Abstract
Head direction (HD) cells have frequently been regarded as an internal “compass” that can be used for navigation, although there is little evidence showing a link between their activity and spatial behaviour. In a navigational task requiring the use of internal cues to return to a home base location without vision (path integration), we found a robust correlation between HD cell activity and the rat's heading error in their homing behaviour. Furthermore, we observed two different correction processes that animals used to improve performance after an error. The more frequent one consists of `resetting' the cell whenever the animal returns to the home location. However, we found that when large errors occur the HD system has the ability to `remap' and set a new reference frame, which is then used in subsequent trials. We also offer some insight into how these two correction processes operate when animals make an error.
Path integration, first hypothesized by Darwin1, is an animals' ability to continuously update their position relying on self-motion cues (vestibular, proprioceptive or motor cues)2. This strategy permits a direct return to the nest (homing) at any time and has been studied in a wide variety of species from insects to humans3. Various types of spatially tuned neurons (place cells4, head direction cells5 and grid cells6) have been studied extensively within the limbic system, but how these cells continually compute distance and direction, and direct path integration, remains unclear7,8. Head direction (HD) cells, which provide a constant signal of the rat's heading5,9- and are modulated by self-motion cues10 - are commonly viewed as the best candidate for integrating angular head motion over time (angular integrator)11–15. However, to fully establish that these cells underlie the neural basis for path integration, the HD signal should (1) match the heading error, reflecting the accumulation of error over time that is characteristic of path integration16–18, and (2) reflect the reorientation process when an animal uses landmarks to take a “fix” after making an error17–19. Here we show that the HD signal recorded from the anterodorsal thalamic nucleus20 of rats performing a path integration task, satisfies these two conditions. First, we observed that the shift of the HD cell's preferred firing direction (PFD) was correlated with the heading error of the animal. Second, we report two types of correction processes used by the animals. After large heading errors the HD system `remaps' and takes a new bearing for the following trials, but in most cases, the HD cells' PFDs are `reset' to stable values anchored to the refuge. This coupling of the HD signal and homing behaviour suggests that the HD signal provides the directional heading component to the path integrator.
RESULTS
Seven Long-evans rats were trained to perform the food-carrying task (forage for a large food pellet and bring it back to the refuge for consumption21). Once they were proficient at performing the task with blindfolds, the animals were implanted with recording electrodes in the anterodorsal thalamus20 (Supplementary Fig. 1 & 2). We recorded a total of 27 HD cells (Fig.1a) in at least one foraging trial, and for each trial we evaluated the homing accuracy of the animals. A trial was considered “correct” when the animal's return trip reached the edge of the arena within the two barriers framing the refuge (Fig. 1b–c). A trial was counted as an error when the homing trip ended outside these two barriers. Among 263 trials, 135 were classified as correct trials (51.3 %), with a mean heading error of 14.78 ± 0.08° from a straight return to the refuge and 128 trials were classified as errors, with a mean heading error of 39.43 ± 2.41°. To determine whether the overall directional firing properties were different between correct and error trials, we calculated the Rayleigh r (mean vector length) for correct (mean: 0.632 ± 0.020) vs. error trials (mean: 0.626 ± 0.019); no significant difference was found between these two types of trials (student's t-test P >0.05).
Figure 1.
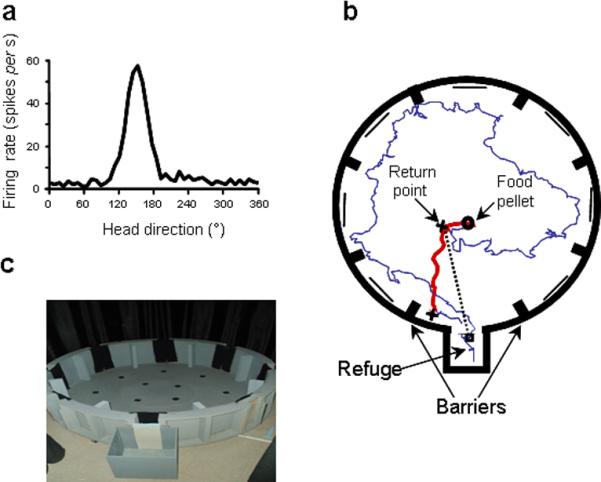
a, Example of a HD cell tuning curve (8 min. session in the cylinder, PFD = 150°). b, Example of path taken by a rat: the foraging (outward) trip (in blue) ends when the animal finds the food pellet. The heading of the return trip (in red) was measured from the return point (1st cross) to the edge of the arena (2nd cross). c, The food-carrying task apparatus.
Are the animals using path integration?
To verify that the animals were path integrating, we analysed whether the complexity of their outward trip (searching for the pellet) affected the accuracy of their return to the refuge (homing). We compared foraging excursions resulting in correct or incorrect (error) trials. Analyses revealed significant differences in the duration of the outward trip (t261 = − 2.07, P < 0.05) and in the distance covered by the rats (t261 = − 3.14, P < 0.01), suggesting that the longer the outward trip was, the higher the probability was to make an error on the return trip. Further analyses showed that rats were more likely to make an error if the outbound trip contained more head turns, suggesting that errors may also result from inaccurate integration of head turns (t261 = − 2.12, P < 0.05). We also observed that correct returns usually followed outbound paths that had a higher mean angular head velocity (t261 = − 2.02, P < 0.05) indicating that path integration errors were more likely to occur after a succession of smooth large turns (see details in Supplementary Fig. 3).
In sum, we did not find any defining characteristic of the outbound trip that predicted whether or not the animal would make a heading error (see Supplementary Fig. 4a–d). Nonetheless, the differences observed between correct and error trials confirmed that the complexity of their foraging trips influences the animals' accuracy in their homing behaviour and demonstrates that the rats were likely path integrating when performing the task.
The shift in the cell's PFD correlates with heading error
To determine whether the amount of shift in HD cell PFDs (Supplementary Fig. 5) was correlated with the heading error of the rats during their homing behaviour, we first calculated the amount the PFD shifted between the initial 6 min refuge session and each subsequent foraging trip. Our initial view was that for every trip, the refuge would serve as a unique and stable spatial reference point, which the rat would recognize and then use when resetting its orientation after each trial 17,22 (Fig. 2a–e). In agreement with this hypothesis, there was (Fig. 2f) a linear correlation (Pearson r = 0.45, P < 0.0001) between the amount the PFD shifted from the initial refuge session and the rat's heading error. Further analyses, however, revealed a mean difference of 44.58 ± 2.12° between the heading error of the animals and the cells' PFD shifts, indicating that the PFD shift gave a poor prediction of the heading error.
Figure 2.
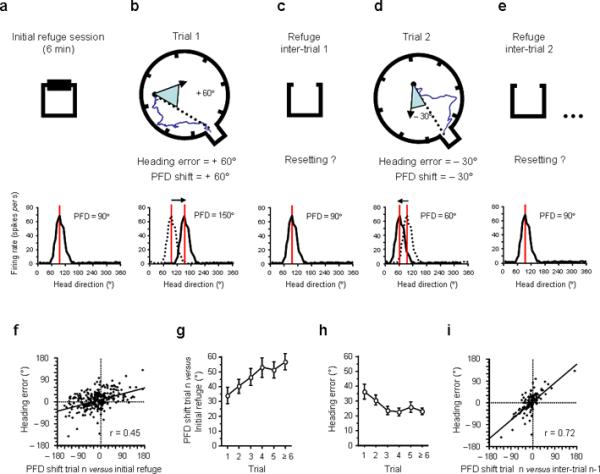
Hypothesized relationship between the PFD shift and the rat's heading error. a–e, Two consecutive trials are depicted in a–e, with inter-trial intervals in the refuge (a, c, e) in between each foraging trial (b, d). Beneath each figure are firing rate vs. HD plots of a hypothetical HD cell, which has a PFD of 90° in the refuge. If the heading error of the animal on its return trip is +60° (b, blue triangle) the PFD recorded at the same time (trial 1) should show a +60° shift (PFD value: 150°) compared to the initial refuge value (dashed line in b, bottom row). Previous theories suggested that a resetting of the cell's PFD would occur after each trip when the animal returns to the refuge (in c and e the cell's PFD shifts back to the initial refuge value: 90°). f, Heading error as a function of the cell's PFD shift from its initial value in the refuge. g, Mean PFD shift between trial n and the initial refuge value as a function of the trial number in the session. h, Heading error as a function of the trial number in the session. i, Heading error as a function of PFD shift on trial n compared to the previous inter-trial interval (n-1). All error bars indicate s.e.m.
We also found that the PFD shift on each trial compared to the initial refuge increased within a session (F5,277 = 2.65, P < 0.05; Fig. 2g), suggesting a gradual drift in the cell's PFD during the course of the session. Does this drift affect the animal's performance? Surprisingly, the homing accuracy improved significantly within a session (F5,257 = 2.28, P < 0.05; Fig. 2h) and indicates that either there is a progressive disconnection between the HD signal and the animal's behaviour within a session, or that the resetting phenomenon is more complex than a simple return of the cells' PFDs to their initial refuge values.
Is the cell's PFD reset after each foraging trip?
To determine whether the cells' PFDs return to their initial refuge values after a foraging trip (during inter-trial intervals, Fig. 2c–e), we assessed the cells' PFDs during 98 inter-trial intervals. Notably, we found that the mean shift of the PFDs between the inter-trial intervals and the initial refuge values was 36.76 ± 3.41° (the mean shift after a correct trial = 36.64 ± 4.73°; the mean shift after an error trial = 36.86 ± 4.91°). This ~ 36° shift cannot be accounted for by shorter inter-trial sampling times because a comparable analysis using sub-sampled data from the initial refuge session gave similar values (mean shift = 37.26 ± 3.51°). Further, if we used the cell's PFD during the inter-trial interval preceding each foraging trip, we obtained a more accurate prediction of the animal's heading error (heading error vs. cell shift ± 20.48°) than if we used the initial refuge value (heading error vs. cell shift ± 43.35°, n = 98, Paired t97 = 6.57, P < 0.0001). Indeed, confirming that the inter-trial interval PFD was a better reference for predicting the animal's behaviour than the initial refuge value, we observed (Fig. 2i) a strong correlation between the heading error of the animal and the cell's PFD shift using the preceding inter-trial interval value (r = 0.72, P < 0.0001; see examples in Fig. 3 and Supplementary Fig. 6 for separate analyses on each animal). In addition, supporting the view that disorientation in path integration results from a slow accumulation of errors, we show several examples where the cell's PFD slowly drifted away from its initial value, and gave an accurate prediction of the animal's heading error (Supplementary Fig. 7–9).
Figure 3.
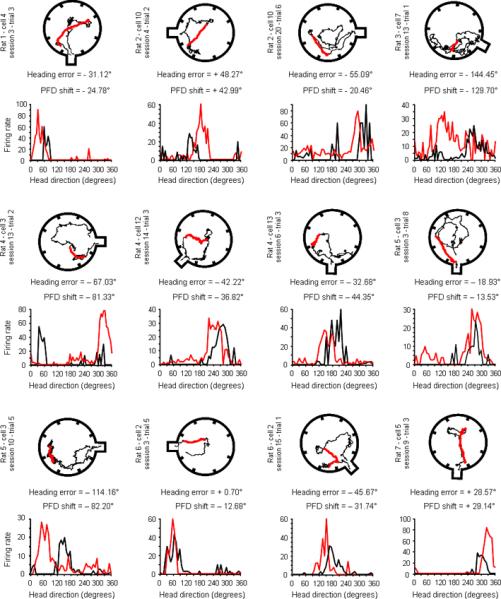
Examples of the foraging path with the corresponding tuning curve of a HD cell recorded during that trial. In each of these twelve examples, the return path of the animal, which was used to calculate its mean heading error, is shown in red. The cell's tuning curve recorded during the entire foraging trip is shown underneath in red and can be compared with the preceding inter-trial interval tuning curve (in black).
In sum, we observed that using the most immediate inter-trial interval as a reference, as opposed to the initial refuge period, the PFD shift observed during foraging accurately reflects the heading error of the animal. But the question remains as to how the signal's preferred orientation can change from one inter-trial interval to another without altering the animal's performance (cf., Fig. 2g–h).
Do HD cells remap during each refuge period?
What process accounts for the better correlation observed when using the refuge PFD from the preceding inter-trial interval compared to the PFD in the original refuge (Fig. 2f vs. 2i)? Is the cell's PFD gradually drifting around the initial refuge value within a session or is it characterized by large shifts (remapping)? The distribution of PFD shifts across successive inter-trial intervals (Fig. 4a) revealed that in several refuge periods the cells' PFDs showed large shifts compared to the previous refuge period. In order to understand what caused these large shifts, we separated the distribution into two modes: mode 1, referred to as `resetting', consisted of inter-trial intervals in which the PFD shifted < 35° (n = 50, grey), and mode 2, referred to as `remapping', included the remaining cases where the PFD shifted > 35° (n = 10, blue). We plotted the inter-trial interval PFD shift against the inter-trial order number in the session (Fig. 4b, open circles). The separation of mode 1 (blue circles) from mode 2 (grey circles) for the first two trials of a session showed that large shifts only occurred in the first or second inter-trial interval. Trials 3 to 5 only contained small PFD shifts (mode 1). Excluding the mode 2 shifts during the first two trials, there was a stable PFD shift from one refuge trial session to another (mean = 13.10°).
Figure 4.
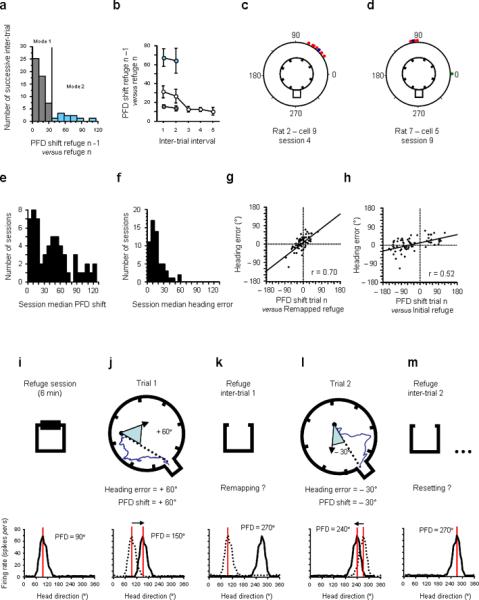
a, Distribution of all PFD shifts between successive refuge periods across all trials. b, PFD shift between successive refuge periods within a session (open circles). For inter-trial intervals 1 and 2, mode 1 trials (resetting, grey circles) and mode 2 trials (remapping, blue circles) are separated from one another. There were no mode 2 inter-trial PFD shifts observed for inter-trial intervals 3–5. Mode 2 inter-trial intervals (PFD shift > 35°, remapping) are represented in blue. c, Circular histogram of PFD shifts showing an example of a session where the cell's PFD remapped after the first trial (blue dot). The cell's PFD shifted + 51.2° from the initial refuge value (represented by zero). The PFD shifts on the following trials (red dots) are all clustered around the remapped value, indicating that subsequent PFD shifts were all reset to the new remapped value rather than the original refuge value. d, Circular histogram of PFD shifts showing an example of a session where the cell's PFD initially reset to the refuge on the first trial (green dot) and then remapped after the second trial (blue dot) and then maintained this new value in all the following trials (red dots). e, Distribution of the session median PFD shifts (from the initial refuge value). f, Distribution of the session median heading errors. g–h, Heading error as a function of the cell's PFD shift from its remapped refuge value (g) and from its initial refuge value (h). Note that the correlation is higher and contains a steeper slope for the remapped refuge values. i–m, Schematic of the remapping hypothesis. Similar to Figure 2a–e, the sequential behavioural episodes are represented in the first row. Beneath each figure are firing rate vs. HD plots of a hypothetical HD cell. i, In the initial refuge the cell's PFD is 90°. If the heading error of the animal on its return trip is +60° (j, blue triangle) the PFD recorded at the same time (trial 1) should show a +60° shift (PFD value: 150°) compared to the initial refuge value (dashed line in j, bottom row). In k, the cell's PFD does not return to its initial refuge value, but remaps to a random PFD (e.g. 270°). In l, the remapped PFD is the new reference and the amount the PFD will shift during trial 2 is compared to that remapped value (− 30°). Thus, the amount the PFD shifts from the new remapped PFD value (− 30° to 240°) predicts the heading error (− 30°) of the animal. m, in the following inter-trial interval, the PFD resets to the value established after remapping (270°). The data partially confirm this remapping hypothesis. One important difference is that the value of the remapped PFD is not random like that shown in k, but is influenced by the shift observed in the previous foraging trip. In this case (j), the remapped value would be ~150°.
We found no difference between the PFD shift occurring after correct trials (mean = 13.90°) vs. after error trials (mean = 12.57°) (t48 = 0.56, P > 0.05). These results suggest that in the majority of the inter-trial intervals sampled (mode 1), the cell's PFD was stable from one refuge to another. The stability of the cell's PFD has been documented in previous studies20,23,24. Thus, it is not surprising that in our experimental conditions, where animals are blindfolded24 and must rely on tactile and olfactory cues to recognize the refuge, resetting was less accurate than when animals have access to visual landmarks (mean = 4–8° 20,23). In the relatively small number of trials per session we recorded, we never observed remapping more than once per session. Notably, the mean PFD shift across inter-trials intervals following remapping (mean = 21.09°) was significantly lower than the mean shift in mode 2 inter-trial intervals (± 68.18°, t15 = 4.82, P < 0.001), suggesting that after remapping the cell's PFD stabilized around the remapped refuge value.
In sum, we observed a relative stability of the cells' PFDs in the refuge, suggesting that resetting is the most common strategy used by the animals. However, in some cases (mode 2) the cells' PFDs remapped to a new orientation (Fig. 4k), and thereafter became stable, such that on subsequent trials they reset to their new “remapped values” (Fig 4m). Although informative, the number of trials that could be analysed using this method (comparing two successive inter-trial intervals) was limited to 21% of the trials because of frequent insufficient sampling in the refuge (i.e., during short inter-trial intervals, the rats sometimes only turned in one direction and sampling was confined to ~180°). We therefore sought a second method to identify trials in which remapping occurred.
Identification of remapped sessions
If remapping only occurred once during a session (Fig. 4b), then we should be able to identify the sessions in which it occurred. Using the amount the PFD shifted during foraging trips (compared to the initial refuge value), we sought instances where there was a consistent bias in the distribution of PFD shifts across session trials. For example, if a cell's PFD remapped and shifted +70° compared to its initial refuge value after the first trial, then we should observe a consistent +70° shift in the cell's PFD for all foraging trips and inter-trial intervals following remapping (see examples in Fig. 4c: +50°, Fig. 4d: + 90°). To identify sessions showing this pattern, we examined the median PFD shift (to avoid the influence of outliers) for each session containing at least two trials (n = 51; see Suppl. Fig. 10 for similar results using the session mean PFD shift). The distribution of the session median shifts (Fig. 4e) revealed a clear bimodality in the values (mode 1 around 0°, mode 2 around 50°). Consistent with Figure 4a, this pattern indicated that in mode 1 sessions (median PFD shift < 35°) the cell's PFD drifted around the initial refuge value, but that in mode 2 sessions (median PFD shift > 35°) the cell's PFD consistently shifted by about ± 50° (e.g., some PFDs shifted > 90°). For comparison, the distribution of the session median heading errors (Fig. 4f) showed that behaviour could not account for these large and consistent shifts.
When does remapping occur?
For each session, we identified the moment when the PFD shifted using the session median shift and searched for the trial after which the PFD shift occurred consistently. In the examples shown (Fig. 4c–d), the blue dots represent the remapping trials (trial 1 in Fig. 4c, trial 2 in Fig. 4d). We then used the following inter-trial value (remapped refuge value) to test whether the remapped value was used as the new stable reference in the following trials. For trials following remapping, a much better correlation between PFD shift and heading error was obtained when using the remapped refuge value as a reference (Fig. 4g; n = 66, r = 0.70, P < 0.0001) than when using the initial refuge value (Fig. 4h; r = 0.52). This result confirms that after remapping occurs, a new `zero' point (the remapped refuge value) was set for the remainder of the session (Fig. 4m). Together with the inter-trial interval analyses above, these remapping trials explain the apparent discrepancy we observed between a good correlation when using the preceding inter-trial interval as reference (Fig. 2i) vs. a poorer correlation when using the initial refuge value (Fig. 2f).
What determines the remapped refuge value?
Did it result from a random shift in the PFD (Fig. 4k), or was there a reason that the cell remapped to a particular value? We addressed this issue using our two indicators of remapping: (1) the PFD shift between two successive inter-trial intervals, when available (n = 10), and (2) the session median shift (n = 26) to identify remapping trials. Both measures revealed a significant correlation with the animal's heading error in the trial preceding remapping (Fig. 5a, r = 0.70, P < 0.05; Fig. 5b, r = 0.73, P < 0.0001; see Suppl. Fig. 11, for the same results using the session mean shift). These results indicate that the remapped PFD was not arbitrary, but instead, was influenced by the animal's behaviour in the trial preceding remapping. In essence, this analysis suggests that the shifted PFD value that just lead to an error on trial n becomes the new reference point for the cell. This new PFD value is then maintained during the subsequent inter-trial interval in the refuge and remains the new referenced value for the next trial (n+1). An example of remapping is as follows: if a cell is initially tuned to 100° in the refuge, and drifts 60° CCW during the foraging trip, then the animal's return path will have a 60° CCW error (PFD = 160°), but importantly, the cell's PFD in the subsequent inter-trial interval will remain about 160° (see examples Fig. 6a–b and Suppl. Fig. 12).
Figure 5.
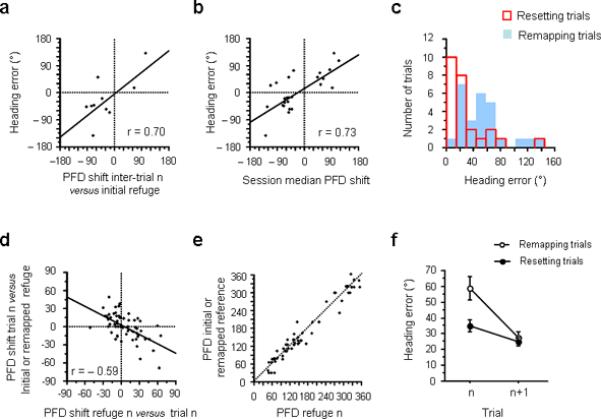
The correction process. a, Heading error as a function of the cell's PFD shift between the current inter-trial interval and the initial refuge (Mode 2 inter-trials). b, Heading error observed in remapping trials, as a function of the session median PFD shift compared to its initial refuge value. c, Distribution of heading errors for resetting (red) and remapping (blue) trials. d, PFD shift in trial n (compared to initial or remapped refuge value) as a function of the PFD shift between trial n and the following inter-trial interval (refuge n). e, PFD recorded during the inter-trial interval n compared to the initial or remapped refuge value. f, Comparison of heading errors made by the animals before (trial n) and after (trial n+1) remapping (white dots) and resetting (black dots).
Figure 6.
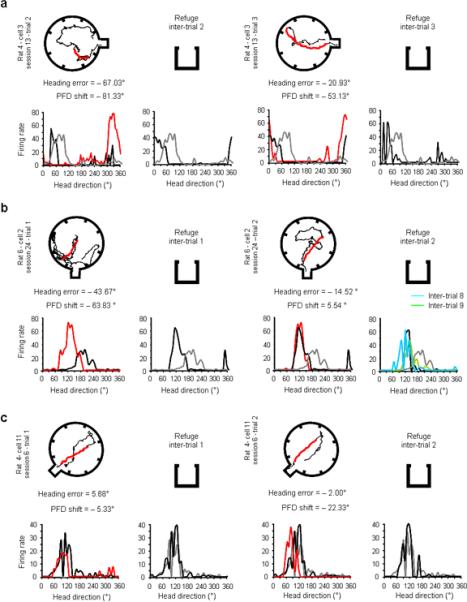
Examples of remapping (a–b) and resetting sessions (c). Each pair of rows depicts successive behavioural trials in a single session, along with the responses of a HD cell recorded during the trial. Similar to Figure 4, columns 1 and 3 show examples of foraging paths (outbound trips in black, return trips in red) above the firing rate vs. HD tuning curves recorded during the same trial (red trace), as well as the cell's response in the preceding refuge episode (black traces). Columns 2 and 4 show the cell's tuning curve during the inter-trial interval after the foraging trial (black traces). To illustrate the changes of the cell's PFD in the refuge after remapping, the HD cell's response during the initial refuge period is shown for all plots as the grey trace. Column 4 shows the cell's response during the following inter-trial interval(s) in the refuge after the foraging trial shown in column 3 (black trace). a, In this session, remapping occurs during trial 2. After shifting −81.33° during the second trial (compare red and black traces, column 1), the cell's PFD in the refuge during the next inter-trial interval (column 2) does not return to its initial refuge value (compare black and grey traces), but remains close to the value it had during the foraging trip (in this case trial 2). Note that in the following foraging trip (column 3) the shift of the cell's PFD to the remapped refuge value (black trace) gives a better prediction of behaviour than the PFD shift calculated using the initial refuge value (grey trace). Also, note that in the inter-trial interval following trial 3, the cell's PFD returns to this remapped refuge value (column 4 plot). Thus, the cell's PFD is reset to the new established reference. b, Remapping: in this example remapping occurs on trial 1 (column 1). In the following inter-trial interval (column 2), instead of returning to the initial refuge value (grey trace), the cell's PFD (see black trace) is closely aligned to the value recorded in the previous foraging trip (trial 1). As in the previous example, after remapping occurred, the cell's PFD resets to this new value in the refuge after subsequent foraging trials (column 4 shows inter-trial intervals 2 in black, 8 in blue, and 9 in green), and shows that the cell's PFD maintained this new refuge value for the remainder of the session. Note that for this example the initial refuge period is the same as the previous inter-trial interval since column 1 depicts the first trial. c, Resetting: the cell's PFD remains stable across all inter-trial refuge periods.
Beyond the simple description of this phenomenon, two questions remain about remapping. First, what causes remapping? Does remapping follow large heading errors? Second, why does the cell remap to the PFD observed in the preceding foraging trip? Why would a PFD that just lead to an error be kept as the new reference for subsequent foraging trips? We observed (Fig. 5c) that remapping tended to follow larger heading errors (blue bars, remapping trials: 50.12 ± 6.58°, red bars, resetting trials: 29.67 ± 6.58°, t49 = 2.27, P < 0.05). However, no systematic prediction about whether the rat would remap or reset could be made solely based on the animal's behaviour. Notably, most of the remapped values (PFD shifts, Fig. 4e) were situated around ± 50°, which is approximately the space that separates two consecutive doors in the arena (45°), which suggests that remapping was influenced by the structure of the apparatus.
In sum, our data suggest that the remapped PFD results from (1) the state of the HD network in the preceding foraging trip (which in most cases lead to a large heading error) and (2) from the geometry of the apparatus. Further, it is noteworthy that the animal could reset its orientation upon return to the refuge, but apparently they ignored the cues in the refuge and resetting did not occur.
Resetting trials
Our analyses of successive refuge periods suggested that for most trials, the cells' PFDs remained stable from one refuge period to another (Fig. 4a). Even in sessions when remapping occurred the cells' PFDs tended to reset to the new reference point for the remainder of the session (Fig. 4h). Another way to verify if resetting occurred is to determine whether a shift in the PFD that occurred during a foraging trip was corrected when the animal reached the refuge. For example, if the PFD shifted +60° during an outbound trip (PFD trial n – PFD refuge n-1), would the PFD shift −60° in the following inter-trial interval in the refuge (PFD refuge n – PFD trial n)? We observed (Fig. 5d) that the amount the PFD shifted after a foraging trip was negatively correlated with the amount of shift observed during that same trial (compared to the initial or remapped value; n = 61, r = −0.59, P < 0.0001). Thus, after each foraging trip for non-remapping trials, the cell's PFD returned either to a stable refuge value or to the remapped refuge value. Another way of representing this resetting phenomenon (Fig. 5e) shows that the PFD in refuge n was very close to the initial/remapped refuge value (mean difference = ±17.31°, see Fig. 6c and Supplementary Fig. 13).
Behavioural benefit of remapping and resetting
We compared the effect of remapping and resetting on the animal's performance for the subsequent foraging trip after an error (Fig. 5f). A two-way ANOVA with repeated measures (trial × process type) revealed a global effect for trial (F1,76 = 24.34, P < 0.0001), process type (F1,76 = 7.59, P < 0.01), and an interaction between the two factors (F1,76 = 6.66, P < 0.05). But importantly, when the analyses were separated based on remapping vs. resetting, the animals improved their homing behaviour after both remapping (F1,19 = 18.83, P < 0.001) and resetting (F1,57 = 5.41, P < 0.05). Notably, performance reached a comparable level after both processes, with the mean heading errors after remapping and resetting = 27.72° and 25.15°, respectively (t76 = 0.52, P > 0.05). For comparison, recall that the mean heading errors prior to remapping and resetting were 50.12° and 29.67°, respectively. In sum, these results indicate that both processes tend to correct the animals' orientation after an error and improve their performance on the next trial.
Error trials and cell resetting
In theory, resetting could result from two different strategies: (1) the cell's PFD could reset upon return to the refuge, where the animals can use the features of this familiar location to correct its orientation, or (2) since despite their errors, the animals were able to find the refuge, it is possible that re-orientation occurs “on-line” on their way back to the refuge. Indeed, if the HD signal codes for the animal's behaviour, then the active re-orientation of the animal after reaching the apparatus edge and perceiving that it made an error should be coupled with a coherent shift in the cell's PFD.
When animals perceived they made an error by reaching the apparatus periphery without successfully attaining the refuge, they quickly turned and changed their trajectory. These turns were sometimes in the correct direction of the refuge (e.g., Fig. 3 row 1, examples 1, 3), but could also be in the wrong direction (e.g., Fig. 3, row 1 examples 2, 4). [Note that turning in the wrong direction provides evidence that the animals were unlikely using olfactory cues emanating from the refuge to guide their return.] Although there were a limited number of trials to analyse in which the animal made a sizeable heading error, and in which we also had sufficient sampling of different head directions during these short correction episodes, there were three trials that could be sufficiently analysed. We analysed the moment-to-moment changes of the cell's PFD (Figs. 7–8) during trials that the animal made an error and had to change course on its return trip. Besides the gradual drifting of the cell's PFD, which is apparent in both figures, these trials suggest that the animal can use both strategies. In the first example (Fig. 7a–b), the cell's PFD appeared to reset before the animal reached the refuge, while in the second example (Fig. 7c–d), the shifted PFD was maintained throughout the course correction and was reset only when the animal entered the refuge upon its return. Consistent with the second hypothesis, in a remapping trial (Fig. 8), the cell's PFD appears to change with the animal's orientation (see panel 3, the PFD shift reflects the animal's re-orientation in the wrong direction) until the cell's PFD remains stable around a new value. This new PFD value will not be reset in the refuge and will remain the `reference' for subsequent trials.
Figure 7.
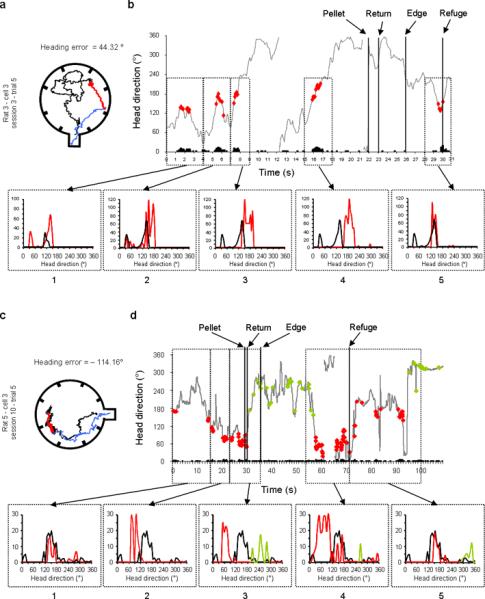
Moment-to-moment changes of the cell's PFD in two examples of resetting trials. For each example the foraging path is shown in the left panels (a, c), and the moment-to-moment HD of the animal coupled with cell activity (black bars) (Firing rate × HD × Time) is shown in the right panels (b, d). a & c, The animal's path has been divided into three parts: the outbound path is shown in black, the return path (from the pellet to the edge of the arena) is represented in red, and the correction path (from the apparatus edge to the refuge) is shown in blue. b & d, The red dots indicate the HD and time at which the cell's firing rate reached 50% of its maximum firing rate. Successive sampling episodes were isolated and the tuning curves for each episode (red trace) are compared with a reference (black trace). b, In panel 1 the cell's PFD at the beginning of the trial is compared to the only inter-trial value that could be recorded in that session (inter-trial value 2). In panels 2 to 4 the cell's PFD is compared to the PFD recorded at the beginning of the trial, and shows a slow drift counter-clockwise while the rat forages for the food pellet. Panel 5 shows the cell's PFD while the rat is correcting its orientation after reaching the wall of the arena (a, blue path) and presumably first perceives that it made an error. At that time, the PFD is reset to the value it had at the beginning of the trip (black). Notably, the cell does not fire when the rat is facing 170° (29th sec), which suggests that it was reset before reaching the refuge. This result would suggest that when the rats correct their homing direction (from apparatus edge to refuge), the cell's PFD is reset at the same time on their way to the refuge. d, Panels 1 to 3 show the cell's PFD drifting clockwise away from the preceding inter-trial value (black curve). Importantly, panel 4 shows that however the rat is correcting its error on its return to the refuge, the cell did not reset until the rat entered the refuge (panel 5). In this example, a second cell is visible with a PFD around 260° (green dots). Notably, these two cells are shifting in register: when the animal reaches the refuge, the first cell resets (red dots) and the PFD of the second cell (green dots) shifts approximately the same amount.
Figure 8.
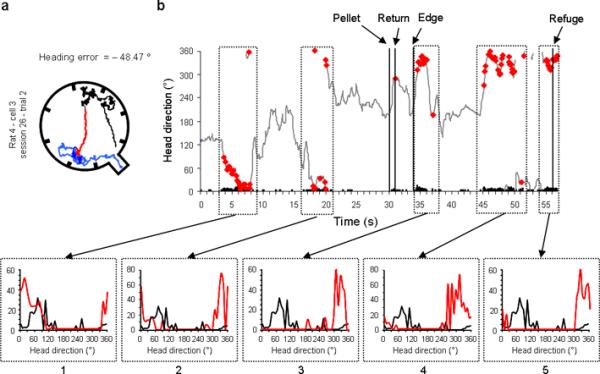
Moment-to-moment changes of the cell's PFD in an example of a remapping trial. a, Similar to Figure 7, the outbound path of the animal is shown in black, the return path in red, and the correction path in blue. b, Moment-to-moment HD of the animal coupled with the cell's activity (Firing rate × HD × Time). Similar to Figure 7, the tuning curves for the successively sampled episodes are represented below. Panels 1 and 2 show the cell's PFD drifting clockwise away from the value recorded in the preceding inter-trial value (black). The third panel depicts the cell's PFD after the animal reached the edge of the arena. Notably, in this trial and visible in a, the rat goes in the wrong direction (south-west door), while trying to find the refuge (located in south-east). The cell's PFD recorded at the same time (panel 3) reflects this error in the re-orientation process, by drifting farther away from its original value on the outbound foraging trip. Therefore, the re-orientation of the rat appears to be coupled with a predictable change in the cell's PFD. Panels 4 and 5 show a representative example of the remapping process, where the cell's PFD remains at the shifted value even after the rat returns to the refuge. In this case, however the rat manages to find the refuge, the cell's PFD is not reset when the animal reaches the refuge, but remains stable around 325°, which will be the new reference for subsequent trials.
DISCUSSION
The present study demonstrates that in a path integration task, the HD cells' PFD shifts are clearly correlated with the animal's behavioural trajectory. The apparent disconnection that is occasionally observed between the cells' PFD shifts and behaviour results from a remapping phenomenon, which appears to be one way the animals re-orient themselves after making an error. Indeed, our data provide evidence for two correction processes in order to avoid accumulation of errors and maintain accurate orientation: remapping and resetting. Most of the time the cells' PFDs reset, either to the initial or to the remapped refuge value, when the animal returns to the refuge, but we also observed that after large heading errors, the HD cells shift to a new PFD that becomes the new `zero value' for the following foraging trips.
What differentiates remapping from resetting? If resetting is the return of the cells' PFDs to their initial or remapped values, is it possible that remapping is simply the failure of the resetting process and we artificially separated them into two different processes? We feel this possibility is unlikely for two reasons. First, remapping was beneficial to the animals' performance, which suggests that remapping is more of an active correction process than an uncontrolled shift of the cell. Second, we observed that remapping was not a random shift, but rather the animal's new reference was influenced by two elements: the PFD observed in the preceding foraging trip and the geometric structure of the apparatus. Previous studies have shown that the geometry of the environment can drive both the HD signal25,26, and the animals' spatial behaviour27. In this case, our finding suggests that the animals re-oriented using external landmarks during remapping trials in order to establish a new bearing for the following trials.
This sequential use of internal and external information is necessary to perform path integration and it has been observed in several other species17,18,28. The use of fixes has usually been referred to as resetting29 and viewed as a process by which the path integrator adjusts to the perceived environment. Notably, a resetting phenomenon comparable to the one reported here, was observed in place cells30,31, and a recent report on grid cells could also be interpreted as containing home-based resetting32. Further, in place cells, two types of adjustment have been reported when idiothetic and external cues gave conflicting information30 - in cases of small mismatches between idiothetic and external cues, “the internal representation caught up with the real world coordinate”. But in addition to this smooth correction process, in cases of large mismatches the authors observed “abrupt shifts in the hippocampal representation”. These observations clearly parallel the two correction processes we describe in HD cells. Further, the observed remapping of the HD cell can be viewed as a result of a failure to reach the refuge using idiothetic cues. Indeed, it appears that after large errors and experiencing `misorientation', the animals established and maintained a new reference point - they set a new `zero' that anchored their current PFDs to the available landmarks.
The fact that the animals use external information is not what differentiates remapping from resetting. In both cases, the animals use a fix point to anchor their spatial representation - the refuge in the case of resetting versus some tactile or geometric features of the apparatus in the case of remapping. It is important to note that even if the HD signal is strongly influenced by self-motion information, it does not code for an egocentric representation of space, but rather for an allocentric one33. Therefore, what characterizes remapping is the fact that the allocentric-based representation of the initial refuge is abandoned, and that the animals establish a new reference, a new zero, which they maintain in the refuge and during subsequent trials. A key characteristic of remapping is the absence of resetting when the animals reach the refuge, suggesting that the spatial representation based on the initial refuge has been discarded and replaced by a new reference frame. Our data suggest that in this highly familiar environment, this new reference frame is influenced by the geometry of the apparatus, and in most occasions, consists of a simple rotation of the initial reference frame from 45° (one doorway). Similar to our results, this reference frame change could explain the apparent disconnection between behaviour and the HD signal observed in previous studies13,26.
How is the remapping process we observed related to the remapping phenomenon usually evoked for hippocampal place cells? Three types of remapping have been observed in place cells: (1) rotational remapping, when place cells keep their spatial characteristics and relationships, but all the place fields rotate together, (2) global remapping, which consists of a complete reorganization of the place code following significant changes in the environment34 or in task demand35, and (3) rate remapping, when place cells maintain their place field, but show firing rate changes based on subtle changes in a given environment36. A tight relationship between HD cells and place cells was observed in two studies where they were recorded simultaneously37,38. Further, these studies show that when idiothetic cues were in conflict with visual cues, place and HD cells showed rotational remapping until the conflicting information reached a difference of 45° - at which point global remapping occurred. These results parallel our observations and suggest that place cells would show rotational remapping in our resetting trials, but would show global remapping when we observed large PFD shifts (remapped trials).
Our results showing that animals used two different correction processes, presumably depending on the size of the most recent heading error, suggests that the HD system receives information feedback about the outcome of its current performance. Numerous studies exploring the neural processes involved in reward, particularly in the mesolimbic dopaminergic system39, have shown that the dopamine signal encodes for “prediction error”40. Is this signal somehow conveyed to the HD system? One possibility is via the lateral habenula, which projects to the dorsal tegmental nucleus, a key point in the HD circuitry41,42. Importantly, unexpected omission of an anticipated reward results in a transient cessation of dopaminergic activity in the ventral tegmental area (VTA)43, which is mediated by an indirect inhibitory projection from the lateral habenula→VTA44. In the food-carrying task, an error on the return trip could be viewed as an omitted reward, which would require a reconsideration of the animal's orientation. Alternatively, the error signal could be conveyed from the anterior cingulate cortex45,46 to the postsubiculum via the retrosplenial cortex. In agreement with this hypothesis some studies have shown that lesions of retrosplenial cortex induce impairments in the food carrying task47. Another possibility is that given the tight relationship between the hippocampus and the HD network37, remapping in the HD network results from remapping in the hippocampus. Indeed, the locus coeruleus, which is also activated by unexpected outcomes39, has been hypothesized to initiate remapping in the hippocampus48. Understanding what causes this switch in the reference frame, which permits re-orientation, as well as the brain areas involved, is critical for discerning how navigational errors are corrected.
METHODS
Subjects and apparatus
Seven food-deprived Long-Evans female rats (250–400 g) were placed on a food restricted diet (10–15 g/day) and trained in a food-carrying task, which involves searching for food in an open field and returning to a refuge to consume it21. The food-carrying task apparatus consisted of a large grey, circular (1.83 m diameter) open field with 12 black food cups placed uniformly around the surface (Fig. 1c). The open field was surrounded by a wall (38-cm high) containing eight uniformly distributed doorways, each separated by 45° with the ones adjacent to it. There was a refuge (29×30 cm) behind one doorway. The other seven doorways served as false refuges and were closed. All screening for HD cells took place in a grey cylinder (76 cm diameter, 51 cm high) containing a white cue card covering 110° of arc. All procedures involving the rats were performed in compliance with institutional standards as set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Behavioural procedure
Before being implanted with an electrode, the rats were trained in the food-carrying task. This task relies on the rat's proclivity to carry large food pellets back to a shelter for eating, rather than consuming them out in the open field21. The aim of this task is to test how accurately rats can return to the refuge (homing) after a random search for a food pellet when they are deprived of visual cues and must rely on self-motion cues and path integration for their homeward trip. Detailed training procedures have been described previously49. There were three training stages. In the first stage (3 days), rats were acclimated to the apparatus by placing them in the refuge for 10 min with the door closed and 10 food pellets in it. In the second stage, rats were trained in the task with visual cues available. In the third stage, rats were blindfolded and they had to complete the task without the use of visual cues. A typical session started with the rat placed in the refuge. Then the door was opened and the rat eventually left the refuge and went out into the open field to forage for large sugar pellets (750 mg), which were randomly placed in a food cup on the arena's floor. Once it found a food pellet, the rat had to bring it back to the refuge in order to consume it there. To aid in their motivation that the only safe way to consume the food was to bring it back to the refuge, the experimenter took the food pellet away from them whenever they tried to consume it in the arena. The homing accuracy of the animals was not assessed during the training phase. The behavioural criterion to go to the third stage was the completion of four trials (4 pellets brought back to the refuge) in < 10 min for two consecutive training sessions. The animals required 12.57 ± 0.68 sessions to complete this second training stage (range: 9 to 14 sessions). At the end of the first training stage, the rats were habituated to wear the blindfold: 20 min/day in their home cage for at least one week. Once habituated, and able to complete four trials in < 10 min with the visual cues available, the rats were trained to perform the task blindfolded50. The animals required 16.29 ± 1.21 training sessions to complete this third training phase (range: 12 to 22 sessions).
Surgery and Electrophysiological recordings
When proficient at performing the task, the animals were implanted with an array of recording electrodes just dorsal to the anterodorsal thalamic nucleus (1.5 mm posterior to bregma, 1.3 mm lateral to bregma and 3.7 mm ventral to the cortical surface). Following recovery (7 d), the activity on each of the 10 electrode wires was monitored while the animal foraged for food pellets thrown randomly inside the grey cylinder apparatus. When a cellular waveform was well isolated from the background noise (signal-to-noise ratio > 2:1) of an identified HD cell, a standard session (8 min) was recorded in the cylinder. Two light-emitting diodes (LEDs), spaced ~ 12 cm apart and positioned above the rats' head along its longitudinal axis, were used to indicate the rat's directional heading. Their positions were monitored at 60 Hz with a two-spot video tracking system. The firing rate of the cell in relation to the rat's HD was computed and analysed off-line (LabView, National Instruments, Austin, TX). If the cell was classified as directional (Rayleigh r > 0.4), the animal was blindfolded and underwent a disorientation procedure, which consisted of gently spinning them back-and-forth in an opaque cardboard box for about 1 min, while the experimenter walked around the periphery of the curtain and cylinder. The animal was then placed in the refuge area, and the cell was recorded for 6 min with the door closed. A second video camera was positioned above the refuge in order to track the rat's directional heading while in the refuge. In cases when there was an obvious shift in the cell's preferred firing direction (PFD) during the 6 min refuge session, the PFD value during the last minute was taken as the refuge value for the next session and used to calculate the PFD shift. Then the door of the refuge was opened and the rat was allowed to forage for food pellets for 10 min. Twelve food cups were randomly scattered on the floor of the apparatus away from the walls. The pellet locations were varied pseudo-randomly and only one cup was baited at a time. Animals typically completed 2 – 10 trials in a 10 min recording session.
HD cell analyses
The cell's PFD was analysed and plotted off-line (firing rate × head direction) for each recording condition (cylinder, refuge and arena). Analysis of directionality was assessed using the Rayleigh test. A cell was classified as a HD cell in the cylinder if the Raleigh r ≥ 0.4. All HD cells were then recorded in the food-foraging task. To determine the cell's mean PFD on each trial (foraging trip) or during inter-trial intervals, the mean firing direction was calculated from the firing rate vs. HD tuning function by determining the centre of mass (COM) using ± 8 bins (64°) on either side of the bin with the maximal firing rate. COM = [Σ(fri × αi)] / FR, where fri is the firing rate in the ith bin, αi is the angle of the ith bin, and FR is the sum of the firing rates in all bins. The PFD shift was defined as the difference in the cell's PFD between one episode and the previous episode (see details in Supplementary Fig. 5). On five occasions (5 sessions, 20 trials), two HD cells were recorded simultaneously during the task; the analyses of the coherence of these simultaneously recorded cell is shown in Supplementary Fig. 14. A complete excursion included both an outbound and an inbound trip. The outbound trip was defined as the animal's path from the refuge to the cup containing the food. The return trip was defined as the animal's path from the food cup to the wall of the apparatus, but not its path from the peripheral wall to the refuge when it made an error (Supplementary Fig. 3).
Behavioural analyses
The behavioural analyses (errors, mean HD of the return trip, number of turns) were measured offline using LabView software (Supplementary Fig. 3). A trial was counted as correct, if the rat reached the periphery of the arena between the two vertical barriers that framed the refuge (see Fig. 1c). For all analyses, clockwise heading errors and PFD shifts were defined as negative, counter-clockwise deviations were defined as positive. In some trials the rats returned to the refuge without having found the food pellet; these trials were included in the analyses when a clear return path was identified (n=64, see details and examples in Supplementary Figure 15).
Statistical analyses
We used unpaired Student t-tests to test for behavioural differences between correct vs. incorrect trials, and used a one-way ANOVA to test for within session affects of heading error and PFD shift. Correlations between different measures were computed using a Pearson correlation (Supplementary Fig. 16).
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Leaton, P. Trifieff, and E. Lesburgueres for their valuable comments on the manuscript. These experiments were supported by NIH grants DC009318 and NS053907.
Footnotes
AUTHOUR CONTRIBUTIONS S.V. and J.S.T. conceived and designed the study, discussed the findings, and wrote the manuscript. S.V. performed the experiments and analysed the results.
Bibliography
- 1.Darwin C. Origin of certain instincts. Nature. 1873;VII:417–418. [Google Scholar]
- 2.Mittelstaedt H, Mittelstaedt M-L. In: Avian Navigation. Papi/Wallraff, editor. Springer-Verlag; Berlin: 1982. [Google Scholar]
- 3.Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–92. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- 4.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 5.Ranck JB., Jr. Neuroscience, S. f., editor. 1984.
- 6.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–6. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 7.Muir GM, Taube JS. The neural correlates of navigation: do head direction and place cells guide spatial behavior? Behav Cogn Neurosci Rev. 2002;1:297–317. doi: 10.1177/1534582302238339. [DOI] [PubMed] [Google Scholar]
- 8.Save E, Poucet B. In: Hippocampal Place fields, Relevance to learning and memory. Mizumori SJ, editor. Oxford University Press; New-York: 2008. pp. 138–149. [Google Scholar]
- 9.Wiener SI, Taube JS, editors. Head Direction Cells and the Neural Mechanisms of Spatial Orientation. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- 10.Taube JS. The head direction signal: origins and sensory-motor integration. Ann Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- 11.McNaughton BL, Chen LL, Markus EJ. “Dead reckoning”, landmark learning, and the sense of direction: a neurphysiological and computational hypothesis. J Cogn Neurosci. 1991;3:190–202. doi: 10.1162/jocn.1991.3.2.190. [DOI] [PubMed] [Google Scholar]
- 12.Taube JS. Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol. 1998;55:225–56. doi: 10.1016/s0301-0082(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 13.van der Meer MA, Richmond Z, Braga RM, Wood ER, Dudchenko PA. Evidence for the use of an internal sense of direction in homing. Behav Neurosci. 2010;124:164–9. doi: 10.1037/a0018446. [DOI] [PubMed] [Google Scholar]
- 14.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the `cognitive map'. Nat Rev Neurosci. 2006;7:663–78. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 15.Hasselmo ME. Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus. 2008;18:1213–29. doi: 10.1002/hipo.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallistel CR. The organization of learning. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- 17.Souman JL, Frissen I, Sreenivasa MN, Ernst MO. Walking straight into circles. Curr Biol. 2009;19:1538–42. doi: 10.1016/j.cub.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 18.Etienne AS, Maurer R, Boulens V, Levy A, Rowe T. Resetting the path integrator: a basic condition for route-based navigation. J Exp Biol. 2004;207:1491–508. doi: 10.1242/jeb.00906. [DOI] [PubMed] [Google Scholar]
- 19.Knaden M, Wehner R. Ant navigation: resetting the path integrator. J Exp Biol. 2006;209:26–31. doi: 10.1242/jeb.01976. [DOI] [PubMed] [Google Scholar]
- 20.Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whishaw IQ, Coles BL, Bellerive CH. Food carrying: a new method for naturalistic studies of spontaneous and forced alternation. J Neurosci Methods. 1995;61:139–43. doi: 10.1016/0165-0270(95)00035-s. [DOI] [PubMed] [Google Scholar]
- 22.Etienne AS, Boulens V, Maurer R, Rowe T, Siegrist C. A brief view of known landmarks reorientates path integration in hamsters. Naturwissenschaften. 2000;87:494–8. doi: 10.1007/s001140050766. [DOI] [PubMed] [Google Scholar]
- 23.Goodridge JP, Taube JS. Preferential use of the landmark navigational system by head direction cells in rats. Behav Neurosci. 1995;109:49–61. doi: 10.1037//0735-7044.109.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS. Cue control and head direction cells. Behav Neurosci. 1998;112:749–61. doi: 10.1037//0735-7044.112.4.749. [DOI] [PubMed] [Google Scholar]
- 25.Clark BJ, Harris MJ, Taube JS. Control of anterodorsal thalamic head direction cells by environmental boundaries: Comparison with conflicting distal landmarks. Hippocampus. 2010 doi: 10.1002/hipo.20880. [DOI] [PubMed] [Google Scholar]
- 26.Golob EJ, Stackman RW, Wong AC, Taube JS. On the behavioral significance of head direction cells: neural and behavioral dynamics during spatial memory tasks. Behav Neurosci. 2001;115:285–304. [PubMed] [Google Scholar]
- 27.Cheng K. Whither geometry? Troubles of the geometric module. Trends Cogn Sci. 2008;12:355–61. doi: 10.1016/j.tics.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Steck K, Hansson BS, Knaden M. Desert ants benefit from combining visual and olfactory landmarks. J Exp Biol. 2011;214:1307–12. doi: 10.1242/jeb.053579. [DOI] [PubMed] [Google Scholar]
- 29.Collett TS, Graham P. Animal navigation: path integration, visual landmarks and cognitive maps. Curr Biol. 2004;14:R475–7. doi: 10.1016/j.cub.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Gothard KM, Skaggs WE, McNaughton BL. Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J Neurosci. 1996;16:8027–40. doi: 10.1523/JNEUROSCI.16-24-08027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci. 1996;16:823–35. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derdikman D, et al. Fragmentation of grid cell maps in a multicompartment environment. Nat Neurosci. 2009;12:1325–32. doi: 10.1038/nn.2396. [DOI] [PubMed] [Google Scholar]
- 33.Taube JS, Muller RU, Ranck JB., Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990;10:436–47. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–68. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markus EJ, et al. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–94. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leutgeb S, et al. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–23. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- 37.Knierim JJ, Kudrimoti HS, McNaughton BL. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J Neurophysiol. 1998;80:425–46. doi: 10.1152/jn.1998.80.1.425. [DOI] [PubMed] [Google Scholar]
- 38.Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci. 1995;15:1648–59. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz W, Dickinson A. Neuronal coding of prediction errors. Ann Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- 40.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 41.Sharp PE, Tinkelman A, Cho J. Angular velocity and head direction signals recorded from the dorsal tegmental nucleus of gudden in the rat: implications for path integration in the head direction cell circuit. Behav Neurosci. 2001;115:571–88. [PubMed] [Google Scholar]
- 42.Bassett JP, Taube JS. Neural correlates for angular head velocity in the rat dorsal tegmental nucleus. J Neurosci. 2001;21:5740–51. doi: 10.1523/JNEUROSCI.21-15-05740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–5. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 44.Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 519:1143–64. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–2. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 46.Michelet T, Bioulac B, Guehl D, Goillandeau M, Burbaud P. Single medial prefrontal neurons cope with error. PLoS One. 2009;4:e6240. doi: 10.1371/journal.pone.0006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whishaw IQ, Maaswinkel H, Gonzalez CL, Kolb B. Deficits in allothetic and idiothetic spatial behavior in rats with posterior cingulate cortex lesions. Behav Brainv Res. 2001;118:67–76. doi: 10.1016/s0166-4328(00)00312-0. [DOI] [PubMed] [Google Scholar]
- 48.Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–82. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Frohardt RJ, Bassett JP, Taube JS. Path integration and lesions within the head direction cell circuit: comparison between the roles of the anterodorsal thalamus and dorsal tegmental nucleus. Behav Neurosci. 2006;120:135–49. doi: 10.1037/0735-7044.120.1.135. [DOI] [PubMed] [Google Scholar]
- 50.Whishaw IQ, Maaswinkel H. Rats with fimbria-fornix lesions are impaired in path integration: a role for the hippocampus in “sense of direction”. J Neurosci. 1998;18:3050–8. doi: 10.1523/JNEUROSCI.18-08-03050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


