Abstract
Objective
Chronic kidney disease (CKD) is histologically characterized by interstitial fibrosis, which may be driven by peritubular capillary dropout and hypoxia. Surprisingly, peritubular capillaries have little repair capacity. We sought to establish long-term cultures of rat kidney endothelial cells to investigate their growth regulatory properties.
Methods
Kidney endothelial cells from adult rats (AKEC) or young rats (YKEC) were isolated using CD31 based isolation techniques and sustained in long-term cultures.
Results
Although YKEC grew slightly better than AKEC, both performed poorly compared to endothelial cells of the rat adult pulmonary microvasculature (PMVEC), pulmonary artery (PAEC), or HUVEC cells. PMVEC and PAEC contained a large percentage of cells with high colony forming potential. By contrast, KECs were incapable of forming large colonies and most remained as single non-dividing cells. KEC expressed high levels of mRNA for VEGF receptors but were surprisingly insensitive to VEGF stimulation. KECs did not form branching structures on Matrigel when cultured alone, but in mixed cultures KECs incorporated into branching structures with PMVECs.
Conclusions
These data suggest that the intrinsic growth of rat kidney endothelial cells is limited by unknown mechanisms. The low growth rate may relate to the minimal intrinsic regenerative capacity of renal capillaries.
Keywords: angiogenesis, progenitor, hypoxia, CKD
Introduction
The stability of the renal vascular structure is important in the maintenance of normal renal function but instable vascular structure may promote chronic kidney disease. Very little is known regarding the control of renal vascular growth and stability following completion of embryonic development [1]. In some disease models such as diabetes, immune complex mediated disease [2–5] or following reductions in renal mass [6] there is evidence for new glomerular capillary sprouting and endothelial cell proliferation. However, the vast majority of chronic kidney disease (CKD) models are associated with a reduction in both glomerular and peritubular capillary networks [7]. This reduction in renal capillaries is consistently seen in human kidney disease [7, 8]. The reduction in peritubular capillaries plays a central role in decreased medullary perfusion, promotes hypoxia, interstitial fibrosis and therefore participates in the progression of CKD [8]. Importantly, although hypoxia is a well-known stimulator of angiogenesis, nearly all models of CKD associated with hypoxia are ultimately characterized by predominant vessel rarefaction without significant vascular repair [8].
There is little evidence to suggest that the renal peritubular capillary system can be replenished with new growth. For example, acute kidney injury (AKI) induced by ischemia reperfusion results in a rapid and sustained loss of peritubular capillaries, representing a potential link between AKI and CKD [9]. In recent studies by our group, the in vivo administration of VEGF-121 shortly following ischemia reperfusion preserved peritubular capillary loss, but this was not attributable to increased endothelial cell proliferation [10]. Indeed, following AKI, the number of proliferating endothelial cells is remarkably small [10]. Moreover, VEGF-121 does not restore vessel numbers when administered after the initial resolution of AKI [11]. These results suggest that impaired growth response of the rat kidney vasculature underlies predisposition to CKD following AKI.
Despite the lack of evidence for renal vascular growth, many vascular beds have significant angiogenic and/or reparative potential. Although the endothelial proliferative response following injury to the aortic intimal layer is well known [12], careful analysis during normal homeostatic growth suggests that the source of new endothelial cells arises from small local niches, and not heterogeneously across the endothelium [13]. Moreover, certain vascular beds are characterized for their robust resident angiogenic potential including the pulmonary microvasculature following lung injury [14], and in response to physiological needs in the corpus luteum [15], placenta [16], and skeletal and cardiac muscle [17]. While it is clear that the local micro-environment participates in regulating these responses, the endothelial cells residing within these beds have the potential for significant growth and repair.
Studies carried out over the last 5 years have demonstrated that circulating and resident vascular endothelial cells possess varying growth potentials. Endothelial colony forming cells (ECFC) have been isolated from adult peripheral blood and human cord blood. These cells display a wide range of clonal growth potentials, including cells capable of forming large (>2,000 cells) colonies (i.e., high proliferative potential cells) [18–21]. High telomerase activity in the cord blood-derived ECFC reflects an enrichment of the high proliferative potential (HPP) cells compared to adult peripheral blood ECFC [18–21]. Similar analysis can be extended to cells isolated from vascular beds. For example, rat pulmonary microvascular endothelial cells (PMVEC) display a range of colony forming potential, with a large proportion of cells having high-proliferative capacity, while endothelial cells isolated from the pulmonary artery (PAEC) display a more limited range of clonogenic potential [22]. Of interest, the PMVECs retained their unique high permeability barrier properties reflecting the mature differentiated status of the cells with retained high proliferative potential [22].
As an extension of these observations, the tendency toward microvascular rarefaction in the kidney may reflect a modest progenitor potential intrinsic to renal endothelia. Historically speaking, with notable exceptions [23], there been few studies describing the establishment of successful long-term cultures of rodent renal endothelial cells. Therefore, in order to understand the growth potential of renal endothelial cells and to increase understanding of the predisposition toward renal microvascular rarefaction, we sought to establish long-term cultures of rat kidney endothelial cells and to compare their growth and colony forming potential with rapidly growing endothelial cells derived from the rat pulmonary microvasculature.
Methods
Animals
Male Sprague-Dawley rats were obtained from Harlan labs at 8–10 weeks of age. For studies involving the isolation of young, neonatal rat kidney cells, pregnant dams were obtained at 18 days of gestation and isolation of kidney endothelial cells carried out on pups between 9–11 days of postnatal age. Transgenic Sprague Dawley rats expressing green fluorescent protein SD-Tg(GFP)2BalRrrc, were obtained from the University of Missouri Rat Resource Center. All studies were approved by the Institutional Animal Care and Use Committee of Indiana University.
Isolation and culture of rat kidney endothelial cells and pulmonary endothelial cells
PAECs and PMVECs were isolated and cultured in Dulbecco’s modified Eagles medium and 10% fetal bovine serum as previously described [24]. These cells were previously characterized by Alvarez et al. [22], and were shown to express CD31, CD34, KDR, vWF, VE-Cadherin and E-Cadherin, but were negative for CD45 and CD133 by FACS analysis. Additional FACS analyses for CD31 were repeated to verify that these cells retained their endothelial phenotype as described previously [24] (not shown).
For kidney endothelial cells (KECs), anesthetized rats were cannulated through the aorta to flush the kidney with sterile saline followed by infusion with 1% low-melting agarose (Sigma). The kidneys were excised from the body cavity, placed on ice for 2 min and the cortex and medulla were dissected with a sterile scalpel. The respective sections were minced and incubated for 10 min at room temperature with dispase I (Roche). The tissue slurry was then strained through filter meshes (70 and 40 μm, sequentially) and suspended in EGM-2 media (Lonza) at room temperature. Dispersed cells were incubated with biotylated anti-CD31 (BD Biosciences) followed by incubation in streptavidin coated magnetic beads (Invitrogen, Cat # 112.05D) and positive cells separated using a DynaMag device (Invitrogen). Isolated cells were gently washed multiple times in 0.1% bovine serum albumen in phosphate buffered saline (PBS) and plated at a density 3 × 106 cells/cm2 in 6-well plates in EGM-2 containing 20% FBS and 1% penicillin/streptomycin. Plated cells were allowed to grow for 7 days in 5% O2/5% CO2 (this oxygen tension was determined to provide the most optimal growth potential of KECs; data not shown).
To enrich for CD31 expressing endothelial cells, adherent kidney cells following 7 days in culture were released with 0.1% Trypsin (Invitrogen) and underwent a second round of anti-CD31 magnetic separation and replating. In some cases, individual endothelial colonies were isolated using cloning rings, released from attachment with Trypsin, and were replated. The growth media was changed to fresh EGM-2 media supplemented with 10% fetal bovine serum to propagate cultures. Growth profile analysis and functional characterization studies described below were carried out between passages 2–3 of the KEC.
Cell Growth Studies
Cells were seeded at 1 × 104 cells/cm2 in separate six-well plates and growth rates of were determined as described previously [22]. In some studies, VEGF-165 (Isokine) was administered to cell cultures for up to 3 days and cell proliferation was measured either using an MTS assay (Promega) or standard cell counting.
Immunostaining and other characterization of endothelial cells
KECs, PMVECs or HUVEC were cultured in 6-well plates; incorporation of PE-conjugated acetylated low-density lipoprotein (Alexa Fluor 488 Ac LDL, Molecular Probes) at a concentration of 4 μg/ml for 2 hours at 37°C. Subsequently, cells were prepared for staining by rinsing with cold PBS and incubation with anti-CD31 (mouse anti-rat or anti human CD31, BD Biosciences, PE conjugated for rat) followed by visualization using fluorescent microscopy.
mRNA analysis
Total RNA was isolated from KEC, RPAEC and RPMVEC using Trizol (Invitrogen). In some studies, freshly isolated cells enriched for either kidney of pulmonary microvascular cells were used to evaluate mRNA expression prior to culture. Reverse transcriptase and PCR master mix was also obtained from Invitrogen. Primers sequences and melting temperature (Tm) for each set is shown in Table 1. Quantitative Real-time PCR was conducted using the rat “VEGF-pathway” RT2 Profiler PCR array (rat, # PARN-091, SA Biosciences, Frederick MD) according to the manufacturer’s instructions [25]. To determine the potential expression of the Flt-1 variant that produces the expression of the soluble inhibitor sFlt-1, rat specific primers were designed based on the approach described by Heydarian et al.[26], in which the sFlt amplified region was extended between exon 13/14 of full length Flt-1 (See Figure 6). Primers were obtained from Invitrogen; the 5′ primer for full length Flt-1 was TTACCTGGATCCTGCTACGG and the 3′ primer was CCTCGTGGTCACTGAGGTTT. For sFlt-1, the 5′ primer sequence was AAGACTCGGGCACCTATGC and the 3′ primer sequence was CACAACCACTCCTCCTGCTT.
Table 1.
PCR primers used to characterize endothelial cell cultures (5′ to 3′)
| Forward | Reverse | Anealing Temp | product size | |
|---|---|---|---|---|
| VEGF | TGCACCCACGACAGAAGGGGA | TCACCGCCTTGGCTTGTCACA | 62 | 360/492 |
| Flt-1 | GCTCTGTGGTTCTGCGTGGA | CATGGGATCACCACAGTTTT | 60 | 422 |
| Flk-1/KDR | GCAGCACCTTGACCTTGAAC | AGGATTGTATTGGTCTGCCG | 60 | 424 |
| Tie-2 | ATGGACTCTTTAGCCGGCTTA | CCTTATAGCCTGTCCTCGAA | 55 | 337 |
| beta-actin | AGCCATGTACGTAGCCAT | CTCTCAGCTGTGGTGGTGAA | 55 | 228 |
| Nrp1 | TAATGTGTCTGCGGCCCAG | TTGGTGTGGTCAGAGCCAAG | 62 | 297 |
| NOS-3 | CGAGATATCTTCAGTCCCAAGC | GTGGATTTGCTGCTCTCTAGG | 60 | 200 |
| Nrp2 | ACCCTTGCTCTGAATGTTG | TCCAGTAGTACCACTTGATAC | 55 | 225 |
| Ang-1 | GGAGCATGTGATGGAAAATTA | TGTGTTTTCCCTCCATTTCTA | 55 | 360 |
| Ang-2 | AAAGAGTACAAAGAGGGCTTA | TCCAGTAGTACCACTTGATAC | 55 | 415 |
Figure 6.
Q PCR results for Flt-1 and sFlt mRNA in PMVEC vs KEC cultured cells. Top Panel: schematic illustration demonstrating the region from which primers were designed to detect full length Flt-1 mRNA and sFlt mRNA, in which the 3′ primer targeted sequence is within intron-13 of the sFlt gene. Bottom panel: Log(2) ratio of Flt-1 and sFlt-1. The log (2) ratios were determined from 3 replicate determinations per group. * indicates log(2) ratio was outside 99% C.I. (= 3.28) determined in Figure 5A.
Telomerase Assay
Telomerase activity was measured in 2 μg protein extract of KEC, PAEC and PMVECs by the Trapeze Telomerase Detection Kit (Chemicon) according to the manufacturers instructions.
In vitro angiogenic activity
KEC or PMVEC were seeded at 2 × 104 cells/cm2 in Matrigel-coated (100 μl/cm2) 96-well plates (~20,000 cells/well) supplemented with standard culture medium (400 μl total volume/well). After seeding, the plates were incubated at 37°C with 5% CO2-5% O2 for up to 6 hours. For studies using mixed populations, KECs were incubated with PMVEC; in order to distinguish cell types, KECs were stained with Cell Tracker-Green (Invitrogen) just prior to placing the cells in culture. Alternatively, one study utilized KECs that were derived from transgenic GFP expressing rats (SD-Tg(GFP)2Bal). Cells were plated in phenol-free Matrigel and EGM-2 media to avoid confounding problems associated with fluorescence microscopy. Confocal images were obtained by acquiring consecutive Z-stack images with an Olympus FV1000-MPE Confocal microscope equipped with an Argon laser (515 nm excitation lines for GFP), and a diode laser with the capacity to excite at 405 nm for DAPI (405 excitable for DAPI). Twenty consecutive Z-stack images were obtained per acquisition and 3-D reconstruction was carried out using Voxx software [27].
Statistical Analysis
Quantitative data are depicted as the mean ± SE. Comparison among groups was made using one way-ANOVA, and Student Newman Kuels post hoc test was used to identify intergroup differences. In other comparisons, Student’s t-test was used and P < 0.05 was considered statistically significant. For data analysis of Q-PCR arrays, the ΔΔCt method. Log-normalized ratios were used to calculate the 95% confidence interval and values used to determine statistically significant changes in expression between cell types [25].
Results
Isolation and growth of kidney endothelial cells
Rat kidney endothelial cell cultures (KECs) were isolated using anti-CD31 magnetic separation and propagated in culture. Both adult KEC (AKEC, from rat ~12–16 weeks of age) and young KEC (YKEC, derived from rats ~9–11 postnatal days of age) were used in these studies. Because of the limited growth rates of KEC, the successful establishment of cultures required a secondary isolation following 7 days in culture to alleviate overgrowth from contaminating cell types (ie., fibroblasts, epithelial cells, and blood cells) with faster growth rates. Once purified, rat KECs manifested many features typically attributed to endothelial cells. For example, KECs display a classic cobblestone morphology (Fig 1A). KEC demonstrate CD31 along the surface of the cells and incorporate acetylated LDL, similar to the pattern seen with HUVECs (Figure 1B and C). KECs also strongly expressed the mRNA for classic endothelial cells markers such as the VEGF receptors (fms-like tyrosine kinase, Flt-1; and kinase insert domain receptor, KDR), neuropilin 1 and 2, (Nrp1 and 2), the angiopoetin 1 receptor (Tie-2), and nitric oxide synthase-3 (NOS-3). PMVECs also strongly express Nrp 1, Nrp 2, Tie-2. Immunochemical characterization by FACS of PMVECs has previously demonstrated that they are positive for KDR and NOS3 [22], but the mRNA expression of these genes was minimal (KDR) or undetectable (NOS3), when compared to KECs (Figure 1D).
Figure 1.
Characterization of the rat kidney derived endothelial cells. Figure 1A shows phase contrast image of cultured rat kidney endothelial cells from adult rat (RKEC) indicative of an endothelial morphology. Panel B and inset shows double-label imaging of CD31 immunostaining (green) and incorporation of acetylated LDL (red). For comparison, a similar staining pattern is demonstrated in human umbilical vein endothelial cells (HUVEC, panel C). Figure 1D shows representative reverse transcriptase-PCR results for common endothelial cell markers in rat KEC and rat PMVEC. Shown are results for the VEGF receptors KDR/Flk, and Flt-1 and VEGR-accessory proteins neuropilin-1 and 2 (NRP). Note the relatively high expression of VEGF receptors in AKEC and YKEC. Also shown are results for Tie-2, Angiopoietin-1 and -2 (Ang-1, 2), and nitric oxide synthase-3 (NOS3). The control in which reverse transcriptase was omitted is indicated by “-RT”.
The growth potential of KECs in culture was then compared to the well-characterized and rapidly growing rat PMVECs, rat PAECs, and HUVECs [22]. Rat PMVECs grow rapidly in culture with nearly 10-doublings within 5 days of plating, while PAEC grow at a significantly slower rate (Figure 2A). For comparison, the growth rate of HUVECs was also evaluated and shown to be similar to PMVECs over the first 3 days; at this point HUVEC cultures became confluent and began to growth arrest (data not shown) prohibiting additional time points. In contrast, KEC grow at a dramatically slower rate of growth relative to these other cell types. AKECs averaged less than 2 population doublings within a 7-day period. YKECs grew slightly better than AKECs, but still significantly more slowly than PMVEC and PAECs (Figure 2A). We did not observe differences in growth potential of kidney endothelial cells based on whether the source of endothelial cells was renal cortex or renal medulla (data not shown).
Figure 2. Growth rates and colony forming potential of kidney endothelial vs pulmonary microvascular enthelial cells.
A) Endothelial cells from kidneys (RKEC) from young or adult rats (AKEC, YKEC) were compared with pulmonary micrvascular (PMVEC) and pulmonary artery endothelial cells (PAEC). Cells were plated on Day 0 at a density of 50K per 24-well plate and each cell type was grown under respective optimal conditions (See Methods). Cells were counted at the indicated times. Data derived are from triplicate determinations of multiple independent cultures per group, indicated by the N. * P < 0.05 vs RPMVEC at the indicated times, by ANOVA and Student-Newman Keuls post hoc. B) Clone forming potential was determined from single cell colony forming assay. Colony sizes determined after 14 days of growth after plating at a density of 1 cell/well. Note: KEC remain as single cells or form only small colonies, C) while PMVEC form large colonies.
Previous studies demonstrated that the rat PMVECs have a large percentage of cells with high proliferative potential, which are capable of forming large colonies [22]. In contrast, PAECs have a higher percentage of cells with low proliferative potential [22]. We therefore analyzed the proliferative potential of KECs by carrying out single cell colony forming assays and comparing the potential to PMVECs. Interestingly, greater than 90% of KECs remained as single viable plated cells, while the remaining percentage formed colonies of very small sizes (Figure 2B). Less than 1 % of the colonies grew to > 500 cells and no colonies were > 2000 cells. By contrast (and similar to previous findings) [22], the majority of PMVEC formed colonies > 2000 cells, with some forming colonies greater than 10,000 cells (Figure 2C).
Impaired angioigenic activity of kidney endothelial cells
Endothelial cells typically form branching tubular structures in Matrigel-coated plates in vitro and this activity is a commonly assayed feature of angiogenic cells. The angiogenic activity of rat KECs was therefore examined in Matrigel assays. Figure 3A illustrates robust branching capacity of PMVECs placed on Matrigel for 6 hours, however, when KECs are cultured on the Matrigel, there is no branching even after 24 hours (Figure 3B). Interestingly, additional studies using mixed co-cultures in Matrigel showed that labeled KECs at a 1:100 ratio (i.e., 1 KEC to 100 PMVECs), did not remain as isolated cells, but rather incorporated into PMVEC branching structures (green cells in Figure 3C′ and inset). Similar data were obtained when using KEC that were-derived from GFP-transgenic rats (Figure 3D). Moreover, three-dimensional confocal imaging demonstrated that KEC resided in the same plane as the PMVECs with which they associated (Figure 3D′ inset).
Figure 3. Kidney endothelial cells incorporate into tubular structures in vitro, but inhibit angiogenesis of pulmonary microvascular cells.
Branching capacity of endothelial cell cultures was investigated and is illustrated using phase contrast microscopy following plating on Matrigel-coated plates for 6 hours for PMVEC (A) or adult KEC (B). High branching capacity of PMVEC is apparent whil e KEC have no such capacity. The effects of co-culture was determined following labeling of KEC with Cell Tracker Green and incubation with unlabeled PMVEC at a density of 1:100 (C and C′ inset). Note the incorporation of green-labeled KEC into PMVEC branches (arrows). Panel D shows KEC-derived from transgenic GFP rats were co-cultured on Matrigel with unlabeled with PMVEC. For confocal microscopy, all cells were stained with DAPI prior to imaging. As in panel C, GFP expressing KEC incorporate into PMVEC. An optical cross section based on Z-stack images were was generated VOXX software and is shown in the inset. Arrows indicate incorporation into tubular structures in the same plane as PMVECs.
Impaired VEGF responses in KECs
The impaired growth potential of KEC could be due to early senescence; however we were unable to detect presence of beta-galactosidase, a common feature of cells undergoing senescence (data not shown). Telomerase activity has been shown to be altered in states of impaired endothelial growth and in cells undergoing replicative senescence, however, no difference in telomerase activity was observed in AKECs or YKECs relative to the activity observed in PMVECs (Figure 4). Impaired growth of KEC could also be related to a down-regulation of the molecules in the VEGF pathway; a well recognized pathway critical for endothelial growth and survival. A Q-RT-PCR VEGF pathway array was utilized to evaluate the expression of genes associated with VEGF signaling. Many of the VEGF signaling genes were paradoxically and markedly elevated in AKECs relative to PMVECs (Figure 5A). Most notably, the expression of the VEGF receptors Flt-1 and Flk-1/KDR were among the most highly expressed mRNAs in KECs vs. PMVECs. The mRNA concentration of the VEGF receptor accessory protein Nrp-1 was also significantly elevated in KEC, as were down-stream signaling molecules such as phosphatidylinositol-4,5-bisphosphate 3-kinase (gamma, PIK3CG) MAP kinase-12 and -13, and nitric oxide synthase-3 (NOS-3). The expression of VEGF pathway genes in YKEC was essentially identical that of AKECs (not shown).
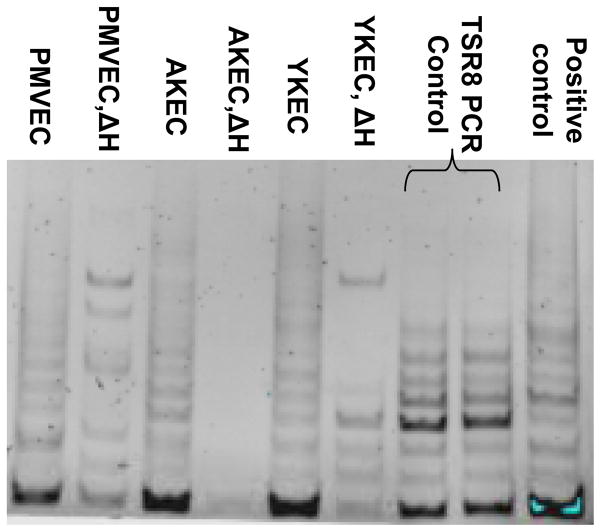
Figure 4.
Telomerase activity in kidney endothelial cells (AKEC and YKEC) and pulmonary microvascular endothelial cells (PMVEC). Cell extracts were assayed for telomerase activity assays as described in “Methods” and activity measured by gel electrophoresis of the PCR product. Telomerase activity was similar in PMVEC, AKEC and YKEC and was sharply attenuated by heating the samples (ΔH).
Figure 5. mRNA expression of VEGF-pathway genes in KEC vs PMVEC using PCR expression arrays.
Panel A: mRNA expression data was derived using “VEGF response” pathway arrays (SA-Biosciences, See Methods) comparing expression in AKEC and RPMVEC. mRNA expression data are expressed as Log(2) normalized ratios relative to the expression in PMVEC; therefore, greater expression in AKEC vs. PMVEC corresponds to a positive value and a lower expression in AKEC is represented by negative value. Of the 84 test genes, 73 genes were detectable and the 95% CI of Log(2) ratios was set at ± 2.25. Shown are data from 19 genes with log (2) ratios > 2.25. Panel B: similar comparison was carried out on freshly isolated endothelial cells derived from the pulmonary microvasculature and kidney endothelial cells obtained prior to plating and outgrowth. Of 84 test genes, 65 were detectable. Shown are data from 8 most differentially expressed genes with an absolute value of the Log(2) ratio > 1.8. The 95% C.I. was determined to ± 2.1; and values beyond this range are indicated by *. Red arrows indicate genes expressed at higher levels in both KEC cultures (panel A) and freshly isolated KEC (panel B).
We further evaluated whether the differences in expression of these cultured kidney and pulmonary microvascular cells reflected expression patterns in vivo or were due to sustained maintenance in culture. To address this, VEGF-pathway gene expression was compared in freshly isolated KEC samples vs freshly isolated PMVEC samples. In these comparisons, fewer differences in gene expression between the two sample types were observed. However, mRNA expression from several genes was at higher levels in freshly isolated KECs vs freshly isolated PMVECs (Figure 5B). Some of these gene transcripts were elevated in both the freshly isolated cell comparison (Figure 5B) and sustained culture comparison (indicated by arrows in Fig 5A and 5B). These elevated gene transcripts included Flk-1/KDR, Nrp-1 and NOS-3. Taken together, the data suggest that neither accelerated senescence nor the impaired expression of the VEGF pathway genes accounts for the slow growth of KECs.
The elevated expression of the VEGF receptor Flt1 in KEC motivated us to investigate whether this high expression may be due, in part, to a contribution of sFlt-1, the soluble VEGF inhibitor produced by differential mRNA splicing. We further analyzed Flt-1 and sFlt-1 mRNA using Q PCR primers specific for each type of transcript (Figure 6A). Similar to results observed in Figure 5A, Flt-1 mRNA was substantially elevated in KEC relative to PMVECs, as was the expression of sFlt (Figure 6B).
Based on the high level of VEGF-associated signaling molecules in KECs, and the potential that the competitive VEGF-inhibitor s-Flt may released in the media of KECs, the growth potential of KECs was then compared with PMVECs under VEGF-stimulated conditions. PMVECs grew rapidly and the addition of VEGF-165 significantly enhanced the growth rate of these cells (Figure 7A). KECs underwent significantly less growth and VEGF-165 provided no additional trophic activity (Figure 7B, represents KEC data from Figure 7A). To determine if this insensitivity of KEC was attributable to a shift in the dose response, as would be expected by competitive inhibition, we tested a wide range of VEGF concentrations (10–250 ng/ml). However, no dose of VEGF influenced the growth rate of KECs (Figure 7C).
Figure 7. Effect of VEGF on the growth potential of kidney endothelial cells (KEC) and pulmonary microvascular endothelial cells.
A) Growth rates for KEC from adult and young animals and PMVEC were followed for 3 days with or without supplementation of VEGF-165 (50 ng/ml). VEGF significantly enhanced the growth of PMVEC over 3 days, but did not influence the growth rate of KEC from either adult or young rats. B) Data from from panel A, rescaled to show KEC growth and impaired response to 50 ng/ml VEGF. C; VEGF dose response of KEC proliferation (10–250 ng/ml) followed for 3 consecutive days demonstrated no responsiveness at any concentration. Each experiment was carried out in triplicate samples. In Panel A,* indicates P < 0.05 in VEGF treated vs. non-treated PMVEC, by Student’s t-test.
Discussion
In the kidney, vascular remodeling, or lack thereof, has significant importance in the pathogenesis of chronic kidney disease. Some data from animal models of kidney injury report evidence of increased renal angiogenesis. For example, there is new glomerular capillary loop formation and/or endothelial proliferation in early stages of diabetes induced by streptozotocin or immune complex disease [2–5]. However, these changes typically represent early steps of the disease process, while progressive disease is associated with loss of capillary density in both the glomerular and/or peritubular compartments [8].
In the setting of CKD, the loss of peritubular capillaries has been linked with exacerbated hypoxia, a key mediator in the fibrotic process [7, 8]. Despite this persistence in this classic angiogenic stimulus, there is very little evidence supporting the existence of vascular growth or endothelial cell proliferation under these conditions. Following an acute ischemic injury which can lead to capillary rarefaction, anti-angiogenic factors such as angiostatin, TGF-β, and ADAMTS-1 are transiently elevated in kidney and may impede normal growth or regenerative responses [10, 28]. Moreover, the loss of vessels may be attributable to reduced VEGF activity from adjacent renal parenchyma [29, 30]. Thus, VEGF-based therapies have been utilized to preserve renal vessels in several models of CKD [11, 31, 32]. However, whether exogenous VEGF stimulates de novo endothelial cell proliferation in the kidney is unclear. In our report on renal ischemia, endothelial proliferation was minimal and not influenced by exogenous VEGF [10]. Thus, even when the trophic environment is altered to favor endothelial cell proliferation, renal endothelial cell growth remains impaired.
A significant amount of information regarding the growth and angiogenic properties of endothelial cells relies on data from a limited number of endothelial cell types, such as HUVECs, which are chosen typically for their robust growth properties. Our studies demonstrate the KEC grow significantly more slowly than HUVECs and other rat endothelial cells such as PMVECs. Thus, it is reasonable to raise caution regarding conclusions drawn from studies on rapidly growing endothelial cells (e.g., HUVEC, rat PMVEC) insofar as the regulatory factors mediating growth may differ in specific vascular beds, such as the kidney. Based on the relatively consistent observation of impaired renal vascular growth, the motivation to study growth properties of renal endothelial cells seems justified.
Ballermann in 1989 described one of the first examples of long-term endothelial cell cultures from kidney [23]. In these studies, glomerular endothelial cells were derived from bovine kidneys and individual clones were propagated in culture for up to 15 passages. Based on that report, there appears to be one important similarity between the KECs described in our study and the glomerular endothelial cells described by Ballermann; i.e., both cell types are prone to overgrowth by contaminating cell types, such as mesangial cells, a feature likely due to the slow growth relative to other kidney cell types. In the current study, a second round of KEC re-selection and re-plating from the primary cultures was determined to be essential for establishment of pure KEC populations.
In contrast, the bovine glomerular endothelial cells described by Ballermann clearly grow better than the rat KEC reported here. It should be noted that bovine endothelial cells from numerous tissues display significantly greater proliferative potential than circulating or resident endothelial cells from mouse, rat, pig, rhesus monkey, and man [33]. For example, bovine corneal endothelial cells display robust clonal proliferative potential that is significantly greater than that of human corneal endothelium [34]. Bovine aortic endothelial cells are known to display greater proliferative potential than human aorta when compared at a clonal level [19, 34]. The underlying mechanism that permits greater proliferative potential in bovine endothelial cells from other studied species remains unknown. However, the choice of the bovine species to isolate the glomerular capillary cells by Ballermann [23], may have contributed to the success in establishing cultures that could be passaged for some time in vitro.
Several other investigators have attempted to culture renal endothelial cells from either mouse or rat sources with limited success. Most studies on rat or mouse renal endothelial cells have utilized viral transformation with the SV40 T antigen or immorto-mouse approaches [35–37] and are therefore not practical for use to understand intrinsic growth mechanisms. At the current time, we are aware of only one other study in which a single kidney endothelial cell clone from mouse has been propagated in long-term culture (over 1 year and 100 passages), without known intervening transformation [38].
The current study was not undertaken to establish an individual cell line, but rather to characterize the growth potential across the resident population of endothelial cells within the rat kidney. When viewed globally, primary cultures were obtained demonstrating slow but consistent growth rates across multiple different cultures, when compared to previously studied PMVECs [22], a finding that is consistent with the lack of an effective renal vascular regeneration in response to injury.
With regard to limited growth and impaired angioigenic response of kidney derived endothelial cells, several features are noteworthy. First, we did not observe any consistent differences in cultures derived from renal cortex versus renal medulla. While our approach does not allow us to identify which endothelial cells within the kidney are being propagated, our preparative steps (sieving pieces larger than 40 microns) likely minimized the contribution of glomerular cells. Although we cannot exclude that glomerular endothelial cells are present in these cultures, the majority likely derive from the peritubular capillary system. Glomerular endothelial cell proliferation has been reported in some models [39]; if glomerular endothelial cells did contribute to our cortical cultures, they did not influence overall growth rate.
Secondly, several elements of this study support the conclusion that these cells represent true kidney endothelial cells. These cells fulfill most of the features typically ascribed to endothelial cells including “cobblestone morphology”, expression of VEGF receptors and CD31, and do not express CD45. However, these cells did not form branches when plated on Matrigel, a feature typically observed in endothelial cell cultures. It is possible that KEC lack the ability to generate trophic signals driving branch formation, but might incorporate into these structures in the presence of the proper environmental cues. Therefore, the incorporation of KECs with PMVEC derived branching structures is consistent with, but not definitive of their endothelial nature (Fig 3).
Finally, the lack of cell growth or angioigenic activity does not appear to be a function of aging per se, since cultures derived from young rats (YKECs) were only moderately more potent than adult KECs, and neither AKEC nor YKEC can independently form branched structures on Matrigel. Moreover, there was no indication that the impaired growth rates were due to premature senescence, since telomerase activity was comparable in AKEC, YKEC and PMVECs.
The slow growth potential in KEC remains to be fully elucidated. Interestingly, and unexpectedly, both AKEC and YKEC demonstrated robust expression of VEGF receptor mRNA and multiple downstream signaling molecules, relative to the VEGF-sensitive PMVECs. It is important to emphasize that different culture conditions were used for comparison in these studies, such that empirically optimal conditions were employed for each respective cell type. KEC grew most optimally in a low oxygen environment (i.e., 3–5% O2) relative to PMVECs, which grow optimally under normoxic conditions. Whether the low oxygen environment of KEC contributed to the apparent higher expression of VEGF related genes relative to PMVEC is not known since we were not able to sustain KEC cultures under normoxic conditions (not shown). In addition, we also carried out studies on freshly isolated KEC and PMVEC prior to culture. While contaminating cell types likely contributed to these samples, VEGF signaling molecules were also higher in the KEC-enriched samples relative to the PMVEC-enriched samples, supporting the suggestion that these differences may be reflected in the in vivo condition.
Initially, we speculated that the slow growth may be the result of sFlt-1 because of its high mRNA expression in KEC. sFlt-1 is a soluble VEGFR1 that has been shown to act as a competitive inhibitor of VEGF-activity [40]. However, regardless of whether sFlt-1 is contained in the media, our functional studies argue against the likelihood that sFlt-1 is primarily responsible for impaired KEC growth. KEC proliferation remained unchanged throughout a wide and increasing range of VEGF concentrations, which is not consistent with a competitive inhibition mechanism. In summary, our data indicate that KECs are under the control of strong negative regulatory signaling, of a thus far unknown nature. Because of the dominant nature of this negative regulatory signaling, until this pathway can be identified and inhibited, it will be difficult to determine if s-Flt1 provides additional negative growth regulation in KECs.
Lastly, the most unique aspect of the current study relates to the results from clonal analysis suggesting that the vast majority of KEC remain as viable but non-dividing cells. Only a small number of KECs (<1%) were capable of forming moderately sized colonies (>500 cells), while many of PMVECs form colonies in excess of 10,000 cells. We suggest that the slow KEC growth rate reflects the relatively poor potential of individual cells distributed across the population, despite retaining telomerase activity similar to the PMVECs.
It is likely that new endothelial cells derive from a source of progenitor cells that arise either from the circulation or from adjacent areas within the vascular bed. Non-bone marrow derived endothelial progenitor cells have been described that can be isolated from either the circulation or vasculature [33]. Clonal analysis has revealed that these endothelial cells contain a hierarchy of progenitor potential in which some cells have high proliferative potential (HPP) capable of forming large colonies, while others form colonies with low proliferative potential (LPP) or remain as single cells [19, 21]. In human cord blood, HPP-ECFC are found in abundance, while adult human blood has lower percentage of HPP-ECFC (17). Moreover, disease states such as diabetes reduces the ECFC potential [41].
It is reasonable to suggest that the proportion of HPP-ECFC residing within a given vascular bed represents its growth or regenerative potential. Recent studies by Alvarez et al., noted that pulmonary microvascular beds associated with significant repair potential have greater HPPs than cells isolated from pulmonary macrovasculature (i.e., pulmonary artery) [22]. From this perspective, it is remarkable that not a single KEC possessed progenitor potential similar to that which is routinely observed in PMVECs (ie., > 2,000 cells). Indeed, the most potent KECs formed colonies of ~ 500 cells and this represents only a very small percentage of clones studied. If one recognizes that the culturing process selects for the most proliferatively competent cells within a population, these results suggest that the basis for impaired regeneration in the kidney relates to the relatively low ECFC potential intrinsic to the renal endothelium.
It is worth noting that Muczynski et al., hypothesized that human kidney endothelial cells comprise a heterogeneous distribution of endothelial colony forming proliferative potential. In contrast to our results, this group identified heterogeneous ECFC proliferative potential in kidney endothelial cells, including some characterized as “high” in proliferative potential [42]. However, the proliferative potential of kidney ECFC was still significantly lower than the ECFC potential reported for human aorta [19]. Based on these reports, it is reasonable to conclude, that while proliferative capacity of human kidney endothelial cells is greater than rat, the proliferative capacity of KEC within a species is low relative to other EC types. We suggest that within a species, the kidney has relatively low ECFC potential and that investigation of the factors limiting progenitor potential may provide a greater insight or a therapeutic target in the treatment of chronic kidney disease.
Perspectives
Chronic kidney disease (CKD) is characterized by progressive interstitial fibrosis and is exacerbated by hypoxia and capillary rarefaction. Renal peritubular capillaries have little regenerative capacity and the basis of this impaired regenerative potential is not known. We show that primary cultures of rat kidney endothelial cells grow slowly and have limited angioigenic potential, despite having abundant levels of VEGF signaling molecules. The data indicate that KEC are growth inhibited by an unknown mechanism, and we suggest that this intrinsic property of KECs is the basis for irreversible capillary rarefaction in progressive CKD.
Acknowledgments
This work was supported by NIH grants DK063114 (D.B.), an ARRA supplement to this same grant. Additional funding for this study was from Riley Children’s Foundation (M.Y.), pilot funding from the George M O’Brien grant, P30 DK79312 (Bruce Molitoris, Program Director), and Bridge Funding from the Indiana University Research Foundation. We would like to thank Dr. Paul Critser for help with the Matrigel assay and Peter Corridon and Jeff Mclendon for help with Image analysis of data collected on the confocal microscope.
Grant support: DK063114
Footnotes
Portions of this work were presented at the Experimental Biology Meeting, Washington DC, April 2011.
References
- 1.Freeburg PB, Abrahamson DR. Hypoxia-Inducible Factors and Kidney Vascular Development. Journal of the American Society of Nephrology. 2003;14:2723–30. doi: 10.1097/01.asn.0000092794.37534.01. [DOI] [PubMed] [Google Scholar]
- 2.Nyengaard J, Rasch R. The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia. 1993;36:189–94. doi: 10.1007/BF00399948. [DOI] [PubMed] [Google Scholar]
- 3.Ostendorf T, Kunter U, Eitner F, Loos A, Regele H, Kerjaschki D, Henninger DD, Janjic N, Floege VEGF165 mediates glomerular endothelial repair. The Journal of Clinical Investigation. 1999;104:913–23. doi: 10.1172/JCI6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nangaku M, Alpers CE, Pippin J, Shankland SJ, Adler S, Kurokawa K, Couser WG, Johnson RJ. A new model of renal microvascular endothelial injury. Kidney Int. 1997;52:182–94. doi: 10.1038/ki.1997.318. [DOI] [PubMed] [Google Scholar]
- 5.Iruela-Arispe L, Gordon K, Hugo C, Duijvestijn A, Claffey K, Reilly M, Couser W, Alpers C, Johnson R. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol. 1995;147:1715–27. [PMC free article] [PubMed] [Google Scholar]
- 6.Floege J, Burns MW, Alpers CE, Yoshimura A, Pritzl P, Gordon K, Seifert RA, Bowen-Pope DF, Couser WG, Johnson RJ. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992;41:297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- 7.Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrology Dialysis Transplantation. 2011;26:1132–37. doi: 10.1093/ndt/gfq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–72. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 9.Basile DP, Donohoe DL, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. American Journal of Physiology. 2001;281:F887–F99. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 10.Basile DP, Friedrich JL, Spahic J, Knipe NL, Mang HE, Leonard EC, Ashtiyani SC, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. American Journal of Physiology - Renal Physiology. 2011;300:F721–33. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard EC, Friderich J, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol-Renal Physiol. 2008;295:F1648–57. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reidy M, Silver M. Endothelial regeneration. VII. Lack of intimal proliferation after defined injury to rat aorta. Am J Pathol. 1985;118:173–77. [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz S, Benditt E. Clustering of replicating cells in aortic endothelium. Proc Natl Acad Sci. 1976;73:651–53. doi: 10.1073/pnas.73.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohsenin A, Burdick MD, Molina JG, Keane MP, Blackburn MR. Enhanced CXCL1 production and angiogenesis in adenosine-mediated lung disease. The FASEB Journal. 2007;21:1026–36. doi: 10.1096/fj.06-7301com. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds L, Grazul-Bilska A, Redmer D. Angiogenesis in the corpus luteum. Endocrine. 2000;12:1–9. doi: 10.1385/ENDO:12:1:1. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds LP, Redmer DA. Angiogenesis in the Placenta. Biology of Reproduction. 2001;64:1033–40. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- 17.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiological reviews. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 18.Yoder MC. Defining human endothelial progenitor cells. Journal of Thrombosis and Haemostasis. 2009;7:49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- 19.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 20.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–31. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 21.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 22.Alverez D, Huang L, King JA, Khair ElZarrad M, Yoder M. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol. 2008;294:L419–30. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 23.Ballermann BJ. Regulation of bovine glomerular endothelial cell growth in vitro. American Journal of Physiology. 1989;256 doi: 10.1152/ajpcell.1989.256.1.C182. [DOI] [PubMed] [Google Scholar]; Cell Physiol. (25):C182–89. [Google Scholar]
- 24.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvascular Research. 2004;67:139–51. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SA, Pechman KR, Leonard EC, Friedrich JL, Bian J-T, Beal AG, Basile DP. Increased ANG II sensitivity following recovery from acute kidney injury: role of oxidant stress in skeletal muscle resistance arteries. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1682–91. doi: 10.1152/ajpregu.00448.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heydarian M, McCaffrey T, Florea L, Yang Z, Ross M, Zhou W, Maynard S. Novel splice variants are upregulated in pre-eclampsia. Placenta. 2009;30:250–55. doi: 10.1016/j.placenta.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Clendenon JL, Phillips CL, Sandoval RM, Fang S, Dunn KW. Voxx: a PC-based, near real-time volume rendering system for biological microscopy. American Journal of Physiology - Cell Physiology. 2002;282:C213–C18. doi: 10.1152/ajpcell.2002.282.1.C213. [DOI] [PubMed] [Google Scholar]
- 28.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–56. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. Journal of the American Society of Nephrology. 2001;12:1434–47. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 30.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol. 2008:294. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 31.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. Journal of the American Society of Nephrology. 2001;12:1448–57. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 32.Suga S, Kim Y, Joly A, Puchacz E, Kang DH, Jefferson JA, Abraham JA, Hughes J, Johnson R, Schreiner GF. Vascular endothelial growth factor (VEGF-121) protects rats from renal infarction in thrombotic microangiopathy. Kidney International - 2001. 60 doi: 10.1046/j.1523-1755.2001.00935.x. [DOI] [PubMed] [Google Scholar]
- 33.Yoder MC. Is Endothelium the Origin of Endothelial Progenitor Cells? Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1094–103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 34.Huang L, Harkenrider M, Thompson M, Zeng P, Tanaka H, Gilley D, Ingram DA, Bonanno JA, Yoder MC. A Hierarchy of Endothelial Colony Forming Cell Activity Displayed by Bovine Corneal Endothelial Cells. Investigative Ophthalmology & Visual Science. 2010;51:3943–49. doi: 10.1167/iovs.09-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukahara H, Gordienko DV, Tonshoff B, Gelato MC, Goligorsky MS. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994;45:598–604. doi: 10.1038/ki.1994.78. [DOI] [PubMed] [Google Scholar]
- 36.MacKay K, Striker L, Elliot S, Pinkert C, Brinster R, Striker G. Glomerular epithelial, mesangial and endothlelial cell lines from transgenic mice. Kidney Intl. 1988;33:677–84. doi: 10.1038/ki.1988.53. [DOI] [PubMed] [Google Scholar]
- 37.Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. American Journal of Physiology - Cell Physiology. 2007;292:C2070–C83. doi: 10.1152/ajpcell.00489.2006. [DOI] [PubMed] [Google Scholar]
- 38.Gazzaniga S, Gonzalez L, Mantovani A, Vecchi A, Wianstok R. Isolation and molecular characterization of a mouse renal microvascular endothelial cell line. In Vitro Cell Dev Biol Animal. 2004;40:82–88. doi: 10.1290/1543-706x(2004)040<0082:iamcoa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Shao J, Miyata T, Yamada K, Hanafusa N, Wada T, Gordon KL, Inagi R, Kurokawa K, Fujita T, Johnson RJ, Nangaku M. Protective role of nitric oxide in a model of thrombotic microangiopathy in rats. Journal of the American Society of Nephrology. 2001;12:2088–97. doi: 10.1681/ASN.V12102088. [DOI] [PubMed] [Google Scholar]
- 40.Maynard SE, Min J, Merchan J, Lim K, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt) may contribut to endothelial dysfunction, hypertension and proteinuria in pre-eclampsia. Journal of Clinical Investigation. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingram D, Krier T, Mead LE, McGuire C, Prater D, Bhavsar J, Saadatzadeh M, Bijangi-Vishehsaraei K, Yoder MC, Haneline LS. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25:297–304. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- 42.Muczynski K, Leca N, Anderson S. Proliferative potential of human kidney endothelial cells: bone marrow derived cells may not be required for high proliferation. Nephrol Dial Transplant. 2010;25:2953–60. doi: 10.1093/ndt/gfq130. [DOI] [PubMed] [Google Scholar]