Abstract
Animal studies have found that deficits in brain docosahexaenoic acid (DHA, 22:6n-3) accrual during perinatal development leads to transient and enduring abnormalities in brain development and function. Determining the relevance of this evidence to brain disorders in humans has been hampered by an inability to determine antimortem brain DHA levels and limitations associated with a postmortem approach. Accordingly, there is a need for alternate or complementary apsroaches to better understand the role of DHA in cortical function and pathology, and conventional magnetic resonance imaging (MRI) techniques may be ideally suited for this application. A major advantage of neuroimaging is that it permits prospective evaluation of the effects of manipulating DHA status on both clinical and neuroimaging variables. Emerging evidence from MRI studies suggest that greater DHA status is associated with cortical structural and functional integrity, and suggest that reduced DHA status and abnormalities in cortical function observed in psychiatric disorders may be interrelated phenomenon. Preliminary evidence from animal MRI studies support a critical role of DHA in brain maturation. Neuroimaging research in both human and animals therefore holds tremendous promise for developing a better understanding of the role of DHA status in cortical function, as well as for elucidating the impact of DHA deficiency on neuropathological processes implicated in the etiology and progression of neurodevelopmental and psychiatric disorders.
Keywords: Omega-3 fatty acids, Docosahexaenoic acid (DHA), Gray matter, White matter, Magnetic resonance imaging, Magnetic resonance spectroscopy, Diffusion tensor imaging
1. Introduction
Mammalian brain tissue is predominantly composed of lipids (60–65% of brain dry weight) which are comprised of saturated, monounsaturated, and polyunsaturated fatty acids. The principle omega-3 polyunsaturated fatty acid in mammalian brain is docosahexaenoic acid (DHA, 22:6n-3), which comprises approximately 10–20% of gray matter, and approximately 2% of white matter, fatty acid composition depending on brain region, age, and habitual dietary omega-3 fatty acid intake [1–6]. Although omega-3 fatty acid precursors of DHA, including α-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA< 20:5n-3), and docosapentaenoic acid (22:5n-3), cross the blood-brain barrier, they are rapidly β-oxidized [7,8] and consequently comprise <1% of total brain fatty acid composition [1–6]. Mammals require a dietary source of omega-3 fatty acids to procure and maintain adequate concentrations of DHA in peripheral and central tissues, and healthy adult human exhibit limited or negligible ALA→EPA, ALA→DHA, and EPA→DHA biosynthesis [9]. However, preformed DHA can be obtained directly from the diet, particularly from fatty cold water fish or fish oil supplements [10], and preformed DHA is significantly more effective than ALA for increasing DHA levels in erythrocytes [11], breast milk [12,13], and brain gray matter [14].
Unesterified DHA rapidly diffuses from plasma to brain [15], and at a rate that is equilibrated with brain DHA consumption [16]. DHA preferentially accumulates in gray matter [16,17], and is enriched in synaptic and mitochondrial membranes [18]. DHA is acetylated into the sn-2 position of membrane phospholipids phosphatidylethanolamine and phosphatidylserine [19], and is mobilized preferentially by the calcium-independent phospholipase A2 (iPLA2) isoform [20]. It has been estimated that approximately 2–8% of rat brain DHA is replaced daily due to metabolism, and has a loss half-life in total rat brain phospholipids of 33 days under steady state ALA intake [21,22]. Dietary ALA insufficiency resulting in deficits in rat brain DHA composition are associated with a reduction in iPLA2 expression and activity [23], and an increase in the brain DHA half-life [21]. Preliminary estimates of the DHA half-life in human brain phospholipids are 2.5 years [17].
During perinatal rat brain development, cortical DHA concentrations increase sharply in parallel with active periods of neurogenesis, neuroblast migration, differentiation and synaptogenesis [1]. In human brain, DHA accumulates at a rapid rate initiating at approximately the third trimester in utero, and increases to approximately 9% of total cortical fatty acid composition in term-birth infants [24,25]. Infants born preterm exhibit lower postmortem cortical DHA concentrations relative to term infants maintained on the same ALA-fortified formula [25–28]. Non-human primates born preterm similarly exhibit lower postmortem brain DHA concentrations relative to term-born primates [29,30]. During human childhood and adolescence, there is a linear increase in postmortem frontal cortex DHA composition, which stabilizes at ~15% of total cortical fatty acid composition by ~20 years of age [4].
Preclinical studies have provided evidence that brain DHA accrual during perinatal maturation is required for normal neurotrophic factor expression, neurite outgrowth, neurogenesis and migration, neuronal differentiation and dendritic arborization, embryonic cortical plate expansion, nerve growth cone membrane signaling dynamics, and synaptogenesis and plasticity [31–42]. Moreover, early cortical DHA accrual during perinatal development is required for the normal functional maturation of multiple neurotransmitter systems, including dopamine, serotonin, and acetylcholine [43–45]. Behavioral studies have demonstrated that deficits in cortical DHA accrual during perinatal development are associated with enduring impairments in different cognitive tasks [46], and elevated indices of depression and aggression [47]. In addition to the demonstrated neurotrophic effects of DHA, emerging evidence suggests that DHA is protective against neuronal degenerative processes in response to a variety of excitotoxic insults [48–54], and increases resilience of axons and white matter in experimental injury and inflammation models [55–57].
While the importance of cortical DHA accrual in human brain development and function is poorly understood and controversial, a body of evidence suggests that higher maternal DHA status during and following pregnancy is associated with improved infant cognitive development, particularly in the realm of attention [58–65]. Moreover, a growing body of evidence suggests that deficits in attention during childhood frequently precede and predict the subsequent emergence of psychopathology in high-risk populations [66–70]. Importantly, the initial onset of psychiatric disorders, including attention deficit hyperactivity disorder (ADHD), schizophrenia, bipolar disorder, and major depressive disorder (MDD), most frequently occurs during childhood and adolescence [71–73]. It is relevant, therefore, that cross-sectional studies have repeatedly found that patients with ADHD [74–77], schizophrenia [78–80], bipolar disorder [81–83], and MDD [83–89] exhibit peripheral (plasma, erythrocyte) DHA deficits compared with healthy controls. Together, these data suggest that low DHA status may contribute to impaired development of brain circuits that mediate attention.
While the contribution of DHA deficiency to the progressive abnormalities in cortical structure and function observed in psychiatric patients is not known, investigations of the relationship between cortical DHA status and psychopathology have relied on case-control studies of postmortem brain tissue. Some postmortem brain studies have observed significant cortical DHA deficits in patients with psychiatric disorders [90–93] whereas others have not [94–97]. The discrepancy in these findings may be attributable in part to methodological challenges and limitations associated with this approach [98]. Nevertheless, evidence from primary and secondary intervention studies suggest that elevating DHA status through dietary supplementation is efficacious for preventing and/or treating psychopathology in adolescent [99–102] and adult [103–105] patients, and prospective longitudinal studies have found that lower baseline DHA status is a significant predictor of future suicidal attempts in medication-free MDD patients [106] and cytokine-induced MDD [107]. However, it is currently not known if central mechanisms mediate the psychopathogenic effects of low DHA status, and new approaches are required to more definitively elucidate such mechanisms.
2. Clinical MRI studies
Conventional magnetic resonance imaging (MRI) techniques may be well-suited to elucidate the role of DHA in human cortical function and pathology. Modern MRI techniques permit investigation of dynamic changes in cortical structure, chemistry, and functional activity, as well as associated changes in clinical symptoms and DHA status. Because cortical DHA status cannot be determined in living human subjects, peripheral indices (i.e., erythrocyte membrane DHA composition) may serve as a surrogate measure of DHA status. In human subjects, erythrocyte DHA levels provide a valid and reliable index of habitual DHA intake [108–111]. Additionally, non-human primate and human postmortem studies suggest that cortical and erythrocyte DHA levels are positively correlated under steady state dietary conditions [4,5], though erythrocyte DHA levels change more rapidly than brain cortex levels in response to changes in dietary omega-3 fatty acid intake.
Potential neuroimaging techniques available to evaluate the role of DHA status in psychopathology include: (1) structural MRI, which determines cortical and subcortical gray and white matter volumes, (2) diffusion tension imaging (DTI), which determines white matter structural integrity, (3) MRI T2 relaxometery which determines membrane water content as an index of fluidity, (4) functional magnetic resonance imaging (fMRI), which determines resting and task-elicited changes in cortical activation patterns, (5) proton magnetic resonance spectroscopy (1H MRS), which determines concentrations of different chemical markers associated with cortical metabolic integrity, and (6) phosphorous magnetic resonance spectroscopy (31P MRS), which determines chemical indices of phospholipid membrane turnover. Additionally, positron emission tomography (PET) can determine changes in multiple metabolic processes including cortical fatty acid incorporation and turnover rates and glucose metabolism. While these different imaging techniques have been used extensively in clinical and animal research, only recently have they been employed to investigate the role of DHA status on cortical structural and functional integrity.
The present review will focus on MRI studies investigating the role of DHA in neurodevelopmental and psychiatric disorders, and readers are referred to a separate review in this issue that is focused on the role of DHA in age-related neurodegenerative processes [112]. In the following sections, evidence for abnormalities in cortical structural and functional integrity in psychiatric disorders associated with DHA deficiency is briefly reviewed, and evidence from MRI studies investigating relationships with peripheral indices of DHA status and/or the effects of long-chain omega-3 fatty acid supplementation on MRI outcomes are presented.
2.1. Structural magnetic resonance imaging
Longitudinal and cross-sectional structural MRI studies have begun to characterize gray and white matter maturational patterns in typically developing youth [113]. The childhood and adolescent period is associated with dynamic changes in both regressive (synaptic pruning) and progressive (i.e., myelination) cellular events. Longitudinal structural MRI studies have found that the period between childhood and early adolescence (7–12 years) is associated with a rapid expansion of cortical gray matter density, whereas the period between adolescence (13–18 years) and young adulthood (≥ 8 years) is associated with a progressive loss of cortical gray matter density, which stabilizes in the third decade of life [114]. These age-related changes in cortical volume are sexually dimorphic, peaking later in males than females [115], and are governed by both genetic and environmental factors [116,117]. Postmortem human and non-human primate histological studies suggest that the decrease in cortical gray matter volume during adolescence is attributable in part to reductions in synaptic density rather than neuronal loss [118–121]. Frontal gray matter density loss is associated with reciprocal increases in white matter density in fiber tracts including frontotemporal pathways [122–123], the expansion of which is positively correlated with cognitive development (i.e., performance on working memory tasks)[124].
Although the effect of deficits in prenatal brain DHA accrual on human neuroanatomical trajectories are not known, structural MRI studies have found that children/adolescents born preterm, which is associated with early deficits in cortical DHA accrual, exhibit significant reductions in regional cortical and striatal gray matter volumes, reduced amygdala and hippocampal volumes, reduced corpus callosum and white matter volumes, and larger cerebral ventricles compared with age- and sex-matched term born controls [125]. Importantly, a placebo-controlled structural MRI study found that postnatal DHA supplementation did not significantly alter age-related changes in white matter volume in premature infants [126]. It is also relevant that preterm children are at increased risk for developing ADHD [127–131], and structural MRI studies have found that ADHD children exhibit patterns of cortical gray and white matter volume deficits similar to those observed in preterm children [132].
Emerging evidence from cross-sectional structural MRI studies suggest that patients with MDD exhibit lateral ventricle enlargement and smaller volumes of the basal ganglia, thalamus, hippocampus, frontal lobe, cingulate cortex, and orbitofrontal cortex compared with healthy controls [133]. Bipolar disorder is associated with increased volumes in lateral ventricles, temporal lobe, and putamen [134] as well as progressive frontal white matter pathology [135–138]. Moreover, reductions in amygdala volume have consistently been observed in pediatric and adolescent, but not adult, bipolar patients [139], and chronic lithium exposure is associated with increased hippocampal and amygdala volumes compared with unmedicated patients and healthy controls [134]. A longitudinal structural MRI study further suggests that bipolar disorder is associated with accelerated loss in gray matter volume in subregions of the prefrontal cortex during adolescence compared with typically developing controls [140]. Longitudinal structural MRI studies have also found that schizophrenic patients exhibit greater decreases over time in whole brain volume, whole brain gray matter, frontal gray and white matter, parietal white matter, and temporal white matter volume, as well as greater increases in lateral ventricular volume, compared with healthy controls [141]. Postmortem histological studies suggest that the deficits in gray matter volume observed in patients with psychiatric disorders is attributable in part to lamina-specific reductions in neuronal size and density, dendritic arborization, and/or dendritic spine density rather than neuronal loss [142–149].
Whether these region-specific deficits in gray and white matter volumes observed in psychiatric patients by MRI are associated with lower DHA status, or are preventable or reversible with adequate dietary DHA supplementation, is not known. However, prospective structural MRI is ideally suited to evaluate this relationship. For example, patients with generalized peroxisomal disorders exhibit significant erythrocyte and postmortem cortex DHA deficits [150] and impaired central myelinogenesis [151], and a preliminary structural MRI study found that treatment with DHA ethyl ester (100–600 mg/d) normalized or significantly improved brain white matter volumes in peroxisomal disorder patients [152]. A second preliminary structural MRI study found that greater habitual intake of long-chain omega-3 fatty acids, which are positively correlated with erythrocyte DHA composition [108–111], was associated with larger gray matter volumes in the anterior cingulate cortex, the right hippocampus, and the right amygdala [153]. In a prospective randomized placebo-controlled structural MRI trial, chronic (1 year) treatment with ethyl-EPA was found to slow volume loss in the caudate and thalamus of patients with Huntington's disease [154]. Together, these preliminary MRI findings suggest that DHA status is positively associated with regional gray and white matter volumes.
Neuronal membrane fluidity can also be evaluated using MRI by investigating changes in brain water proton transverse relaxation times (T2). A preliminary T2 MRI study found that four week treatment with EPA+DHA decreased whole brain T2 relaxation time in bipolar patients compared with healthy controls, and was interpreted to reflect an increase cortical membrane fluidity [155]. In a second study, 12-week ethyl-EPA treatment prevented progressive increases in hippocampal T2 relaxation time observed in placebo-treated first-episode psychosis patients, and reductions in negative symptom severity were associated with smaller increases in T2 relaxation time [156].
2.2. Diffusion tension imaging (DTI)
As discussed, white matter abnormalities are one of the most consistently reported neuroimaging findings in psychiatric disorders, and preclinical evidence suggests that DHA increases myelin resilience to inflammation and injury. Complimenting MRI determinations of white matter volumes, DTI additionally permits investigation of white matter structural integrity as indexed by fractional anisotropy. A reduction in fractional anisotropy is indicative of loss of white matter integrity, and has been found to be correlated with inter-regional functional connectivity. For example, deficits in functional prefrontal-amygdala connectivity in bipolar patients are correlated with reduced fractional anisotropy in the uncinate fasciculus [157], the principal white matter axonal bundle connecting prefrontal regions with anterior temporal lobe structures [158–160]. While the relationship between DHA status and DTI measures of white matter integrity are not known, a preliminary DTI study (n=12) observed a positive correlation between total plasma polyunsaturated fatty acid concentrations and fractional anisotropy in the uncinate fasciculus white matter tract in medicated psychotic patients [161].
2.3. Functional magnetic resonance imaging
Functional magnetic resonance imaging (fMRI) determines relative changes in blood oxygen level-dependent (BOLD) activity. Increases in BOLD signal are attributable to greater oxygen-dependent synaptic activity and associated increases in local blood volume [162], and reductions in BOLD signal are thought to reflect a suppression of neural activity and shunting of blood from less active to more active regions [163]. fMRI studies can be performed during resting state or during performance of cognitive tasks designed to activate specific brain regions of interest, including the identical pairs continuous performance task. In general, evidence from fMRI studies suggest that psychiatric disorders including MDD and bipolar disorder are associated with greater resting activity in the prefrontal cortex, deficits in task-elicited prefrontal activation, and greater amygdala activation during both resting and active states using different tasks [164–169].
In a dose-ranging controlled fMRI trial, our group investigated the effects of 8-week DHA supplementation on functional cortical activity during performance of sustained attention task (CPT-IP) in healthy male children (8–10 years) [170]. At 8 weeks, erythrocyte membrane DHA composition increased significantly from baseline in subjects receiving low-dose (+47%) and high-dose DHA (+70%), but not placebo (−11%). During sustained attention, both DHA dose groups exhibited significantly greater change from baseline in the activation of dorsolateral prefrontal cortex (DLPFC, BA9), and low-dose and high-dose DHA groups exhibited greater decreases in the occipital cortex and cerebellar cortex, respectively, relative to placebo. Relative to low-dose DHA, high-dose DHA resulted in greater decreases in activation of bilateral cerebellum. Using a less stringent statistical threshold, greater decreases in temporal lobe and cerebellar cortices were observed in the high-dose DHA group compared with placebo. Among all subjects, erythrocyte DHA composition was positively correlated with DLPFC activation at baseline and endpoint. This fMRI study therefore provides proof-of-concept evidence that increasing dietary DHA intake alters functional activity in cortical attention networks in healthy male children. It is relevant, therefore, that mediation-naïve pediatric ADHD patients exhibited reduced prefrontal cortex activation and greater cerebellar cortex activation while performing a sustained attention task [171], a pattern that is opposite to that observed in healthy children receiving DHA supplmentation.
Prior case-control studies have found that MDD patients exhibit DHA deficits in erythrocytes [89] and postmortem prefrontal cortex [91], and EPA+DHA supplementation increases erythrocyte DHA levels and decrease mood symptom severity [100–102]. In view of these findings, our group conducted a prospective 10-week open-label supplementation trial to evaluate the effects of two doses of fish oil (EPA+DHA) (2.4 or 15 g/d) on functional cortical activity patterns in adolescent (10–18 years) MDD patients during performance of a sustained attention task (CPT-IP) [172]. We found that adolescent MDD patients exhibited significantly lower erythrocyte and plasma DHA levels compared with a nested healthy adolescent control group. At baseline, MDD patient erythrocyte DHA composition was positively correlated with functional activation of the prefrontal cortex and anterior cingulate cortex during sustained attention (Fig. 1). Fish oil supplementation significantly increased erythrocyte DHA composition in low- and high-dose groups, and baseline depression symptom severity scores declined significantly in both dose groups. However, we did not observe any significant baseline-endpoint changes in functional activity in either low-dose or high-dose groups, or when both groups were combined. These preliminary fMRI findings suggest that DHA status of adolescent MDD patients is positively associated with functional activity in the prefrontal cortex, and that increasing DHA status reduces depression symptom severity independent of changes in functional cortical activity.
Fig. 1.
Statistical parametric map illustrating the relationship between erythrocyte DHA composition and regional activation patterns during performance of the CPT-IP task at baseline in adolescent MDD patients (n=21). Erythroycte DHA composition is positively correlated with functional activation clustered in the left medial frontal gyrus (BA9, dorsolateral prefrontal cortex) and left anterior cingulate cortex during sustained attention. Significant correlations were defined as an r-value equivalent to p≤0.05 (corrected).
2.4. Proton magnetic resonance spectroscopy (1H MRS)
Proton magnetic resonance spectroscopy (1H MRS) is a voxel-based technique that determines cortical concentrations of different compounds including glutamine/glutamate/γ-aminobutyric acid (Glx), myo-inositol (mI), and N-acetyl aspartate (NAA). The Glx peak detected by 1H MRS is primarily composed of neuronal and astrocyte glutamine, a glutamate precursor, and glutamate. NAA is primarily localized to neurons [173,174] and is positively correlated with mitochondrial metabolism [175,176], and cortical NAA concentrations decrease following excitotoxic injury [177–179]. mI is a product of Gαq-coupled receptor-generated phosphoinositide biosynthesis [180], and is a carbohydrate metabolized from glucose via 1L-myo-inositol 1-phosphate synthase that is predominantly concentrated in astroctyes [173,174]. The mI peak detectable by 1H MRS likely reflects this astrocyte mI pool.
In general, case-control 1H MRS studies have observed increased Glx concentrations in the prefrontal cortex of patients with bipolar disorder and schizophrenia, and reduced prefrontal Glx concentrations in patients with MDD [181,182]. 1H MRS studies have observed decreased NAA concentrations in the prefrontal cortex of patients with bipolar disorder [183–185] and decreased NAA in medial temporal lobe structures of schizophrenic patients [181]. Postmortem studies have observed lower mI concentrations in the prefrontal cortex of patients with unipolar or bipolar depression [186], and some, but not all, 1H MRS studies have observed reduced mI concentrations in the prefrontal cortex of patients with mood disorders [187–190].
A preliminary placebo-controlled 1H MRS study found that 12-week supplementation with ethyl-EPA selectively increased NAA concentrations in the anterior cingulate cortex of medicated patients with bipolar disorder [191]. A second placebo-controlled 1H MRS study found that 12-week ethyl-EPA supplementation increased Glx concentrations in the temporal lobes of first-episode psychotic patients [192]. A third placebo-controlled, dose-ranging, multi-voxel 1H MRS study, found that 8-week DHA supplementation did not significantly alter any chemical peak including mI, Glx, NAA in the right or left dorsolateral prefrontal cortex (BA9) and anterior cingulate gyrus of healthy male children (8–10 years) [McNamara et al., unpublished data].
2.5. Phosphorous magnetic resonance spectroscopy (31P MRS)
31P MRS permits central determination of concentrations of phosphorus-containing metabolites, including phospholipid anabolites (i.e., phosphomonoesters, PME) and catabolites (i.e., phosphodiesters, PDE). Reductions in the PME:PDE ratio, secondary to either elevations in PDE or reductions in PME, are thought to reflect a decrease in the synthesis and/or an increase in the breakdown of membrane phospholipids. 31P MRS studies have repeatedly observed significant reductions in regional brain PME and concomitant elevations in PDE in the brains of schizophrenic patients [193]. Abnormalities in 31P MRS measures of membrane phospholipid metabolism have been observed in medication-naïve first-episode psychosis patients [194–196], and are correlated with both symptom severity [197] and ventricle-to-brain ratio [198]. A meta-analyses of 31P MRS studies in patients with bipolar disorder found that PME levels are lower in euthymic patients, and are higher in depressed patients, compared with healthy controls [199].
A preliminary 31P MRS study found that cortical PDE levels were correlated with erythrocyte DHA and EPA in healthy subjects [200]. A second study found that erythrocyte total polyunsaturated fatty acids and arachidonic acid (20:4n-6) were positively correlated with bilateral prefrontal cortex PME levels, and linoleic acid (18:2n-6) was positively correlated with PDE levels, in neuroleptic-naïve first-episode psychosis patients [201]. In this study, these fatty acids were not correlated with PME levels in other regions including the basal ganglia, occipital, inferior parietal, or superior temporal cortex. Lastly, a case study found that 6 month treatment with ethyl-EPA resulted in the normalization of brain membrane phospholipid metabolism in a patient with schizophrenia [202].
2.6. Positron emission tomography (PET)
A pioneering PET study using radiolabeled DHA ([1-11C]DHA) investigated incorporation and turnover rates in healthy adult human subjects [17]. This study found that DHA incorporation was greater in gray versus white matter regions, and was positively correlated with regional cerebral blood flow. A second PET study using radiolabeled glucose ([18F]-fluoro-2-deoxyglucose) evaluated the relationship between plasma DHA composition and resting state cerebral glucose metabolism in adult medication-free MDD patients [203]. Plasma DHA composition was positively correlated with glucose metabolism in the temporoparietal cortex, and negatively correlated with glucose metabolism in prefrontal cortex and anterior cingulate cortex. This study also found that plasma EPA (20:5n-3) levels were not significantly correlated with regional cerebral glucose metabolism. These preliminary data suggest that DHA status is correlated with regional cortical glucose metabolic activity, and that DHA status may have opposing effects on resting activity in prefrontal and temporal cortices.
3. Animal MRI findings
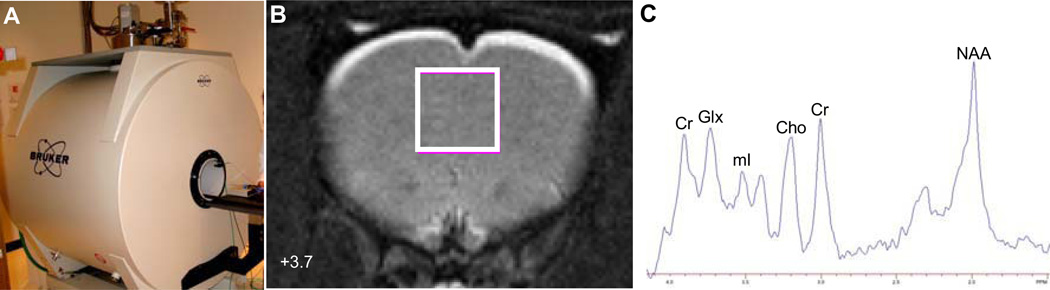
Because it is not possible to directly investigate cortical gray matter DHA composition in living human subjects, analogous MRI techniques are available for rodents so that brain DHA status can be manipulated and quantitated. A rodent 7T Bruker Biospec MRI system is presented in Figure 2A. Moreover, other postmortem brain variables can be directly quantified, including histology (i.e., spine density), gene expression, and neurochemical concentrations to evaluate relationships with MRI measures. Additionally, pharmacological MRI (phMRI) can be used to evaluate dynamic changes in cortical activation patterns following pharmacological challenge [204], and seed-region analysis can be performed to measure functional connectivity between different brain regions [205]. Rodent MRI is therefore well-suited for extending and interpreting clinical MRI findings.
Fig. 2.
The 7T Bruker Biospec rodent imaging system (A), localization of a 1H MRS voxel in rat bilateral medial prefrontal cortex in the coronal view (+3.7 cm anterior of Bregma)(B), and a representative 1H MRS spectrum from a control rat medial prefrontal cortex (C). Cr, creatine; Glx, glutamate+glutamine; mI, myo-inositol; Cho, choline; NAA, N-acetyl aspartate.
To date there have been few studies investigating the effects of brain DHA deficits or enrichment on MRI outcomes. A structural MRI study did not find alterations in brain gray or white volumes in aged (>15 months) omega-3 fatty acid-deficient rats relative to aged controls [206]. In a structural MRI study, our group recently found that young adult rats subjected to deficits in cortical DHA accrual during postnatal development exhibited elevations in volume in prefrontal cortex, nucleus accumbens, and amygdala in young adulthood compared with controls [207]. In an 1H MRS study, our group investigated the effects of perinatal deficits in DHA accrual on Glx, mI, and NAA concentrations in rat medial prefrontal cortex. The position of the voxel in the rat medial prefrontal cortex and a representative 1H MRS spectrum is presented in Figure 2. We found that perinatal, but not postnatal, deficits in brain DHA accrual were associated with reductions in baseline concentrations of mI, but not Glx or NAA, in the medial prefrontal cortex [208]. Additionally, acute treatment with SKF83959, a selective agonist at dopamine D1 phosphoinositide-coupled receptors, increased mI concentrations in the perinatal deficiency group but not in controls [208]. To date, there have been no animal 31P-MRS, phMRI, or DTI studies conducted to investigate membrane turnover, functional activity and connectivity, or white matter integrity, respectively, in DHA-deficient or DHA-enriched rat brain.
4. Discussion
Emerging evidence from both human and animal MRI studies are beginning to develop a clearer understanding of the role of DHA in cortical structure and function, and may provide critical insight into the contribution of DHA deficiency to the progressive neuropathological brain changes implicated in psychiatric disorders. An advantage of clinical MRI is that it permits prospective and concomitant evaluation of the effects of manipulating DHA status on both symptom severity and different neuroimaging variables to investigate potential central mediating mechanisms. A limitation associated with clinical MRI studies includes reliance on peripheral measures of DHA status which may not accurately reflect brain DHA levels [209]. However, emerging evidence suggests that plasma and/or erythrocyte DHA levels are correlated with functional cortical activity by fMRI [170], membrane phospholipid turnover by 31P MRS [200], and resting cortical glucose metabolism by PET [203]. Although rodent MRI studies permit systematic manipulation of brain DHA composition, rodent brain DHA deficiency models frequently produce large reductions in brain DHA levels which may not be clinically relevant. Another limitation of rodent MRI studies is the requirement for general anesthesia to obviate motion artifacts, and different general anesthetics have been found to alter blood blow dynamics [204] and neuronal morphology [210]. Nevertheless, combining evidence from both clinical and animal MRI approaches is anticipated to accelerate our understanding of the role of DHA in cortical structure and function.
Regarding future MRI studies, evaluation of the relationship between DHA status and functional brain maturation requires a prospective longitudinal study design. By analogy, prior prospective longitudinal MRI studies have found robust effects of psychostimulant medications on cortical developmetal trajectories in ADHD patients [211], and that semi-chronic lithium treatment increased prefrontal cortical volumes in bipolar patients responding to treatment [212]. Using this approach, it will be of considerable interest to determine whether increasing DHA status can normalize abnormlities in cortical gray and white matter volumes in psychiatric patients, and whether increasing DHA status can prevent or forestall progression of neuropathology in high-risk subjects. In an ongoing placebo-controlled trial (NCT00917501) we are evaluating whether 12-week EPA+DHA (fish oil) supplementation influences functional connectivity by fMRI and chemical indices of metabolic integrity by 1H MRS in medication-free adolescents at ultra high-risk for developing mania (i.e., they have a biological parent with bipolar disorder and are diagnosed with DSM-IV MDD).
An issue for future omega-3 fatty acid intervention trials is brain bioavailability. Although preformed DHA is effective for increasing cortical gray matter DHA levels [14], unesterified DHA, but not phospholipid-esterified DHA, diffuses from plasma to brain more efficiency [16]. By analogy, peripheral bioavailability of EPA+DHA from re-esterified triglycerides was found to have greater incorporation into peripheral membranes compared with natural fish oil [213]. Moreover, our preliminary fMRI data suggest that supplementation with triglyceride DHA [170], but not natural fish oil [172], increases functional prefrontal cortical activity. Future fMRI studies are therefore warranted to determine which DHA carriers are most effective for altering functional cortical activity. Moreover, EPA is rapidly oxidized following entry into brain [7], plasma EPA levels are not correlated with resting cortical glucose metabolism in MDD patients [199], and EPA→DHA biosynthesis is negligible in human subjects [9]. However, MRI studies have observed effects of ethyl-EPA supplementation on different aspects of cortical metabolic function [154,156,191,192,202], raising the possibility that peripheral actions of long-chain omega-3 fatty acids may also contribute to central changes observed by MRI.
In view of the clinical observation that patients with psychiatric disorders exhibit deficits in frontal white matter volume by structural MRI and reduced frontal white matter integrity by DTI, and preclinical evidence that increasing brain DHA status increases resilience of white matter to experimental injury and inflammation, it will be of considerable interest to determine in future studies whether increasing DHA status can reverse deficits in frontal white matter integrity in patients with psychiatric disorders. Furthermore, preclinical evidence that increasing brain DHA status increases dendritic spine density [41], and it will be of interest to determine whether DHA supplementation can alter developmental trajectories in human gray matter volume and associated cognitive function in typically developing youth, as well as slow accelerated gray matter atrophy observed in patients with psychiatric disorders.
These preliminary observations support a role for MRI research to develop a clearer understanding of the role of DHA in cortical functional maturation as well as for elucidating the contribution of DHA deficiency to neuropathological processes implicated in the pathoetiology of psychiatric disorders. While human and animal MRI techniques both have unique limitations, combining these approaches is anticipated to accelerate our understanding of the role of DHA in cortical structure and function as well as guide the optimization of treatment and prevention strategies.
Acknowledgments
This work was supported in part by National Institute of Health grants MH083924 and AG03617, and a NARSAD Independent Investigator Award to R.K.M. R.K.M. has received research support from Martek Biosciences Inc, Inflammation Research Foundation, Ortho-McNeil Janssen, AstraZeneca, Eli Lilly, and is a member of the Inflammation Research Foundation scientific advisory board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids. 1996;31:859–865. doi: 10.1007/BF02522981. [DOI] [PubMed] [Google Scholar]
- 2.Xiao Y, Huang Y, Chen ZY. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br. J. Nutr. 2005;94:544–550. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]
- 3.McNamara RK, Carlson SE. Role of omega-fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 5.Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J. Lipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- 6.Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CT, Liu Z, Bazinet RP. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. J Neurochem. 2011;116:363–373. doi: 10.1111/j.1471-4159.2010.07116.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80:157–163. doi: 10.1016/j.plefa.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. International Society for the Study of Fatty Acids and Lipids, ISSFAL. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 11.Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr. 2008;88:801–809. doi: 10.1093/ajcn/88.3.801. [DOI] [PubMed] [Google Scholar]
- 12.Francois CA, Connor SL, Bolewicz LC, Connor WE. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003;77:226–233. doi: 10.1093/ajcn/77.1.226. [DOI] [PubMed] [Google Scholar]
- 13.Jensen CL, Maude M, Anderson RE, Heird WC. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am J Clin Nutr. 2000;71:292–299. doi: 10.1093/ajcn/71.1.292s. [DOI] [PubMed] [Google Scholar]
- 14.Su HM, Bernardo L, Mirmiran M, Ma XH, Corso TN, Nathanielsz PW, Brenna JT. Bioequivalence of dietary alpha-linolenic and docosahexaenoic acids as sources of docosahexaenoate accretion in brain and associated organs of neonatal baboons. Pediatr Res. 1999;45:87–93. doi: 10.1203/00006450-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Ouellet M, Emond V, Chen CT, Julien C, Bourasset F, Oddo S, LaFerla F, Bazinet RP, Calon F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the bloodbrain barrier: An in situ cerebral perfusion study. Neurochem Int. 2009;55:476–482. doi: 10.1016/j.neuint.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Rapoport SI, Ramadan E, Basselin M. Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain DHA metabolism and neurotransmission. Prostaglandins Other Lipid Mediat. 2011;96:109–113. doi: 10.1016/j.prostaglandins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umhau JC, Zhou W, Carson RE, Rapoport SI, Polozova A, Demar J, Hussein N, Bhattacharjee AK, Ma K, Esposito G, Majchrzak S, Herscovitch P, Eckelman WC, Kurdziel KA, Salem N., Jr Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res. 2009;50:1259–1268. doi: 10.1194/jlr.M800530-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki H, Manabe S, Wada O, Crawford MA. Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int. J Vitam. Nutr. Res. 1997;67:272–278. [PubMed] [Google Scholar]
- 19.Lee CH, Hajra AK. Molecular species of diacylglycerols and phosphoglycerides and the postmortem changes in the molecular species of diacylglycerols in rat brains. J. Neurochem. 1991;56:370–379. doi: 10.1111/j.1471-4159.1991.tb08161.x. [DOI] [PubMed] [Google Scholar]
- 20.Farooqui AA, Horrocks LA. Brain phospholipases A2: a perspective on the history. Prostaglandins Leukot Essent Fatty Acids. 2004;71:161–169. doi: 10.1016/j.plefa.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 21.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J. Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 22.Rapoport SI, Chang MC, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J. Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- 23.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 24.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:131–138. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- 25.Martínez M, Mougan I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998;71:2528–2533. doi: 10.1046/j.1471-4159.1998.71062528.x. [DOI] [PubMed] [Google Scholar]
- 26.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 27.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340:810–813. doi: 10.1016/0140-6736(92)92684-8. [DOI] [PubMed] [Google Scholar]
- 28.Farquharson J, Jamieson EC, Abbasi KA, Patrick WJ, Logan RW, Cockburn F. Effect of diet on the fatty acid composition of the major phospholipids of infant cerebral cortex. Arch Dis Child. 1995;72:198–203. doi: 10.1136/adc.72.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkadi-Nagy E, Wijendran V, Diau GY, Chao AC, Hsieh AT, Turpeinen A, Nathanielsz PW, Brenna JT. The influence of prematurity and long chain polyunsaturate supplementation in 4-week adjusted age baboon neonate brain and related tissues. Pediatr. Res. 2003;54:244–252. doi: 10.1203/01.PDR.0000072795.38990.F2. [DOI] [PubMed] [Google Scholar]
- 30.Sarkadi-Nagy E, Wijendran V, Diau GY, Chao AC, Hsieh AT, Turpeinen A, Lawrence P, Nathanielsz PW, Brenna JT. Formula feeding potentiates docosahexaenoic and arachidonic acid biosynthesis in term and preterm baboon neonates. J. Lipid. Res. 2004;45:71–80. doi: 10.1194/jlr.M300106-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007;415:154–158. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Coti Bertrand P, O'Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136:1570–1575. doi: 10.1093/jn/136.6.1570. [DOI] [PubMed] [Google Scholar]
- 34.Yavin E, Himovichi E, Eilam R. Delayed cell migration in the developing rat brain following maternal omega 3 alpha linolenic acid dietary deficiency. Neuroscience. 2009;162:1011–1122. doi: 10.1016/j.neuroscience.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 36.Ikemoto A, Nitta A, Furukawa S, Ohishi M, Nakamura A, Fujii Y, Okuyama H. Dietary n-3 fatty acid deficiency decreases nerve growth factor content in rat hippocampus. Neurosci Lett. 2000;285:99–102. doi: 10.1016/s0304-3940(00)01035-1. [DOI] [PubMed] [Google Scholar]
- 3.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 38.Innis SM, de La Presa Owens S. Dietary fatty acid composition in pregnancy alters neurite membrane fatty acids and dopamine in newborn rat brain. J Nutr. 2001;131:118–122. doi: 10.1093/jn/131.1.118. [DOI] [PubMed] [Google Scholar]
- 39.Martin RE, Bazan NG. Changing fatty acid content of growth cone lipids prior to synaptogenesis. J Neurochem. 1992;59:318–325. doi: 10.1111/j.1471-4159.1992.tb08906.x. [DOI] [PubMed] [Google Scholar]
- 40.Ikemoto A, Kobayashi T, Watanabe S, Okuyama H. Membrane fatty acid modifications of PC12 cells by arachidonate or docosahexaenoate affect neurite outgrowth but not norepinephrine release. Neurochem Res. 1997;22:671–678. doi: 10.1023/a:1027393724676. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto T, Cansev M, Wurtman RJ. Oral supplementation with docosahexaenoic acid and uridine-5'-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 2007;1182:50–59. doi: 10.1016/j.brainres.2007.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 44.McNamara RK, Able J, Liu Y, Jandacek R, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: dissociation from estrogenic effects. J Psychiatr Res. 2009;43:656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aïd S, Vancassel S, Poumès-Ballihaut C, Chalon S, Guesnet P, Lavialle M. Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. J Lipid Res. 2003;44:1545–1551. doi: 10.1194/jlr.M300079-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Fedorova I, Salem N., Jr Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot Essent Fatty Acids. 2006;75:271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 47.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot Essent Fatty Acids. 2009;81:205–211. doi: 10.1016/j.plefa.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL, Raung SL, Wu CW, Chiang AN, Chen CJ. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem. 2009;20:715–725. doi: 10.1016/j.jnutbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Green P, Glozman S, Weiner L, Yavin E. Enhanced free radical scavenging and decreased lipid peroxidation in the rat fetal brain after treatment with ethyl docosahexaenoate. Biochim Biophys Acta. 2001;1532:203–212. doi: 10.1016/s1388-1981(01)00132-9. [DOI] [PubMed] [Google Scholar]
- 52.Högyes E, Nyakas C, Kiliaan A, Farkas T, Penke B, Luiten PG. Neuroprotective effect of developmental docosahexaenoic acid supplement against excitotoxic brain damage in infant rats. Neuroscience. 2003;119:999–1012. doi: 10.1016/s0306-4522(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 53.Ozyurt B, Sarsilmaz M, Akpolat N, Ozyurt H, Akyol O, Herken H, Kus I. The protective effects of omega-3 fatty acids against MK-801-induced neurotoxicity in prefrontal cortex of rat. Neurochem Int. 2007;50:196–202. doi: 10.1016/j.neuint.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 55.Bailes JE, Mills JD. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J Neurotrauma. 2010;27:1617–1624. doi: 10.1089/neu.2009.1239. [DOI] [PubMed] [Google Scholar]
- 56.Tuzun F, Kumral A, Dilek M, Ozbal S, Ergur B, Yesilirmak DC, Duman N, Yilmaz O, Ozkan H. Maternal omega-3 fatty acid supplementation protects against lipopolysaccharide-induced white matter injury in the neonatal rat brain. J Matern Fetal Neonatal Med. 2011 Sep 6; doi: 10.3109/14767058.2011.587917. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Ward RE, Huang W, Curran OE, Priestley JV, Michael-Titus AT. Docosahexaenoic acid prevents white matter damage after spinal cord injury. J Neurotrauma. 2010;27:1769–1780. doi: 10.1089/neu.2010.1348. [DOI] [PubMed] [Google Scholar]
- 58.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:39–44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 59.Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J Clin Nutr. 2002;76:608–613. doi: 10.1093/ajcn/76.3.608. [DOI] [PubMed] [Google Scholar]
- 60.Williams C, Birch EE, Emmett PM, Northstone K. Stereoacuity at age 3.5 y in children born full-term is associated with prenatal and postnatal dietary factors: a report from a population-based cohort study. Am J Clin Nutr. 2001;73:316–322. doi: 10.1093/ajcn/73.2.316. [DOI] [PubMed] [Google Scholar]
- 61.Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–1267. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 62.Innis SM. Polyunsaturated fatty acids in human milk: an essential role in infant development. Adv Exp Med Biol. 2004;554:27–43. doi: 10.1007/978-1-4757-4242-8_5. [DOI] [PubMed] [Google Scholar]
- 63.Westerberg AC, Schei R, Henriksen C, Smith L, Veierød MB, Drevon CA, Iversen PO. Attention among very low birth weight infants following early supplementation with docosahexaenoic and arachidonic acid. Acta Paediatr. 2011;100:47–52. doi: 10.1111/j.1651-2227.2010.01946.x. [DOI] [PubMed] [Google Scholar]
- 64.Daniels JL, Longnecker MP, Rowland AS, Golding J the ALSPAC Study Team. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394–402. doi: 10.1097/01.ede.0000129514.46451.ce. [DOI] [PubMed] [Google Scholar]
- 65.Jensen CL, Voigt RG, Llorente AM, Peters SU, Prager TC, Zou YL, Rozelle JC, Turcich MR, Fraley JK, Anderson RE, Heird WC. Effects of early maternal docosahexaenoic acid intake on neuropsychological status and visual acuity at five years of age of breast-fed term infants. J Pediatr. 2010;157:900–905. doi: 10.1016/j.jpeds.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Klein KL, Price JE, Faraone SV. Psychopathology in females with attention-deficit/hyperactivity disorder: A Controlled, five-year prospective study. Biol Psychiatry. 2006;60:1098–1105. doi: 10.1016/j.biopsych.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 67.Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, Adamo UH, Gottesman II. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 68.Keshavan MS, Sujata M, Mehra A, Montrose DM, Sweeney JA. Psychosis proneness and ADHD in young relatives of schizophrenia patients. Schizophr Res. 2003;59:85–92. doi: 10.1016/s0920-9964(01)00400-5. [DOI] [PubMed] [Google Scholar]
- 69.Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: a selective review and update. Schizophr Res. 2005;79:45–57. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Singh MK, DelBello MP, Kowatch RA, Strakowski SM. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8:710–720. doi: 10.1111/j.1399-5618.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 71.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 72.Perlis RH, Miyahara S, Marangell LB, et al. STEP-BD Investigators. Long-Term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) . Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 73.Chengappa KN, Kupfer DJ, Frank E, Houck PR, Grochocinski VJ, Cluss PA, Stapf DA. Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. Am J Psychiatry. 2003;160:1636–1642. doi: 10.1176/appi.ajp.160.9.1636. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell EA. Clinical characteristics and serum essential fatty acid levels in hyperactive children. Clin Pediatrics. 1987;26:406–411. doi: 10.1177/000992288702600805. [DOI] [PubMed] [Google Scholar]
- 75.Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, Burgess JR. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 76.Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem. 2004;15:467–472. doi: 10.1016/j.jnutbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Young GS, Maharaj NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids. 2004;39:117–123. doi: 10.1007/s11745-004-1209-3. [DOI] [PubMed] [Google Scholar]
- 78.Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 79.Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30:901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- 80.Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69:393–399. doi: 10.1016/j.plefa.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 81.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- 82.Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, Shen WW. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 83.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 86.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20: :5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 87.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 88.Riemer S, Maes M, Christophe A, Rief W. Lowered omega-3 PUFAs are related to major depression, but not to somatization syndrome. J Affect Disord. 2010;123:173–180. doi: 10.1016/j.jad.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 90.Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins Leukot Essent Fatty Acids. 2010;82:111–119. doi: 10.1016/j.plefa.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford K, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 92.McNamara RK, Jandacek R, Rider T, Tso P, Stanford K, Hahn C-G, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatric Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McNamara RK, Jandacek R, Rider T, Tso P, Richtand NM, Stanford K. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: Gender differences and partial normalization with antipsychotic medications. Schizophr Res. 2007;91:37–50. doi: 10.1016/j.schres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamazaki K, Choi KH, Kim HY. Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: no changes in docosahexaenoic acid species. J Psychiatr Res. 2010;44:688–693. doi: 10.1016/j.jpsychires.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Igarashi M, Ma K, Gao F, Kim HW, Greenstein D, Rapoport SI, Rao JS. Brain lipid concentrations in bipolar disorder. J Psychiatr Res. 2010;44:177–182. doi: 10.1016/j.jpsychires.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lalovic A, Levy E, Canetti L, Sequeira A, Montoudis A, Turecki G. Fatty acid composition in postmortem brains of people who completed suicide. J Psychiatry Neurosci. 2007;32:363–370. [PMC free article] [PubMed] [Google Scholar]
- 97.Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42:7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]
- 98.McNamara RK, Jandacek R. Investigation of postmortem brain polyunsaturated fatty acid composition in psychiatric disorders: Limitations, challenges, and future directions. J Psychiatry Res. 2011;45:44–46. doi: 10.1016/j.jpsychires.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 100.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 101.Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 102.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;162:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 103.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 104.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr, Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 105.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 106.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 107.Su KP, Huang SY, Peng CY, Lai HC, Huang CL, Chen YC, Aitchison KJ, Pariante CM. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alphainduced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67:550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fekete K, Marosvölgyi T, Jakobik V, Decsi T. Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: a systematic review. Am J Clin Nutr. 2009;89:2070–2084. doi: 10.3945/ajcn.2009.27230I. [DOI] [PubMed] [Google Scholar]
- 109.Kuratko CN, Salem N., Jr Biomarkers of DHA status. Prostaglandins Leukot Essent Fatty Acids. 2009;81:111–118. doi: 10.1016/j.plefa.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 110.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 111.Itomura M, Fujioka S, Hamazaki K, Kobayashi K, Nagasawa T, Sawazaki S, Kirihara Y, Hamazaki T. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–135. [PubMed] [Google Scholar]
- 112.Cunnane S, Chouinard-Watkins R, Castellano CA, Barberger-Gateau P. Docosahexaenoic acid homeostasis, brain aging and Alzheimer’s disease: Can we reconcile the evidence? Prostaglandins Leukot Essent Fatty Acids. 2012 doi: 10.1016/j.plefa.2012.04.006. [in press] [DOI] [PubMed] [Google Scholar]
- 113.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res Hum Genet. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 120.Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 121.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 122.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 123.Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 125.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 126.van Wezel-Meijler G, van der Knaap MS, Huisman J, Jonkman EJ, Valk J, Lafeber HN. Dietary supplementation of long-chain polyunsaturated fatty acids in preterm infants: effects on cerebral maturation. Acta Paediatr. 2002;91:942–950. doi: 10.1080/080352502760272632. [DOI] [PubMed] [Google Scholar]
- 127.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 128.Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38:931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 129.Cherkes-Julkowski M. Learning disability, attention-deficit disorder, and language impairment as outcomes of prematurity: a longitudinal descriptive study. J Learn Disabil. 1998;31:294–306. doi: 10.1177/002221949803100309. [DOI] [PubMed] [Google Scholar]
- 130.Cooke RW, Foulder-Hughes L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Arch Dis Child. 2003;88:482–487. doi: 10.1136/adc.88.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Foulder-Hughes LA, Cooke RW. Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med Child Neurol. 2003;45:97–103. [PubMed] [Google Scholar]
- 132.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 133.Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 134.Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, Kieseppä T, Altshuler LL, Fornito A, Malhi GS, McIntosh AM, Yurgelun-Todd DA, Labar KS, Sharma V, MacQueen GM, Murray RM, McDonald C. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry. 2011;69:326–335. doi: 10.1016/j.biopsych.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 135.Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163:322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 136.Beyer JL, Taylor WD, MacFall JR, Kuchibhatla M, Payne ME, Provenzale JM, Cassidy F, Krishnan KR. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30:2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 137.Mahon K, Wu J, Malhotra AK, Burdick KE, DeRosse P, Ardekani BA, Szeszko PR. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34:1590–1600. doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kafantaris V, Kingsley P, Ardekani B, Saito E, Lencz T, Lim K, Szeszko P. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:79–86. doi: 10.1097/CHI.0b013e3181900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1289–1298. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- 140.Kalmar JH, Wang F, Spencer L, Edmiston E, Lacadie CM, Martin A, Constable RT, Duncan JS, Staib LH, Papademetris X, Blumberg HP. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol Soc. 2009;15:476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 142.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 144.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 145.Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 146.Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- 147.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 148.Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005;7:358–369. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 149.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 150.Martinez M. Polyunsaturated fatty acids in the developing human brain, erythrocytes and plasma in peroxisomal disease: therapeutic implications. J Inherit Metab Dis. 1995;18:61–75. doi: 10.1007/BF00711429. [DOI] [PubMed] [Google Scholar]
- 151.Powers JM, Moser HW. Peroxisomal disorders: genotype, phenotype, major neuropathologic lesions, and pathogenesis. Brain Pathol. 1998;8:101–120. doi: 10.1111/j.1750-3639.1998.tb00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martinez M, Vazquez E. MRI evidence that docosahexaenoic acid ethyl ester improves myelination in generalized peroxisomal disorders. Neurology. 1998;51:26–32. doi: 10.1212/wnl.51.1.26. [DOI] [PubMed] [Google Scholar]
- 153.Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, Muldoon MF. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 154.Puri BK, Bydder GM, Manku MS, Clarke A, Waldman AD, Beckmann CF. Reduction in cerebral atrophy associated with ethyl-eicosapentaenoic acid treatment in patients with Huntington's disease. J Int Med Res. 2008;36:896–905. doi: 10.1177/147323000803600505. [DOI] [PubMed] [Google Scholar]
- 155.Hirashima F, Parow AM, Stoll AL, Demopulos CM, Damico KE, Rohan ML, Eskesen JG, Zuo CS, Cohen BM, Renshaw PF. Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am J Psychiatry. 2004;161:1922–1924. doi: 10.1176/ajp.161.10.1922. [DOI] [PubMed] [Google Scholar]
- 156.Wood SJ, Cocchi L, Proffitt TM, McConchie M, Jackson GD, Takahashi T, Pantelis C, McGorry PD, Berger GE. Neuroprotective effects of ethyl-eicosapentaenoic acid in first episode psychosis: a longitudinal T2 relaxometry pilot study. Psychiatry Res. 2010;182:180–182. doi: 10.1016/j.pscychresns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 157.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- 159.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York, Oxford: [Google Scholar]
- 160.Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 161.Peters BD, Duran M, Vlieger EJ, Majoie CB, den Heeten GJ, Linszen DH, de Haan L. Polyunsaturated fatty acids and brain white matter anisotropy in recent-onset schizophrenia: a preliminary study. Prostaglandins Leukot Essent Fatty Acids. 2009;81:61–63. doi: 10.1016/j.plefa.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 162.Hess A, Stiller D, Kaulisch T, Heil P, Scheich H. New insights into the hemodynamic blood oxygenation level-dependent response through combination of functional magnetic resonance imaging and optical recording in gerbil barrel cortex. J Neurosci. 2000;20:3328–3338. doi: 10.1523/JNEUROSCI.20-09-03328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 164.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 165.Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 166.Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Mintz J, Cohen MS. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 167.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 168.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 170.McNamara RK, Able JA, Jandacek R, Rider T, Tso P, Eliassen J, Alfieri D, Weber W, Jarvis K, DelBello MP, Strakowski SM, Adler CM. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, Brammer MJ. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 172.McNamara RK, Adler C, Strawn J, Strimpfel J, Jandacek R, Rider T, Tso P, Weber W, Strakowski SM, DelBello MP. Adolescents with major depressive disorder exhibit reversible erythrocyte long-chain omega-3 fatty acid deficits: Associations with mood symptoms and functional cortical activity. Society of Biological Psychiatry Meeting; Philadelphia, PA. 2012. [Google Scholar]
- 173.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 174.Griffin JL, Bollard M, Nicholson JK, Bhakoo K. Spectral profiles of cultured neuronal and glial cells derived from HRMAS (1)H NMR spectroscopy. NMR Biomed. 2002;15:375–384. doi: 10.1002/nbm.792. [DOI] [PubMed] [Google Scholar]
- 175.Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of Nacetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- 176.Ciccarelli O, Toosy AT, De Stefano N, Wheeler-Kingshott CA, Miller DH, Thompson AJ. Assessing neuronal metabolism in vivo by modeling imaging measures. J Neurosci. 2010;30:15030–15033. doi: 10.1523/JNEUROSCI.3330-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bjartmar C, Kidd G, Mörk S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- 178.Demougeot C, Marie C, Giroud M, Beley A. N-acetylaspartate: a literature review of animal research on brain ischaemia. J Neurochem. 2004;90:776–783. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- 179.Woo CW, Lee BS, Kim ST, Kim KS. Correlation between lactate and neuronal cell damage in the rat brain after focal ischemia: An in vivo 1H magnetic resonance spectroscopic (1H-MRS) study. Acta Radiol. 2010;51:344–350. doi: 10.3109/02841850903515395. [DOI] [PubMed] [Google Scholar]
- 180.Fukami K, Inanobe S, Kanemaru K, Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res. 2010;49:429–437. doi: 10.1016/j.plipres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 181.Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: A focused review and meta-analysis of 1H-MRS studies. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr069. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ross BD. Biochemical considerations in 1H spectroscopy. Glutamate and glutamine; myo-inositol and related metabolites. NMR Biomed. 1991;4:59–63. doi: 10.1002/nbm.1940040205. [DOI] [PubMed] [Google Scholar]
- 184.Chang K, Adleman N, Dienes K, et al. Decreased N-acetylaspartate in children with familial bipolar disorder. Biol Psychiatry. 2003;53:1059–1065. doi: 10.1016/s0006-3223(02)01744-4. [DOI] [PubMed] [Google Scholar]
- 185.Winsberg ME, Sach N, Tate DL, et al. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biol Psychiatry. 2000;47:475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- 186.Shimon H, Agam G, Belmaker RH, Hyde TM, Kleinman JE. Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. Am. J. Psychiatry. 1997;154:1148–1150. doi: 10.1176/ajp.154.8.1148. [DOI] [PubMed] [Google Scholar]
- 187.Coupland NJ, Ogilvie CJ, Hegadoren KM, Seres P, Hanstock CC, Allen PS. Decreased prefrontal myo-inositol in major depressive disorder. Biol. Psychiatry. 2005;57:1526–1534. doi: 10.1016/j.biopsych.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 188.Frey R, Metzler D, Fischer P, Heiden A, Scharfetter J, Moser E, Kasper S. Myo-inositol in depressive and healthy subjects determined by frontal 1H-magnetic resonance spectroscopy at 1.5 tesla. J. Psychiatr Res. 1998;32:411–420. doi: 10.1016/s0022-3956(98)00033-8. [DOI] [PubMed] [Google Scholar]
- 189.Gruber S, Frey R, Mlynárik V, Stadlbauer A, Heiden A, Kasper S, Kemp GJ, Moser E. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Invest. Radiol. 2003;38:403–408. doi: 10.1097/01.rli.0000073446.43445.20. [DOI] [PubMed] [Google Scholar]
- 190.Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders-focus on magnetic resonance spectroscopy (MRS) studies. Hum. Psychopharmacol. 2005;20:309–326. doi: 10.1002/hup.693. [DOI] [PubMed] [Google Scholar]
- 191.Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21:435–439. doi: 10.1177/0269881106067787. [DOI] [PubMed] [Google Scholar]
- 192.Berger GE, Wood SJ, Wellard RM, Proffitt TM, McConchie M, Amminger GP, Jackson GD, Velakoulis D, Pantelis C, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33:2467–2473. doi: 10.1038/sj.npp.1301628. [DOI] [PubMed] [Google Scholar]
- 193.Keshavan MS, Stanley JA, Pettegrew JW. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings--part--II. Biol. Psychiatry. 2000;48:369–380. doi: 10.1016/s0006-3223(00)00940-9. [DOI] [PubMed] [Google Scholar]
- 194.Fukuzako H, Fukuzako T, Hashiguchi T, Kodama S, Takigawa M, Fujimoto T. Changes in levels of phosphorus metabolites in temporal lobes of drug-naive schizophrenic patients. Am. J. Psychiatry. 1999;156:1205–1208. doi: 10.1176/ajp.156.8.1205. [DOI] [PubMed] [Google Scholar]
- 195.Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, Tretta MG, Allen M. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 1991;48:563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- 196.Stanley JA, Williamson PC, Drost DJ, Carr TJ, Rylett RJ, Malla A, Thompson RT. An in vivo study of the prefrontal cortex of schizophrenic patients at different stages of illness via phosphorus magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 1995;52:399–406. doi: 10.1001/archpsyc.1995.03950170073010. [DOI] [PubMed] [Google Scholar]
- 197.Deicken RF, Calabrese G, Merrin EL, Meyerhoff DJ, Dillon WP, Weiner MW, Fein G. 31phosphorus magnetic resonance spectroscopy of the frontal and parietal lobes in chronic schizophrenia. Biol. Psychiatry. 1994;36:503–510. doi: 10.1016/0006-3223(94)90613-0. [DOI] [PubMed] [Google Scholar]
- 198.Shioiri T, Hamakawa H, Kato T, Fujii K, Murashita J, Inubushi T, Someya T. Frontal lobe membrane phospholipid metabolism and ventricle to brain ratio in schizophrenia: preliminary 31P-MRS and CT studies. Eur Arch Psychiatry Clin Neurosci. 2000;250:169–174. doi: 10.1007/s004060070021. [DOI] [PubMed] [Google Scholar]
- 199.Yildiz A, Sachs GS, Dorer DJ, Renshaw PF. 31P Nuclear magnetic resonance spectroscopy findings in bipolar illness: a meta-analysis. Psychiatry Res. 2001;106:181–191. doi: 10.1016/s0925-4927(01)00082-8. [DOI] [PubMed] [Google Scholar]
- 200.Richardson AJ, Allen SJ, Hajnal JV, Cox IJ, Easton T, Puri BK. Associations between central and peripheral measures of phospholipid breakdown revealed by cerebral 31- phosphorus magnetic resonance spectroscopy and fatty acid composition of erythrocyte membranes. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1513–1521. doi: 10.1016/s0278-5846(01)00211-1. [DOI] [PubMed] [Google Scholar]
- 201.Yao J, Stanley JA, Reddy RD, Keshavan MS, Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol Psychiatry. 2002;52:823–830. doi: 10.1016/s0006-3223(02)01397-5. [DOI] [PubMed] [Google Scholar]
- 202.Puri BK, Richardson AJ, Horrobin DF, Easton T, Saeed N, Oatridge A, Hajnal JV, Bydder GM. Eicosapentaenoic acid treatment in schizophrenia associated with symptom remission, normalisation of blood fatty acids, reduced neuronal membrane phospholipid turnover and structural brain changes. Int. J. Clin. Pract. 2000;54:57–63. [PubMed] [Google Scholar]
- 203.Sublette ME, Milak MS, Hibbeln JR, Freed PJ, Oquendo MA, Malone KM, Parsey RV, Mann JJ. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins Leukot Essent Fatty Acids. 2009;80:57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Steward CA, Marsden CA, Prior MJ, Morris PG, Shah YB. Methodological considerations in rat brain BOLD contrast pharmacological MRI. Psychopharmacology (Berl) 2005;180:687–704. doi: 10.1007/s00213-005-2213-7. [DOI] [PubMed] [Google Scholar]
- 205.Schwarz AJ, Gozzi A, Bifone A. Community structure in networks of functional connectivity: resolving functional organization in the rat brain with pharmacological MRI. Neuroimage. 2009;47:302–311. doi: 10.1016/j.neuroimage.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 206.Ahmad A, Momenan R, van Gelderen P, Moriguchi T, Greiner RS, Salem N., Jr Gray and white matter brain volume in aged rats raised on n-3 fatty acid deficient diets. Nutr Neurosci. 2004;7:13–20. doi: 10.1080/1028415042000202009. [DOI] [PubMed] [Google Scholar]
- 207.Lindquist DM, Jandacek R, Rider T, Tso P, McNamara RK. Omega-3 fatty acid deficiency during adolescent development leads to structural alterations in the adult rat limbic forebrain: An in vivo magnetic resonance imaging study. Society of Biological Psychiatry Meeting; Philadelphia, PA. 2012. [Google Scholar]
- 208.McNamara RK, Able JA, Jandacek R, Rider T, Tso P, Lindquist DM. Perinatal omega-3 fatty acid deficiency selectively reduces myo-inositol levels in the adult rat prefrontal cortex: An in vivo 1H-MRS study. J Lipid Res. 2009;50:405–411. doi: 10.1194/jlr.M800382-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, Morris MC. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2012 doi: 10.3233/JAD-2012-110629. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–556. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 211.Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, Evans AC, Rapoport JL. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Moore GJ, Cortese BM, Glitz DA, Zajac-Benitez C, Quiroz JA, Uhde TW, Drevets WC, Manji HK. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry. 2009;70:699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- 213.Dyerberg J, Madsen P, Møller JM, Aardestrup I, Schmidt EB. Bioavailability of marine n-3-fatty acid formulations. Prostaglandins Leukot Essent Fatty Acids. 2010;83:137–141. doi: 10.1016/j.plefa.2010.06.007. [DOI] [PubMed] [Google Scholar]