Abstract
Background & Aims
T cells are an important component for development of a vaccine against hepatitis C virus (HCV), but little is known about the features of successful vaccine-induced T cells.
Methods
We compared the phenotype, function, and kinetics of vaccine-induced and infection-induced T cells in chimpanzees with HCV infection using multicolor flow cytometry and real-time PCR.
Results
In chimpanzees successfully vaccinated with recombinant adenovirus and DNA against HCV NS3-NS5, HCV-specific T cells appeared earlier, maintained better functionality, and persisted at higher frequencies, for a longer time after HCV-challenge, than those of mock-vaccinated chimpanzees. Vaccine-induced T cells displayed higher levels of CD127, a marker of memory precursors, and lower levels of programmed death (PD)-1 than infection-induced T cells. Vaccine-induced, but not infection-induced T cells, were multifunctional; their ability to secrete interferon-γ and tumor necrosis factor-α correlated with early expression of CD127 but not PD-1. Based on a comparison of vaccine-induced and infection-induced T cells from the same chimpanzee, the CD127+ memory precursor phenotype was induced by the vaccine itself, rather than by low viremia. In contrast, PD-1 induction correlated with viremia, and levels of intrahepatic PD-1, PD-L1, and 2,5-OAS-1 mRNAs correlated with peak titers of HCV.
Conclusions
Compared with infection, vaccination induced HCV-specific CD127+ T cells with high functionality that persisted at higher levels for a longer time. Control of viremia prevented upregulation of PD-1 on T cells, and induction of PD-1, PD-L1, and 2,5-OAS-1 in the liver. Early development of a memory T-cell phenotype and, via control of viremia, attenuation of the inhibitory PD1–PD-L1 pathway might be necessary components of successful vaccine-induced protection against HCV.
Keywords: CD8+ T cells, IFN, TNF, vaccine development
INTRODUCTION
Hepatitis C virus (HCV) establishes persistence t 60–80% of infected patients and is a leading cause of liver cirrhosis and hepatocellular carcinoma1. Although more than 170 million people worldwide are infected with HCV, a prophylactic vaccine is not yet available.
Spontaneous HCV clearance depends on a vigorous and sustained T cell responses as demonstrated in HCV-infected patients 2, 3 and in chimpanzees, the sole animal model of HCV infection4, 5. The appearance of HCV-specific T cells coincides with the decrease in viral titer in the acute phase of infection [reviewed in 1], and HCV-specific T cells rather than antibodies remain detectable in the blood for decades 6. HCV clearance may result in protective immunity as evidenced by lower peak viremia, more rapid viral clearance and less liver damage when spontaneously recovered chimpanzees are challenged with homologous and heterologous HCV strains 7–10. This protection is T cell-mediated because depletion of CD4 T cells prior to challenge results in chronic HCV infection 9, and depletion of CD8 T cells delays HCV clearance10. Thus, T cells play a critical role in both HCV clearance and protection.
To date, candidate vaccines that employ recombinant DNA, replication-defective recombinant adenovirus, replicating recombinant vaccinia virus, recombinant proteins and virus-like particles 11–15 have been tested in the chimpanzee model with most being only partly successful in preventing chronic HCV infection 13, 15–17. A candidate vaccine that is currently in clinical studies is based on adenoviral vectors encoding the HCV NS3-5 sequence of HCV genotype 1b 12. When chimpanzees were vaccinated with this adenovirus-based prime and DNA boost regimen and challenged with heterologous genotype 1a HCV, four of five displayed strong T cell responses and cleared HCV 12. While three of the five mock-vaccinated control chimpanzees also cleared HCV in the presence of T cell responses these three chimpanzees had a significantly longer period of viremia, higher peak HCV titers and elevated ALT levels. The accelerated HCV clearance and the stable ALT levels in the vaccinated chimpanzees therefore suggest that vaccine-induced T cells have unique features compared to infection-induced T cells.
To evaluate the phenotype of vaccine-induced T cells during HCV-challenge we compared successful vaccine-induced CD8 T cells with infection-induced CD8 T cells. We identified minimal CD8 T cell epitopes, determined their Pan troglodytes (Patr) restriction and synthesized Patr tetramers to detect HCV-specific CD8 T cells. Furthermore, using Patr tetramers we were able to characterize kinetic changes in the HCV-specific CD8 T cell population size, phenotype and function during the course of HCV infection. In addition, intrahepatic mRNA levels of T cell markers and their ligands were studied.
MATERIALS AND METHODS
Chimpanzees, vaccination and HCV-challenge
Chimpanzees were studied at New Iberia Research Center under protocols approved by its Animal Care and Use Committee. Five chimpanzees were vaccinated with replication-defective recombinant adenoviral vectors encoding the HCV NS3-5B (genotype 1b BK strain) and with NS3-5B–encoding plasmid DNA in a combined modality regimen 12. Five control chimpanzees received a control adenovirus and DNA plasmids encoding HIV gag. All were challenged intravenously with 100 chimpanzee infectious dose 50 of the heterologous HCV H77 (genotype 1a) 12.
Determination of minimal T cell epitopes and Patr class I restriction
PBMCs were stimulated in 96-well round-bottom plates (3 × 105 per well) with 10 μg/ml 15-mer HCV peptides, 10 ng/ml IL-7 (PeproTech) and 300 pg/ml IL-12 (PeproTech) in 10% FBS-RPMI 1640 (Mediatech). Restimulation with 10 μg/ml peptide, 20 U/ml IL-2 and 105/well irradiated (3,000 rads) autologous PBMCs was performed in the presence of 20 ng/ml IL-7 on day 7 and in the absence of IL-7 on day 14. On days 3, 10 and 17, 100 μl of 10% FBS-RPMI with 20 U/ml of IL-2 was added. To determine minimal epitopes, the generated T cell lines were subjected to 51Cr-release assays 18 using EBV-transformed autologous B cells pulsed with 8- to 11-mer peptides as targets. T cells cocultured with nonradioactive targets were assessed for IFN-γ, TNF-α, GM-CSF and MIP-1β release by Cytometric Bead Array (BD Biosciences). Patr-restriction was determined with peptide-pulsed Patr-transfected 721.221 targets 18.
Determination of Patr class I alleles, generation of Patr class I tetramers, and multi-color flow cytometry
Patr-class I alleles were determined by sequence-based typing 19. Patr monomers were folded, purified, biotinylated and tetramerized with streptavidin-PE or streptavidin-APC (Invitrogen).
Ethidium monoazide (EMA)-stained PBMC (4×106) were incubated with PE- or APC-conjugated tetramers for 20 min at room temperature, and subsequently stained with anti-CD3-Alexa Fluor 700, anti-CD19-PE-Cy5 (both BD Biosciences), anti-CD14-PE-Cy5 (Serotec) and with either anti-CD8-FITC (BD Biosciences) and anti-PD-1-PE (BioLegend), or anti-CD8-Pacific Blue (BD Biosciences) and anti-CD127-FITC (eBioscience). Fluorescence-minus-one control samples were included. A minimum of 2 million events per tube were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed using FacsDiva Version 4.1 (BD Biosciences) and FlowJo software (Tree Star).
Cytokine secretion assays
PBMCs were stained with PE-conjugated Patr-tetramers for 10 min at room temperature, washed twice and stimulated with 1 μg/ml HCV epitope or an irrelevant HBV peptide for 5.5 h. After subsequent incubation with IFN-γ and TNF-α catch reagents (Miltenyi Biotec) for 55 min at 37°C on a rotator, cells were stained with FITC-labeled anti-IFN-γ and APC-labeled anti-TNF-α detection reagents (Miltenyi Biotec) for 10 min on ice. Additional staining with 5 μg/ml EMA (Sigma), PE-conjugated Patr tetramers (as above) washed, and anti-CD3-Alexa Fluor 700, anti-CD8-APC-Cy7, anti-CD19-PE-Cy5 (all BD Biosciences) and anti-CD14-PE-Cy5 (Serotec) antibodies was performed prior to acquisition on an LSR II flow cytometer.
RNA extraction, cDNA synthesis and TaqMan real-time PCR
Total RNA was isolated from mechanically homogenized liver biopsies using the RNeasy Mini Kit (Qiagen) with on-column DNase digestion, and reverse transcribed using the First Strand cDNA Synthesis Kit (Marligen Biosciences). TaqMan Gene Expression Assays (Applied Biosystems) were performed in duplicate to determine CD4, CD8, PD-1, PD-L1 and 2,5-OAS-1 mRNA levels. The amount of specific mRNA was calculated using comparative cycle threshold values and standard curves, and normalized to mean levels of β-actin, GAPDH and β7 mRNA. Relative mRNA levels represent the fold-increase over median expression in 5 chimpanzees’ pre-infection liver biopsies of 5 chimpanzees of each group.
Statistical analysis
Mann Whitney U tests, linear regression analyses and serial measures ANOVA were performed with GraphPad Prism Version 5.0 (GraphPad Software Inc, San Diego, CA) and JMP (SAS Inc. Cary, NC) software. A p-value < 0.05 was considered significant.
RESULTS
Epitope mapping, Patr-restriction analysis and Patr-tetramer selection
The present study includes five vaccinated and five control chimpanzees challenged with HCV genotype 1a. The vaccinees were primed three times with recombinant adenoviral vectors and boosted by three immunizations with a DNA plasmids encoding the NS3-5 sequence of HCV genotype 1b (BK strain). Controls were mock-vaccinated with the same vectors encoding an unrelated HIV antigen 12.
To characterize HCV-specific immune responses we first determined the chimpanzees’ Patr class I haplotypes and identified CD8 T cell epitopes by stimulating PBMC with overlapping 15-mer peptides in IFN-γ Elispot assays. Minimal optimal epitopes and their Patr-restriction were subsequently identified by analyzing CD8 T cell responses against single Patr-transfected target cells pulsed with amino- and carboxy-terminally truncated peptides. Overall, eight Patr-tetramers were generated, which were suitable for examination of four control and four vaccinated chimpanzees. As shown in table 1, all but one vaccine-induced T cell population recognized the sequence of the HCV-challenge inoculum.
Late appearance of HCV-specific CD8 T cells during HCV-challenge of mock-vaccinated control chimpanzees
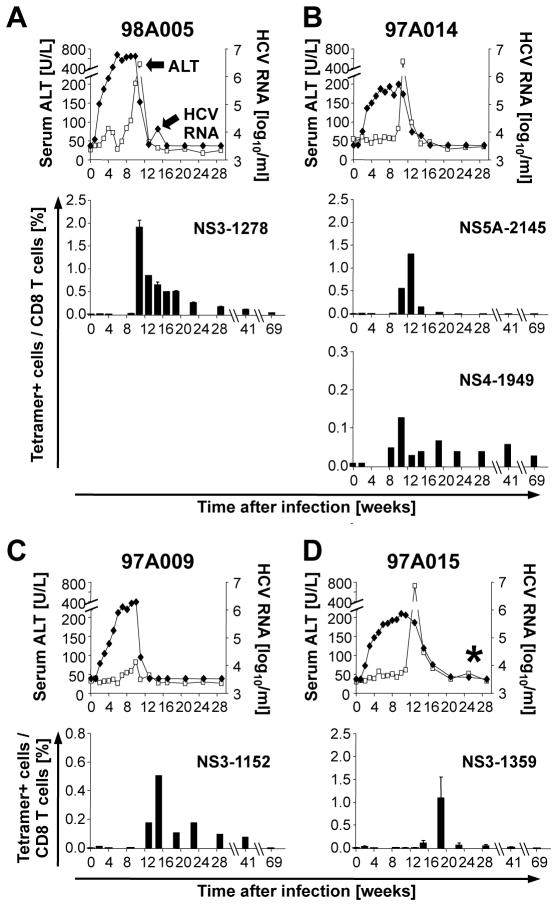
To characterize the kinetics and vigor of the HCV-specific CD8 T cell response of mock-vaccinated control chimpanzees, serial PBMC samples were stained ex vivo with the generated Patr-tetramers. The previously reported virological and clinical course of self- limited and chronic HCV infection 12 are shown in figure 1 for reference purposes. Within a week of HCV infection, the mock vaccinated control chimps developed high levels of viremia; however, HCV-specific CD8 T cells were undetectable for at least 11 weeks after HCV-challenge. Their frequency peaked at week 11–14 in the 3 chimpanzees with self-limited infection (Fig. 1A–C, see Suppl. Fig. 1A for FACS dot plots), as compared to week 19 in chimpanzee 97A015 with a chronic course of HCV infection (Fig. 1D, Suppl. Fig. 1A). The appearance of tetramer+ T cells coincided with peak ALT levels and decrease in viremia for all chimpanzees, and declined to undetectable levels after the acute phase of hepatitis.
Figure 1. Late and transient appearance of HCV-specific CD8 T cells in mock-vaccinated control chimpanzees during HCV-challenge.
Serum HCV RNA titers (filled diamonds) and ALT levels (open squares) have previously been reported 12 and are shown for reference purposes. HCV RNA titers became undetectable by quantitative and qualitative PCR for three chimpanzees (A–C), but remained just above the detection limit for chimp 97A015 (marked by *, D). Error bars represent the standard error of the mean from 3 experiments. The HCV protein and first amino acid position of the epitope presented by the tetramer is indicated in each panel
Early expansion HCV-specific CD8 T cells during HCV-challenge of vaccinees
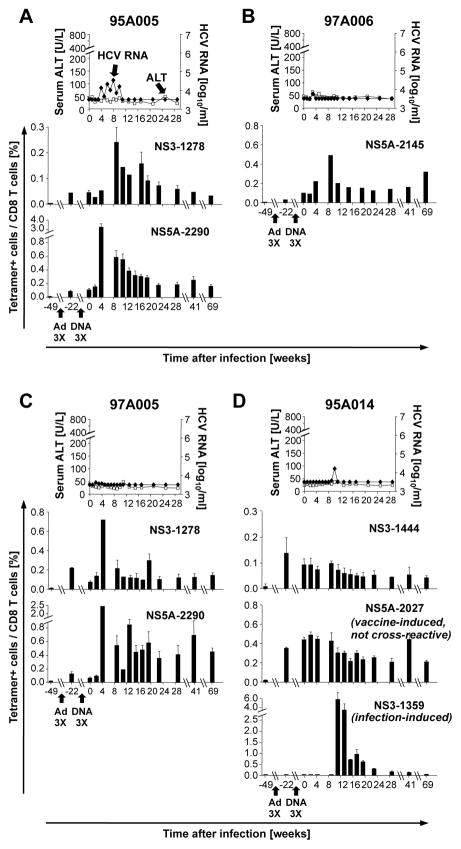
In contrast to the control chimpanzees all vaccinated chimpanzees experienced low HCV titers and stable ALT levels (Fig. 2). HCV-specific tetramer+ T cells were detectable after vaccination with recombinant adenovirus (Fig. 2, Suppl. Fig. 1B) and reached peak levels as early as week 4 after HCV-challenge, which was 7–15 weeks earlier than in the mock-vaccinated control chimpanzees. This is confirmatory of an earlier study which employed stimulation with pools of overlapping peptides and intracellular cytokine staining to study the immune responses of these animals 12.
Figure 2. Early expansion and long-term persistence of HCV-specific CD8 T cells during HCV-challenge of vaccinees.
Serum HCV RNA titers (filled diamonds) and ALT levels (open squares) of four vaccinated chimpanzees have previously been reported 12 and are shown for reference purposes. Error bars represent the standard error of the mean from 3 experiments. The HCV protein and first amino acid position of the epitope presented by the tetramer is indicated in each panel. Ad3X, 3 consecutive vaccinations with a replication-defective recombinant adenovirus encoding HCV NS3-5B; DNA3X, 3 consecutive boosts with recombinant DNA plasmid encoding HCV NS3-5B.
Vaccine-induced T cells persisted in the circulation for at least 69 weeks after HCV-challenge and at higher levels than infection-induced T cells (Fig. 2A–C). Thus, this tetramer study revealed that the differential cytokine responses observed by Folgori et al. 12 were not solely due to differential functionalities but also due to the superior persistence of vaccine-induced T cells after HCV clearance.
The use of individual epitopes rather than overlapping peptides as in the original study by Folgori et al. 12 allowed us to study the kinetics of vaccine-induced and infection-induced HCV-specific T cell responses side-by-side in a single animal (Fig. 2D, Suppl. Fig. 1B). Chimpanzee 95A014 had three populations of tetramer-positive T cells. The vaccine-induced NS31444-specific T cells, which cross-reacted with an epitope in the HCV-challenge inoculum, were maintained for at least one year after HCV-challenge. Another population of vaccine-induced T cells (NS5A2027-specific T cells) did not react with the HCV-challenge inoculum (Table 1) but was maintained a similar length of time. This suggests that persistence of the vaccine-induced T cells after HCV-challenge did not require restimulation with cognate antigen. The third population of HCV-specific T cells (NS3-1359-specific) was not induced by the vaccine and became detectable late after HCV-challenge suggesting that they were infection-induced. In fact, their kinetics resembled those of infection-induced HCV-specific T cells in the control chimpanzees with high viremia (Fig. 1). This result demonstrates that the late and transient appearance of infection-induced T cells was not improved by early control of viremia.
Vaccine-induced HCV-specific CD8 T cells exhibit higher CD127 levels than infection-induced HCV-specific T cells
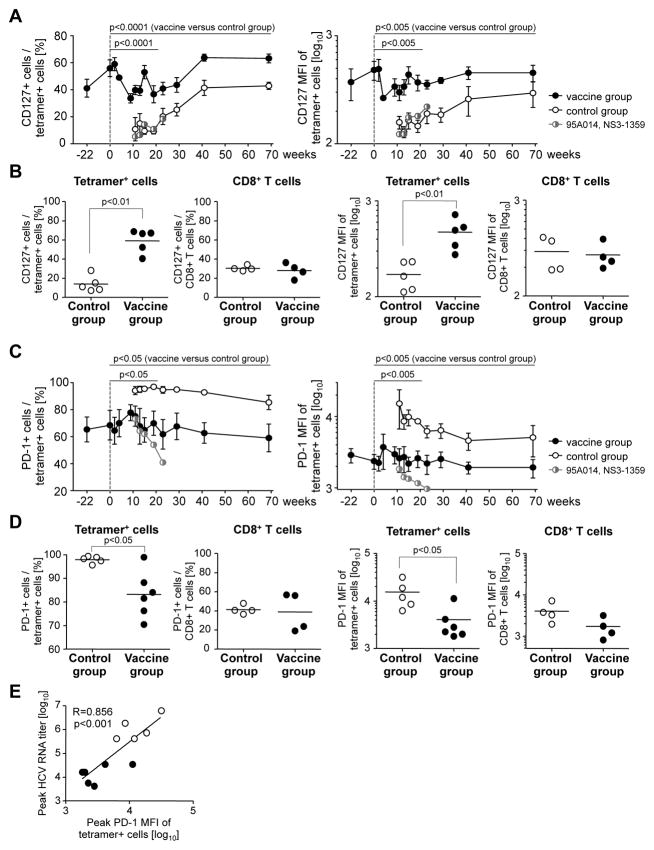
Multi-color flow cytometry were used to compare the phenotype of infection-induced and vaccine-induced HCV-specific T cells. CD127, the IL-7Rα-chain, is known as a marker of memory cell precursors 20. Vaccinated chimpanzees displayed a higher percentage of CD127+ tetramer+ T cells than control chimpanzees from the onset of HCV-challenge throughout the follow up (p<0.0001, Fig. 3A). The persistence of these cells for more than one year did not depend on stimulation with cognate antigen because NS5A2027-specific CD127+ T cells from chimpanzee 95A014, which did not cross-recognize the sequence of the HCV-challenge inoculum, were maintained for the same length of time as HCV-specific CD127+ memory T cells that recognized the challenge virus not shown. In addition, generation of CD127+ T cells was not the result of effective control of HCV viremia at the time of T cell priming because the infection-induced NS31359-specific cells in the vaccinated chimpanzee 95A014 did not increase their CD127 levels even though viremia was controlled. Instead, NS31359-specific T cells displayed the CD127-negative phenotype of of infection-induced tetramer+ T cells in the control chimpanzees (Fig. 3A).
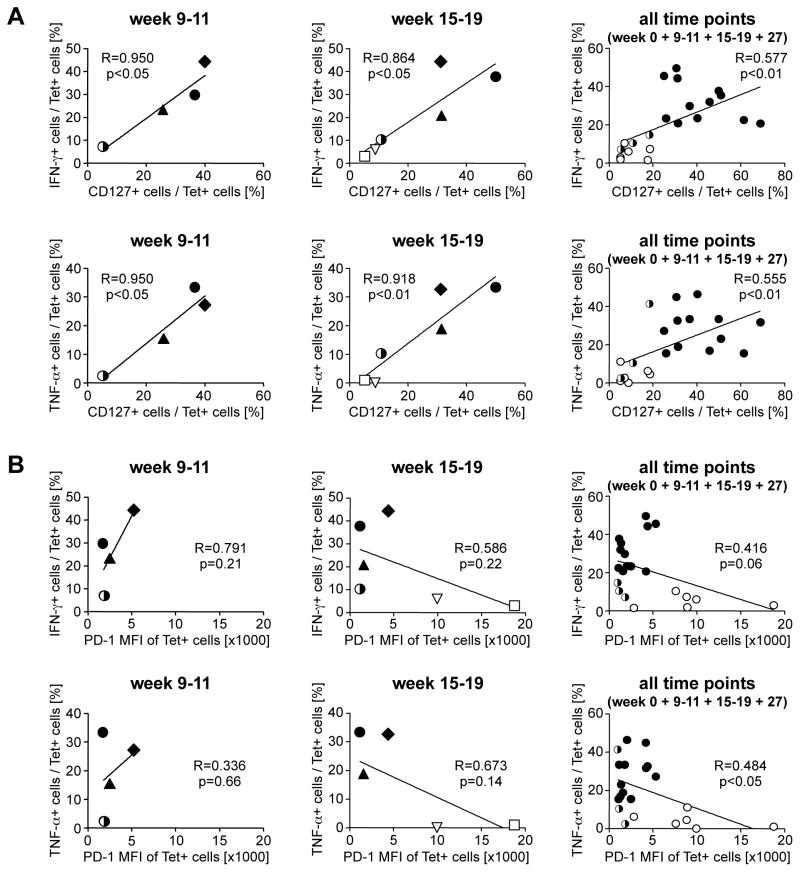
Figure 3. Vaccine-induced HCV-specific CD8 T cells express more CD127 and less PD-1 than infection-induced HCV-specific T cells.
(A, C) Frequency and MFI of CD127+ cells and CD127 MFI (A) and frequency of PD-1+ cells and PD-1 MFI (C) in the HCV-specific tetramer+ CD8 T cell population. Graphs show mean + SEM for each group. Serial measures ANOVA is used to compare these parameters for vaccine-induced HCV-specific CD8 T cells from the vaccinated chimpanzees (filled circles) and infection-induced HCV-specific CD8 T cells from the control chimpanzees (open circles) in the acute phase of hepatitis (up to week 19) and the entire time course. Infection-induced NS31359-specific T cells of the vaccinated chimpanzee 95A014 are shown by half-filled, half-open circles. (B, D) Increased CD127 (B) and decreased PD-1 expression (D) is limited to HCV-specific T cells of the vaccinated chimpanzees and not seen on bulk CD8 T cells. Vaccinated and control chimpanzees are compared at the time with the highest percentage of CD127+ HCV-specific T cells (B) and the highest MFI of PD-1 + HCV-specific T cells (D). The horizontal line indicates the geometric mean. NS31359-specific T cells of vaccinee 95A014 were excluded from the analysis, because these cells were induced by infection rather than the vaccine. See supplementary figure 2 for FACS dot plots. (E) Correlation between PD-1 expression and viremia. Filled symbols, vaccines; open symbols, mock-vaccinated control chimpanzees. R, correlation coefficient.
Differential CD127 expression by vaccine-induced and infection-induced tetramer+ CD8 T cells was most evident when peak CD127 levels were compared during the first 20 weeks of acute HCV infection (p<0.0001), but was not observed for the total CD8 T cell population (p<0.01, Fig. 3B; see Suppl. Fig. 2A for FACS dot plots).
Vaccine-induced HCV-specific CD8 T cells express lower levels of PD-1 during acute HCV infection than infection-induced HCV-specific T cells
Next, we studied PD-1 expression on infection-induced and vaccine-induced HCV-specific CD8 T cells. PD-1 is known to be expressed on exhausted T cells in chronic viral infection and is induced by both TCR and cytokine stimulation 21, 22. For the infection-induced T cells of mock-vaccinated control chimpanzees PD-1 expression was highest when they first appeared in the blood and decreased thereafter (Fig. 3C). In contrast, for vaccine-induced tetramer+ CD8 T cells PD-1 levels were much lower and stable after HCV-challenge (p<0.005, Fig. 3C). The differences in PD-1 expression by infection-induced and vaccine-induced tetramer+ CD8 T cells were most evident comparing the peak levels during the first 20 weeks of acute HCV infection (Fig. 3D, p<0.05) but were not observed for the total CD8 T cell population (Fig. 3D). Notably, vaccine-induced NS5A2027-specific T cells of vaccinated chimpanzee 95A014, which did not cross-recognize the HCV-challenge inoculum, displayed the lowest PD-1 levels (Suppl. Fig. 2B) suggesting that PD-1 expression required cognate T cell receptor stimulation rather than cytokines. This is consistent with the observation that infection-induced NS31359-specific T cells of the vaccinated chimpanzee 95A014 exhibited much lower peak PD-1 levels than the infection-induced HCV-specific T cells of the control chimpanzees (Fig. 3). This result suggests that PD-1 expression depended on the level of HCV viremia, which was effectively controlled in this vaccinated chimpanzee. This interpretation is supported by a significant correlation between peak HCV RNA titer and peak PD-1 MFI (R=0.856, p<0.001, Fig. 3E).
Vaccine-induced HCV-specific CD8 T cells maintain greater functionality during acute HCV infection than infection-induced HCV-specific T cells
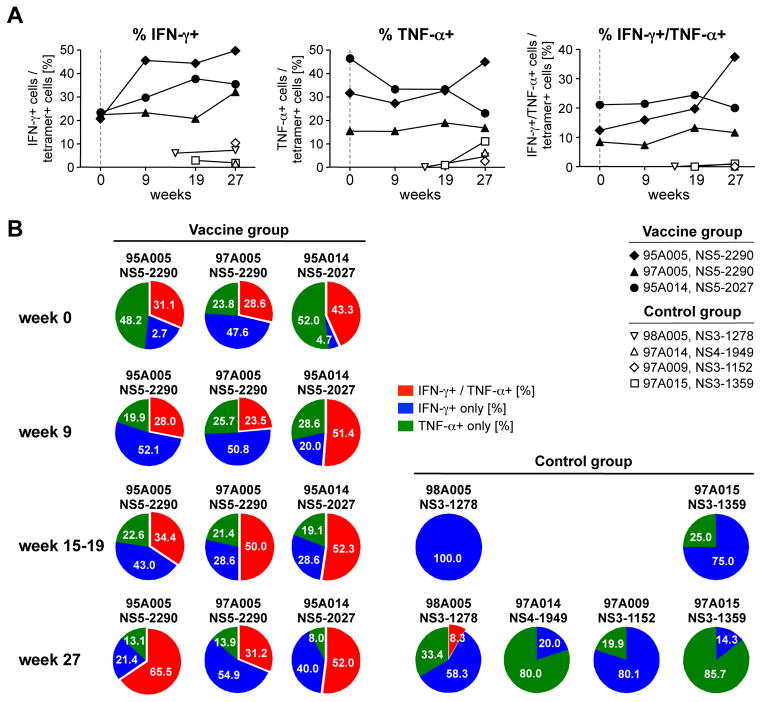
We also studied the function of tetramer+ T cells in response to in vitro stimulation with cognate antigen at the time of HCV challenge (week 0), the time of peak viral load, (week 9–11), just after HCV clearance (week 15–19) and long-term after HCV challenge (week 27). As shown in figure 4A, the vaccinated chimpanzees had a higher percentage of IFN-γ and TNF-α-secreting T cells within the tetramer+ T cell population than the control chimpanzees at all time points. Moreover, vaccinated chimpanzees possessed a high percentage of IFN-γ/TNF-α-double positive T cells that are rarely seen during natural HCV infection (Fig. 4B, see Suppl. Fig. 3 for FACS dot plots). Cytokine secretion correlated closely with CD127 expression on tetramer+ T cells (Fig. 5A), which was higher on the vaccine-induced HCV-specific T cells of the vaccinated chimpanzees than on those of the control chimpanzees. Interestingly, cytokines were poorly produced by the population of infection-induced tetramer+ T cells of the vaccinated chimpanzee 95A014, which displayed the same CD127-low phenotype as the tetramer+ T cells of the control chimpanzees (Fig. 5A). These results suggest that the vaccine-induced T cells had superior functionality due to early CD127 expression, and not due to the vaccine-induced control of viral load.
Figure 4. Vaccine-induced but not infection induced CD8 T cells secrete multiple cytokines.
(A) The percentage of IFN-γ and/or TNF-α-secreting in the population of tetramer+ CD8 T cells is higher in vaccinated chimpanzees than in control chimpanzees at all studied time points. (B) The percentage of multifunctional IFN-γ/TNF-α-double positive cells in the population of cytokine-secreting HCV-specific CD8 T cells is higher in vaccinated chimpanzees than in control chimpanzees. The selected time points represent the time of HCV-challenge (week 0), peak viral load (weeks 9–11) the time just after HCV clearance (weeks 15–19) and long-term follow up (week 27). The sum of all IFN-γ and/or TNF-α-secreting cells is 100%.
Figure 5. Strong correlation between cytokine secretion and expression of CD127 (A) but not PD-1 (B) early after HCV-challenge.
Vaccine-induced tetramer+ T cells are indicated by filled symbols and infection-induced T cells of the control chimpanzees are indicated by open symbols. The infection-induced NS31359-specific tetramer+ T cells in the vaccinated chimpanzee 95A014 (indicated by half filled, half open circles) did not display the CD127 phenotype and superior cytokine production of the vaccine-induced tetramer+ T cells but instead resembled the infection-induced T cells of the control chimpanzees.
In contrast to the strong correlation between cytokine secretion and CD127 expression, there was no correlation between cytokine secretion and PD-1 expression at the early and intermediate time points (Fig. 5B). Rather, PD-1 expression followed viremia, (Fig. 3E) because both the vaccine-induced and the infection-induced T cells of all chimpanzees with low viremia were PD-1 low whereas tetramer+ T cells of the nonvaccinated control chimpanzees were PD-1 high (Fig. 5B).
Vaccinated chimpanzees expressed lower PD-1, PD-L1, and 2,5-OAS-1 mRNA levels in the liver than control chimpanzees during HCV-challenge
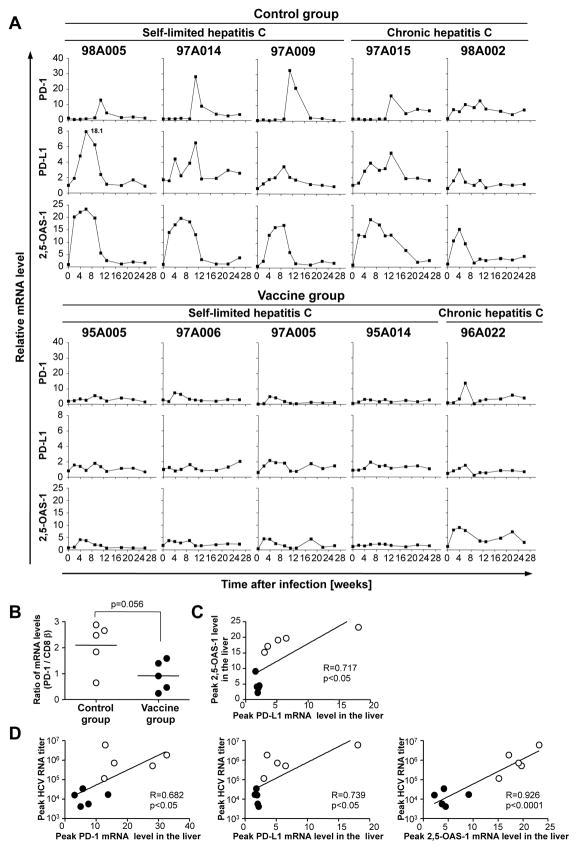
To study the kinetics of immune responses in the liver, the site of infection, we prospectively analyzed intrahepatic mRNA levels of selected markers. Because this analysis did not depend on specific Patr-haplotypes and tetramers, all five control chimpanzees and all five vaccinees were examined throughout the course of acute HCV-challenge. Whereas intrahepatic PD-1 mRNA levels increased during acute HCV infection in the control chimpanzees, they remained stable or slightly increased in the vaccinees (Fig. 6A). Because the majority of liver-infiltrating T cells were CD8 T cells 23 we normalized PD-1 mRNA levels to CD8β mRNA levels. As shown in figure 6B there was a trend of higher peak PD-1 mRNA levels in mock-vaccinated control chimpanzees than in vaccinees (p=0.056, Fig. 6B).
Figure 6. Intrahepatic PD-1, PD-L1, and 2,5-OAS-1 mRNA expression levels throughout the course of HCV-challenge.
(A) Intrahepatic PD-1, PD-L1, and 2,5-OAS-1 mRNA levels normalized to endogenous references (β-actin, GAPDH and β7) and expressed as fold-increase over pre-infection levels. (B) Peak PD-1 mRNA levels, normalized to CD8β mRNA levels, respectively, differed between control mock-vaccinated chimpanzees and vaccinees. (C, D) Linear regression analysis between peak intrahepatic PD-L1 and 2,5-OAS-1 mRNA levels (C), and between peak HCV RNA titers and peak intrahepatic PD-1, PD-L1 and 2,5-OAS-1 mRNA levels respectively (D). Open circles, mock-vaccinated control chimpanzees; filled circles, vaccinees. R, correlation coefficient.
To further study the PD-1/PD-L1 inhibitory pathway intrahepatic PD-L1 mRNA levels were quantified throughout the course of the HCV-challenge. The kinetics of PD-L1 mRNA expression in the liver correlated with the kinetics of the intrahepatic type I IFN response, as evidenced by mRNA levels of the type I IFN-inducible gene 2,5-OAS-1 (Fig. 6A) and the correlation between peak PD-L1 and peak 2,5-OAS-1 levels (Fig. 6C). Both, PD-L1 and 2,5-OAS-1 mRNA levels were higher in control chimpanzees than in vaccinees throughout the course of acute HCV infection (Fig. 6A). Furthermore, peak PD-1, PD-L1 and 2,5-OAS-1 mRNA levels correlated with peak HCV RNA titers and were higher in control chimpanzees than in vaccinees (Fig. 6C, DE, closed circles). Thus, efficient control of viremia by vaccination resulted in the attenuation of the PD-1/PD-L1 inhibitory pathway by reducing both PD-1 expression on HCV-specific CD8 T cells in the blood and PD-L1 mRNA expression in the liver. These coordinated effects, along with a vaccine-induced CD127+ early memory phenotype, may allow virus-specific CD8 T cells to exert and maintain optimal antiviral function during the acute phase of HCV-challenge and the long-term follow-up.
DISCUSSION
The key feature of many vaccines is the induction of neutralizing antibodies. Although antibody-based neutralization of HCV has been described both in vitro 24, 25 and in vivo 26, the humoral immune response typically selects HCV escape mutants and therefore fails to eradicate HCV 26, 27. Moreover, HCV antibody titers decline and often become undetectable years after spontaneous HCV clearance 6 raising questions about the longevity of protective antibody responses.
Recent studies have therefore emphasized the role of the T cell response in protection against HCV 8–10 and explored the feasibility of inducing efficient HCV-specific T cells via vaccination. However, the quality and kinetics of a successful, vaccine-induced T cell response is still not known. Here we prospectively studied the quality and kinetics of vaccine-induced CD8 T cells in comparison to infection-induced T cells. The use of genetic vaccination with exclusion of HCV structural antigen sequences allowed for the unique opportunity to study protective T cell responses in the absence of neutralizing antibodies, and to compare vaccine- and infection-induced T cells in the same host.
The timing of HCV-specific T cell responses is considered to be an important determinant of the outcome of acute HCV infection 5, 28. Consistent with an earlier study that used stimulation of PBMC with overlapping peptides and intracellular cytokine staining rather than epitope-specific tetramers as a readout 12 vaccine-induced HCV-specific T cells were detectable prior to HCV-challenge and rapidly expanded after HCV infection reaching peak levels as early as week 4, i.e. 7–15 weeks earlier than infection-induced T cells (Fig. 1, 2). This accelerated memory T cell response, likely the most important component of vaccine-induced protective immunity against HCV, may be attribured to the early expression of CD127, which binds IL-7. Recent studies of acutely infected HCV patients also demonstrate a correlation between CD127 expression on HCV-specific T cells and a self-limited course of infection 29–31. In addition, early antiviral treatment with IFN-α has been shown to rescue a small population of HCV-specific CD8 T cells that maintains high CD127 levels and polyfunctionality 29.
It is difficult to prove in a translational study whether the early CD127+ memory precursor phenotype of the tetramer+ T cells was the result of vaccination and an essential factor in the control of viremia, or whether it was simply the result of controlled viremia. Because viremia was lower in vaccinated chimps than in control chimps throughout this study it was not possible to compare both chimpanzee groups at time points with similar levels of viremia. However, one vaccinated chimpanzee had populations of both vaccine-induced and infection-induced tetramer+ T cells. Despite the early control of viremia in this chimpanzee, the infection-induced tetramer+ T cells appeared late in a similar manner to those of infection-induced T cells in the control chimpanzees. Furthermore, despite early control of viremia these infection-induced T cells never developed the CD127+ phenotype characteristic of polyfunctional IFN-γ/TNF-α-secreting vaccine-induced T cells. Thus, it appears that increased CD127 expression and improved T cell functionality were the result of the vaccination itself and due to low viremia. In contrast to CD127 expression, PD-1 expression followed viremia, because both the vaccine-induced and the infection-induced T cells of all chimpanzees with low viremia were PD-1low whereas tetramer+ T cells of the nonvaccinated control chimpanzees were PD-1high.
Attenuation of the PD-1/PD-L1 inhibitory pathway is another feature of this successful vaccines. The PD-1 MFI of vaccine-induced tetramer+ cells in the periphery (Fig. 3E) and the PD-1 mRNA level in the liver (Fig. 6) correlated with the peak HCV RNA titer during acute HCV infection, which was controlled by prior vaccination. These data are reminiscent of a previous study that showed a correlation between intrahepatic PD-1 levels and outcome of acute HCV infection 32. Our data are also consistent with a study on simian immunodeficiency virus (SIV) infection 33. In that study, vaccinated macaques expressed lower levels of PD-1 on SIV-specific CD8 T cells than non-vaccinated control macaques during SIV challenge 33. Thus, immediate control of virus replication in vaccinees precludes virus-specific T cells from upregulating PD-1, whereas viral persistence stimulates PD-1 expression and exhausts virus-specific T cells. This was illustrated by the unique case of NS31359-specific T cells in chimpanzee 95A014. Even though NS31359-specific T cells were induced by HCV-challenge and not by vaccination they maintained a very low PD-1 MFI (Fig. 3D, Suppl. Fig. 2). Presumably, early viral control by vaccination-induced T cells limited the expression of PD-1 on these infection-induced T cells.
Not only the expression of PD-1 on HCV-specific T cells, but also the expression of its ligand, PD-L1 in the liver was attenuated in vaccinees. HCV-induced type I IFN 34 stimulates the expression of PD-L1 on hepatocytes and liver sinusoidal endothelial cells in HCV infection 35. Vaccination limits the intrahepatic type I IFN response and PD-L1 expression due to the early and efficient control of viremia (Fig. 6A). Attenuation of PD-L1 expression in the liver may thereby allow HCV-specific CD8 T cells to avoid PD-1-mediated inhibition and to preserve their effector function.
In summary, the phenotypic characteristics of successful vaccine-induced CD8 T cells during acute HCV infection are preserved effector functions, high CD127 expression and low PD-1 expression. This is complemented by reduced expression of PD-L1 in the liver. Thus, a major inhibitory pathways is attenuated during vaccine-mediated HCV clearance, which in combination with the early induction of a CD127+ memory precursor phenotype may ensure optimal antiviral effector functions of vaccine-induced T cells during HCV-challenge and their persistence at relatively high levels after HCV clearance.
Supplementary Material
Table I.
Minimal optimal CD8 T-cell epitopes used for the synthesis of Patr class I tetramers
| HCV protein | Amino acid sequence
|
Cross-reactivityb | Minimal optimal epitope
|
Patr-restriction | Chimpanzees responding to the epitope
|
|||
|---|---|---|---|---|---|---|---|---|
| Vaccine (genotype 1b) | Infection (genotype 1a)a | Location | Sequence | Vaccinated | Non-vaccinated | |||
| NS3 | SLLSPRPVSYLKGSS | SLLSPRPISYLKGSS | N.D. | 1152–1162 | LLSPRPISYLK | A0101 | - | 97A009 |
| NS3 | AHGIDPNIRTGVRTI DPNIRTGVRTITTGA |
AHGVDPNIRTGVRTI DPNIRTGVRTITTGS |
Cross-reactive | 1278–1285 | IRTGVRTI | B0301/02 | 95A005 & 97A005 | 98A005 |
| NS3 | VTVPHPNIEEVALSN HPNIEEVALSNTGEI |
VTVSHPNIEEVALST HPNIEEVALSTTGEI |
Cross-reactive | 1359–1367 | HPNIEEVAL | B1301 | 95A014 | 97A015 |
| NS3 | ALMTGYTGDFDSVID | ALMTGFTGDFDSVID | Cross-reactive | 1444–1452 | YTGDFDSVI | B0101 | 95A014 | - |
| NS4 | QILSSLTITQLLKRL | AILSSLTVTQLLRRL_ | N.D. | 1949–1958 | SSLTVTQLLR | A0301 | - | 97A014 |
| NS5A | TTCPCGAQITGHVKN | TRCHCGAEITGHVKN | Not cross-reactive | 2027–2035 | TTCPCGAQI | B0101 | 95A014 | - |
| NS5A | LREEVSFQVGLNQYL | LREEVSFRVGLHEYP | Cross-reactive | 2145–2154 | VSFRVGLHEY | A0301 | 97A006 | 97A014 |
| NS5A | IWARPDYNPPLLESW | VWARPDYNPPLVETW | Cross-reactive | 2290–2298 | RPDYNPPLL | B0301/02 | 95A005 & 97A005 | - |
Amino acids that differ between the vaccine (genotype 1b) and the HCV challenge (genotype 1a) sequences are marked in bold; The minimal optimal epitopes are underlined;
T cell cross-recognition of the 15mer peptides with the sequence of either the vaccine (genotype 1b, BK strain) or the challenge inoculum (genotype 1a, H77 strain) was determined by IFN-γ ICS and reported previously (12), or, in the case of the NS5A-response of chimpanzee 97A006, by detection of MIP-1b and TNF-α in the supernatant of peptide-stimulated PBMC (CBA assays).
N.D., not determined.
Acknowledgments
We thank Dr. Xiongce Zhao, NIDDK for statistical advice, Dr. Christopher M. Walker, Center for Vaccines and Immunity, Columbus Children’s Research Institute, Columbus, OH for providing several of the Patr-transfected cell lines, the NIAID Tetramer Facility of the NIH AIDS Research and Reference Reagent Program for synthesis of Patr class I tetramers, and Dr. Lauren Holz and Mr. Warren Pan for critical reading of the manuscript. This work was supported by the NIDDK, NIH intramural research program to BR and by grant AIRC-10266 to VdR.
Financial Support: This study was supported by the NIDDK, NIH intramural research program.
Abbreviations
- HCV
hepatitis C virus
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffer saline
- ALT
alanine aminotransferase
Footnotes
Author Contributions: All authors designed and analyzed the study and critiqued the manuscript. ES, SC, SP, LC, VDR, AF performed experiments.
Financial Disclosures and Conflict of Interest Statement: A. Folgori, and A. Nicosia are named inventors on patent applications covering HCV vectored vaccines (WO 2006133911 (A3) Hepatitis C virus nucleic acid vaccine). Authors from Okairos are employees of and/or shareholders in Okairos, which is developing vectored HCV vaccines. The other authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Su-Hyung Park, Email: parksuhyung@niddk.nih.gov.
Eui-Cheol Shin, Email: ecshin@kaist.ac.kr.
Stefania Capone, Email: capone@okairos.it.
Laura Caggiari, Email: Lcaggiari@cro.it.
Valli De Re, Email: vdere@cro.it.
Alfredo Nicosia, Email: nicosia@okairos.it.
Antonella Folgori, Email: folgori@okairos.it.
Barbara Rehermann, Email: Rehermann@nih.gov.
References
- 1.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, Houghton M, Parham P, Walker CM. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–49. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 5.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99:15661–8. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 7.Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang XH, Ray SC, Thomas DL. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575–81. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, Rehermann B. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781–93. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–62. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 10.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–55. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmowalid GA, Qiao M, Jeong SH, Borg BB, Baumert TF, Sapp RK, Hu Z, Murthy K, Liang TJ. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci U S A. 2007;104:8427–32. doi: 10.1073/pnas.0702162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, Lahm A, Luzzago A, Vitelli A, Colloca S, Cortese R, Nicosia A. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–7. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 13.Rollier C, Depla E, Drexhage JA, Verschoor EJ, Verstrepen BE, Fatmi A, Brinster C, Fournillier A, Whelan JA, Whelan M, Jacobs D, Maertens G, Inchauspe G, Heeney JL. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J Virol. 2004;78:187–96. doi: 10.1128/JVI.78.1.187-196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youn JW, Hu YW, Tricoche N, Pfahler W, Shata MT, Dreux M, Cosset FL, Folgori A, Lee DH, Brotman B, Prince AM. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J Virol. 2008;82:10896–905. doi: 10.1128/JVI.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C virus: closing in on an evasive target. Expert Rev Vaccines. 2011;10:659–72. doi: 10.1586/erv.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puig M, Mihalik K, Tilton JC, Williams O, Merchlinsky M, Connors M, Feinstone SM, Major ME. CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology. 2006;44:736–45. doi: 10.1002/hep.21319. [DOI] [PubMed] [Google Scholar]
- 17.Shiina M, Rehermann B. Hepatitis C vaccines: Inducing and challenging memory T cells. Hepatology. 2006;43:1395–8. doi: 10.1002/hep.21210. [DOI] [PubMed] [Google Scholar]
- 18.Mizukoshi E, Nascimbeni M, Blaustein JB, Mihalik K, Rice CM, Liang TJ, Feinstone SM, Rehermann B. Molecular and immunological significance of chimpanzee major histocompatibility complex haplotypes for hepatitis C virus immune response and vaccination studies. J Virol. 2002;76:6093–103. doi: 10.1128/JVI.76.12.6093-6103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caggiari L, Simula MP, Marzotto A, Shiina M, Rehermann B, De Re V. Identification of novel chimpanzee MHC class I and II alleles using an improved sequence-based typing strategy. Hum Immunol. 2006;67:63–72. doi: 10.1016/j.humimm.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 21.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–72. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 22.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 23.Shin EC, Capone S, Cortese R, Colloca S, Nicosia A, Folgori A, Rehermann B. The kinetics of hepatitis C virus-specific CD8 T-cell responses in the blood mirror those in the liver in acute hepatitis C virus infection. J Virol. 2008;82:9782–8. doi: 10.1128/JVI.00475-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1–E2 envelope protein complexes. J Exp Med. 2003;197:633–42. [Google Scholar]
- 25.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A. 2004;101:10149–54. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farci P, Alter HJ, Wong DC, Miller RH, Govindarajan S, Engle R, Shapiro M, Purcell RH. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci U S A. 1994;91:7792–6. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–78. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Shin EC, Park SH, Demino M, Nascimbeni M, Mihalik K, Major M, Veerapu NS, Heller T, Feinstone SM, Rice CM, Rehermann B. Delayed induction, not impaired recruitment, of specific CD8 T cells causes the late onset of acute hepatitis C. Gastroenterology. 2011;141:686–95. 695, e1. doi: 10.1053/j.gastro.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sekaly RP, Bruneau J, Shoukry NH. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008;82:10017–31. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golden-Mason L, Burton JR, Jr, Castelblanco N, Klarquist J, Benlloch S, Wang C, Rosen HR. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44:1098–109. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- 31.Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–39. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 32.Rollier CS, Paranhos-Baccala G, Verschoor EJ, Verstrepen BE, Drexhage JA, Fagrouch Z, Berland JL, Komurian-Pradel F, Duverger B, Himoudi N, Staib C, Meyr M, Whelan M, Whelan JA, Adams VC, Larrea E, Riezu JI, Lasarte JJ, Bartosch B, Cosset FL, Spaan WJ, Diepolder HM, Pape GR, Sutter G, Inchauspe G, Heeney JL. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology. 2007;45:602–13. doi: 10.1002/hep.21573. [DOI] [PubMed] [Google Scholar]
- 33.Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, Amara RR. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–28. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin EC, Seifert U, Kato T, Rice CM, Feinstone SM, Kloetzel PM, Rehermann B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006–14. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–8. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.