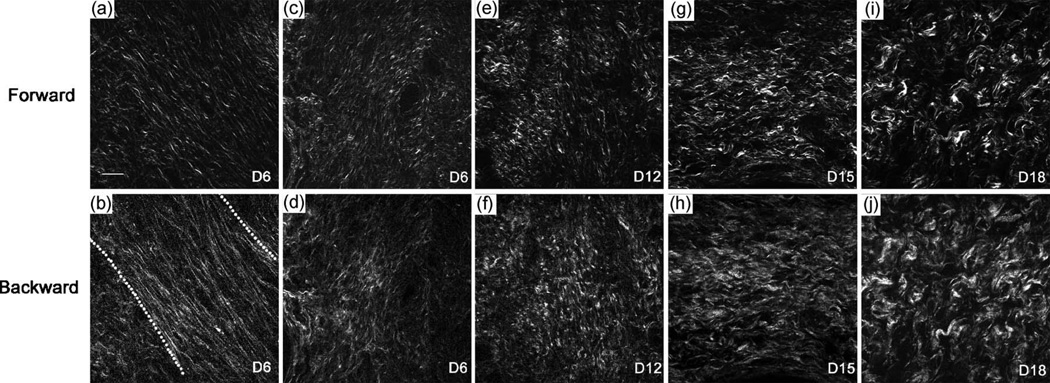
Figure 5. Second Harmonic Generation.
SHG signal of collagen in the rat cervix shows marked changes in morphology throughout gestation (a–d are day 6, e–f day 12, g–h day 15 and i–j day 18). Note the prominent central circumferential band of fibers in the earlier images becomes more random as gestation progresses. (Reprinted with permission from Timmons et al, Cervical remodeling during pregnancy and parturition. Trends Endocrin Metabol 2010; 21(6):353–361.)
Raman spectroscopy (RS)
This optical technique measures subtle microstructural component changes based on the Raman effect, a description of energy exchange between photons and scattering molecules. In a windowless room (no ambient light), energy (light) is sent to the tissue through a fiber optic probe. When a photon collides with a molecule, the energy causes a change in the molecular vibration, and energy differences between the incident and the scattered photons are indicative of particular molecules. Therefore the spectrum of scattered photon energies provides information about the composition of tissues and allows observation over time (Raman shifts) of specific components such as collagen.
Investigators obtained measurements from the distal mouse cervix throughout gestation.38 Trends early in pregnancy followed a “logical process”, but those late in pregnancy were difficult to recognize, “without a clear pattern”. Much greater variation was noted in a pilot study in humans.
The advantage of this method is the precision with which it may be able to identify collagen changes. A disadvantage is its highly specialized equipment and stringent conditions. Also, the authors note that the significant variability noted in women could “hinder” the “ability to find clear Raman shifts of statistical significance”.38
Backscattered power loss (BSPL)
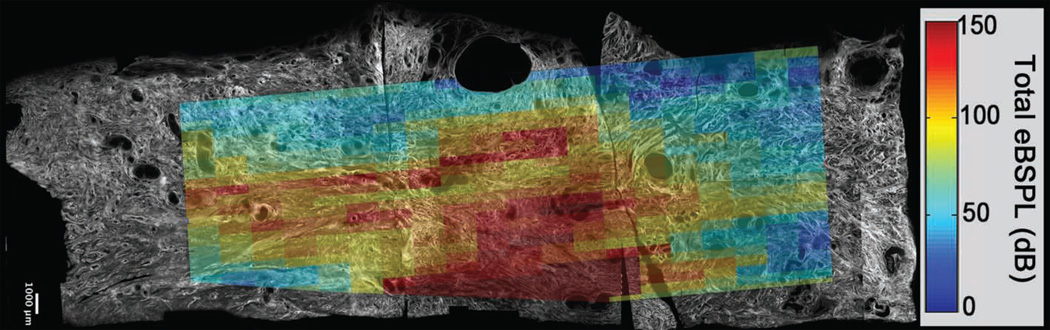
When an ultrasound wave encounters a scatterer (eg collagen), the modes of vibration excited in it causes backscatter (“echo”), and these modes can vary as the incident angle of the acoustic beam changes. Measuring BSPL as a function of the beam angle thus provides information about the shape and organization of scatterers such as collagen. Raw radiofrequency (RF) echo signals are recorded while electronically steering the ultrasound beam, and the power spectrum of the echo signal is computed for each angle to estimate the BSPL.39 We have used this technique to assess cervical microstructure in (ripened vs unripened) human hysterectomy specimens, corroborating findings with micron-scale maps of collagen distribution and alignment from SHG microscopy images. We find that BSPL is sensitive to collagen microstructure organization; when the ultrasound B-mode images are spatially registered and superimposed with the SHG images and quantitative ultrasound measurements, the BSPL correlates well with collagen fiber organization and orientation. (Figure 6)
Figure 6. Backscattered Power Loss.
A composite SHG image of a 43mm × 13mm longitudinal slice of human cervix showing the B-mode and QUS data registered with the SHG image data. The excess backscattered power loss is highest (red) in the region of the central layer, indicating collagen is mostly highly aligned in this area (confirmed by visual inspection of the underlying SHG microscope image).
The advantage of this quantitative method is that it seems able to detect small changes in tissue properties within heterogeneous areas. The disadvantage is that the current implementation utilizes a prototype transducer and specialized software. Also, progress toward a viable clinical tool has been slow because of the need to map out the entire cervix to determine the most appropriate and reproducible location(s) for measurement.
Tissue Elasticity
Cervical consistency: cervical consistency index (CCI) or mean gray-level (MGL)
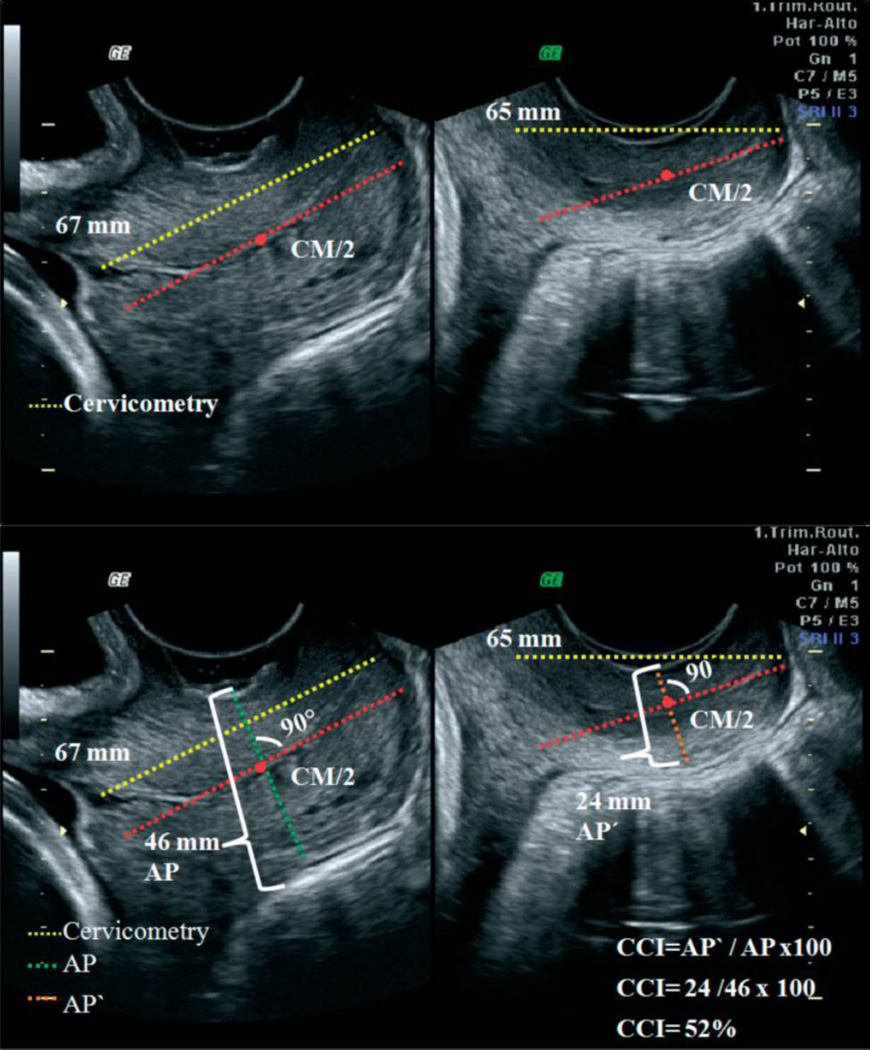
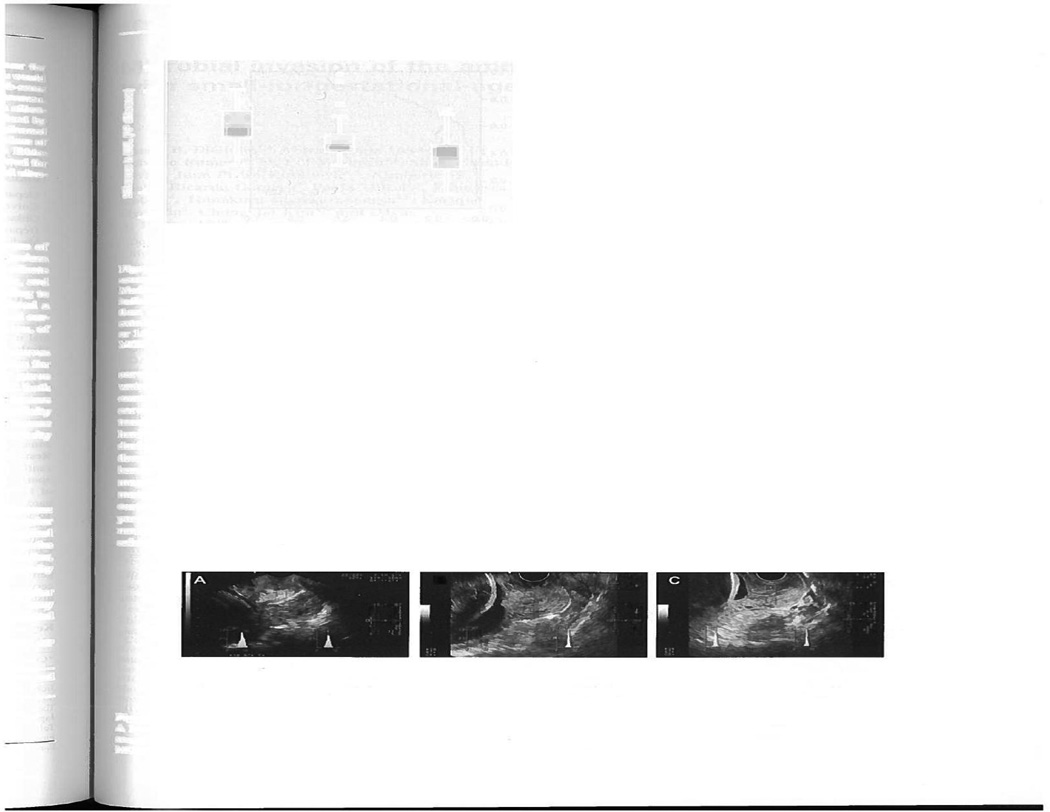
These techniques address cervical softening. The CCI evaluates “compressibility” (softer tissue deforms more easily than stiffer).31 An image of the cervix is obtained in the standard manner, then a 2nd image is obtained after pressure is applied with the transducer until no more shortening of the anterior-posterior diameter is observed (eg the cervix will not compress further). The ratio of the AP diameter on the pre- and post-deformation image is the CCI, and a lower CCI indicates softer tissue. (Figure 7) In a cross-section of 1031 women measured at 5–36 weeks, a lower CCI was seen with increasing gestational age, and the sensitivity for prediction of preterm birth prior to 32, 34 and 37 weeks was 67%, 64%, and 45%, which was superior to the sensitivity of prediction by cervical length. They proposed this technique may be useful for preterm birth prediction.
Figure 7. Cervical Consistency Index.
Ultrasound images with annotation illustrating the assessment of cervical consistency index (CCI). Images on the left are without pressure and those on the right are with pressure from the probe. (Reprinted with permission from Parra-Saavedra et al, Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet Gynecol 2011; 38:44–51.)
The MGL addresses differences between the anterior and posterior cervical wall. From a B-mode image of the cervix obtained in the usual manner, a grayscale histogram is developed from a midsection of the anterior and posterior cervix, and the 2 histograms compared.40 (Figure 8) According to the authors, a greater difference in echogenicity between the anterior and posterior region of interest indicates a “harder” cervix. Measurements were performed in 214 women at 27–30 weeks of gestation. A greater AP difference was associated with a lower Bishop score and the method had a sensitivity of 71% to “identify a hard cervix”. The authors suggested that this measurement may allow evaluation of cervical consistency.
Figure 8. Mean Gray Level.
Examples of hard, moderate consistency, and soft cervix. A (the hardest) shows a more echogenic anterior than posterior cervix. C (the softest) shows a less echogenic anterior than posterior cervix. (Reprinted with permission from Kuwata et al, A novel method for evaluating uterine cervical consistency using vaginal ultrasound gray-level histogram. J Perinat Med 2010; 38:491–494.)
An advantage of both methods is their simplicity; they utilize standard transvaginal cervical imaging. The primary disadvantage is their relatively low performance. For MGL, this may be related to a common drawback of grayscale histograms: adjustments to most front panel controls on an ultrasound system can significantly change the values. Also, there is little physical relationship between the shear modulus (‘stiffness’) of a material and its acoustic scattering properties (that cause ‘echogenicity’ in grayscale images).
Elastography
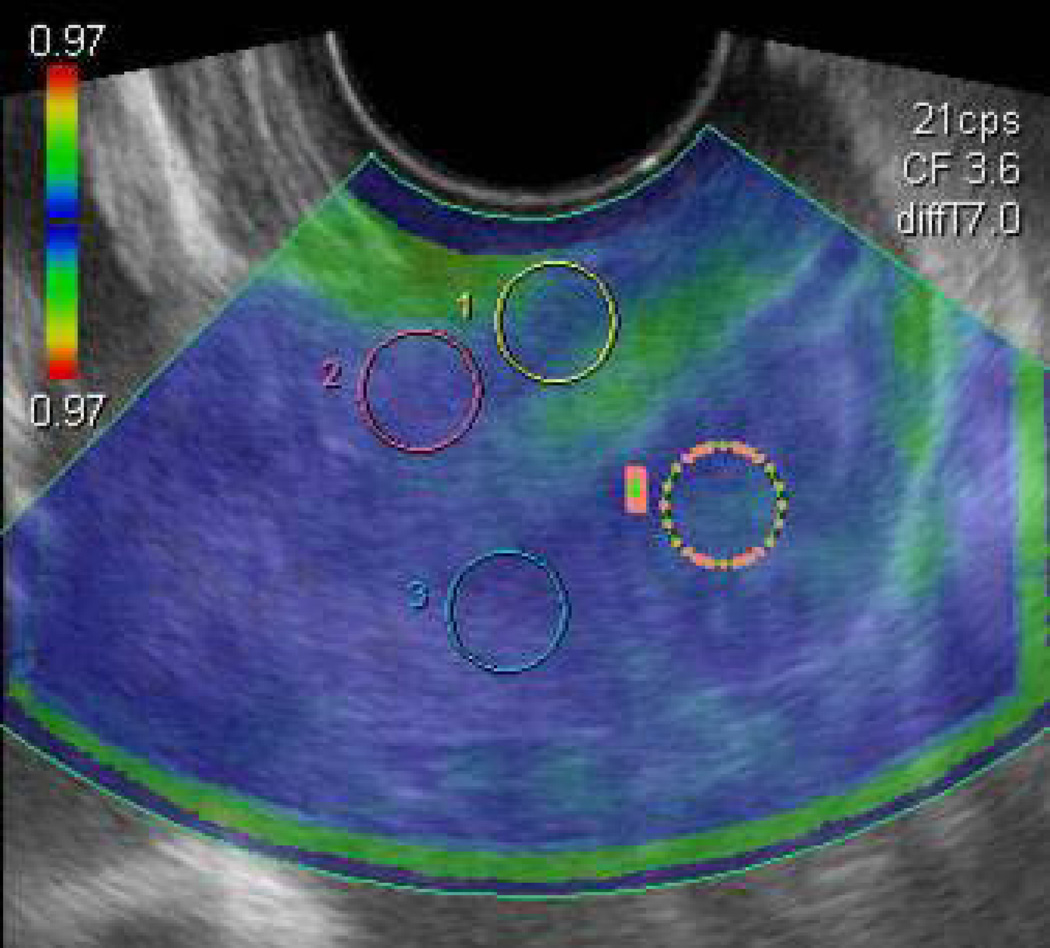
This method is based on determining motion in areas of the cervix relative to other areas, described by a color map. The cervix is scanned in the usual manner, slight pressure is applied with the transducer to deform the tissue, and specialized software is utilized to produce a color map that describes deformation of the tissue relative to neighboring areas. (Figure 9) In one protocol, points are assigned to the map to obtain an elastography index (EI): purple (0, hardest) → blue (1) → green (2) → yellow (3) → red (4, softest).41 Twenty-nine women undergoing term labor induction were evaluated. The mean EI of the internal os demonstrated a statistically significantly different EI of 1.32 vs 0.39 for patients with successful vs failed induction, respectively. The EI from tissue near the external os and the middle of the canal was not predictive. The authors suggested that elastography may guide decisions about need for cervical ripening prior to labor induction, but note this would be most useful if measurements could be standardized between subjects.
Figure 9. Elastography.
An elastographic image of the cervix. The circled areas are the regions of interest evaluated. Region A: External and superior lip of the cervix. Region B: Internal and superior lip of the cervix. Region C: Internal and inferior lip of the cervix. Region D: External and inferior lip of the cervix. (Reprinted with permission from Molina et al, Molina FS, Gomez LF, Florido J, Padilla MC, Nicolaides KH. Quantification of cervical elastography. A reproducibility study. Ultrasound Obstet Gynecol (accepted, online December 2011)
A reproducibility study was performed in a cross-section of 112 pregnant women at all points in gestation.42 There were no statistically significant differences between cervices except in the area that received direct force from the transducer, and the authors concluded that measurements may be “a mere reflection of the force being applied by the transducer”. They cautioned it is premature to conclude that “measurements of rate-of-change in tissue displacement reflect histological changes that could provide a measure of cervical ripening.”
The major advantage of elastography is its ease; although specialized system software is required, the technique is compatible with standard equipment. The disadvantages are that measurements are dependent upon transducer pressure by the operator, thus there is no control for variability or adequate means for standardization.
Aspiration
Tissue compliance is related to tissue softness, thus tissue displacement provides a measure of softening. One group uses a 10mm diameter tube pushed against the distal end of the cervix while a vacuum sucks tissue into it and an optical fiber illuminates the inner part of the tube.43–45 Images of the side-view of the tissue before and after suction are reflected by a mirror, and displacement calculated from the images.
Initial studies noted marked variability among subjects, suggesting that the method may not be useful.44 However, a more recent study demonstrated successful measurement in 12/20 subjects (technical difficulties inhibited the others), and a 20% decrease in stiffness was shown a month later in all 3 of the women who had subsequent evaluation.45
The advantage of this quantitative method is its potential for monitoring incremental tissue changes. A disadvantage noted by the authors is that there is currently no way to standardize the force applied by the operator. Further, they indicate concern that the measurement cannot adequately address cervical heterogeneity.
Shear wave speed (SWS)
Measurement of shear wave speed (SWS) provides an objective description of tissue softness and microstructure because waves travel more slowly in softer tissue, and shear modulus (stiffness) is dependent on fiber (eg collagen) organization. Our methods are a hybrid of techniques for on-line data acquisition and off-line motion tracking, image formation and parameter estimation.46,47 SWS are slower in ripened versus unripened cervical tissue from hysterectomy specimens, and when SWS measurements are spatially registered and superimposed with ultrasound B-mode images and BSPL measurements (see above), it provides an objective description of tissue softness. (Figure 10)
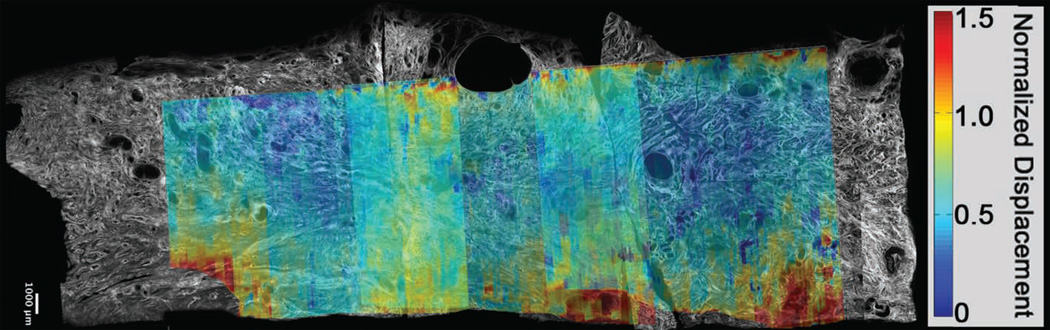
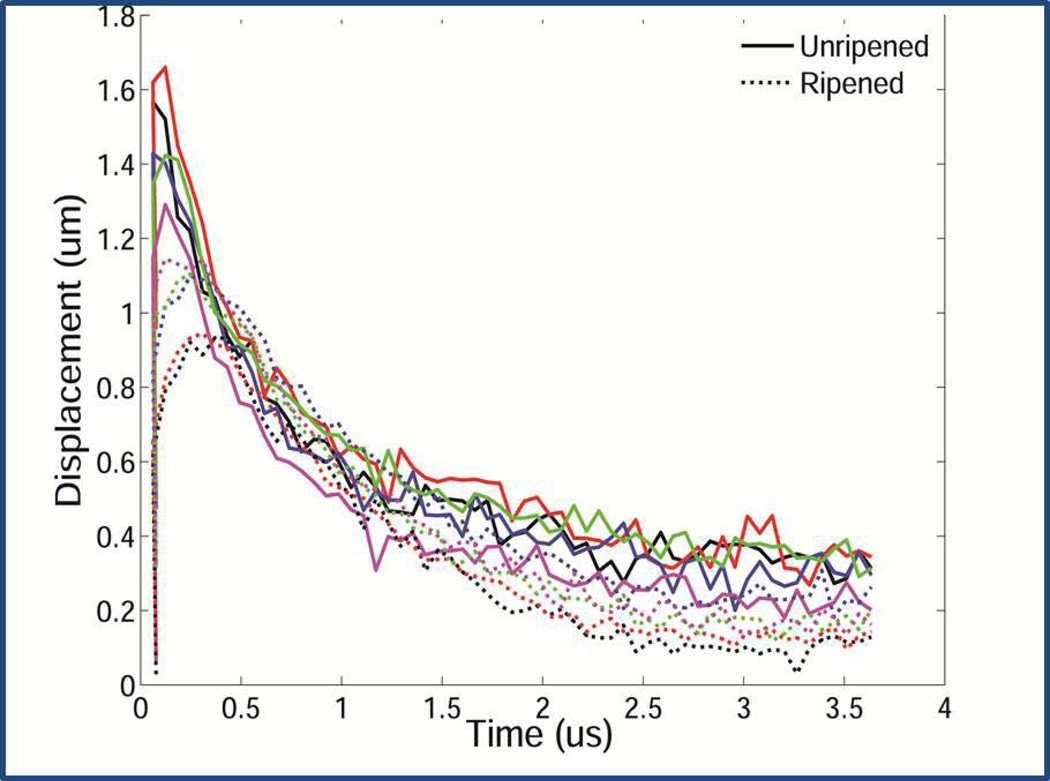
Figure 10.
a. Shear wave speed. The plot shows SWS from ripened versus unripened cervix tissue from hysterectomy specimens. Unripened tissue (solid lines) shows greater displacements (y axis) in less time (x axis) to the peak displacement (highest point for each line on the plot) than ripened tissue, consistent with the latter being softer.
b. Shear wave speed. SHG and normalized displacement image registered and superimposed, showing that the displacements are uniform in the central layer of the cervix. This suggests that similar shear wave excitation could be induced in the central layer anywhere along the endocervical canal to fully assess that specific area.
The advantage of this method is that currently it alone objectively quantifies cervical softness within the context of cervical heterogeneity. The disadvantage is that the current implementation requires a prototype transducer and specialized software; some commercially available systems are capable of SWS estimation, but they use standard transducers that work well in relatively homogeneous tissue (such as liver), but provide biased and inconsistent SWS estimates in complex structures (such as cervix).
THE FUTURE OF CERVICAL ASSESSMENT
Accurate, precise characterization of cervical microstructure is paramount to understanding the pregnant cervix. Tables 1 & 2 summarize the approaches discussed above, and predict their clinical promise. Bringing technology from the bench to the clinic requires careful exploration, including corroborative and reproducibility studies, followed by appropriate clinical exploration and validation. This is time consuming. However, some techniques, such as elastography and acoustic attenuation, are currently under active investigation in pregnant women, and may be clinically useful in the near future. Elastography could be highly promising if data acquisition were standardized, making the measurement less relative. This is also true of acoustic attenuation if the algorithm could be refined to account for tissue heterogeneity.
| Category | Method | Advantages | Disadvantages |
|---|---|---|---|
| Tissue Hydration | Cervical surface area | Easy | Imprecise, semi-quantitative |
| Electrical impedance | None in current form | Little clinical utility | |
| Acoustic attenuation | Relatively easy, quantitative | Requires tissue homogeneity, wide intra-subject variability | |
| Stromal differentiation | Easy | Expensive, semi-quantitative | |
| Collagen Structure | Cervical gland area | Easy | Detection depends on system |
| Light induced fluorescence | Detects collagen crosslinking | Cumbersome | |
| Second harmonic generation | Directly assesses collagen structure | Cumbersome, requires equipment development for in vivo use | |
| Raman spectroscopy | Identifies changes to specific matrix components | Cumbersome, wide intro-subject variability | |
| Backscattered power loss | Addresses tissue heterogeneity | Prototype equipment | |
| Tissue Elasticity | Cervical consistency index | Easy | Low performance |
| Mean gray level | Easy | Low performance | |
| Elastography | Easy | Relative measure, depends on operator pressure | |
| Aspiration | May detect incremental changes | Requires tissue homogeneity, depends on operator pressure | |
| Shear wave speed | Objective measure of softness | Prototype equipment |
| Method | Description of Technique | Clinical Promise |
|---|---|---|
| Cervical surface area | Capture digital image of cervix, calculate surface area | Low. Applicability to human cervix is unclear. |
| Electrical impedance | Place electrodes on the cervix, measure electrical impedance | Low. Predictive values low. |
| Acoustic attenuation | Obtain standard image of cervix, convert to RF data, select regions of interest, use algorithm to estimate attenuation | Moderate. With method adaptations result to standardize measurement and for tissue heterogeneity, potentially High. |
| Stromal differentiation | Visually grade (low, intermediate, high) the difference between the appearance of the inner vs outer cervix on MRI images | Low. Predictive values low. |
| Cervical gland area | Obtain standard image of cervix, visually determine presence/absence of mucosal glands in cervical canal | Low. Relatively subjective. Predictive values low. |
| Light induced fluorescence | Use “collascope” probe on anterior lip of cervix, record spectrum of fluorescence from computer attached to apparatus | Moderate. Potentially High but difficult to estimate because technique does not seem to have been used in humans in recent years |
| Second harmonic generation | Examine tissue (ex vivo) with specialized device | Moderate. With successful development of in vivo device, potentially High. |
| Raman spectroscopy | In darkened room (with no windows), place probe against distal cervix and record spectrum | High. Objective measure of multiple collagen properties. |
| Backscattered power loss | Align transducer with cervical canal, obtain measurements | High. Objective measure of collagen organization. |
| Cervical consistency index | Obtain standard image of cervix, compare to 2nd image obtained during cervical compression (with transducer) | Low. Relatively subjective; challenges include difficulty standardizing transducer pressure. |
| Mean gray level | Compare echogenicity of anterior vs posterior cervix | Low. Relatively subjective; histogram depends on machine settings |
| Elastography | Obtain standard image of cervix, use specialized software to create color map describing relative areas of tissue softness | Moderate. Potentially High with modifications to standardize transducer pressure. |
| Aspiration | Place probe against distal cervix, aspirate tissue into probe with suction, calculate displacement from images pre/during suction | Moderate. Cumbersome. Unable to address tissue heterogeneity |
| Shear wave speed | Align transducer with cervical canal, obtain measurements | High. Quantifies tissue softness |
Methods that can quantitatively and/or directly measure objective microstructural properties hold the greatest promise, but most of these still require significant development. For instance, SHG’s ability to directly visualize collagen could offer remarkable insight, but will require development of an endoscopic tool before human studies can be considered. Raman spectroscopy can evaluate several microstructural components simultaneously, but the method needs standardization and variability reduction for fruitful human studies. SWS and BSPL are promising in the laboratory, but in vivo studies will not begin until fall 2012. SWS could rapidly become clinically useful because objective measurements are generally more informative than subjective, and SWS objectively measures a common obstetrical parameter (cervical softening). However, it will take time to explore its value for preterm birth prediction.
Ultimately, a combination of these methods (and some not yet conceived) will likely be necessary for comprehensive study of the cervix, and in fact, several of the above investigators are establishing new collaborations. An orchestrated approach of complimentary techniques should facilitate novel approaches to the prediction, prevention and treatment of spontaneous PTB.
ACKNOWLEDGEMENTS
The authors thank Dr. Lisa Reusch for her images and careful data analysis (1a and b, 6, and 10a and b). HF gratefully acknowledges Dr. Larry Platt’s mentorship and Dr. Michael House’s contribution to this manuscript. HF and TJH are grateful for the support by NIH Grants R21HD061896 and R21HD063031 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE: The authors report no conflict of interest
REFERENCES
- 1.WHO systematic review on maternal mortality and morbidity: the global burden of preterm birth. Bull World Health Org. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110(2):405–415. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]
- 3.Gravett MG, Rubens CE, Nunes TM and the GAPPS Review Group. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy and Childbirth. 2010;10(Suppl 1):S2. doi: 10.1186/1471-2393-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R. Vaginal progesterone to reduce the rate of preterm birth and neonatal morbidity: a solution at last. Womens Health (LondEngl) 2011 Sep;7(5):501–504. doi: 10.2217/whe.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muglia L, Katz M. The enigma of spontaneous preterm birth. NEJM. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 6.Menon R, Dunlop AL, Kramer MR, Fortunato SJ, Hogue CJ. An overview of racial disparities in preterm birth rates: caused by infection or inflammatory response? Acta Obstetricia et Gyn Scand. 2011;90:1325–1331. doi: 10.1111/j.1600-0412.2011.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutrition Reviews. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 8.Menon R, Torloni MR, Voltolini C, et al. Biomarkers of spontaneous preterm birth: an overview of the literature in the last four decades. Reprod Sci. 2011;18:1046. doi: 10.1177/1933719111415548. [DOI] [PubMed] [Google Scholar]
- 9.Gracie S, Pennell C, Ekman-Ordeberg G, Lye S, McManaman J, Williams S, Palmer L, Kelley M, Menon R, Gravett M and the PREBIC “-omics” Research Group. BMC Pregnancy and Childbirth. 2011;11:71. doi: 10.1186/1471-2393-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol. 2011;117:663–671. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 11.Grobman W. Randomized controlled trial of progesterone treatment for preterm birth prevention in nulliparous women with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;206(Suppl)(1):S367. [Google Scholar]
- 12.Hassan SS, Romero R, Vidyadhan D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Trivedi Y, Soma-Pillay P, Sambarey P, Dayal A, Potapov V, O’Brien J, Astakhov V, Yuzko O, Kinzler W, Dattel B, Sehdev H, Mazheika L, Manchulenko D, Gervasi MT, Sullivan L, Conde-Agudelo A, Phillips JA, Creasy GW for the PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facchinetti F, Paganelli S, Cornitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453.e1–453.e4. doi: 10.1016/j.ajog.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien JM, Defranco EA, Adair CD, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2009;34:653–659. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]
- 15.Kuon RJ, Shi S-Q, Maul H, Sohn C, Balducci J, Maner WL, Garfield RE. Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. Am J Obstet Gynecol. 2010;202:455.e1–455.e9. doi: 10.1016/j.ajog.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durnwald CP, Lynch CD, Walker H, Iams JD. The effect of treatment with 17 alpha-hydroxyprogesterone caproate on changes in cervical length over time. Am J Obstet Gynecol. 2009;201:410.e1–410.e5. doi: 10.1016/j.ajog.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenyon A, Braschi J, Forya F, Peebles D, David A. Cervical length mid trimester in low risk women fails to predict the majority of pre-term birth. Arch Dis Child Fetal Neonatal Ed. 2011;96 Fa128. [Google Scholar]
- 18.Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrin Metabolism. 2010;21(6):353–361. doi: 10.1016/j.tem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akins ML, Luby-Phelps K, Mahendroo M. Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth. J Biomed Optics. 2010;15(2) doi: 10.1117/1.3381184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan S. The molecular basis of sonographic cervical shortening at term: Identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol. 2010;472:e1–e14. doi: 10.1016/j.ajog.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 21.Read CP, Word RA, Ruscheinsky MA, et al. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134:327–340. doi: 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- 22.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Sem Reprod Med. 2007;25(1):69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 23.Danforth D. The morphology of the human cervix. Clin Obstet Gynecol. 1983;26(1):7–13. doi: 10.1097/00003081-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Mahendroo M, Porter A, Russell D, Word A. The parturition defect in steroid 5-alpha-reductase type I knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1995;13(6):981–992. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 25.Rechberg T, Abramson SR, Woessner JF. Onapristone and prostaglandin e(2) induction of delivery in the rat pregnancy: a model for the analysis of cervical softening. Am J Obstet Gyncol. 1996;175(3):719–723. doi: 10.1053/ob.1996.v175.a74254. [DOI] [PubMed] [Google Scholar]
- 26.Kuon RJ, Shi S-Q, Maul H, Sohn C, Balducci J, Shi L, Garfield RE. A novel optical method to assess cervical changes during pregnancy and use to evaluate the effects of progestins on term and preterm labor. Am J Obstet Gynecol. 2011;205:82.e15–82.e20. doi: 10.1016/j.ajog.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Predanic M. Evaluation of a device for objective determination of cervical consistency: a pilot study of device’s validity on uterine specimens obtained by total hysterectomy for benign uterine disease. J Perinat Med. 2002;30:364–366. doi: 10.1515/JPM.2002.056. [DOI] [PubMed] [Google Scholar]
- 28.O’Connell MP, Tidy J, Wisher SJ, Avis NJ, Brown BH, Lindow SW. An in vivo comparative study of the pregnant and nonpregnant cervix using electrical impedance measurements. British J Obstet Gynecol. 2000;107:1040–1041. doi: 10.1111/j.1471-0528.2000.tb10410.x. [DOI] [PubMed] [Google Scholar]
- 29.Jokhi R, Brown B, Do A. The role of cervical electrical impedance spectroscopy in the prediction of the course and outcome of induced labour. BMJ Pregnancy Childbirth. 2009;9:40. doi: 10.1186/1471-2393-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFarlin BL, Bigelow TA, Laybed Y, O’Brien WD, Oelze ML, Abramowicz JS. Ultrasonic attenuation estimation of the pregnant cervix: a preliminary report. Ultrasound Obstet Gynecol. 2010;36:218–225. doi: 10.1002/uog.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parra-Saavedra M, Gomez L, Barrero A, Parra G, Vergara F, Navarro E. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet Gynecol. 2011;38:44–51. doi: 10.1002/uog.9010. [DOI] [PubMed] [Google Scholar]
- 32.de Tejada BM, Faltin DL, Kinkel K, Guittier MJ, Boulvain M, Irion O. Magnetic Resonance imaging of the cervix in women at high risk for preterm delivery. J Matern Fetal Neonatal Med. 2011 Nov;24(11):1392–1397. doi: 10.3109/14767058.2011.552654. [DOI] [PubMed] [Google Scholar]
- 33.Afzali N, Mohajeri M, Malek A, Almatian A. Cervical gland area: a new sonographic marker in predicting preterm delivery. Archives Gynecology Obstetrics. 2012;285(1):255–258. doi: 10.1007/s00404-011-1986-7. [DOI] [PubMed] [Google Scholar]
- 34.Maul H, Olson G, Fittkow CT, Saade GR, Garfield RE. Cervical light-induced fluorescence in humans decreases throughout gestation and before delivery: preliminary observations. Am J Obstet Gynecol. 2003;188:537–541. doi: 10.1067/mob.2003.94. [DOI] [PubMed] [Google Scholar]
- 35.Fittkow CT, Maul H, Olson G, Martin E, MacKay LB, Saade GR, Garfield RE. Light-induced fluorescence of the human cervix decreases after prostaglandin application for induction of labor at term. Eur J Obstet Gynecol Reprod Biol. 2005;123:62–66. doi: 10.1016/j.ejogrb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Maul R, Saade G, Garfield R. Prediction of term and preterm parturition and treatment monitoring by measurement of cervical cross-linked collagen using light-induced fluorescence. Acta Obstetricia et Gynecologica Scandinavica. 2005;84(6):534–536. doi: 10.1111/j.0001-6349.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 37.Schlembach D, Mackay L, Shi L, Maner WL, Garfield RE, Maul H. Cervical ripening and insufficiency: from biochemical and molecular studies to in vivo clinical examination. Eur J Obstet Gynecol Reprod Biol. 2009;144S:S70–S76. doi: 10.1016/j.ejogrb.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 38.Vargis E, Webb CN, Paria BC, Bennett KA, Reese J, Al-Hendy A, Mahadevan-Jansen A. Detecting changes during pregnancy with Raman spectroscopy. Proceedings of the 2011 Biomedical Sciences and Engineering Conference: Image Informatics and Analytics in Biomedicine. 2011:1–4. [Google Scholar]
- 39.Feltovich H, Nam K, Hall TJ. Quantitative ultrasound assessment of cervical microstructure. Ult Imaging. 2010;32:131–142. doi: 10.1177/016173461003200302. [DOI] [PubMed] [Google Scholar]
- 40.Kuwata T, Matsubara S, Taniguchi N, Ohkuchi A, Ohkusa T, Suzuki M. A novel method for evaluating uterine cervical consistency using vaginal ultrasound gray-level histogram. J Perinat Med. 2010;38:491–491. doi: 10.1515/jpm.2010.079. [DOI] [PubMed] [Google Scholar]
- 41.Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol. 2011;38:52–56. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- 42.Molina FS, Gomez LF, Florido J, Padilla MC, Nicolaides KH. Quantification of cervical elastography. A reproducibility study. Ultrasound Obstet Gynecol. 2012 doi: 10.1002/uog.11067. (accepted) [DOI] [PubMed] [Google Scholar]
- 43.Bauer M, Mazza E, Nava A, et al. In vivo characterization of the mechanics of human uterine cervices. Ann NY Acad Sci. 2007;1101:186–202. doi: 10.1196/annals.1389.004. [DOI] [PubMed] [Google Scholar]
- 44.Mazza E, Nava A, Bauer M, Winter R, Bajika M, Holzapfel GA. Mechanical properties of the human uterine cervix: an in vivo study. Medical Image Analysis. 2006;10(2):125–136. doi: 10.1016/j.media.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Bauer M, Mazza E, Jabareen M, Sultan L, Bajka M, Lang U, Zimmermann R, Holzapfel GA. Assessment of the in vivo biomechanical properties of the human uterine cervix in pregnancy using the aspiration test. A feasibility study. Eur J Obstet Gynecol Reprod Biol. 2009;144S:S77–S81. doi: 10.1016/j.ejogrb.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 46.Reusch LM, Anderson JJ, Carlson LC, Simmons HA, Pehlke C, Eliceiri K, Feltovich H, Hall TJ. Detecting cervical microstructure via ultrasound and optical microscopy. IEEE Ultrason Symp Proc. 2010:724–727. [Google Scholar]
- 47.Reusch LM, Palmeri MA, Dahl J, Feltovich H, Hall TJ. Comparison of ultrasonic measurements of nulliparous versus multiparous cervices. IEEE Ultrason Symp Proc. 2011 (in press) [Google Scholar]