Abstract
Background & Aims
Altered gastrointestinal motility is associated with significant morbidity and health care costs. Toll-like receptors regulate intestinal homeostasis. We examined the roles of Toll-like receptor (TLR)4 signaling in survival of enteric neurons and gastrointestinal motility.
Methods
We assessed changes in intestinal motility by assessing stool frequency, bead expulsion, and isometric muscle recordings of colonic longitudinal muscle strips from mice that do not express TLR4 (Tlr4Lps-d or TLR4−/−) or Myd88 (Myd88−/−), in wild-type germ-free mice or wild-type mice depleted of the microbiota, and in mice with neural crest-specific deletion of Myd88 (Wnt1Cre+/−/Myd88fl/fl). We studied the effects of the TLR4 agonist lipopolysaccharide (LPS) on survival of cultured, immortalized fetal enteric neurons (IM-FEN) and enteric neuronal cells isolated from wild-type and Tlr4Lps-d mice at embryonic day 13.5.
Results
There was a significant delay in gastrointestinal motility and reduced numbers of nitrergic neurons in TLR4Lps-d, TLR4−/−, and Myd88−/− mice, compared with wild-type mice. A similar phenotype was observed in germ-free mice, mice depleted of intestinal microbiota, and Wnt1Cre+/−/Myd88fl/fl mice. Incubation of enteric neuronal cells with LPS led to activation of the transcription factor NF-κB and increased cell survival.
Conclusions
Interactions between enteric neurons and microbes increases neuron survival and gastrointestinal motility in mice. LPS activation of TLR4 and NF-κB appears to promote survival of enteric neurons. Factors that regulate TLR4 signaling by neurons might be developed to alter gastrointestinal motility.
Keywords: intestinal transit, microbiota, apoptosis, mouse model
Toll Like receptors (TLRs) are pattern recognition receptors involved in innate immune responses and are highly conserved in species such as Drosophila and humans1. TLRs recognize molecules of microbial origin, called microbial-associated molecular patterns (MAMPs). Recently TLRs have been shown to have a novel function in controlling intestinal epithelial homeostasis and protecting epithelial injury2. Inflammation and altered intestinal homeostasis underlie several diseases affecting the gastrointestinal tract including diabetes and inflammatory bowel disease. During a study performed to understand the role of TLRs in the pathophysiology of gastrointestinal motility disorders in diabetes we noticed a distinct phenotype in the TLR4 mutant mice (Tlr4Lps-d) with a significant reduction in stool pellet frequency compared to controls. This led us to study the role of TLR4 in regulating intestinal enteric neurons and motility. Gastrointestinal motility disorders are associated with significant morbidity and a key reason for outpatient clinic visits.
TLRs are expressed in the cells of the immune system, however recently several studies have demonstrated the presence of TLRs in neurons of the central nervous system3 and they have been implicated in neurodegeneration4. TLR 3, 4, and 7 expression has been demonstrated in the human enteric nervous system5. TLR4 detects lipopolysaccharide (LPS), a major outer membrane component of Gram-negative bacteria and can signal through the NF-κB pathways resulting in the production of proinflammatory cytokines.
Several studies have demonstrated the role of TLRs in mediating neuronal injury. Human neurons express TLR3 and have a role in the neurodegeneration that occurs with inflammation6, 7. Cerebral cortical neurons express TLR2 and TLR4 and are activated in response to energy deprivation and ischemia8. Mouse hippocampal neurons are protected from ischemia induced damage in TLR4−/− mice9. Neurons lacking functional TLR2 or TLR4 exhibit increased resistance to death by energy deprivation and stroke10. TLR4 expression has been identified on sensory neurons and involved in pain modulation11.
TLR4 expression has been demonstrated in the myenteric plexus of the murine jejunum. However, its function in these neurons has not been examined12. Enteric neurons form a complex network of neurons within the gastrointestinal tract, populating the submucosa and muscularis externa. The enteric neurons release neurotransmitters such as acetylcholine, nitric oxide, ATP, VIP and serotonin. The enteric nervous system can also be exposed to bacterial products during times of compromised barrier function. Intestinal bacterial products can translocate, circulate and affect distant organs such as the bone marrow13. In Parkinson’s disease there is evidence for increased intestinal permeability with enhanced bacterial translocation into the intestinal mucosa and circulation14. In the colon bacterial products have been shown to activate nociceptive dorsal root ganglion neurons15.
The role of TLR4 in the enteric nervous system and how it can modulate intestinal motility has not been studied. In rodent models of sepsis damage to enteric neurons due to LPS has been proposed. Rat myenteric neurons exposed to high doses of LPS in culture undergo cell death16. We sought to examine the role of TLR4 signaling in enteric neuronal survival and in the modulation of gastrointestinal motility disturbances. To do this we used both in vitro and in vivo models, Tlr4Lps-d, TLR4−/− and Myd88−/− mice, germ free mice and mice with enteric neuronal specific deletion of Myd88 that we generated. In these models we assessed TLR4 signaling, enteric neuronal apoptosis and associated changes in intestinal motility.
Materials and Methods
Animals
C3H/HeJ (spontaneous mutation in TLR4 gene, Tlr4lps-d), C3H/HeOuJ mice (controls for TLR4 mutant mice), and TLR4−/− mice on BL/10 background were obtained from Jackson Laboratories (Bar Harbor, Maine). Myd88−/− mice on BL/6 background and their controls were bred in Emory animal facility. Swiss Webster Germfree mice and conventional mice were obtained from Taconic (Hudson, NY). Hemizygous for Tg (Wnt1-cre) male mice were bred with noncarrier (wild type) female mice to obtain Wnt1-Cre transgenic mice that express Cre recombinase. Female or male Heterozygous for Myd88tm1Defr were bred to obtain heterozygous or homozygous mice that possess loxP sites on either side of exon 3 of the targeted gene. These mutant mice were bred to mice that express Cre recombinase to obtain offspring with exon 3 deleted in the cre-expressing tissue (Wnt1Cre+/−/Myd88fl/fl). Wnt1Cre−/−/Myd88fl/fl or Wnt1Cre−/−/Myd88fl/− mice were used as controls. The original breeding pairs were obtained from Jackson Laboratories. All experimental mice used were 8–12 weeks old male mice. All animal studies were approved by the Emory University Institutional Review Board.
Reagents
Guanethidine Sulfate (TCI America, Portland, OR), DNAse I (Worthington Biochemical, Lakewood, NJ), Limulus Amebocyte Lysate (LAL) kit (Lonza, Walkersville, MD), Histogene LCM staining kit and Picopure RNA isolation kit (Arcturus, Life Technologies Corporation, Carlsbad, CA), Sensiscript RT kit, QIAamp DNA Stool Mini Kit, QuantiFast SYBR Green PCR Kit (Qiagen, Valencia, CA), and Duoset ELISA kits (R & D Systems). All the other reagents were obtained from Sigma (St. Louis, MO).
Antibodies
TUJ1 (Covance, Princeton, NJ), PGP9.5 (Abcam, Cambridge, MA), MyD88 (ENZO Life Sciences, Farmingdale, NY), nNOS and ChAT (Millipore, Billerica, MA), Cleaved caspase-3, NF-κB p65 and Phospho-NF-κB p65 (Ser536) (93H1) (Cell Signaling Technology, Danvers, MA). Alexa fluor secondary antibodies (Life Technologies Corporation).
Assessment of colonic emptying by stool frequency and bead expulsion test
Stool output measurement was done as described previously17. Distal colonic transit and emptying was assessed by bead expulsion test, done as described previously18.
Intestinal transit assessment
Intestinal transit was determined by assessing the distribution of 70-KDa FITC conjugated dextran in the intestines of mice as described previously19. Cecum was collected as the 11th segment. Transit was analyzed using the intestinal geometric center of the distribution of dextran throughout the intestine or colon and was calculated20.
Isometric colonic muscle strip recording
Longitudinal muscle strips (with myenteric plexus) were obtained from the proximal colon of mice. Recording parameters for longitudinal muscle strips has been established in our laboratory. Colonic relaxation was recorded by electrical field stimulation (EFS) (24 V, 4 Hz, 0.03 msec pulse for duration of 20 s). Strips were pre-contracted with 5-hydroxytryptamine (10 μM) for 30 s before EFS. Colonic contraction was measured using EFS (24 V, 10 Hz, 0.3 msec pulse for duration of 20 seconds). Contraction or relaxation was expressed as a % change from baseline muscle tone21.
Whole mount tissue staining
Proximal colon was used for NADPH Diaphorase and Acetyl Choline staining and was performed and analyzed as described previously21. Proximal, mid and distal colon diameter was assessed in WT and Tlr4Lps-d mice (n=3 mice in each group). Longitudinal muscle strips (with myenteric plexus) from distal ileum were fixed in 4% paraformaldehyde, blocked for 1h in PBS containing 0.3% Triton X-100 and 5% Normal Donkey Serum (NDS) and incubated with Rb nNOS (1:200), Rb TUJ1 (1:750) or Gt ChAT (1:100) antibodies in PBS containing 1.5% NDS, 0.3% Triton X-100 and 0.01% sodium azide,72h at RT. Secondary detection was performed by incubation with Anti-Gt IgG (1:500) or Anti-Rb IgG (1:200) conjugated to Alexa Fluor 594 antibody.
Immunohistochemistry
Paraffin sections of distal ileum from Wnt1Cre+/−/Myd88fl/fl, Wnt1Cre−/−/Myd88fl/fl, Wnt1Cre−/−/Myd88fl/− mice were blocked in PBS containing 3% BSA and 0.02% Triton X-100 and then incubated with MyD88 antibody (1:200) overnight, 4°C. Secondary detection was performed by incubation with Anti-Rb IgG (1:500) conjugated to Alexa Fluor 594 antibody.
Neuronal cell preparation
The intestines of embryos from E13.5 pregnant WT and Tlr4lps-d mice were used for the enteric neuronal preparation as described previously with slight modification of the protocol as mentioned22.
Immunocytochemistry
Enteric neurons were fixed and stained using standard immunostaining protocols as described previously18. Primary antibodies, Cleaved caspase-3 (1:200) and PGP9.5 (1:500) were incubated overnight at 4°C. Secondary detection was performed by incubation with appropriate Alexa Fluor conjugated antibodies.
Measurement of LPS in mouse serum
The concentration of LPS in mouse serum was detected by using Limulus amebocyte lysate (LAL) assay according to the instructions provided by the manufacturer. Endotoxin concentration of the serum samples was measured by plotting endotoxin standard graph (0.1–1.0 EU/ml), using standards provided in the kit.
Quantification of total bacterial content in mouse stool
Bacterial total DNA was isolated from WT and Tlr4lps-d mice (treated with antibiotics for 2 weeks) stool using QIAamp DNA Stool Mini Kit. DNA was subjected to qPCR using global 16S rRNA primers: forward primer (8F) 5′-AGAGTTTGATCCTGGCTCAG-3′, and reverse primer (338R) 5′-CTGCTGCCTCCCGTAGGAGT-3′ to measure the total bacteria23.
Laser capture microscopy (LCM)
Proximal colon cryosections from Wnt1Cre−/−/Myd88fl/fl and Wnt1Cre+/−/Myd88fl/fl mice were stained using the Histogene LCM staining kit (Arcturus) to visualize the ganglia. The microdissection laser (PixCell IIe; Arcturus) was used as described previously to isolate ganglia19. Myd88 expression in the enteric ganglia was measured by real time PCR using Myd88 primers (FW 5′-AGAAAAGGTGTCGCCGCATGGT-3′, RV 5′-AGTCGCTTCTGTTGGACA CCTGGA-3′).
Western Blotting
Cell lysates obtained from IM-FEN cells were treated with or without LPS (10–1000 ng/ml) for 24 hr and Western blot analysis performed using standard methods. A semiquantitative measurement of the band intensity was performed using the Scion Image computer software program.
Food intake and body weight
Tlr4Lps-d mice, Myd88−/− mice, and age and gender matched WT mice were placed in individual cages and given a known amount of food. Food intake was monitored daily over a one week period. Throughout the experiment, mice were monitored for body weight.
Statistical Analysis
Statistics was done with the Student t-test or with the one-way analysis of variance (ANOVA) (GraphPad software). P value ≤0.05 was considered statistically significant.
Results
Tlr4Lps-d mice exhibit delayed intestinal motility
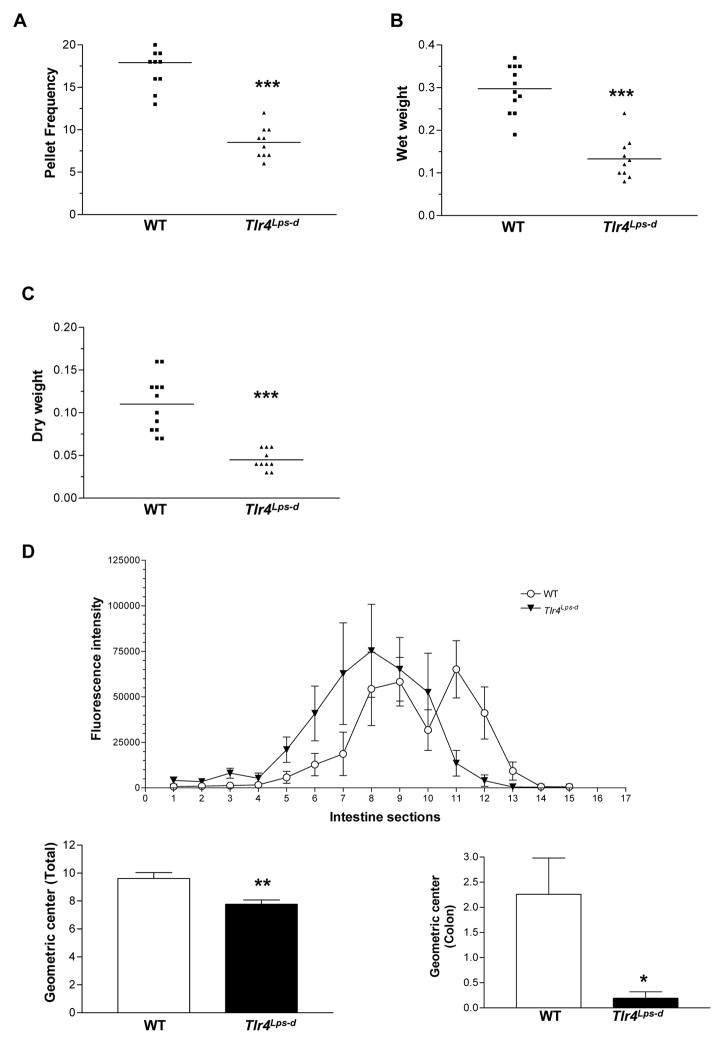
We first examined the role of TLR4 in modulating gastrointestinal motility in vivo. We used the C3H/HeJ mice, which have a spontaneous mutation at the LPS response locus (mutation in toll-like receptor 4 gene, Tlr4Lps-d) making them hyporesponsive to LPS. We observed that even though the body weight of Tlr4Lps-d mice was significantly lower than controls (C3H/HeOuJ, WT) (Supplemental Figure 1A), there was no difference in their food intake (Supplemental Figure 1B). The frequency of bowel movements, measured as an average number of fecal pellets extruded per hour, was significantly lower in Tlr4Lps-d mice compared to WT mice. Accordingly, the wet weight and the dry weight were also significantly lower in Tlr4Lps-d mice (Figure 1, A–C). Difference in wet weight of Tlr4Lps-d was 55.30 ± 5.09 % compared to WT and dry weight of Tlr4Lps-d was 59.10 ± 3.39 % compared to WT mice, P>0.05. Intestinal transit (measured as a relative distribution of FITC-dextran fluorescence) was also found to be delayed in these mice, as noted by a significantly lower intestinal geometric center (Figure 1D). In the WT mice, the geometric center was shifted to the right, indicating a better rate of intestinal and colonic propulsion with the peak accumulation of dye in the cecum, compared to the 8th segment of the small intestine in the Tlr4Lps-d mice. The bead expulsion time, a measure of colonic motility was longer in the Tlr4Lps-d mice compared to the WT mice (Data not shown). To examine the relative contribution of hematopoietic cells in inducing motility changes we performed bone marrow transplantation experiments. Four weeks after the bone marrow transplantation, the frequency of bowel movements, colonic motility as assessed by the colonic bead expulsion (WT to WT, 5.2 ± 1.2 min; Tlr4Lps-d to WT, 5.1 min ± 1.2 min, n=5) and intestinal transit was similar in WT mice given either WT or Tlr4Lps-d bone marrow. Stool frequency, colonic motility and intestinal transit was lower in Tlr4Lps-d given marrow from Tlr4Lps-d mice (Supplemental Figure 2, A and B). Similar results were obtained at 6 and 8 weeks after transplantation. These results suggest that TLR4 expressed in non-hematopoietic cells is involved in the gut motility functioning.
Figure 1. Delayed intestinal transit in Tlr4Lps-d mice.
(A–C) Stool characteristics studied to assess rate of intestinal motility include stool frequency, wet weight and dry weight. (D) Distribution of FITC in the segments of the small intestine (1–10), cecum (11), and colon (12–15), n=10. Results are mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Lack of TLR4 is associated with reduced nitrergic colonic relaxation and decreased number of nNOS neurons
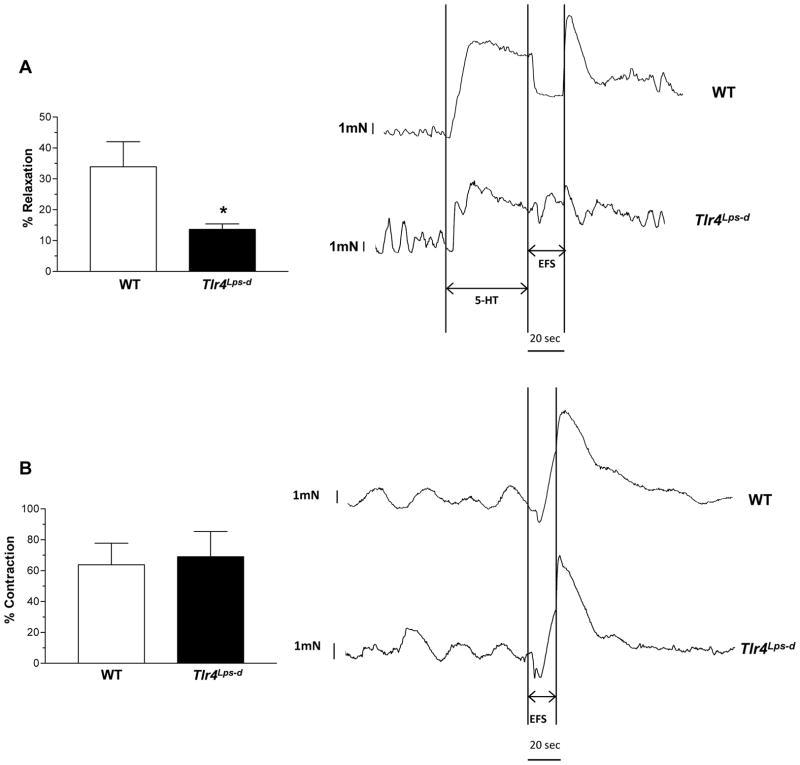
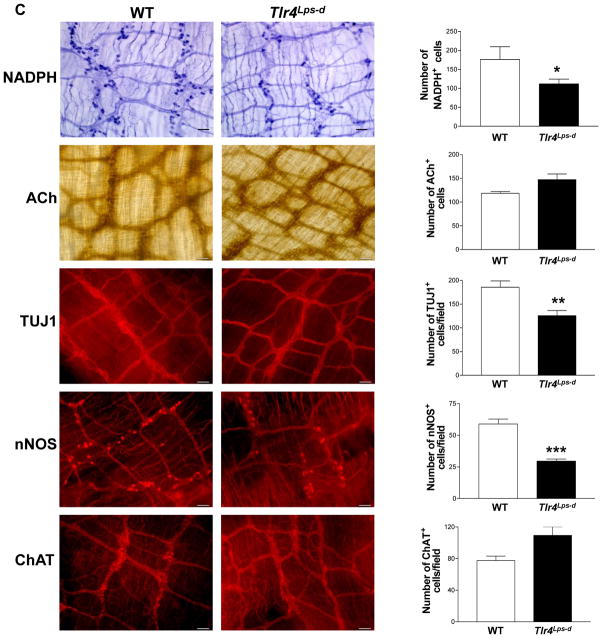
To determine whether the delayed intestinal transit is due to selective changes of specific subtypes of neurons we assessed the nitrergic and cholinergic components of colonic longitudinal muscle strips from WT and Tlr4Lps-d mice. We found a reduction in electrical field stimulation (EFS) induced relaxation in Tlr4Lps-d mice compared to WT mice (Figure 2A) but no difference in contractile responses (Figure 2B). Consistent with this observation we found a reduction in intestinal nitrergic neurons stained with NADPH diaphorase (colon) or nNOS (ileum) specific antibody, and the total number of intestinal neurons (neuronal marker, TUJ1) in Tlr4Lps-d mice compared to WT mice, with no difference in cholinergic neurons assessed by Acetyl choline (ACh) or ChAT staining (Figure 2C). There was no difference in the mid (WT, 1.90 ± 0.06 mm; Tlr4Lps-d, 1.91 ± 0.16 mm) and distal colon diameter of the mice (WT, 2.21 ± 0.06 mm; Tlr4Lps-d, 2.07 ± 0.24 mm). Proximal colon diameter of Tlr4Lps-d mice was slightly higher than the WT mice, but not statistically significant (WT, 1.60 ± 0.01; Tlr4Lps-d, 1.91 ± 0.18, n=3).
Figure 2. Reduced nitrergic responses in Tlr4Lps-d mice.
(A) Tlr4Lps-d mice exhibit decreased colonic nitrergic relaxation in response to nerve stimulation compared to WT mice. (B) No difference in contractile responses was seen between WT and Tlr4Lps-d mice. Representative tracings are shown next to the histograms. The y axis represents force (mN); x axis represents time. Arrows indicate time points at which electrical field stimulus (EFS) was switched on and off. Results are expressed as percentage relaxation or contraction with respect to basal tone. (C) Representative photographs and histograms for proximal colon whole mount staining for NADPH Diaphorase, Acetylcholinesterase, and distal ileum whole mount staining for TUJ1, nNOS and ChAT are shown. The number of neurons stained for a specific marker was determined per unit area. Scale bar: 50 μm. Results are mean ± SE. n=6. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine if the delayed gastrointestinal motility in the Tlr4Lps-d mice could also be seen in the TLR4 knock-out mice (TLR4−/−) on BL/10 background we performed similar experiments for assessment of gastrointestinal motility. We found the number of pellets extruded per hour to be significantly lower in TLR4−/− mice on BL/10 background similar to the Tlr4Lps-d mice (Supplemental Figure 3).
Lack of MyD88 is associated with loss of nNOS neurons and delayed gastrointestinal motility
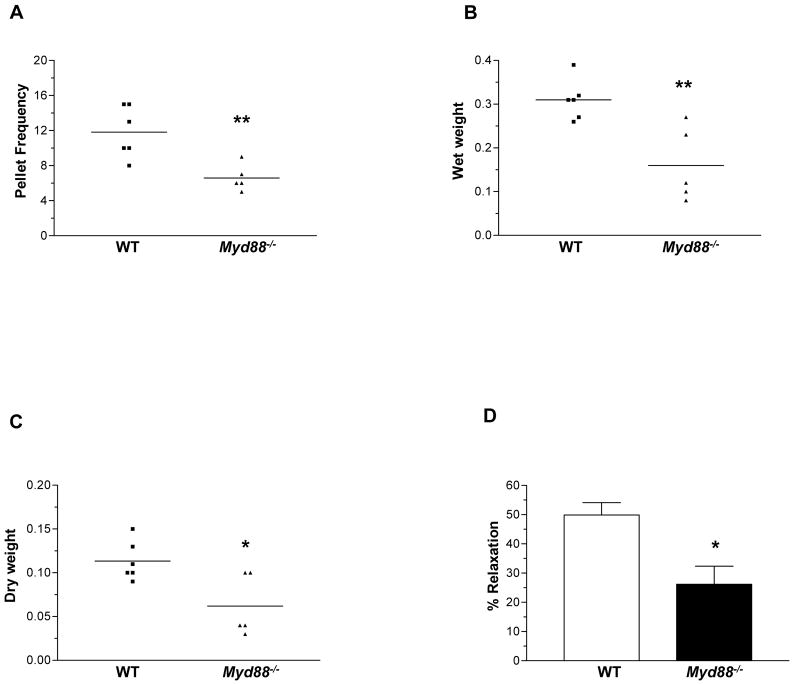
TLR4 is known to signal predominantly through the adaptor molecule Myd88. We next determined if the motility phenotype seen in Tlr4Lps-d mice and TLR4−/− mice was also seen in the Myd88−/− mice. There was no difference in the food intake between WT and Myd88−/− mice (Supplemental Figure 4A). We found lower stool frequency, stool wet and dry weight in the Myd88−/− compared to WT mice (Figure 3, A–C). In addition, Myd88−/− mice exhibited decreased colonic muscle relaxation (Figure 3D). The number of nitrergic and total neurons but not cholinergic neurons was reduced in the Myd88−/− mice compared to controls, similar to the TLR4−/− mice (Supplemental Figure 4, B–D).
Figure 3. Myd88−/− mice have delayed colonic transit and reduced nitrergic neurons.
(A–C) Stool characteristics studied to assess rate of intestinal motility include stool frequency, wet weight and dry weight. (D) Myd88−/− mice exhibit decreased nitrergic relaxation in response to Electrical Field stimulation. Results are mean ± SE. n=5 or 6. *P < 0.05, **P < 0.01.
Germ free mice have reduced colonic nitrergic neurons
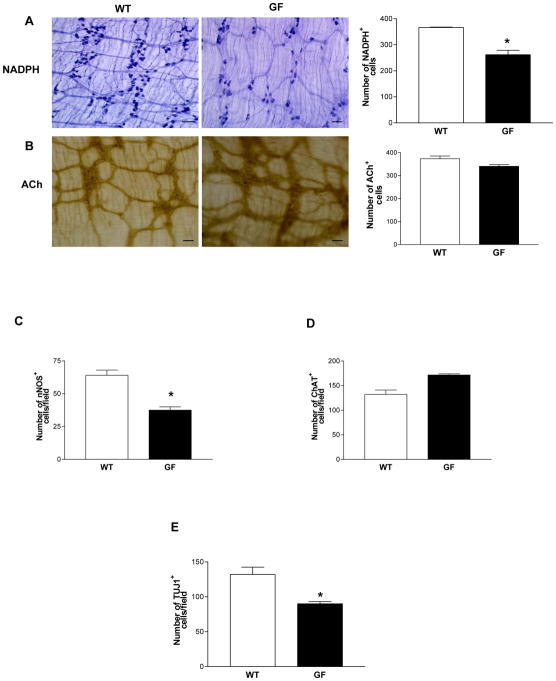
Gut microbiota can signal through TLRs. Our data demonstrated a role for altered TLR4 signaling in influencing enteric neuronal survival and modulating motility. To further examine the role of gut microbiota on the enteric neuronal survival, we assessed enteric neuronal subtypes in four week old germ free mice. We found reduced nitrergic and total neurons (Figure 4, A, C and E) with no change in the cholinergic neurons (Figure 4, B and D) in germ free mice compared to their controls.
Figure 4. Germ free mice have reduced colonic nitrergic neurons.
(A and B) Proximal colonic whole mount preparations stained for NADPH diaphorase and Acetylcholinesterase. (C–E) Distal ileum immunohistochemistry for the expression of nNOS, ChAT and TUJ1. The number of neurons stained for a specific marker was determined per unit area. At least 10 random fields were scored in a blinded fashion. Scale bar: 50 μm. Results are mean ± SE. n=6. *P < 0.05.
Reduction of intestinal microbiota leads to reduced nNOS expression and delayed GI motility
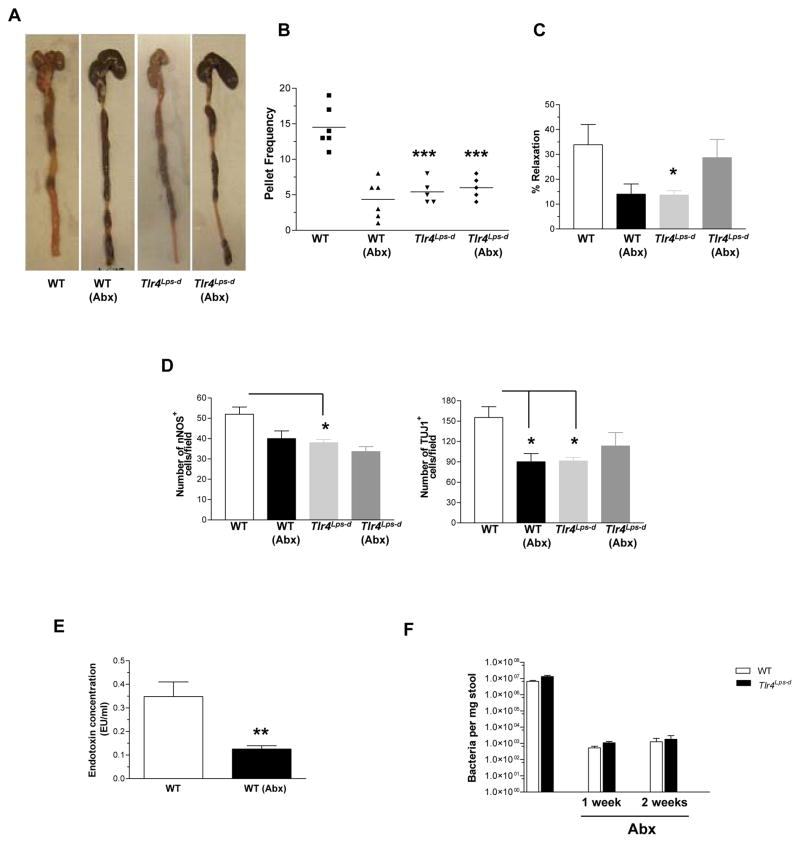
To further examine the role of gut microbiota on gastrointestinal motility, we treated WT and Tlr4Lps-d mice with broad spectrum antibiotics (ampicillin 1g/L and neomycin 0.5g/L) for twelve weeks. We hypothesized that changes in the gut microbiota may lead to alterations in gastrointestinal motility through reduced TLR4 activation. After twelve weeks of treatment with antibiotics the WT mice had slower gastrointestinal motility. This was not seen in the Tlr4Lps-d mice treated with antibiotics (Figure 5B and supplemental figure 5 A–C). There was evidence of stool retention in the WT mice treated with antibiotics consistent with delayed colonic motility (Figure 5A). Further the phenotype of the WT mice treated with antibiotics was similar to the Tlr4Lps-d mice in that they had lower nitrergic relaxation (Figure 5C) and reduced nitrergic and total neurons (Figure 5D). There was no difference in the cholinergic neuron expression (data not shown). These data indicate that bacterial products through activation of TLR4 can mediate changes in gastrointestinal motility. To correlate the effects of antibiotics on modulating endotoxin levels we measured the concentration of endotoxin in the serum of control and antibiotic treated mice. As seen in Figure 5E the endotoxin levels in antibiotic treated WT mice was significantly lower than untreated, WT mice. The levels of endotoxin in the WT mice are reflective of the normal gut microbiota and are significantly less than the levels seen in sepsis24. The efficacy of the antibiotics was verified by the dramatic (> 1000 fold reduction) lowering of total bacterial loads in mice stool (Figure 5F). Studies have shown that antibiotic treatment reduced the bacterial load by approximately 90%23.
Figure 5. Alteration of intestinal microbiota results in delayed colonic motility.
WT and Tlr4Lps-d mice treated with water or antibiotics (ampicillin and neomycin) for twelve weeks were studied to assess rate of gastrointestinal motility. Parameters measured include (A) Stool retention, (B) Pellet number, and (C) Nitrergic relaxation in response to EFS. (D) Whole mount staining data for nNOS and TUJ1 positive neurons. (E) Endotoxin measurement in WT mice in the presence or absence of antibiotic treatment. (F) Total bacterial content in the stool of mice treated with or without antibiotics. Values are mean ± SE. n = 5 or 6. *P < 0.05; **P < 0.01; ***P < 0.001.
Enteric neuronal specific loss of TLR4 signaling leads to delayed colonic motility
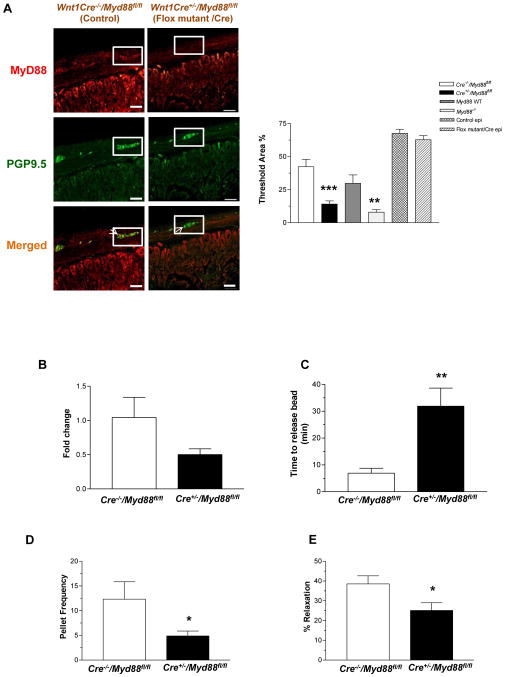
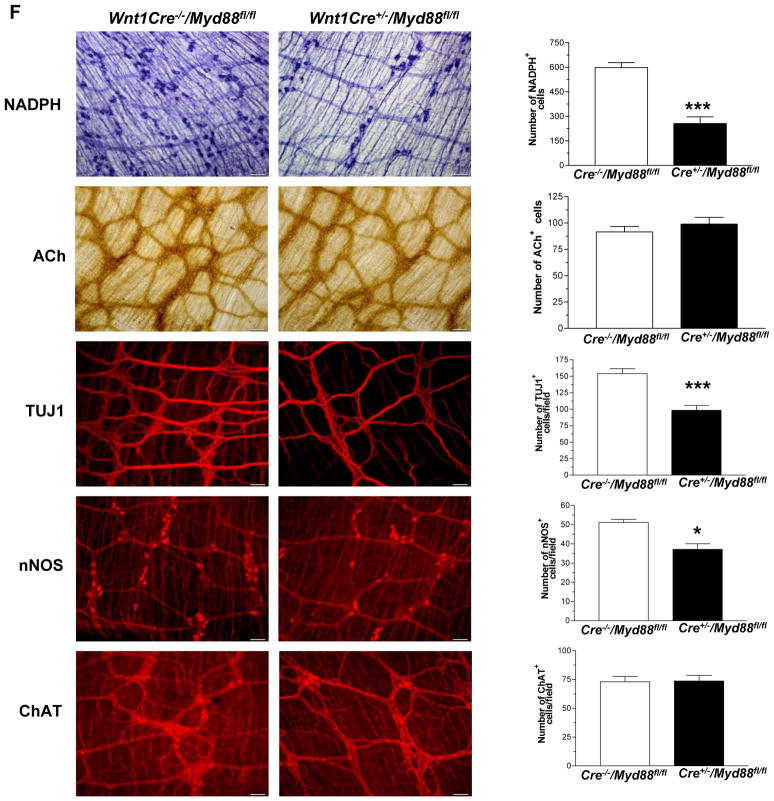
Enteric neurons originate from the neural crest cells. To determine if the changes in gastrointestinal motility were due to lack of TLR4 signaling specifically in enteric neurons we created a neural crest specific knock down of Myd88 by crossing the Wnt1-cre mice and the floxed Myd88 mice. To do this we used mice in which Cre-recombinase, driven by the neural crest specific Wnt1 promoter25 that is expressed at about embryonic day E8. These Wnt1-Cre transgenic mice express Cre recombinase under the control of the neural crest specific wingless-related MMTV integration site 1 (Wnt1) promoter. When crossed with a strain containing loxP site-flanked sequences, Cre-mediated recombination results in deletion of the flanked sequences in the developing neural crest cells. Using Cre-recombinase driven by the Wnt1 promoter we generated mice with excision of the Myd88 in mouse neural crest cells26. The specificity of targeting neural crest cells was demonstrated in this paper by the authors using mice homozygous for floxed stop YFP26. In these mice crossed with the neural crest specific Wnt-1 mice, fluorescence was noted in the neural crest cells and not smooth muscle precursors. In our studies, the knock down of Myd88 was verified in the enteric ganglia by immunostaining for Myd88. PGP9.5 was used as a neuronal marker. Using quantitative morphometry we found the expression levels of Myd88 significantly lower in the ganglion of Wnt1Cre+/−/Myd88fl/fl mice compared to controls. The expression level in enteric ganglia was similar to Myd88−/− mice. There was no change in the Myd88 expression in the epithelial cells indicating specificity of knock down in enteric ganglia (Figure 6A). Immunostaining data was supported by real time PCR of laser capture isolated enteric ganglia. This showed a 50% reduction in the Myd88 expression in the ganglia of Wnt1Cre+/−/Myd88fl/fl mice (Figure 6B) compared to Wnt1Cre−/−/Myd88fl/fl. The body weights of the Wnt1Cre+/−/Myd88fl/fl mice were comparable to their WT counterparts (Wnt1Cre−/−/Myd88fl/fl). Examination of gastrointestinal motility in the Wnt1Cre+/−/Myd88fl/fl mice demonstrated delayed colonic transit, reduced stool frequency, and impaired nitrergic relaxation compared to controls, similar to the TLR4Lps-d and TLR4−/− mice (Figure 6, C–E). These mice also had reduced number of nitrergic and total neurons with no change in cholinergic neurons (Figure 6F) similar to the TLR4Lps-d and TLR4−/−, germ free and antibiotic treated mice.
Figure 6. Wnt1Cre+/−/Myd88fl/fl mice have delayed colonic transit and reduced nitrergic neurons.
(A) Wnt1Cre−/−/Myd88fl/fl and Wnt1Cre+/−/Myd88fl/fl mice, and Myd88−/− mice were stained for Myd88 and neuronal marker PGP9.5. Histogram shows percentage of area stained for Myd88 in enteric ganglion or epithelial cells determined by morphometry. (B) Expression of Myd88, by real time-PCR in the enteric ganglia of Wnt1Cre+/−/Myd88fl/fl mice relative to Wnt1Cre−/−/Myd88fl/fl mice. (C) Colonic motility was determined by time to release bead as described in Methods section, and (D) Pellet frequency per hour was assessed in Wnt1Cre−/−/Myd88fl/fl and Wnt1Cre+/−/Myd88fl/fl. (E) Nitrergic relaxation in response to nerve stimulation was assessed in proximal colon of the mice. (F) Representative photographs and histograms of proximal colonic whole mount staining for NADPH Diaphorase, Acetylcholinesterase and terminal ileum whole mount staining for TUJ1, nNOS and ChAT. The number of neurons stained for a specific marker was determined per unit area. Scale bar: 50 μm. Results are mean ± SE. n=6. *P < 0.05, **P < 0.01, ***P < 0.001.
TLR4 activation promotes enteric neuronal survival
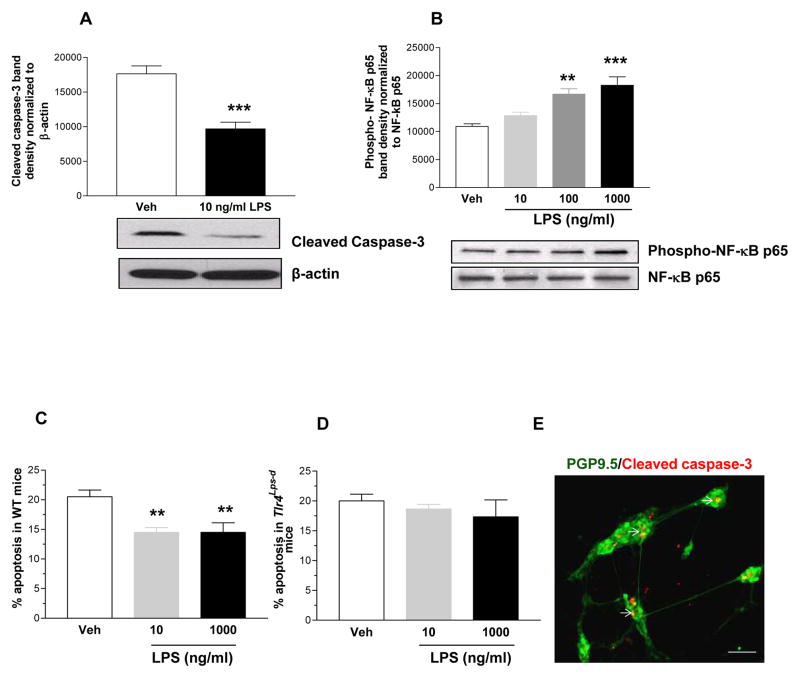
Our in vivo findings demonstrated a role for TLR4 signaling in modulating gastrointestinal motility and influencing nNOS neurons. We sought to determine if activation of TLR4 can modulate enteric neuronal survival. TLR4 expression was verified in enteric neurons using RT-PCR (data not shown). We used primary enteric neurons from E13.5, WT and Tlr4Lps-d mice and IM-FEN cells 18 to determine the effects of LPS on influencing enteric neuronal survival. Low dose LPS treatment (10 ng/ml) resulted in decreased cleaved caspase-3 expression with a dose dependent (10–1000 ng/ml) increase in phosphorylation of NF-κB p65 subunit by Western blot analysis (Figure 7, A and B). Further LPS resulted in improved survival of primary enteric neurons isolated from WT mice (Figure 7C) but not from Tlr4Lps-d mice (Figure 7D). These data suggest a role for TLR4 activation in promoting enteric neuronal survival.
Figure 7. LPS stimulates NF-κB activation and enteric neuronal survival.
(A) Western blot analysis for cleaved caspase-3 (17 KDa band) in neuronal cells treated with or without LPS (10 ng/ml), and (B) Phospho-NF-κB p65 in neuronal cells treated with or without LPS (10–1000 ng/ml). (C–E) Immunostaining of primary culture cells isolated from E13.5, WT and Tlr4Lps-d mice for cleaved caspase-3 costained with neuronal marker PGP9.5. A total of 200 neurons were assessed to determine the percentage of cleaved caspase-3 positive neurons. Arrows represent cleaved caspase-3 positive neurons. Scale bar: 50 μm. Results are mean ± SE. n=3. **P < 0.01, ***P < 0.001.
Discussion
We have demonstrated a role for enteric neuronal TLR4 in the regulation of intestinal motility. Lack of TLR4 signaling led to delayed gastrointestinal motility as demonstrated by reduced pellet frequency and delayed intestinal transit. In addition, in the absence of TLR4 signaling we found a reduction in nitrergic neurons and resultant reduced nitrergic relaxation. Reduction of the microbiota with antibiotics led to a delayed intestinal transit in WT mice but not in the TLR4Lps-d mice indicating the importance of luminal LPS and TLR4. Myd88 is the key adaptor signaling molecule for TLRs. Myd88−/− mice and mice with neural crest specific knock down of Myd88 had a similar phenotype as the TLR4−/− mice. Finally, to understand the mechanism of TLR4 modulation of enteric neuronal survival, we assessed the effects of the TLR4 agonist, LPS on enteric neurons in vitro. TLR4 was expressed in enteric neurons and stimulation with LPS resulted in activation of NF-κB. Further stimulation with LPS promoted enteric neuronal survival in WT but not Tlr4Lps-d mice. Taken together our findings suggest that TLR4 signaling is important for the survival of enteric neurons. These findings implicate the role of intestinal microbiota in regulating gastrointestinal motility.
Studies have demonstrated that in models of sepsis the mechanism of ileus involves the inflammation response induced by bacterial flora and lipopolysaccharide from Gram negative bacteria27. These studies have found that inflammatory cytokines result in intestinal smooth muscle dysfunction and resultant delayed motility. In our models of Tlr4Lps-d mice we did not find any defects in intestinal smooth muscle function as measured by isometric muscle strip recordings. In cultured rat myenteric neurons exposure to LPS resulted in increased vasoactive intestinal peptide expression and reduced neuronal survival16. The concentration of LPS used in these studies ranged from 0.1–20 μg and was much higher than what was used in the current study. Our findings suggest that low dose LPS is essential to maintain neuronal survival however at higher doses LPS results in neuronal toxicity. LPS at low dose has been shown to promote survival of hippocampal neurons through increased expression of granulocyte colony stimulating factor28. Activation of NF-κB has been shown to favor neuronal differentiation29 and in our studies low dose LPS through activation of NF-κB promotes the survival of enteric neurons.
Studies have demonstrated that peptidoglycans from microbiota can circulate systemically and modulate bone marrow neutrophils to enhance systemic innate immunity13. Compromised barrier function has been demonstrated in models of psychological stress30. The presence of TLR on the enteric neural network creates a mechanism for the gut luminal environment to communicate with the central nervous system. Modulation of microbiota through the use of probiotics has been shown to alter myenteric neurons31 and benefit patients with irritable bowel syndrome and this maybe through the bacterial neuronal interactions that we find in the intestinal tract. Recent studies examining TLR4 expression in colonic biopsies of patients with irritable bowel syndrome demonstrated increased expression of TLR432. In another study involving irritable bowel syndrome patients a majority of these patients had diarrhea as part of their symptoms33. Our future studies will examine the role of TLR4 in modulating stress related gastrointestinal symptoms as seen in irritable bowel syndrome.
Another interesting finding is the reduced number of nNOS neurons in 4 week old germ free mice compared to their controls. Treatment of piglets with probiotics has been shown to modify the chemical coding in swine enteric neurons, indicating a role for bacterial products in the neurochemical differentiation of the ENS34. Our findings suggest that for enteric neuronal survival, microbial-neuronal interaction is essential. One potential mechanism we propose is through LPS induction of NF-κ-B and resulting activation of prosurvival pathways. In the central nervous system (CNS) TLR2 has been shown to modulate adult hippocampal neurogenesis35.
Previous studies in human CNS neurons have demonstrated a role for TLRs for induction of innate immune responses and thus neurons can play a role in defense against neurotropic pathogens3, 36. Our studies demonstrate that TLR4 signaling is important in enteric neurons and can participate in modulating motility. Lack of TLR4 is associated with reduced GI motility and signals through the Myd88 pathway. TLR4 and its downstream signaling molecules could be potential therapeutic targets for the treatment of gastrointestinal motility disorders.
Supplementary Material
Acknowledgments
Grant Support: This work is supported by the NIH-RO1 (DK080684, SS), VA-MERIT award (SS), DK06411 (SVS) and DDRDC (DK064399).
This work is supported by the NIH-RO1 (DK080684, SS), VA-MERIT award (SS), DK06411 (SVS) and DDRDC (DK064399). We thank Duke Geem, Jesse Aitken, Gayathri Srinivasan and Benoit Chassaing for technical assistance.
Abbreviations used in this paper
- TLR
Toll-like receptor
- LPS
lipopolysaccharide
- MAMPs
microbial-associated molecular patterns
- WT
wild type
- nNOS
neuronal nitric oxide synthase
- ACh
Acetylcholine
- ChAT
choline acetyltransferase
- NADPH
Nicotinamide adenine dinucleotide phosphate
- ENS
enteric nervous system
- IM-FEN
immorto fetal enteric neurons
- EFS
electrical field stimulation
- LAL
Limulus Amebocyte Lysate
Footnotes
Disclosures:
The authors have nothing to disclose.
Author Contributions:
Mallappa Anitha contributed to the performance of experiments and data analysis and interpretation; Matam Vijay Kumar, Shanthi V. Sitaraman and Andrew Gewirtz contributed to the conception and design and manuscript writing; Shanthi Srinivasan contributed to the concept and design, data analysis and interpretation and manuscript writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res. 2009;175:139–48. doi: 10.1016/S0079-6123(09)17509-X. [DOI] [PubMed] [Google Scholar]
- 4.Okun E, Griffioen KJ, Lathia JD, et al. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–92. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barajon I, Serrao G, Arnaboldi F, et al. Toll-like Receptors 3, 4, and 7 Are Expressed in the Enteric Nervous System and Dorsal Root Ganglia. J Histochem Cytochem. 2009 doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lafon M, Megret F, Lafage M, et al. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29:185–94. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 7.Jackson AC, Rossiter JP, Lafon M. Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol. 2006;12:229–34. doi: 10.1080/13550280600848399. [DOI] [PubMed] [Google Scholar]
- 8.Tang SC, Lathia JD, Selvaraj PK, et al. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua F, Ma J, Ha T, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–11. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rumio C, Besusso D, Arnaboldi F, et al. Activation of smooth muscle and myenteric plexus cells of jejunum via Toll-like receptor 4. Journal of cellular physiology. 2006;208:47–54. doi: 10.1002/jcp.20632. [DOI] [PubMed] [Google Scholar]
- 13.Clarke TB, Davis KM, Lysenko ES, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nature medicine. 2010;16:228–31. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsyth CB, Shannon KM, Kordower JH, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PloS one. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochoa-Cortes F, Ramos-Lomas T, Miranda-Morales M, et al. Bacterial cell products signal to mouse colonic nociceptive dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 299:G723–32. doi: 10.1152/ajpgi.00494.2009. [DOI] [PubMed] [Google Scholar]
- 16.Arciszewski MB, Sand E, Ekblad E. Vasoactive intestinal peptide rescues cultured rat myenteric neurons from lipopolysaccharide induced cell death. Regul Pept. 2008;146:218–23. doi: 10.1016/j.regpep.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Li ZS, Schmauss C, Cuenca A, et al. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci. 2006;26:2798–807. doi: 10.1523/JNEUROSCI.4720-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anitha M, Joseph I, Ding X, et al. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology. 2008;134:1424–35. doi: 10.1053/j.gastro.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrasekharan BP, Kolachala VL, Dalmasso G, et al. Adenosine 2B receptors (A(2B)AR) on enteric neurons regulate murine distal colonic motility. FASEB J. 2009;23:2727–34. doi: 10.1096/fj.09-129544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. Journal of pharmacological methods. 1981;6:211–7. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 21.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–56. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevalier J, Derkinderen P, Gomes P, et al. Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system. J Physiol. 2008;586:1963–75. doi: 10.1113/jphysiol.2007.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uramatsu M, Matsumoto T, Tateda K, et al. Involvement of endotoxin in the mortality of mice with gut-derived sepsis due to methicillin-resitant Staphylococcus aureus. Microbiology and Immunology. 2010;54:330–337. doi: 10.1111/j.1348-0421.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- 25.Danielian PS, Muccino D, Rowitch DH, et al. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Current biology: CB. 1998;8:1323–6. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 26.Druckenbrod NR, Powers PA, Bartley CR, et al. Targeting of endothelin receptor-B to the neural crest. Genesis. 2008;46:396–400. doi: 10.1002/dvg.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overhaus M, Togel S, Pezzone MA, et al. Mechanisms of polymicrobial sepsis-induced ileus. American journal of physiology Gastrointestinal and liver physiology. 2004;287:G685–94. doi: 10.1152/ajpgi.00359.2003. [DOI] [PubMed] [Google Scholar]
- 28.Li ZX, Li QY, Qiao J, et al. Granulocyte-colony stimulating factor is involved in low-dose LPS-induced neuroprotection. Neuroscience letters. 2009;465:128–32. doi: 10.1016/j.neulet.2009.08.069. [DOI] [PubMed] [Google Scholar]
- 29.Bonini SA, Ferrari-Toninelli G, Uberti D, et al. Nuclear factor kappaB-dependent neurite remodeling is mediated by Notch pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:11697–705. doi: 10.1523/JNEUROSCI.1113-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. American journal of physiology Gastrointestinal and liver physiology. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 31.Kamm K, Hoppe S, Breves G, et al. Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2004;16:53–60. doi: 10.1046/j.1365-2982.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang XM, Liu YL. Signal transduction pathway of colonic Toll-like receptor 4 in patients with irritable bowel syndrome. Zhonghua yi xue za zhi. 2008;88:3415–7. [PubMed] [Google Scholar]
- 33.Brint EK, MacSharry J, Fanning A, et al. Differential expression of toll-like receptors in patients with irritable bowel syndrome. The American journal of gastroenterology. 2011;106:329–36. doi: 10.1038/ajg.2010.438. [DOI] [PubMed] [Google Scholar]
- 34.di Giancamillo A, Vitari F, Bosi G, et al. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2010;22:e271–8. doi: 10.1111/j.1365-2982.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 35.Rolls A, Shechter R, London A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nature cell biology. 2007;9:1081–8. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 36.Peltier DC, Simms A, Farmer JR, et al. Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol–3 kinase signaling. Journal of immunology. 2010;184:7010–21. doi: 10.4049/jimmunol.0904133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.