SUMMARY
Adenosine 5′-triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD+) are key intracellular constituents involved in energy transfer and redox homeostasis in the cell. ATP is also released in the extracellular space and in the past half century it has been assumed to be the purinergic neurotransmitter in many systems including smooth muscle. In some smooth muscles (i.e., the human urinary bladder detrusor muscle) ATP does appear to be primarily released from nerves upon action potential firings, but in other smooth muscles (i.e., the human large intestine) ATP does not mimic the endogenous purine neurotransmitter. It was recently found that NAD+, another ubiquitous intracellular adenine nucleotide, also follows a regulated release in neurosecretory cells, vascular and visceral smooth muscles, and the brain. In some cases NAD+ fulfills pre- and postsynaptic criteria for a neurotransmitter better than ATP. Therefore, the purine hypothesis of neural regulation in smooth muscle is in need of reevaluation. This article will briefly review the current understanding of neuronal and extraneuronal release of purines in smooth muscle with emphasis on the roles of extracellular ATP and NAD+, and, further, will discuss more recent information about the likely involvement of multiple purines in smooth muscle neurotransmission.
Keywords: ATP release, NAD release, purine neurotransmission, smooth muscle, purine release, cotransmission, adenine nucleotides, extracellular purines
INTRODUCTION
The adenine nucleotides adenosine 5′-triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD+) are key intracellular constituents involved in energy transfer and redox homeostasis in the cell. In addition, evidence has been accumulating for constitutive and regulated release of adenine nucleotides (frequently referred to as purines) from excitable and non-excitable cells, their participation in cell-to-cell communication, and roles in complex processes including cell motility, cell survival, proliferation, membrane trafficking, and the immune response (1, 2, 3, 4, 5).
ATP is commonly assumed to be the purine neurotransmitter in the central and peripheral nervous systems (5, 6, 7, 8), but in some smooth muscles investigators question the identity of ATP as a neurotransmitter and refer to the actual transmitter as a purine or “purine-like” substance (e.g., 9, 10). The controversy may stem from the facts that i) more than one purine can be stored in and released from nerves in response to action potentials, ii) ATP does not always fulfill pre- and/or postsynaptic criteria for a neurotransmitter, and iii) conclusions about the neurotransmitter role of ATP are commonly based on evaluation of integrated postjunctional responses and not so much on direct measurements of purine release.
The past several decades have witnessed considerable advances in our knowledge about purinergic signaling in living systems (1, 5). The studies on cellular sources, mechanisms of release, postjunctional targets and functions of extracellular purines in smooth muscle, in particular, are hampered by the complex nature of smooth muscle tissues and the many possible roles of extracellular purines. Nevertheless, continual work has, during the last decade, expanded our view of the role of ATP and other purines (i.e., NAD+ and ADP-ribose, ADPR) in neurotransmission, and this analysis suggests that multiple purines likely contribute to the complex neural regulation of smooth muscle (11, 12, 13, 14), moving the field of purine neurotransmission beyond the sovereign role of ATP.
FUNCTIONAL COMPLEXITY OF SMOOTH MUSCLE
Smooth muscle (often referred to as ‘tunica muscularis’ or ‘muscularis propria’) is complex tissue commonly comprised of smooth muscle cells (myocytes), nerve cells and/or processes, glial cells, and several types of interstitial cells and forms the wall of most hollow, smooth muscle organs (i.e., blood vessels, urinary bladder, uterus, airways, and gastro-intestinal tract). The inner surfaces of smooth muscle organs are covered by a variety of epithelial cells (e.g., vascular endothelium, bladder urothelium, endometrium, airway epithelium, and gastric mucosa) that also contribute to the functional complexity of the smooth muscle wall. Virtually all cell types can release extracellular purines in response to mechanical stretch, cell swelling, inflammatory mediators, action potential firings, receptor activation or smooth muscle contraction (2, 4, 15). Purine (i.e., ATP) release pathways range from vesicle exocytosis to release through connexin and pannexin channels, P2X7 receptor pores, maxi ion channels, volume-regulated ion channels, and membrane transport (4, 6, 16). A single cell type could release ATP (and possibly other purines) via multiple pathways (17). Multiple purines are present in the cytosol and subcellular organelles and could potentially be released upon depolarizing or other stimuli. Finally, released transmitters can affect various cell targets (18) leading to complex tissue responses. All these factors contribute to the considerable complexity of purine regulation of smooth muscle.
PURINE-MEDIATED NON-ADRENERGIC NON-CHOLINERGIC NEUROTRANSMISSION
In the early 1960s it became clear that transmission between nerves and smooth muscle is mediated not only by the classical neurotransmitters acetylcholine (ACh) and norepinephrine, but also by non-adrenergic non-cholinergic (NANC) transmitters (19). The first claim regarding the identity of the NANC neurotransmitter in smooth muscle was made for ATP in the early 1970s (20). In the following decades evidence was accumulated for the presence of ATP in synaptic and secretory vesicles, co-release of ATP with classical neurotransmitters, presence of P2 receptors on cell membranes, and extracellular metabolism of ATP (5, 6, 15, 19, 21). Such findings, although originally obtained in single cell-type systems such as invertebrate motor neurons, cultured neurons, astrocytes, neuroendocrine cells, and isolated brain synaptosomes, have commonly been generalized to complex smooth muscle tissues assigning ATP as the universal purine neurotransmitter. In recent years additional purines (e.g., NAD+ and possibly ADPR) have also been proposed as candidate NANC neurotransmitters in smooth muscle (11, 22, 23, 24).
STORAGE OF PURINES IN SYNAPTIC VESICLES
Vesicle exocytosis has evolved as the main means of communication between cells in multicellular organisms. Neurotransmission is initiated at presynaptic terminals by fusion of synaptic vesicles with the plasma membrane and subsequent exocytotic release of chemical transmitters. Though classical criteria for transmitter status have been continually developed and broadened (25), a classical non-gaseous neurotransmitter is expected to be stored in and released from synaptic vesicles.
ATP is probably present in every synaptic and secretory vesicle (8, 15) and, because of this, is sometimes used as a universal tracer of cellular secretion events (26). It is suggested that ATP in vesicles is important for acidification of the vesicle lumen, for creating a proton gradient driving vesicular neurotransmitter uptake and for steps of exocytosis itself (15) in addition to serving neurotransmission (5). ATP is present in large dense-cored (LDCV) and small synaptic vesicles (SSV) as well as in chromaffin vesicles (13, 27). Loading of ATP occurs through all stages of vesicle formation and recycling and ATP is taken up by vesicles of both reserved and readily releasable pools (6). This heterogeneity of ATP-containing vesicles could explain differences in the mechanisms of release of ATP and other neurotransmitters. For example, significant differences between stoichiometry of ACh and ATP in synaptic vesicles and in the extracellular medium after release have been reported (15). Likewise, neural toxins differentially affect the release of ACh and ATP (15). In rat pheochromocytoma PC12 cells we found that both LDCV-like and SSV-like vesicles contained ATP and NAD+, but only the release of NAD+ was inhibited by cleavage of SNAP-25 with botulinum neurotoxin A (13). Together, these findings suggest that the presence of ATP in vesicles is not conclusive evidence for its role as a neurotransmitter. Moreover, ATP is not the only nucleotide localized in synaptic and secretory vesicles: ADP, AMP, GTP, UTP, diadenosine polyphosphates, and NAD+ have also been found present in vesicles (8, 13, 28) and therefore, each of them can be released during vesicle exocytosis. However, surprisingly little is known about the potential release of other nucleotides in response to action potentials and their roles in neural regulation of smooth muscle. Moreover, very little is known about the vesicular storage of NAD+, despite the ample information that is available about its intracellular distribution (e.g., 29).
Until a few years ago it was unclear how ATP is accumulated in secretory vesicles. SLC17A9 was recently proposed to be a vesicular nucleotide transporter (VNUT) and was found to transport ATP, ADP, GTP, Mg2+ and Ca2+ in chromaffin granules, brain vesicles or non-excitable cells (30, 31). It is presently unknown whether SLC17A9 also transports NAD+, ADPR, UTP, UDP, or diadenosine polyphosphates that are present in synaptic vesicles or, rather, these substances utilize additional transport mechanisms such as other specific transport proteins or passive diffusion through nonspecific cation channels. The role of VNUT in smooth muscle remains to be determined.
DETECTION OF RELEASED PURINES
Numerous techniques have been utilized to measure purine concentrations in biological samples (3, 32, 33, 34), but very few of them have been applied to measure release of purines in smooth muscle. In early studies loading tissues with radiolabeled adenosine and monitoring the release of [3H]-adenosine upon electrical field stimulation was the main approach to determining release of ATP in smooth muscle (20, 35). However, it is acknowledged that significant problems with specificity and source arise from relying on radioactive tracers.
The luciferin-luciferase chemiluminescence assay is a standard technique for measuring ATP in solutions, based on analyzing the bioluminescence of the ATP-dependent luciferase-mediated oxidation of luciferin (36). It is a rapid and highly-sensitive method, but is restricted to ATP; other purines that might have been released upon nerve stimulation remain overlooked. This methodology also requires careful calibration and optimization since many factors could interfere with the luciferase activity, including anions, anion transport inhibitors, ion channel blockers, bacterial contamination or P2 receptor antagonists (3, 33). This assay has been utilized to measure the evoked release of ATP in smooth muscles such as rodent vas deferens (37), but has not been commonly adopted in studies in other smooth muscles (7). The chemiluminescence assay in conjunction with single-photon imaging has recently been used to study ATP release from axons (33). The assay appears to be suitable for evaluation of temporal and spatial dynamics of ATP release in single cells, but has not yet been employed in complex tissues such as smooth muscle.
ATP biosensors may provide better spatial and temporal resolution than chemiluminescence measurements. Such probes have been used to measure ATP overflow in the central nervous system, skeletal muscle arterioles or gastric mucosa (34, 38), but purine biosensors have not yet received common utilization for purine measurements in smooth muscle.
A particularly suitable method for detecting neuronal and extraneuronal release of purines in smooth muscle is the reversed-phase gradient HPLC assay in conjunction with fluorescence detection (39), HPLC-FLD. This technique can detect femtomole amounts (40) of endogenous purines in tissue superfusates (11, 12, 22, 41). To increase detection sensitivity as compared with conventional UV absorbance detection methods (32) chloroacetaldehyde is used to form fluorescent 1,N6-etheno purine analogs, which can then be separated simultaneously from the same sample by reverse-phase HPLC-FLD. This ensures about six orders of magnitude greater sensitivity that the UV detection methods. To analyze co-eluting substances the HPLC-FLD method is occasionally coupled with HPLC fraction analysis (11, 12, 22, 23). Use of inhibitors of neural activity can determine the neuronal and extraneuronal fractions of released purines (22, 23). Disruption of members of the soluble NSF attachment receptor family (SNAREs) can be used to discriminate between release of purines by vesicle exocytosis and non-exocytosis mechanisms (e.g, 12). This excellent methodology has some shortcomings: it has relatively low temporal and spatial resolutions, and the absolute amounts of released purines are underestimated due to dilution of released transmitters in tissue superfusates. Nonetheless, HPLC-FLD detection of purine overflow has been instrumental in providing more direct information about the identity of released purines in smooth muscle than the integrated postjunctional responses to nerve stimulation or exogenous purines.
RECEPTOR TARGETS FOR PURINES IN SMOOTH MUSCLE
A typical neurotransmitter is synthesized in presynaptic neurons, is released by exocytosis from vesicles into the synaptic cleft, and elicits a response in the post-synaptic (post-junctional) target cell by binding to receptors on the plasma membrane (5, 25). Extracellular ATP acts on cell-surface receptors divided into two main classes: the ligand-gated ion receptor channels (P2X1–7) and the metabotropic G protein-coupled P2Y1,2,4,6,11–14 receptors (21, 42). P2X and P2Y receptors are expressed in most cell types and mediate a wide range of actions including smooth muscle contraction and relaxation (7, 21, 42). With some exceptions, smooth muscle contractile responses to nerve stimulation or exogenous ATP are mediated primarily by P2X1 receptors, whereas relaxation responses to nerve stimulation or ATP are commonly mediated by P2Y receptors. In the large intestine in particular, inhibitory purine neurotransmission appears to be mediated exclusively by the P2Y1 receptor coupled with activation of small-conductance potassium (SK) channels, causing inhibitory junction potentials, IJP (10, 23, 24, 25, 43). P2 receptors are found expressed not only on smooth muscle cells, but also on cell bodies and nerve terminals of enteric neurons (42) as well as on interstitial cells that are in close proximity to nerve varicosities (44), suggesting that released purines could activate multiple cell types in the effector syncytium. The immediate cell target for released purine neurotransmitter in smooth muscle remains to be elucidated.
In addition to ATP, adenine, pyridine and pyrimidine nucleotides (i.e., ADP, UTP, UDP, UTP-glucose, NAD+ and ADPR) can also activate P2 receptors (21, 22, 45). Therefore, the potential roles of these purines in smooth muscle neurotransmission should also be considered. For example, in the large intestine locally applied ATP and NAD+ cause membrane hyperpolarization similar to the IJPs elicited by the endogenous purine neurotransmitter, but only the responses to NAD+ are inhibited by P2Y1 receptor antagonists. Therefore, NAD+ mimics the effect of the endogenous purine neurotransmitter better than ATP (22, 23). ADPR, a primary extracellular NAD+ metabolite, also shows bioactivity similar to NAD+ and mimics the postjunctional effects of the endogenous neurotransmitter better than ATP (24). An interesting question remains –why is the hyperpolarization response to ATP, a known P2Y1 receptor agonist (21), not blocked by P2Y1 receptor antagonists? It is possible that ATP binds to multiple P2 receptors that mediate membrane hyperpolarization, whereas, despite the fact that NAD+ appears to be a less potent agonists of the guinea-pig P2Y1 receptor overexpressed in HEK293 cells than ATP (22), NAD+ (and ADPR) could be more selective activators of the P2Y1 receptors. Such observations also favor the concept that purine transmitters are released and bind receptors in a limited volume in the interstitium (i.e., neuro-effector junction, NEJ) and the P2Y1 receptors are probably the dominant receptors available for binding on the postjunctional cell membrane. More studies are needed to determine specific mechanisms of storage, uptake, release, receptor complement and action of NAD+ and ADPR in smooth muscle, but the initial investigations suggest that these purines may significantly contribute to the neural control of smooth muscle.
EVIDENCE FOR ATP AND NAD+ AS NEUROTRANSMITTERS IN SMOOTH MUSCLE
Caveats of using postjunctional responses to nerve stimulation as a measure of purine release
Perhaps the most common approach for investigating purine neurotransmission in smooth muscle is by evaluating responses of smooth muscle preparations to nerve stimulation by electrical field stimulation. Assumptions about release of ATP are commonly based on pharmacological characterizations of nerve-evoked smooth muscle contraction or relaxation (7, 46), changes in smooth muscle cell membrane potentials or currents (10, 47) or postjunctional Ca2+ transients (48) that are inhibited by P2 receptor antagonists. Since ATP is a potent agonist of all P2 receptors (21, 42), it is commonly assumed that responses that are blocked by inhibitors of P2 receptors are caused by released ATP. It should be emphasized, however, that these are largely integrated responses to released neurotransmitter(s) and/or their bioactive metabolites, that could be significantly affected by the desensitization of postjunctional receptors, modification of the driving forces for membrane currents, or rapid degradation of released purines (47). Such effects likely represent integrated responses of an effector syncytium that is composed of postjunctional cell targets electrically coupled with neighboring cells of the same or distinct cell types (18, 46, 49). Furthermore, commonly used pharmacological tools (i.e., synthetic receptor agonists and antagonists, enzyme inhibitors or stable ATP analogues) may not have sufficient selectivity or may not demonstrate the same receptor specificity as the endogenous neurotransmitter. Finally, as discussed above, P2 receptors can be stimulated by nucleotides in addition to ATP. Therefore, such methodologies can only provide complementary information about purine release to more direct approaches.
Action potential-evoked overflow of ATP and NAD+
Studies of purine overflow using the small-volume superfusion assay in combination with HPLC-FLD methodologies have made possible a number of key observations in smooth muscle. For example, temporal dissociation of the ATP and norepinephrine overflow in response to electrical field stimulation has suggested that in the guinea-pig vas deferens, in contrast to bovine chromaffin cells, ATP and norepinephrine might be released from different synaptic vesicles (41). Purine overflow measurements also lead to the discovery that stimulation of nerves in mesenteric blood vessels, bladder detrusor smooth muscle, and large intestine evokes concomitant release of NAD+ and ATP (11, 12, 22, 23). Importantly, striking differences in mechanisms of release of ATP and NAD+ were identified. For example, in the human bladder the overflow of ATP evoked by nerve stimulation was frequency-dependent, was significantly reduced by neuronal inhibitors, and required intact vesicle exocytosis machinery similar to the overflow of NAD+ (12); therefore, both ATP and NAD+ met presynaptic criteria for neurotransmitters in this tissue. On the other hand, in colonic muscles from mouse, monkey and human, only the evoked overflow of NAD+, but not of ATP, demonstrated characteristics of neuronal release (22, 23). In blood vessels inhibition of neuronal N-type voltage-dependent Ca2+ channels blocked the release of NAD+ (and norepinephrine), but not of ATP (50). Therefore, purine neurotransmission in smooth muscle is more complex than previously thought.
In smooth muscle, genuine neurotransmitters are expected to be released from synaptic vesicles concentrated in ‘active zones’ (nerve varicosities along the nerve processes), but not from cell bodies. Recent studies in primate colons demonstrated that in preparations containing only nerve processes with varicosities, where the actual release of neurotransmitters occurs, the overflow of NAD+, but not of ATP, showed correlation with neural activity (23) (Fig. 1). Several possibilities could explain why ATP release does not parallel neural activity. First, ATP could be released from both neuronal and non-neuronal sources, the latter being the major source of ATP release in colonic muscle. Second, ATP could be released from neurons, but mainly from cell bodies and less from nerve varicosities. Third, ATP could be released from neurons but by mechanisms not associated with vesicle exocytosis triggered by action potential firings and Ca2+ influx (15, 33). Finally, ATP could be released from non-neuronal sources (i.e., glia, myocytes, interstitial cells of Cajal or fibroblast-like cells) upon stimulation by released neurotransmitter(s) (e.g., receptor activation, smooth muscle contraction or cascade transmission) (15, 51, 52). Further studies are needed to test these possibilities. A more direct determination of neuronal release of purines would be to compare release of ATP and NAD+ from isolated smooth muscle nerve varicosities in response to depolarizing stimuli. Isolated gastrointestinal nerve varicosities have been used in studies on enteric neurotransmission (53), but simultaneous evaluation of depolarization-induced release of ATP, NAD+ and other purines has not been described. Likewise, no data on neurotransmitter storage and release from nerve varicosities or synaptic vesicles in other smooth muscles have been reported.
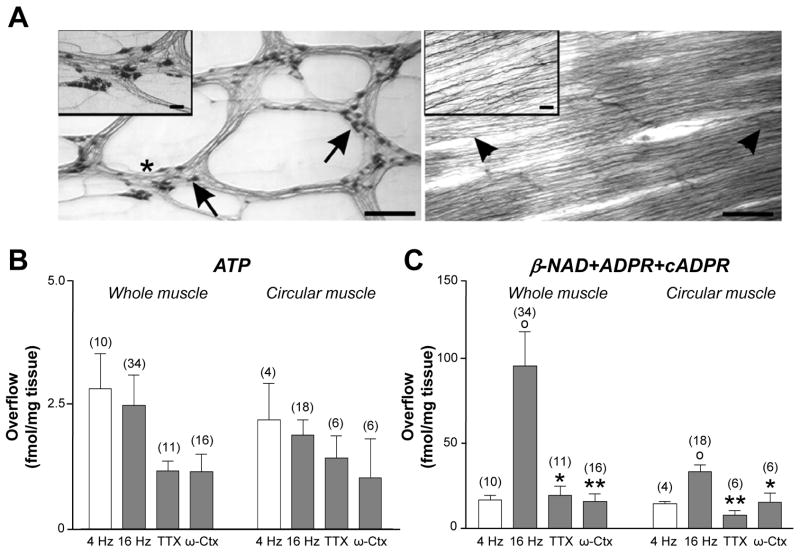
Figure 1. Nerve stimulation evokes release of ATP and NAD+ in whole and circular muscle preparations of primate colon.
(A) NADPH-diaphorase staining of longitudinal and circular muscle of monkey colon. Left panel shows NADPH-diaphorase+ neurons (arrows) in myenteric plexus longitudinal preparation. Inset is magnification showing neurons within myenteric ganglia (*). Right panel shows that circular muscle is free of ganglia. Nerve fibers parallel to circular muscle remain (arrow heads). Inset is magnification showing nerve fibers. Scale bars are 500 μm in both panels and 50 μm in insets. (B and C) ATP and β-NAD+ released from monkey whole muscle and circular muscle in response to electrical field stimulation (4 and 16 Hz, 0.3–0.5 ms pulse width) and with tetrodotoxin (TTX, 0.5 μmol/L) and ω-conotoxin-GVIA (ω-Ctx 50 nmol/L). Averaged data (fmol/mg tissue) are means ± SEM; (°) denote significant differences from 4 Hz controls (P<.05); (*) denote significant differences from 16 Hz controls (*P<.05, **P<0.01). The evoked release of ATP, in contrast to NAD+, showed no frequency-dependence and no sensitivity to neural toxins in both preparations. (Reproduced with permission from 23).
CELL TARGETS FOR NEUROGENIC ATP AND NAD+
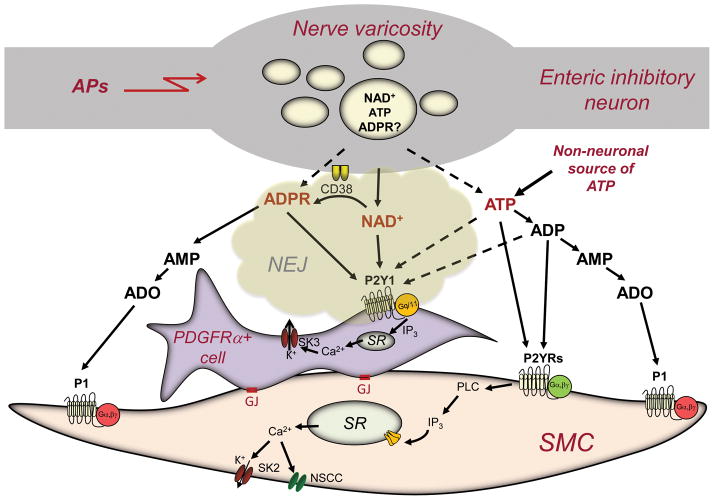
Classical thinking has defined the smooth muscle cells as the immediate targets for neurotransmitter action at the neuroeffector junctions (46), but in many smooth muscle tissues (e.g., gastrointestinal tract and bladder) neuroeffector junctions appear to be much more complicated than simple contact between nerve processes and myocytes (18). In gastrointestinal smooth muscles, in particular, the neuro-effector junction consists of synaptic-like contacts between nerve varicosities and intramuscular interstitial cells of Cajal or PDGFRα (+) fibroblast-like cells (18, 44). Importantly, PDGFRa(+) cells express receptors and ion channels required to mediate purinergic inhibitory responses (44, 49), whereas smooth muscle cells either appear to be far less responsive to purines (44) or respond to purines in manners that are not consistent with purinergic inhibitory neurotransmission (23). Therefore, the enteric purinergic neuroeffector junction may consist of nerve cells and fibroblast-like cells, which then transmit the signal to the smooth muscle cells likely via gap junctions, GJ (Fig. 2). Although some ATP may be released from synaptic vesicles, the main part of ATP appears to be released from non-neuronal sources. After release, both ATP and NAD+ (and possibly ADPR) are degraded to AMP and adenosine. Therefore, multiple purines likely affect multiple cell targets in the neuroeffector syncytium in smooth muscle. Fig. 2 depicts a theoretical model of the purine neuroeffector junction in colonic smooth muscle, based on the findings discussed above.
Figure 2. A simplified model of purine-mediated neurotransmission in the colon smooth muscle neuroeffector junction.
NAD+ is the primary neurotransmitter mediating purinergic inhibitory regulation of colon motility (22, 23). ADP-ribose (ADPR), a metabolite of NAD+, could also be a factor in inhibitory neurotransmission, either as a neurotransmitter or a metabolite of extracellular NAD+ (24). NAD+ is stored in and released from neural vesicles in nerve varicosities into the neuro-effector junction (NEJ) upon action potential firings (APs). NAD+ binds to postjunctional P2Y1 receptors (22, 43) on PDGFRα+ cells (44) and mediate activation of phospholipase (PLC), formation of inositol 1,4,5-trisphosphate (IP3), release of Ca2+ from intracellular stores (i.e., sarcoplasmic reticulum, SR) and activation of small-conductance K+ channels (SK3). Hyperpolarization transients (i.e., inhibitory junction potentials, IJPs) conduct to smooth muscle cells (SMC) via gap junctions (GJ) between PDGFRα+ cells and SMC (43). IJPs reduce excitability and cause muscle relaxation. NAD+ is rapidly degraded to ADPR by CD38 and/or an unknown metabolic pathway (24). ADPR is further degraded to adenosine 5′-monophosphate (AMP) and adenosine (ADO) by well-known pathways (not shown). ADPR also binds to postjunctional P2Y1 receptors and produces responses identical to NAD+ (24). ATP is also released in colonic muscles upon electrical field stimulation, but it appears to originate primarily from non-neuronal sources. ATP is quickly degraded to adenosine 5′-diphosphate (ADP), AMP, and ADO. ADO can activate P1 G-protein-coupled receptors on SMC membrane. ATP and ADP may participate in the post-junctional responses mediated by P2Y1 receptors (dotted arrows), but data shown in 24 suggest that ATP also binds to additional P2Y receptors (P2YRs) expressed by SMCs and possibly other cell types. Purine binding to P2YRs in SMCs activates SK channels and non-selective cation channels (NSCC). With cells at physiological gradients and potentials, the dominant responses of SMCs to ATP and NAD+ are activation of inward current (23). Thus, it is unlikely that IJPs due to transduction of the purinergic neurotransmitter signal could be mediated by SMCs.
CONCLUDING REMARKS
Purine neurotransmission has emerged as an important factor in determining smooth muscle motility. Moreover, ‘purinergic hyperactivity’ appears to be involved in disease states (e.g., 54) and purinergic signaling may contribute to development or maintenance of important human diseases such as hypertension, overactive bladder, and gastrointestinal motility disorders. Despite its importance and potential clinical significance, purinergic signaling in smooth muscle is not adequately understood. Much progress has been made in understanding the extracellular roles of ATP, but new evidence suggests that other purines may also significantly contribute to regulation of smooth muscle motility. With the acceptance of multiple purine involvement come the fundamental questions of how these substances engage in co-regulation of effector systems. Answers to these questions will identify new molecular targets for prevention and therapy of important human diseases.
Acknowledgments
We thank Dr. Kenton Sanders for helpful discussions. This work was supported by National Institute of Health Grant P01 DK41315.
Footnotes
The author declares no conflicts of interest.
References
- 1.Ziegler M, Niere M. NAD+ surfaces again. Biochemical Journal. 2004;382:e5–e6. doi: 10.1042/BJ20041217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billington RA, Bruzzone S, De Flora A, Genazzani AA, Koch-Nolte F, Ziegler M, Zocchi E. Emerging functions of extracellular pyridine nucleotides. Mol Med. 2006;12:324–327. doi: 10.2119/2006-00075.Billington. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012 doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 2010;199:93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 6.Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 7.Ralevic V. Purines as neurotransmitters and neuromodulators in blood vessels. Curr Vasc Pharmacol. 2009;7:3–14. doi: 10.2174/157016109787354123. [DOI] [PubMed] [Google Scholar]
- 8.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Serio R, Alessandro M, Zizzo MG, Tamburello MP, Mule F. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur J Pharmacol. 2003;460:183–190. doi: 10.1016/s0014-2999(02)02923-0. [DOI] [PubMed] [Google Scholar]
- 10.Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. AJP - Gastrointestinal and Liver Physiology. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- 11.Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of {beta}-Nicotinamide Adenine Dinucleotide upon Stimulation of Postganglionic Nerve Terminals in Blood Vessels and Urinary Bladder. Journal of Biological Chemistry. 2004;279:48893–48903. doi: 10.1074/jbc.M407266200. [DOI] [PubMed] [Google Scholar]
- 12.Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. {beta}-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. AJP - Renal Physiology. 2006;290:F486–F495. doi: 10.1152/ajprenal.00314.2005. [DOI] [PubMed] [Google Scholar]
- 13.Yamboliev IA, Smyth LM, Durnin L, Dai Y, Mutafova-Yambolieva VN. Storage and secretion of beta-NAD, ATP and dopamine in NGF-differentiated rat pheochromocytoma PC12 cells. Eur J Neurosci. 2009;30:756–768. doi: 10.1111/j.1460-9568.2009.06869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durnin L, Dai Y, Aiba I, Shuttleworth CW, Yamboliev IA, Mutafova-Yambolieva VN. Release, neuronal effects and removal of extracellular beta-nicotinamide adenine dinucleotide (beta-NAD(+)) in the rat brain. Eur J Neurosci. 2012;35:423–435. doi: 10.1111/j.1460-9568.2011.07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperlagh B, Vizi ES. Seminars in THE NEUROSCIENCES. Vol. 8. Academic Press Ltd; 1996. Neuronal synthesis, storage and release of ATP; pp. 175–186. [Google Scholar]
- 16.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 18.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010;588:4621–4639. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett MR. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Prog Neurobiol. 1997;52:159–195. doi: 10.1016/s0301-0082(97)00012-9. [DOI] [PubMed] [Google Scholar]
- 20.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 21.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. beta-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proceedings of the National Academy of Sciences. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. beta-Nicotinamide Adenine Dinucleotide Is an Enteric Inhibitory Neurotransmitter in Human and Nonhuman Primate Colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5′-diphosphate-ribose (ADPR) is a neural regulator in primate and murine large intestine along with beta-NAD+ J Physiol. 2012;20 doi: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- 26.Aspinwall CA, Yeung ES. Screening populations of individual cells for secretory heterogeneity. Anal Bioanal Chem. 2005;381:660–666. doi: 10.1007/s00216-004-2981-7. [DOI] [PubMed] [Google Scholar]
- 27.Winkler H, Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5:1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 29.Koch-Nolte F, Fischer S, Haag F, Ziegler M. Compartmentation of NAD+-dependent signalling. FEBS Lett. 2011;585:1651–1656. doi: 10.1016/j.febslet.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson M, Sawada K, Morland C, Hiasa M, Ormel L, Moriyama Y, Gundersen V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex. 2012;22:1203–1214. doi: 10.1093/cercor/bhr203. [DOI] [PubMed] [Google Scholar]
- 32.Manfredi G, Yang L, Gajewski CD, Mattiazzi M. Measurements of ATP in mammalian cells. Methods. 2002;26:317–326. doi: 10.1016/S1046-2023(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 33.Fields RD. Imaging single photons and intrinsic optical signals for studies of vesicular and non-vesicular ATP release from axons. Front Neuroanat. 2011;5:32. doi: 10.3389/fnana.2011.00032. Epub;%2011 Jun 6., 32- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dale N, Frenguelli BG. Measurement of purine release with microelectrode biosensors. Purinergic Signal. 2012;8:27–40. doi: 10.1007/s11302-011-9273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White TD. Role of adenine compounds in autonomic neurotransmission. Pharmacol Ther. 1988;38:129–168. doi: 10.1016/0163-7258(88)90095-2. [DOI] [PubMed] [Google Scholar]
- 36.Wood KV, Lam YA, McElroy WD. Introduction to beetle luciferases and their applications. J Biolumin Chemilumin. 1989;4:289–301. doi: 10.1002/bio.1170040141. [DOI] [PubMed] [Google Scholar]
- 37.Kirkpatrick K, Burnstock G. Sympathetic nerve-mediated release of ATP from the guinea-pig vas deferens is unaffected by reserpine. Eur J Pharmacol. 1987;19:138, 207–214. doi: 10.1016/0014-2999(87)90434-1. [DOI] [PubMed] [Google Scholar]
- 38.Patel BA, Rogers M, Wieder T, O’Hare D, Boutelle MG. ATP microelectrode biosensor for stable long-term in vitro monitoring from gastrointestinal tissue. Biosens Bioelectron. 2011;26:2890–2896. doi: 10.1016/j.bios.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 39.Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- 40.Bobalova J, Bobal P, Mutafova-Yambolieva VN. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Analytical Biochemistry. 2002;305:269–276. doi: 10.1006/abio.2002.5667. [DOI] [PubMed] [Google Scholar]
- 41.Todorov LD, Mihaylova-Todorova S, Craviso GL, Bjur RA, Westfall DP. Evidence for the differential release of the cotransmitters ATP and noradrenaline from sympathetic nerves of the guinea-pig vas deferens. J Physiol (Lond) 1996;496:731–748. doi: 10.1113/jphysiol.1996.sp021723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 43.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don KS, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gustafsson AJ, Muraro L, Dahlberg C, Migaud M, Chevallier O, Khanh HN, Krishnan K, Li N, Islam MS. ADP ribose is an endogenous ligand for the purinergic P2Y1 receptor. Mol Cell Endocrinol. 2011;333:8–19. doi: 10.1016/j.mce.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Burnstock G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol. 2009;49:1–30. doi: 10.1146/annurev.pharmtox.052808.102215. [DOI] [PubMed] [Google Scholar]
- 47.Stjarne L. Novel dual ‘small’ vesicle model of ATP- and noradrenaline-mediated sympathetic neuromuscular transmission. Autonomic Neuroscience. 2001;87:16–36. doi: 10.1016/S1566-0702(00)00246-0. [DOI] [PubMed] [Google Scholar]
- 48.Brain KL. Neuroeffector Ca2+ transients for the direct measurement of purine release and indirect measurement of cotransmitters in rodents. Exp Physiol. 2009;94:25–30. doi: 10.1113/expphysiol.2008.043679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanderwinden JM, Rumessen JJ, de Kerchove dA, Jr, Gillard K, Panthier JJ, de Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–358. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- 50.Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. N-type and P/Q-type calcium channels regulate differentially the release of noradrenaline, ATP and [beta]-NAD in blood vessels. Neuropharmacology. 2009;56:368–378. doi: 10.1016/j.neuropharm.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vizi ES, Sperlagh B, Baranyi M. Evidence that ATP released from the postsynaptic site by noradrenaline, is involved in mechanical responses of guinea-pig vas deferens: cascade transmission. Neuroscience. 1992;50:455–465. doi: 10.1016/0306-4522(92)90437-7. [DOI] [PubMed] [Google Scholar]
- 52.Gulbransen BD, Bains JS, Sharkey KA. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci. 2010;30:6801–6809. doi: 10.1523/JNEUROSCI.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jonakait GM, Gintzler AR, Gershon MD. Isolation of axonal varicosities (autonomic synaptosomes) from the enteric nervous system. J Neurochem. 1979;32:1387–1400. doi: 10.1111/j.1471-4159.1979.tb11076.x. [DOI] [PubMed] [Google Scholar]
- 54.Haddock RE, Hill CE. Sympathetic overdrive in obesity involves purinergic hyperactivity in the resistance vasculature. J Physiol. 2011 doi: 10.1113/jphysiol.2011.207944. [DOI] [PMC free article] [PubMed] [Google Scholar]