Abstract
Sufficient sulfate supply has been linked to the development of sulfur induced resistance or sulfur enhanced defense (SIR/SED) in plants. In this study we investigated the effects of sulfate (S) supply on the response of genetically resistant tobacco (Nicotiana tabacum cv. Samsun NN) to Tobacco mosaic virus (TMV). Plants grown with sufficient sulfate (+S plants) developed significantly less necrotic lesions during a hypersensitive response (HR) when compared to plants grown without sulfate (−S plants). In +S plants reduced TMV accumulation was evident on the level of viral RNA. Enhanced virus resistance correlated with elevated levels of cysteine and glutathione and early induction of a Tau class glutathione S-transferase and a salicylic acid-binding catalase gene. These data indicate that the elevated antioxidant capacity of +S plants was able to reduce the effects of HR, leading to enhanced virus resistance. Expression of pathogenesis-related genes was also markedly up-regulated in +S plants after TMV-inoculation. On the subcellular level, comparison of TMV-inoculated +S and −S plants revealed that +S plants contained 55–132 % higher glutathione levels in mitochondria, chloroplasts, nuclei, peroxisomes and the cytosol than −S plants. Interestingly, mitochondria were the only organelles where TMV-inoculation resulted in a decrease of glutathione levels when compared to mock-inoculated plants. This was particularly obvious in −S plants, where the development of necrotic lesions was more pronounced. In summary, the overall higher antioxidative capacity and elevated activation of defense genes in +S plants indicate that sufficient sulfate supply enhances a preexisting plant defense reaction resulting in reduced symptom development and virus accumulation.
Keywords: Cysteine, Glutathione, Nicotiana tabacum, Salicylic acid, Sulfur induced resistance, Tobacco mosaic virus
Abbreviations: APR, adenosine 5′-phosphosulfate reductase; BSA, bovine serum albumin; CATSAB, salicylic acid-binding catalase; CP, coat protein; dpi, days post inoculation; GSH1, γ-glutamyl cysteine synthetase; GSH2, glutathione synthetase; GSTTau1, Tau class glutathione S-transferase; HR, hypersensitive response; PBS, phosphate buffered saline; PCD, programmed cell death; ROS, reactive oxygen species; S, sulfate; SIR, sulfur induced resistance; SED, sulfur enhanced defense; TMV, Tobacco mosaic virus
Highlights
► Sulfate as possible enhancer of plant defense during virus infection. ► Sulfate fertilization reduces symptom severity and virus contents. ► Enhanced cysteine and glutathione metabolism up-regulates defense gene expression. ► Sulfate fertilization enhances plant defense during virus infection.
1. Introduction
The macroelement sulfur is not only essential for plant growth, development and metabolism but also plays important roles in plant defense. Sulfur is mainly obtained by plants through the uptake of inorganic sulfate by roots, which is then transported throughout the plant and after entering plastids reduced enzymatically to sulfide [1–6]. Sulfide is then used in mitochondria, plastids and the cytosol to form the essential amino acid cysteine which is incorporated into proteins and peptides, or serves as a donor of reduced sulfur for synthesis of methionine, iron–sulfur centers, thiols, various coenzymes and secondary metabolites [2,3,6–8]. Many of these compounds play crucial roles in a number of cellular processes which are involved in plant defense, such as redox buffering and signaling, regulation of enzymatic activities, modulation of gene expression, detoxification of reactive oxygen species (ROS), heavy metals and xenobiotics, and metabolism of secondary products [3,4,9]. Thus it is not surprising that sufficient sulfur supply was linked with increased resistance of plants against pathogens - a phenomenon that has been called sulfur induced resistance (SIR) or sulfur enhanced defense (SED). Plants that were supplied with high amounts of sulfate were less susceptible to fungal pathogens [10–16]. It has been postulated that a sulfur induced synthesis of glucosinolates, phytoalexins, thiols, or H2S might be the key factors for the activation of SIR/SED [12,17–19] during fungal infection of plants but clear links still have to be established.
Recently, it was demonstrated that a sufficient sulfate supply was correlated with an increased resistance of plants to virus infections [20]. Increased resistance was connected with higher glutathione and cysteine levels and a stronger activation of defense genes which indicated that SIR/SED is based on the activation of general defense mechanisms through elevated glutathione metabolism. Glutathione fulfills many important roles in plant defense such as the protection against oxidative stress, heavy metals, herbicides, and xenobiotics. It is a key regulator of redox signaling and buffering and plays key roles in plant defense through the activation of defense genes [21,22]. Glutathione is synthesized in plants from glutamate, cysteine and glycine triggered by the enzymes γ-glutamyl cysteine synthetase (GSH1, E.C. 6.3.2.2), and glutathione synthetase (GSH2, E.C. 6.3.2.3) [23–27]. Glutathione contents in plants are strongly influenced by sulfate supply, as it is involved in the regulation of uptake, assimilation, transport and storage of reduced sulfur [3,7,28,29]. High sulfate supply correlates with increased levels of cysteine and, subsequently, glutathione [10,30–33], and sulfur deprivation (especially in young leaves) strongly decreases cysteine and glutathione contents in plants [31,34–36]. Additionally, glutathione synthesis seems to be limited by the availability of cysteine, as glutathione contents could be increased by the supplementation or artificial elevation of cysteine [27,37–39]. Thus, it seems obvious that sufficient sulfate supply is essential for the synthesis of glutathione and for plant defense against abiotic and biotic stress.
In a previous study we have shown that enhanced glutathione metabolism is correlated with SIR/SED in Tobacco mosaic virus-infected, genetically susceptible Nicotiana tabacum cv. Samsun nn plants [20]. In the present study we tested the hypothesis that a sufficient sulfate supply positively influences defense reactions in TMV-infected, genetically resistant N. tabacum cv. Samsun NN plants. N. tabacum cv. Samsun NN carries a resistance gene (N) responsible for the induction of a hypersensitive response (HR) to TMV that results in the formation of localized necrotic lesions restricting the growth and spread of this viral pathogen, and in the activation of plant defense mechanisms ultimately leading to systemic acquired resistance [40,41]. HR is characterized by the production of reactive oxygen species (ROS) leading to lipid peroxidation and eventually cell death. Antioxidants such as glutathione are important antagonists in these reactions as they detoxify ROS and therefore regulate the severity of these events on the cellular level [21]. Thus, the present study was aimed at comparing the severity of virus-induced HR in plants supplied with sufficient amounts of sulfate (+S plants) with that in plants grown without sulfate (–S plants) and to correlate these results with compartment specific cysteine and glutathione status in these plants. Virus contents, defense gene activation and sulfur contents were also assayed in TMV-infected N. tabacum cv. Samsun NN in order to get a deeper insight into the compartment specific roles of glutathione metabolism during the development of SIR/SED in plants.
2. Results
2.1. Symptom characterization and virus contents
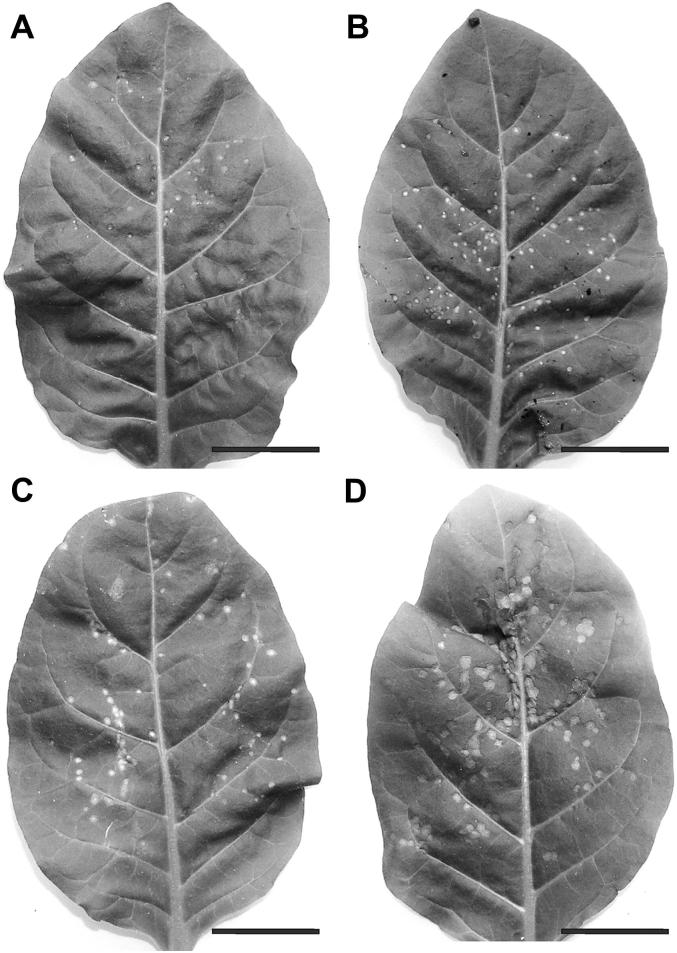
Symptom development was characterized according to the number of necrotic lesions and the total area they cover on TMV-inoculated leaves (Figs. 1 and 2). The numbers of necrotic lesions were significantly lower (−51% and −45%) in +S plants when compared to −S plants 2 and 4 days after TMV-inoculation, respectively. In addition, the total areas these necrotic lesions cover on the leaves were significantly lower (−66% and −47%) in +S plants when compared to −S plants 2 and 4 days after TMV-inoculation, respectively.
Fig. 1.
Leaves of Nicotiana tabacum cv. Samsun NN plants grown with (A, C) or without (B, D) sulfate (+S and −S, respectively) showing necrotic lesions 2 (A, B) and 4 (C, D) days after TMV inoculation. Note that TMV-inoculated leaves of +S plants contain less necrotic lesions which cover less area on the leaves in comparison to leaves of –S plants at the same time point. Bars = 3 cm.
Fig. 2.
Symptom development on Nicotiana tabacum cv. Samsun NN leaves 2 and 4 days post TMV-inoculation (dpi). Plants were grown with (gray columns) or without sulfate (diagonally striped columns). Symptom severity is indicated by a) the amount of necrotic lesions formed per leaf and b) by the percentage of leaf area covered by necrotic lesions. n = 40 for each, +S and −S plants, from 3 different experiments.
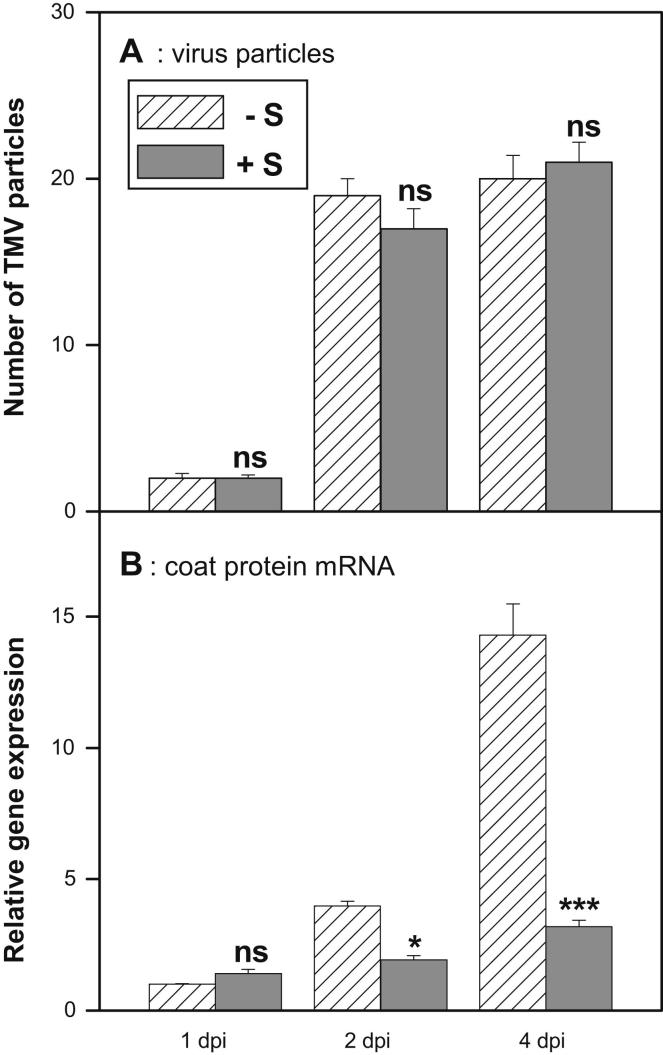
Quantitative analysis of viral particles in the sap of TMV-inoculated leaves revealed no significant changes in virus contents in +S plants as compared to −S plants throughout the investigation period (Fig. 3). In order to clarify whether sulfate treatment affects TMV on the level of viral RNA, we have assessed the accumulation of mRNA encoding the TMV-coat protein (CP) (i.e. TMV-CP gene expression) in inoculated leaves (Fig. 3). One day after inoculation there were no significant differences in TMV-CP mRNA levels between +S and −S plants. However, two and four days after inoculation TMV-CP mRNA levels were significantly lower in TMV-inoculated leaves of +S plants as compared to −S plants.
Fig. 3.
Amount of TMV-particles (A) and coat protein mRNA levels (B) detected in virus-inoculated leaves of Nicotiana tabacum cv. Samsun NN plants 1, 2 and 4 days after TMV-inoculation (dpi). Symbols −S and +S indicate plants grown without sulfate or with sulfate, respectively. A: The amount of TMV-particles per 100 μm2 detected on 20 square areas (for each replicate sample) on the grid after negative staining (n = 6). Significant differences were calculated by using the Mann Whitney U-test. ns indicates non-significance. B: TMV coat protein mRNA levels as determined by real-time RT-qPCR. A relative gene expression of 1 represents TMV coat protein mRNA levels in inoculated leaves at 1 dpi (–S). Means of three independent biological experiments ± SE are shown. Significant differences were calculated by using Student’s t-test, where * and *** indicate significance at the 0.05 and 0.001 levels of confidence, respectively. ns indicates non-significance.
2.2. Subcellular glutathione and cysteine contents
2.2.1. Subcellular glutathione contents
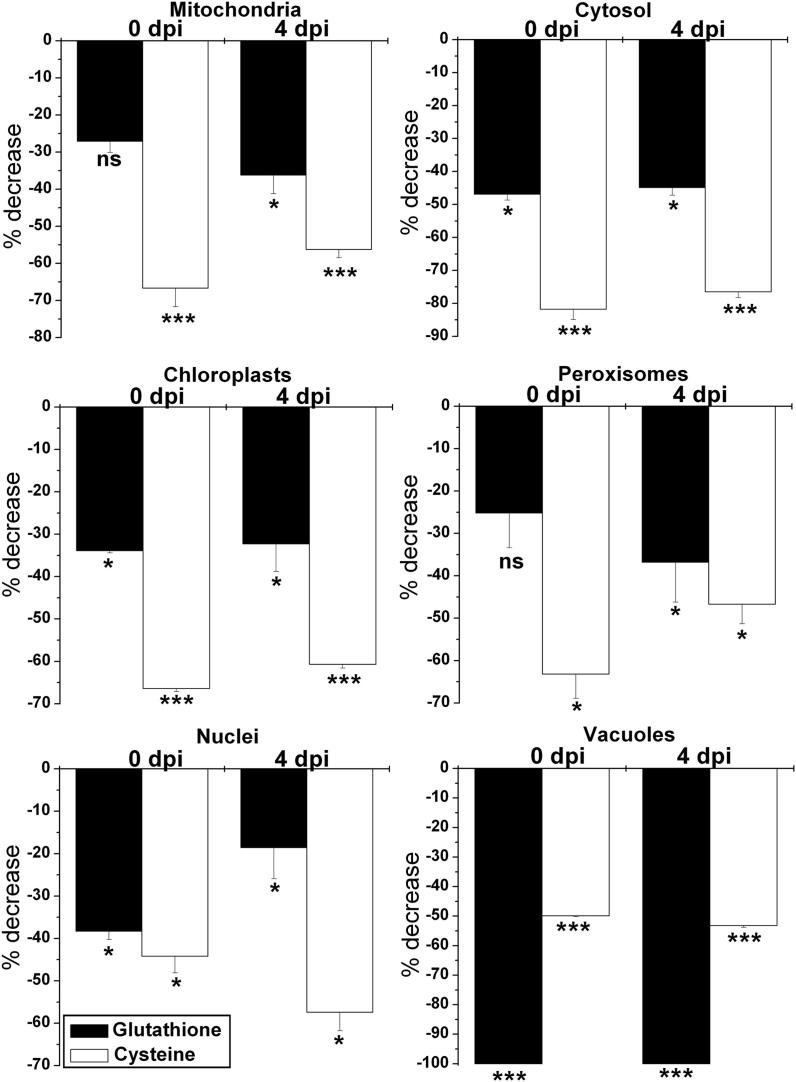
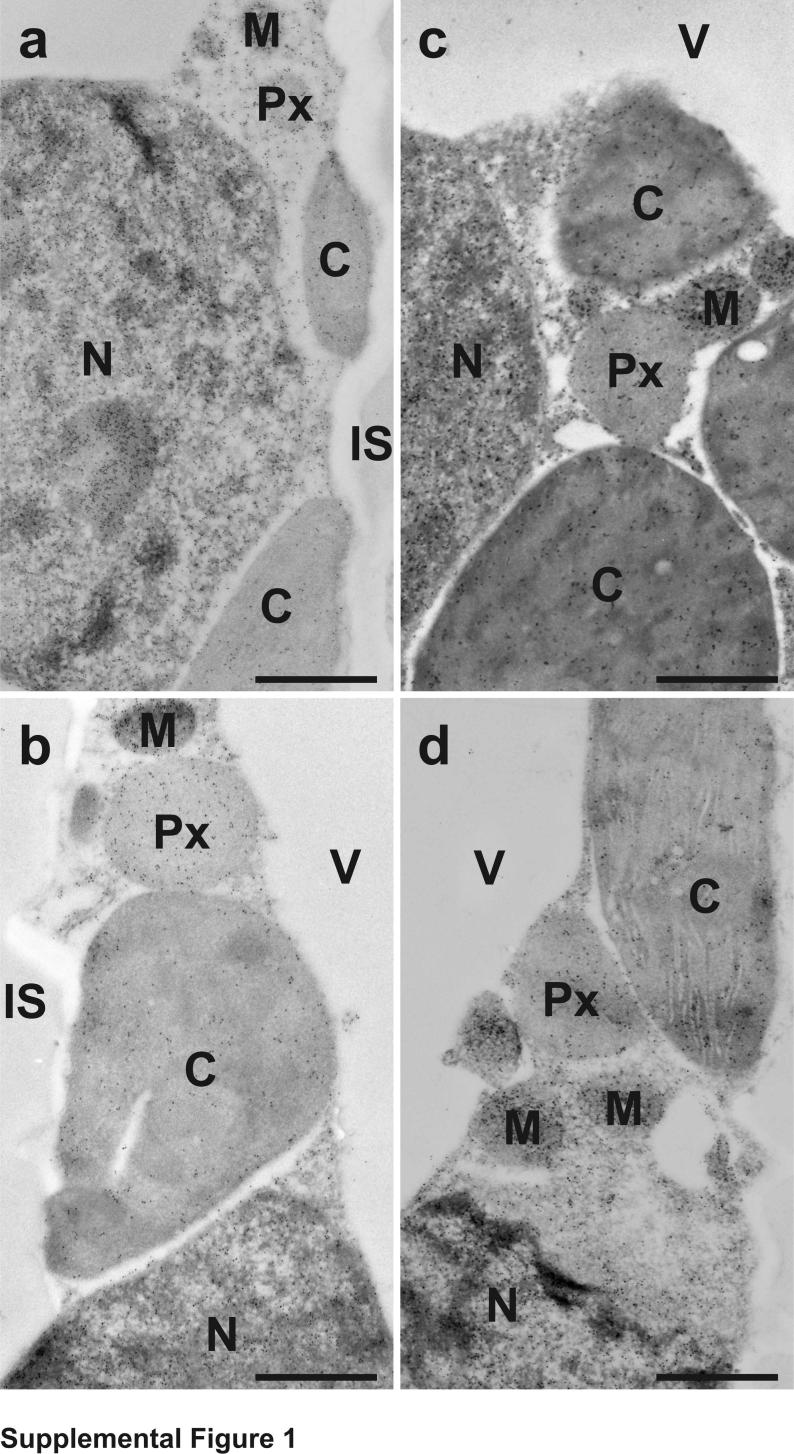
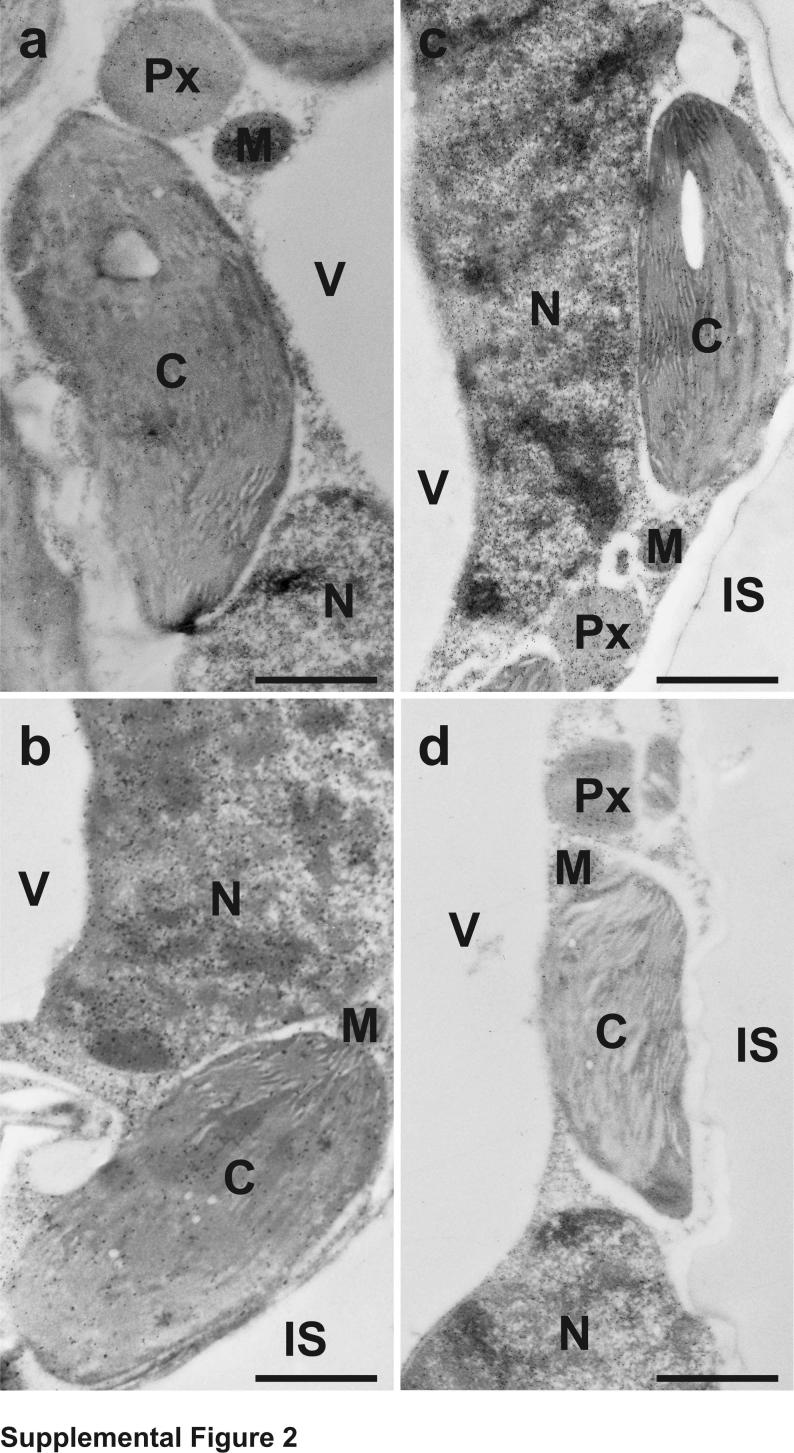
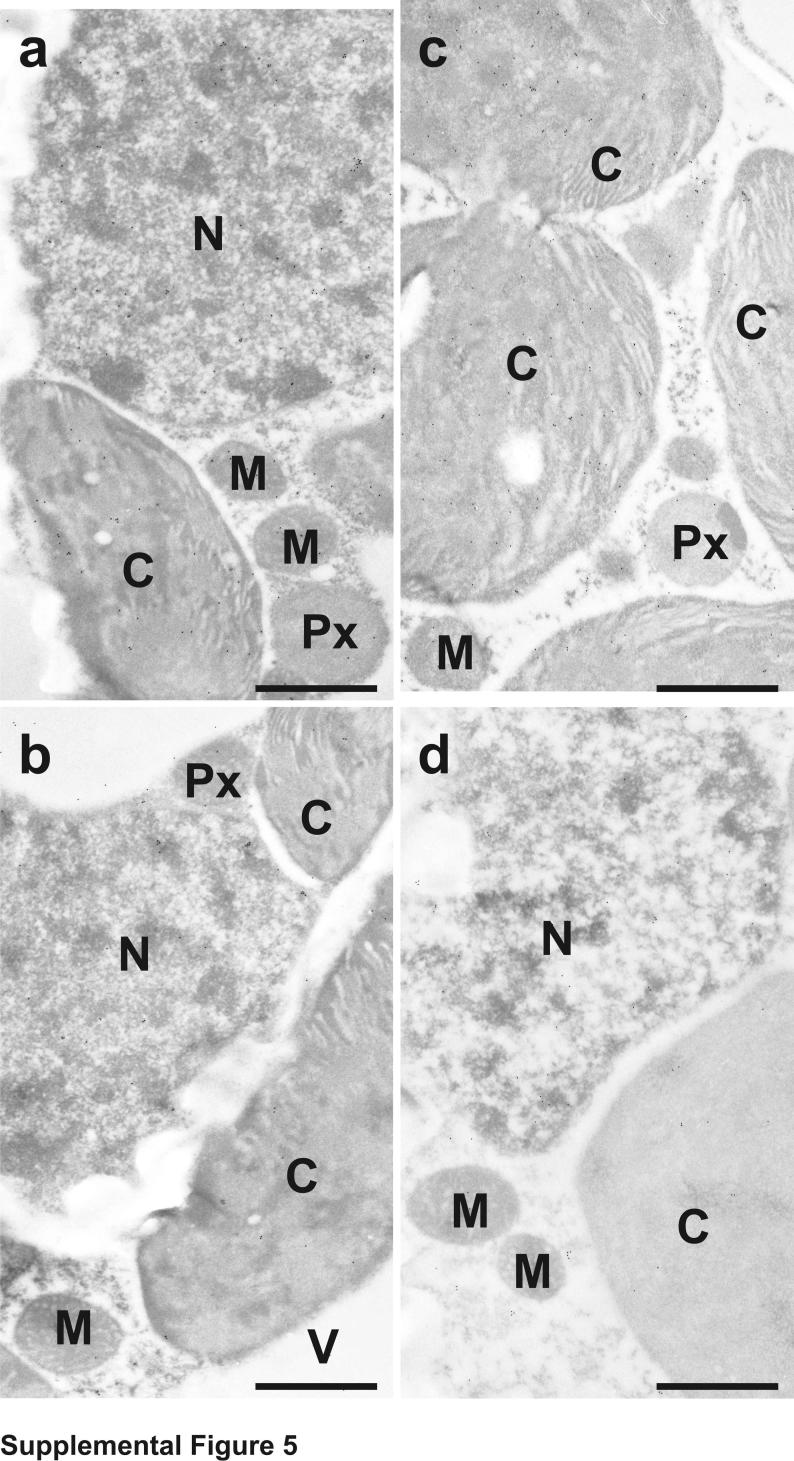
The subcellular distribution of glutathione in tobacco plants was found to be similar to what we have observed previously in tobacco plants genetically susceptible to TMV [20]. Highest levels of glutathione were detected in mitochondria and lowest ones in chloroplasts. Intermediate labeling occurred in nuclei, the cytosol and peroxisomes. No glutathione-specific labeling was observed in cell walls and intercellular spaces. Gold particles bound to glutathione were also observed in vacuoles of +S plants but were not detected in vacuoles of –S plants (Supplemental Figures 1 and 2 and Supplemental Table 1).
Mock inoculated −S plants contained significantly less glutathione in most cell compartments throughout the experiment (Fig. 4). At the time of TMV-inoculation −S plants contained no glutathione in vacuoles, whereas other cell compartments of −S plants contained between 47% (cytosol) and 25% (peroxisomes) less gold particles than +S plants. At the end of the experiment −S plants contained between 45% (cytosol) and 19% (nuclei) less glutathione than +S plants (Fig. 4).
Fig. 4.
Compartment specific changes in glutathione and cysteine labeling density in mesophyll cells of mock inoculated Nicotiana tabacum cv. Samsun NN grown on media without sulfate in comparison to plants grown with sulfate (absolute amounts are shown in Supplemental Tables 1 and 2). Measurements were performed at the time of inoculation and 4 days post inoculation (dpi). n > 20 for peroxisomes and n > 60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; ns indicates non-significance, whereas * and ***, respectively, indicate significance at the 0.05 and 0.001 levels of confidence.
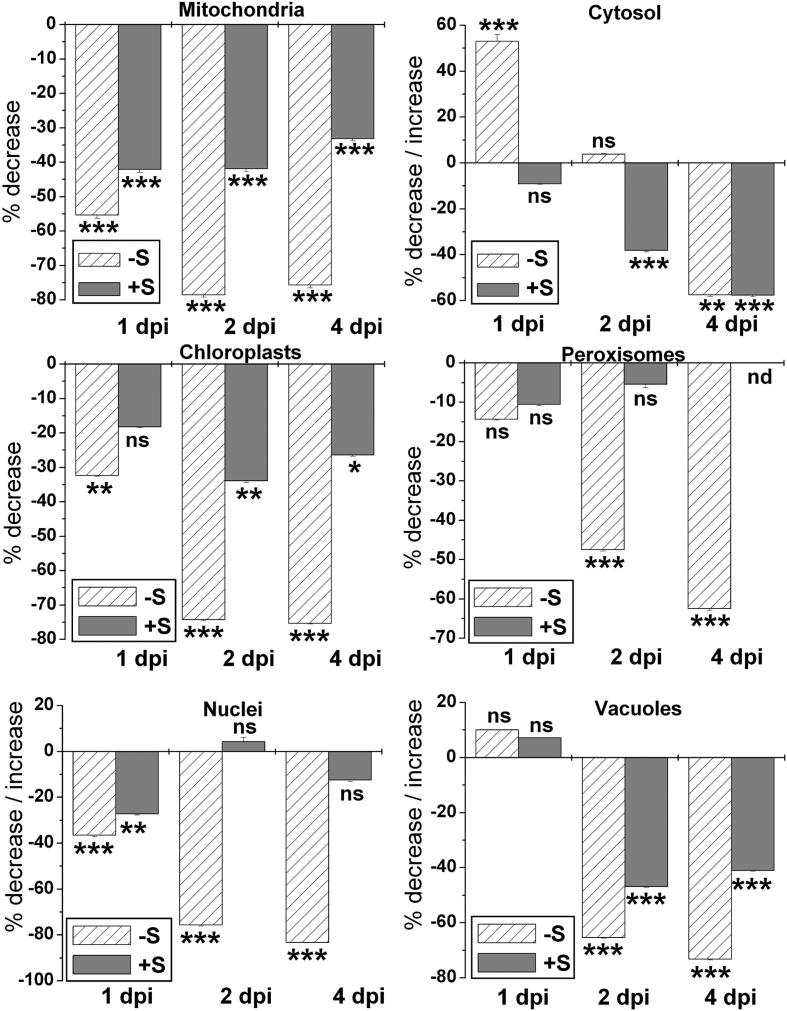
Throughout the whole time period investigated TMV-inoculated +S plants displayed higher glutathione contents of up to 93% in mitochondria (4 dpi), 132% in chloroplasts (4 dpi), 88% in nuclei (4 dpi), 55% in peroxisomes (2 dpi) and 61% in the cytosol (1 dpi) when compared to TMV-inoculated –S plants as shown in Supplemental Figure 3.
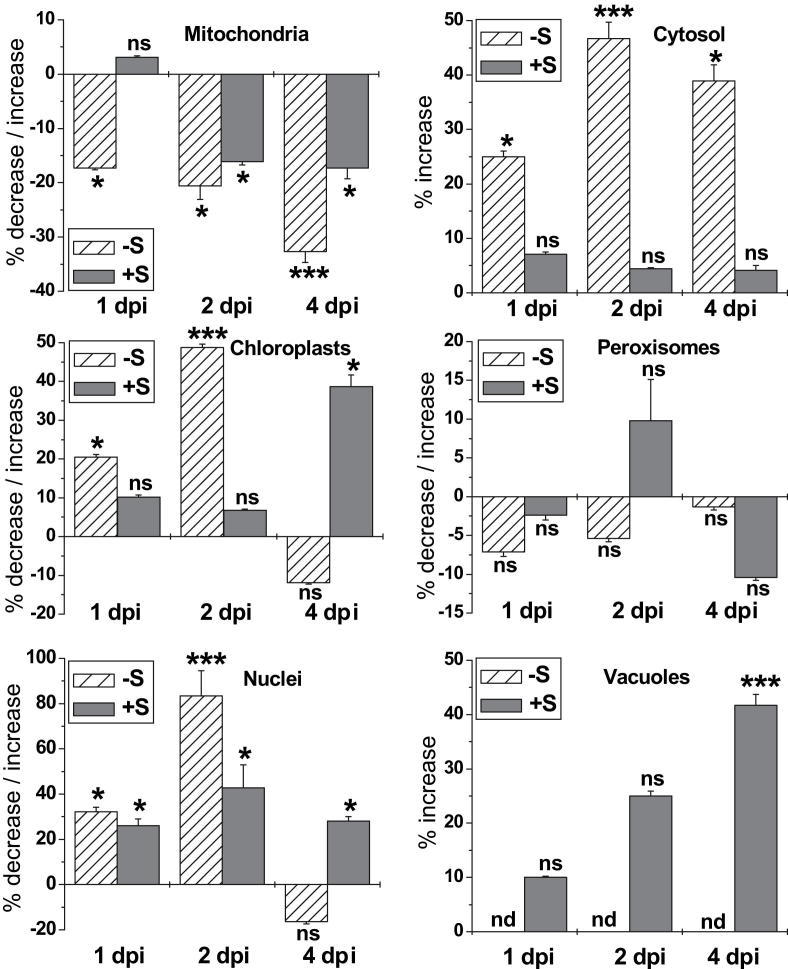
Nevertheless, TMV-inoculation significantly changed compartment specific glutathione labeling in both +S and –S plants when compared to cells of mock inoculated plants grown under the same conditions (Fig. 5). One and two days after TMV-inoculation glutathione contents were significantly increased in chloroplasts (21% and 49%, respectively), nuclei (32% and 84%, respectively) and the cytosol (25% and 47%, respectively) of cells from TMV-inoculated −S plants, whereas in cells of TMV-inoculated +S plants only nuclei showed significantly increased glutathione contents (26% and 43%, respectively) when compared to mock inoculated –S and +S plants, respectively (Fig. 5). Mitochondria in cells of −S plants showed significantly decreased levels of glutathione already at 1 and 2 days (−17% and −21%, respectively) after TMV-inoculation whereas in +S plants decreased levels were found beginning at two days post inoculation. Four days after TMV-inoculation an increase of glutathione contents was observed in chloroplasts (39%), nuclei (28%), and vacuoles (42%) in cells of TMV-inoculated +S plants whereas in cells of TMV-inoculated −S plants increased glutathione levels were observed only in the cytosol (40%) when compared to mock inoculated +S and –S plants, respectively. At this time point mitochondria in cells of TMV-inoculated +S and −S plants showed a significant decrease in glutathione contents of −17% and −33% respectively, when compared to mock-inoculated plants (Fig. 5).
Fig. 5.
Compartment specific changes in glutathione labeling density in mesophyll cells of TMV-inoculated Nicotiana tabacum cv. Samsun NN grown on media with and without sulfate (+S and −S, respectively) when compared to mock inoculated plants grown under the same conditions. Measurements were performed 1, 2 and 4 days post inoculation (dpi). n > 20 for peroxisomes and vacuoles and n > 60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; ns indicates non-significance, whereas * and ***, respectively, indicate significance at the 0.05 and 0.001 levels of confidence.
2.2.2. Subcellular cysteine contents
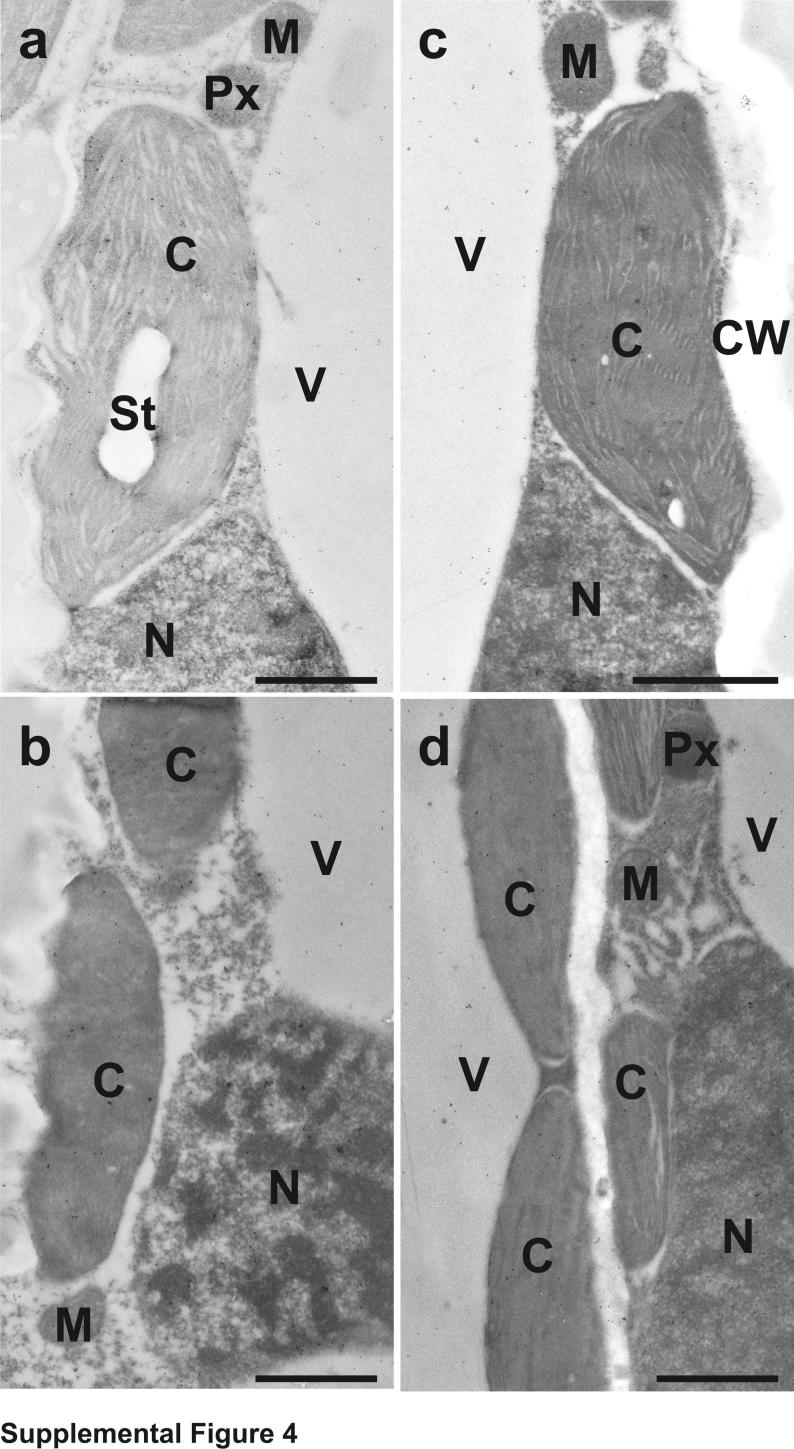
Cysteine contents in cells of +S plants were found to be the highest in mitochondria and the cytosol, with intermediate labeling in nuclei, plastids, peroxisomes and vacuoles. Cysteine was not detected in intercellular spaces and cell walls (Supplemental Figure 4 and 5 and Supplemental Table 2). In cells of −S plants cysteine contents were generally highest in the mitochondria and nuclei, and similar labeling intensities were found in all other cell compartments (Supplemental Figure 4 and 5 and Supplemental Table 2).
Mock inoculated −S plants contained significantly less cysteine labeling in most cell compartments throughout the experiment (Fig. 4). At the time of TMV-inoculation −S plants contained between 82% (cytosol) and 44% (nuclei) less gold particles bound to cysteine than +S plants. At the end of the experiment −S plants contained between 77% (cytosol) and 47% (peroxisomes) less cysteine than +S plants (Fig. 4).
Throughout the whole time period investigated TMV-inoculated +S plants displayed higher cysteine contents of up to 529% in mitochondria (4 dpi), 659% in chloroplasts (4 dpi), 950% in nuclei (4 dpi), 400% in peroxisomes (4 dpi), 324% in the cytosol (4 dpi), and 372 %vacuoles (4 dpi) when compared to TMV-inoculated –S plants as shown in Supplemental Figure 3.
TMV-inoculation induced significant changes in cysteine labeling density in most cell compartments of cells from −S and +S plants when compared to mock-inoculated plants grown in the same conditions (Fig. 6). One day after TMV-inoculation cells of −S plants showed significantly less cysteine in mitochondria (−55%), chloroplast (−32%), and nuclei (−37%), whereas increased cysteine labeling was observed in the cytosol (53%) when compared to mock inoculated –S plants. TMV-inoculated +S plants contained decreased levels of cysteine in mitochondria (−42%), and nuclei (−27%) whereas all other cell compartments showed unchanged levels when compared to mock inoculated +S plants (Fig. 6). Two days after TMV-inoculation cells of –S plants showed decreased levels of cysteine in mitochondria (−79%), chloroplast (−74%), nuclei (−76%), peroxisomes (−48%) and vacuoles (−65%) when compared to mock inoculated –S plants. TMV-inoculated +S plants showed significantly decreased cysteine labeling density in mitochondria (−42%), chloroplasts (−34%), the cytosol (−38%) and vacuoles (−47%) when compared to mock inoculated +S plants at this time point. All other cell compartments of TMV-inoculated +S and –S plants showed unchanged levels of cysteine (Fig. 6). Four days after TMV-inoculation cells of –S plants showed a similar decrease in cysteine labeling in all cell compartments between −58% in the cytosol and −83% in nuclei when compared to the same cell compartments in mock inoculated –S plants. Cells of TMV-inoculated +S plants showed decreased cysteine levels in mitochondria (−33%), chloroplasts (−26%), the cytosol (−58%) and vacuoles (−41%) when compared to mock inoculated +S plants. Unchanged levels of cysteine were observed in nuclei and peroxisomes of cells from TMV-inoculated +S plants at this time point (Fig. 6).
Fig. 6.
Compartment specific changes in cysteine labeling density in mesophyll cells of TMV-inoculated Nicotiana tabacum cv. Samsun NN grown on media with and without sulfate (+S and –S, respectively) when compared to mock inoculated plants grown under the same conditions. Measurements were performed 1, 2 and 4 days post inoculation (dpi). n > 20 for peroxisomes and vacuoles and n > 60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; ns indicates non-significance, whereas *, ** and ***, respectively, indicate significance at the 0.05, 0.01 and 0.001 levels of confidence.
2.3. Expression of genes encoding pathogenesis related-1 defense proteins, key enzymes of cysteine and glutathione biosynthesis and antioxidants
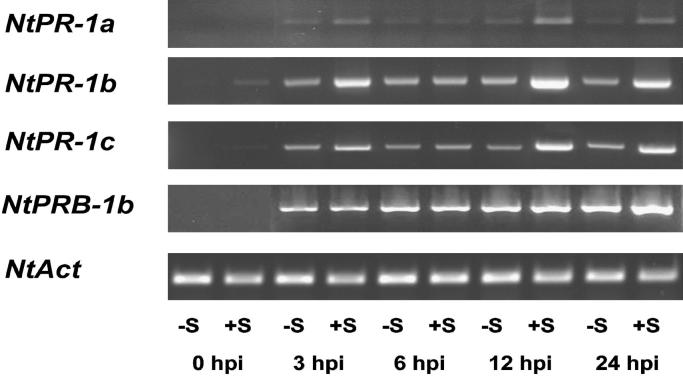
Within 24 h after TMV-inoculation the expression of genes that encode pathogenesis related-1 type defense proteins (NtPR-1a, NtPR-1b, NtPR-1c and NtPRB-1b) was considerably up-regulated in virus-inoculated leaves (Fig. 7). A sufficient sulfate supply resulted in a further enhancement of the expression of these genes.
Fig. 7.
Expression of pathogenesis related-1 genes (NtPR-1a, NtPR-1b, NtPR-1c and NtPRB-1b) in virus-inoculated leaves of Nicotiana tabacum cv. Samsun NN at 3, 6, 12 and 24 h after TMV-inoculation (hpi) as determined by semiquantitative RT-PCR. Symbols −S and +S indicate plants grown without sulfate or with sufficient sulfate, respectively. Expression of an actin gene (NtAct) was used as a constitutive control. Representative results of three independent biological experiments are shown.
In order to test if increased cysteine and glutathione levels in +S plants are correlated with changes in expression of cysteine and glutathione biosynthesis genes, we assayed the expression of adenosine 5′-phosphosulfate reductase (NtAPR), NtGSH1 and NtGSH2. Interestingly, in virus-inoculated leaves of +S plants the expression of these genes did not change significantly, as compared to –S plants (data not shown).
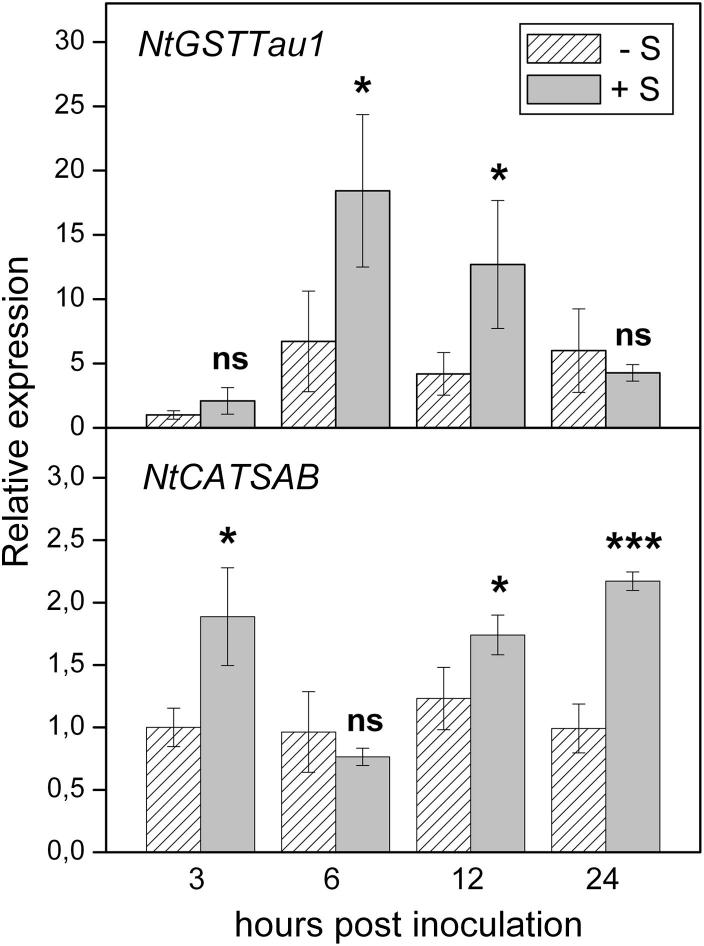
To see if elevated levels of cysteine and glutathione in +S plants correlate with increased expression of stress-related and antioxidant genes, transcript levels of genes encoding a Tau class glutathione S-transferase (NtGSTTau1) and a salicylic acid-binding catalase (NtCATSAB) were checked. Both genes showed an enhanced expression in +S plants, as compared to –S plants, within 24 h after TMV-inoculation. However, the induction pattern of the two genes in TMV-inoculated leaves of +S plants was different: NtGSTTau1 expression peaked at 6 h after inoculation, while NtCATSAB was induced before and after this time point (Fig. 8).
Fig. 8.
Expression of genes encoding a Tau class glutathione S-transferase (NtGSTTau1) and a salicylic acid-binding catalase (NtCATSAB) in virus-inoculated leaves of Nicotiana tabacum cv. Samsun NN at 3, 6, 12 and 24 h after TMV-inoculation as determined by real-time RT-qPCR. Symbols −S and +S indicate plants grown without sulfate or with sufficient sulfate, respectively. A relative gene expression of 1 represents transcript levels in TMV-inoculated leaves at 3 hpi (−S). Means of three independent biological experiments ± SE are shown. Significant differences were calculated by using Student’s t-test. Symbols * and *** indicate significance at the 0.05 and 0.001 levels of confidence, respectively, while ns indicates non significance.
2.4. Biochemical detection of sulfur contents
2.4.1. Sulfur contents in leaves
Leaves of +S plants contained significantly higher amounts of total sulfur (between 38% and 65%) throughout the investigation period than leaves of −S plants (Supplemental Figure 6). Leaves of +S plants contained between 4.2 and 5.4 mg sulfur per gram dry weight whereas −S plants contained about 3.0 and 3.7 mg sulfur per gram dry weight (Supplemental Figure 6). TMV-inoculation did not induce significant changes in sulfur contents (Supplemental Figure 7). Interestingly, TMV-inoculated leaves of +S contained about 63%, 40%, and 50% higher sulfur than TMV-inoculated leaves of –S plants 1, 2, and 4 days after TMV-inoculation (data not shown).
2.4.2. Sulfur contents in soil
Total sulfur contents were significantly higher (up to 514%) in the sand-vermiculite mixtures of +S plants when compared to −S plants throughout the experiment. At the time of inoculation sulfur contents in soil were found to be 478 mg/kg and 110 mg/kg in soil watered with nutrient solution with and without sulfate, respectively. At the end of the experiment slightly more sulfur was detected in the soil watered with nutrient solution containing sulfate (566 mg/kg) and remained similar (115 mg/kg) in soil watered with nutrient solution containing no sulfate. TMV-inoculation did not significantly change sulfur contents in the soil. Sulfur contents were 582 mg/kg and 143 mg/kg in soils containing TMV-inoculated plants watered with nutrient solution with and without sulfate, respectively (Supplemental Figure 8).
3. Discussion
In this study we tested the hypothesis that sufficient sulfate supply reduces virus accumulation and symptom development in correlation with higher glutathione and cysteine contents during Tobacco mosaic virus (TMV) infection in a resistant tobacco cultivar. The data clearly demonstrate that necrotic lesions on leaves of –S plants occurred in larger numbers and covered more area of the leaves than on leaves of +S plants after TMV-inoculation indicating that –S plants went through a more severe hypersensitive response (i.e. less effective resistance) than +S plants. These data could be correlated with much lower cysteine and glutathione levels and a reduced induction of defense marker, stress-related and antioxidant genes in −S plants in comparison to +S plants. Necrotic lesions during a hypersensitive response (HR) are formed through the production of reactive oxygen species (ROS) [40,41]. Therefore, our results indicate that in +S plants the enhanced capacity of antioxidants, primarily cysteine and glutathione, with a possible contribution of glutathione S-transferases and catalases, was able to reduce the effects of HR, thus leading to less necrotic lesions following TMV-inoculation. Interestingly, in +S plants TMV particle numbers did not change in comparison to −S plants. A reduced accumulation of TMV was evident only on the level of viral mRNA that encodes the TMV-coat protein, since +S plants showed a more than 50% lower accumulation of TMV CP mRNA two and four days after inoculation as compared to –S plants. It is known that changes in TMV coat protein levels do not necessarily correlate with changes in TMV CP mRNA. Mutants of TMV that express higher than normal levels of coat protein in planta are not transcribing proportionally higher levels of mRNA [42]. Also, transgenic tobacco that accumulate TMV coat protein display much lower levels of CP at high temperatures while TMV CP mRNA remains constant [43]. We have shown earlier that in a genetically susceptible tobacco cultivar (Samsun nn) TMV inhibition during sulfur induced resistance (SIR/SED) is detectable primarily as a decline in TMV coat protein levels and not TMV CP mRNA [20]. In contrast, the present study suggests that in TMV-resistant (Samsun NN) tobacco SIR/SED is manifested as a significant decline of TMV CP mRNA which points to possible differences in the mechanism of SIR/SED to TMV between susceptible and resistant tobacco.
Sufficient sulfate supply is able to reduce the development of TMV-induced HR-type necrotic lesions and TMV CP mRNA accumulation most probably through elevated levels of cysteine and glutathione along with a stronger activation of defense genes early after virus inoculation. In +S plants, enhanced expression of pathogenesis related-1 type defense markers (NtPR-1a, NtPR-1b, NtPR-1c and NtPRB-1b) was observed during the first 24 h after TMV-inoculation in comparison to –S plants. Similarly, a reduction in symptom development and virus levels and enhanced expression of NtPR-1a induced by elevated glutathione contents has been observed during compatible plant virus interactions [20,39]. The pivotal role of cysteine and glutathione in these processes might be signaled by the enhanced expression of cysteine and glutathione biosynthesis genes like adenosine 5′-phosphosulfate reductase (APR), γ-glutamylcysteine synthetase (GSH1) or glutathione synthetase (GSH2). In compatible tobacco-TMV interactions, NtAPR and NtGSH1 indeed showed elevated expression during SIR/SED [20]. Although the present study demonstrated that in incompatible tobacco-TMV interactions none of these genes were induced during SIR/SED, the role of NtGSH1 cannot be ruled out. According to a recent report, overexpression of a tomato GSH1 gene in tobacco leads to higher salicylic acid-mediated resistance to Pseudomonas syringae pv. tabaci infection [44]. Results of the present study also point to the possible role of glutathione and salicylic acid during SIR/SED to viral infections. In TMV-infected +S tobacco we found an elevated, early induction of a stress-related and an antioxidant gene, Tau class glutathione S-transferase 1 (NtGSTTau1) and a salicylic acid-binding catalase (NtCATSAB), respectively. Catalases are antioxidants typically found in peroxisomes involved in degradation of hydrogen peroxide, thus protecting cells from oxidative damage [45]. Stress inducible GSTs exert a similar effect through their glutathione peroxidase activity by detoxifying fatty acid hydroperoxides generated during abiotic stress and pathogen attack, including the HR [46,47]. Several earlier studies indicate that living plant cells surrounding local lesions during an HR exhibit a significant induction of GST and CAT genes and enzymes, which may contribute to the restriction of cell and tissue death [48,49]. Furthermore, in TMV-resistant transgenic tobacco unable to accumulate salicylic acid (nahG), down-regulation of GST and CAT enzymatic activity occurs and this could be a primary cause of the development of large, spreading lesions during viral infection [50]. Taken together, even though a reduced accumulation of TMV in +S plants was evident only on the level of TMV CP mRNA, it can be concluded that a sufficient supply of sulfate can be linked with an increased antioxidant capacity and an enhanced general plant defense against virus infections.
Compartment specific changes in the subcellular distribution of cysteine and glutathione were observed in TMV-inoculated plants when compared to mock-inoculated plants grown under the same conditions. In general, TMV-inoculated plants showed a continuous decrease in cysteine contents in all cell compartments during the course of the experiment. These observations correlated with a time dependent increase in glutathione contents in most cell compartments within these plants. As cysteine is the limiting factor for glutathione synthesis [24,29] these data indicate that –S and +S plants used significant amounts of cysteine for the production of glutathione. Surprisingly, –S plants reacted with a much stronger decrease of cysteine and an immediate (starting at 1dpi) and stronger increase in glutathione contents in most cell compartments after TMV-inoculation in comparison to +S plants. Additionally, glutathione contents in –S plants were immediately increased in chloroplasts and the cytosol which are considered to be the main centers for glutathione synthesis [24–26,51]. Thus, these results indicate that –S plants, presumably with a much lower antioxidative capacity than +S plants, reacted much faster and stronger with the synthesis of glutathione most probably to assuage the higher demand of antioxidants needed to prevent the negative effects of HR.
In this respect it is also interesting that mitochondria contained significantly decreased levels of glutathione immediately after TMV-inoculation in –S plants and two days after inoculation also in +S plants. A significant decrease of glutathione contents was never observed in other cell compartments after TMV-inoculation. These observations could be linked with the development of necrotic lesions in TMV-inoculated plants. It has been proposed that elevated levels of ROS in mitochondria are involved in the induction of programmed cell death (PCD) [52–54]. Thus, the exclusive decrease of glutathione in mitochondria following TMV-inoculation could indicate that either this organelle lacks the protection conferred by glutathione during TMV-infection which could lead to increased formation of ROS in mitochondria contributing to the induction of programmed cell death events or increased ROS levels in mitochondria during PCD result in decreased glutathione levels.
Furthermore, it is interesting that nuclei showed the strongest increase in glutathione contents after TMV-inoculation both in –S plants and +S plants – in the former elevated glutathione contents were exclusively found in nuclei 1 and 2 dpi. Even though the exact role of glutathione in nuclei during pathogen attack is not completely clear, these data indicate an important role of glutathione during HR in TMV-infected plants. It has been recently addressed that glutathione fulfills pivotal roles in nuclear functions in bacterial, plant and animal tissues as it is important for DNA synthesis, cell proliferation, and regulation of the nuclear matrix organization and proteins [55,56]. Additionally, it has been proposed that nuclear glutathione protects DNA from oxidative modifications, as the latter were negatively correlated with reduced nuclear glutathione content [57]. Thus, it could be possible that the strong elevation of glutathione observed in nuclei during a TMV-infection that results in HR-type resistance could be important for maintaining and preserving function of nuclei during events of oxidative stress.
In our previous study we have observed that during a compatible tobacco-TMV interaction glutathione was withdrawn from vacuoles most probably to assuage the higher demand for antioxidants in the cytoplasm [20]. In contrast, this study shows that during an incompatible tobacco-TMV interaction glutathione contents increased and cysteine contents decreased in vacuoles. In line with our results, large amounts of oxidized glutathione accumulate within vacuoles of an Arabidopsis mutant (cat2) during oxidative stress resulting in a 10-fold increase in glutathione labeling in this cell compartment when compared to the wild-type [58]. As HR is also characterized by a strong oxidative stress [40,41], it is likely that the redox state of glutathione is shifted towards the oxidized form. Our results also indicate that plants can withdraw cysteine from the vacuole if it is needed for plant metabolism (e.g. increased glutathione synthesis). It is known that vacuoles can act as a sink for elemental sulfur, which can be released into the cytosol when needed, e.g., when cysteine and glutathione contents decline [18,36,59]. A similar mechanism might therefore also apply for cysteine.
In summary, the results of this study demonstrate a clear correlation of sufficient sulfate supply and the ability of TMV-infected plants to reduce symptom development and virus accumulation during an incompatible plant–virus interaction. Sufficient sulfate supply correlated with elevated cysteine and glutathione contents which reduced the negative effects of HR and thus led to less necrotic lesions and lower levels of TMV CP mRNA in +S plants. The enhanced activation of defense genes in +S plants in correlation with a higher antioxidative capacity indicates that a sufficient sulfate supply can enhance a preexisting plant virus resistance (i.e. HR) and demonstrates that SIR/SED is indeed characterized by the activation of general plant defense mechanisms.
4. Methods
4.1. Plant material and TMV-inoculation
The cultivation of plant material and the inoculation of plants with TMV were performed almost identical to what was described in Höller et al. [20]. Briefly, three weeks after germination in quartz sand individuals of TMV-resistant Nicotiana tabacum cv. Samsun NN (obtained from the German Resource Centre for Biological Material, DSMZ, Braunschweig, Germany; www.dsmz.de) were grown on a mixture of quartz sand and vermiculite (1:3) in growth chambers under defined conditions. At this stage plants were divided into two groups and treated with a Hoagland nutrient solution containing sulfate (+S plants) or no sulfate (−S plants). −S plants received only one single dose (5 ml) of Hoagland solution with sulfate when transferred onto the sand-vermiculite mixture, in order to assure that they reached the adult stage.
Seven week old plants were inoculated with the sap of TMV-infected, susceptible (cv. Samsun nn) tobacco plants (strain id. for TMV: DSMZ PV-0107; TMV-vulgare/U1, geographical origin: Germany; obtained from the German Resource Centre for Biological Material, DSMZ, Braunschweig, Germany; www.dsmz.de). A homogenate of TMV-infected leaf material containing celite was rubbed on three fully developed leaves of tobacco plants. Mock inoculation was performed with control plants by rubbing on the homogenate without TMV-infected leaf material.
4.2. Symptom characterization
Symptom characterization was performed on a total of 80 plants (40 +S and 40 –S plants) out of three different independent experiments. TMV-inoculated leaves were cut off and photographed 2 and 4 days after inoculation. The image software Cell D (Olympus Soft Imaging Solutions GmbH, Münster, Germany) with the particle detection tool was used to count the necrotic lesions on the leaves and to calculate their total area. The data were then analyzed with the the non-parametric Kruskal–Wallis test, followed by a post-hoc comparison according to Conover [60]. P < 0.05 was regarded as significant.
4.3. Electron microscopical studies
4.3.1. Negative staining and quantification of viral particles
Negative staining and quantification of viral particles was performed as described previously [39]. Briefly, 500 mg leaf samples were homogenized in 200 μl of 0.06 M phosphate buffer. 20 μl of the homogenate was applied on top of a formvar coated grid for 5 min, washed in buffer and stained with 2% phosphotungstic acid for 2 min. For statistical analysis TMV-particles were counted on 20 defined square areas for each replicate sample on the grid after negative staining (n = 6 plants for each data set) and the data were analyzed with the software program Statistica (Stat-Soft, Tulsa, OK, USA) by using the Mann Whitney U-test [60]. Statistical differences were determined at the 0.05, 0.01 and 0.001 levels of confidence.
4.3.2. Sample preparation for electron microscopy/immunogold labeling of glutathione and cysteine
Preparation of samples for transmission electron microscopy and immunogold labeling of glutathione and cysteine was done as described in Zechmann et al. [61,62] and Zechmann and Müller [63]. Briefly, small samples of fully developed first true leaves were fixed in 2.5% paraformaldehyde/0.5% glutardialdehyde in 0.06 M phosphate buffer (pH 7.2) rinsed in the buffer, dehydrated in increasing concentrations of acetone (50%, 70%, and 90%) and infiltrated with increasing concentrations of LR-White resin (30%, 60% and 100%; London Resin Company Ltd., Berkshire, UK). Samples were polymerized at 50 °C. Ultrathin sections were blocked with 2% bovine serum albumine (BSA) in phosphate buffered saline (PBS, pH 7.2), treated with the primary antibodies (anti-glutathione rabbit polyclonal IgG and anti-cysteine rabbit polyclonal IgG, Millipore Corp., Billerica, MA, USA) diluted 1:50 (glutathione antibody) and 1:300 (cysteine antibody), rinsed in PBS, incubated with a 10 nm gold-conjugated secondary antibody (goat anti rabbit IgG, British BioCell International, Cardiff, UK) diluted 1:50 and washed with distilled water. At least four different samples from mock- and TMV-inoculated leaves were examined for statistical evaluation. A minimum of 20 (peroxisomes and vacuoles) to 60 (other cell structures) sectioned cell structures of at least 15 different cells were analyzed for gold particle density. The obtained data were statistically evaluated using Statistica (Stat-Soft, Tulsa, OK, USA, 2002) and presented as the number of gold particles per μm2 or as changes in percentages. For statistical analyses of the total number of gold particles, the non-parametric Kruskal–Wallis test, followed by a post-hoc comparison according to Conover, was used. P < 0.05 was regarded as significant. To determine significant differences of the changes in percentage the Mann Whitney U-test was used [60]. Statistical differences were determined at the 0.05, 0.01 and 0.001 levels of confidence.
4.4. Total RNA extraction and gene expression analysis
At least 200 mg of fresh leaves/sample from TMV- or mock-inoculated leaves were homogenized in liquid nitrogen and used for total RNA isolation and subsequent reverse transcription (i.e. first strand cDNA synthesis) as described in Höller et al. [20].
Semiquantitative PCR for assaying expression of tobacco pathogenesis related-1 genes (NtPR-1a, NtPR-1b, NtPR-1c and NtPRB-1b) was conducted as outlined in Höller et al. [20]. Expression of a tobacco actin gene (NtAct) served as a reference (constitutive control) of gene expression. Quantitative real-time PCR (qPCR) for assaying expression of tobacco cysteine and glutathione biosynthesis genes (adenosine 5′-phosphosulfate reductase, NtAPR, γ-glutamylcysteine synthetase, NtGSH1 and glutathione synthetase, NtGSH2), a stress-related and an antioxidant gene (Tau class glutathione S-transferase 1, NtGSTTau1 and salicylic acid-binding catalase, NtCATSAB, respectively) and the virus gene encoding the TMV coat protein (TMV-CP) was conducted as recently described [20]. Expression of NtAct served as an internal control. Gene expression in TMV-inoculated samples was also normalized to that in mock-inoculated samples.
Oligonucleotide primers used in RT-PCR were the following: 5′-CGGAATCCACGAGACTACATAC-3′ (5′ primer [forward]) and 5′-GGGAAGCCAAGATAGAGC-3′ (3′ primer [reverse]) for a 230-bp NtAct cDNA fragment (GenBank accession X69885); 5′-GCAGATTGTAACCTCGTA-3′ (5′primer) and 5′-CAATTAGTATGGACTTTCG-3′ (3′primer) for a 297-bp NtPR-1a cDNA fragment (D90196); 5′-CAGGGAAGTGGCGATTTTATG-3′ (5′primer) and 5′-AGACCACTTGGACTTTTTACAGAT-3′ (3′primer) for a 400-bp NtPR-1b cDNA fragment (X03465); 5′-GGATGCCCATAACACAGC-3′ (5′primer) and 5′-TTTTTCCCCATAATCAAGAGC-3′ (3′primer) for a 568-bp NtPR-1c cDNA fragment (X12487); 5′- GGGATACTCCACAACATTAG -3′ (5′primer) and 5′- CACATACATATACACACCTC -3′ (3′primer) for a 744-bp NtPRB-1b cDNA fragment (X66942); 5′-CATGTTCCCTGACGCTGTTGA-3′ (5′primer) and 5′-GGCATCTTCCCACCACCATCT-3′ (3′primer) for a 430-bp NtAPR cDNA fragment (AY648056); 5′-TAATGCCGAAGGGGAGATACG-3′ (5′primer) and 5′-GCCGGGAATAGGGGAAAGT-3′ (3′primer) for a 434-bp NtGSH1 cDNA fragment (DQ444219); 5′-AGTGCCCTTCAATTTCCTATCAT-3′ (5′primer) and 5′-CTCCGCATTCAACCCCAGTA-3′ (3′primer) for a putative 574-bp NtGSH2 cDNA fragment (EB437755); 5′-GATGGCAGAAGTGAAGTTG-3′ (5′primer) and 5′- CTCCTAGCCAAAATSCCA-3′ (3′primer) for a putative 487-bp NtGSTTau1 cDNA fragment (AY206006 and X56263); 5′-GCCATATTGTCCCTGGTCTTT-3′ (5′primer) and 5′-GTATCTTTCTCCTGCCTGCTT-3′ (3′primer) for a 326-bp NtCATSAB cDNA fragment (U03473); 5′-CTTGTCATCAGCGTGGGC-3′ (5′primer) and 5′-AAGTCACTGTCAGGGAAC-3′ (3′primer) for a 165-bp TMV-CP cDNA fragment (AJ429078). Oligonucleotide primers were designed with the aid of the Primer Premier 5 program (PREMIER Biosoft International, Palo Alto, CA, USA).
For statistical analysis of gene expression Student’s t-test was used. Significant differences were determined at the 0.05, 0.01 and 0.001 levels of confidence.
4.5. Biochemical studies
4.5.1. Sulfur contents in soil
Sulfur contents in soil were measured according to Kirsten and Nordenmark [64] with an automated elemental analyzer (Carlo Erba NA 1500; Carlo Erba Instruments, Milan, Italy). Briefly, soil samples were dried at 80 °C for 3 days and sieved (corn size < 2 mm). Samples were ground (corn size < 63 μm) and burned in the elemental analyzer over copper oxide with oxygen injected into the carrier gas. Combustion gases were reduced with copper. Water was absorbed, and sulfur dioxide was separated from carbon dioxide and nitrogen. Sulfur contents were then determined using the software Agilent Chemstation 32 (Agilent Technology, Santa Clara CA, USA). The obtained data were statistically evaluated using Statistica (Stat-Soft, Tulsa, OK, USA). For statistical analyses, the non-parametric Kruskal–Wallis test followed by a post-hoc comparison according to Conover was used [60]. P < 0.05 was regarded significant.
4.5.2. Sulfur contents in leaves
Total sulfur contents in leaves were determined according to Pilch and Grill [65]. Briefly, dried leaves (4 days at 80 °C) were combusted at 900 °C in a closed system and the gases were absorbed in 3% hydrogen peroxide. The ash was wetted with distilled water and shaken at 100 °C in a water bath for 3 h. Aliquots of the solutions were used to determine total sulfur contents (as sulfate) for each sample by HPLC (LDC Milton Roy CM 4000, Milton Roy, Ivyland, PA, USA) equipped with a Spark Holland Basic Marathon autosampler (Ions Spark Holland B. V., Emmen, The Netherlands). Ions were separated with an anion exchange column (Hamilton PRP-X100, Hamilton Bonaduz AG, Bonaduz, Switzerland). The ions were detected with a conductivity detector (ESA IonChem Model 5400; ESA, Chelmsford, MA, USA), and the signals were processed with a computer software. Significant differences were calculated with the Mann Whitney U-test by using Statistica (Stat-Soft, Tulsa, OK, USA). Statistical differences were determined at the 0.05, 0.01 and 0.001 levels of confidence [60].
Acknowledgements
This work was supported by the Austrian Science Fund (FWF-Project nr. 20619 to BZ), and grants from the Hungarian Scientific Research Fund (OTKA K 77641, K77705 and K 84002) and by Austrian-Hungarian exchange grants (WTZ HU 10-2009 and AT-3/2008). We would like to thank Ewald Brauner from the University of Natural Resources and Applied Life Sciences in Vienna for soil analysis.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.plaphy.2011.10.020.
Appendix. Supplementary data
Fig. s1.
Transmission electron micrographs showing the subcellular distribution of glutathione in Nicotiana tabacum cv. Samsun NN leaf cells of mock- (a) and TMV-inoculated (b-d) plants grown with sulfate at the day of inoculation (a), 1 (b), 2 (c) and 4 (d) days after inoculation. C = chloroplasts, IS = intercellular spaces, M = mitochondria, N = nuclei, Px = peroxisomes, V = vacuoles. Bars = 1 μm.
Fig. s2.
Transmission electron micrographs showing the subcellular distribution of glutathione in Nicotiana tabacum cv. Samsun NN leaf cells of mock- (a) and TMV-inoculated (b-d) plants grown without sulfate at the day of inoculation (a), 1 (b), 2 (c) and 4 (d) days after inoculation. C = chloroplasts, IS = intercellular spaces, M = mitochondria, N = nuclei, Px = peroxisomes, V = vacuoles. Bars = 1 μm.
Changes in glutathione and cysteine labeling density in mesophyll cells of TMV-inoculated Nicotiana tabacum cv. Samsun NN grown with sulfate when compared to TMV-inoculated plants grown without sulfate. Measurements were performed 1, 2 and 4 days post inoculation (dpi). n > 20 for peroxisomes and n > 60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; ns indicates non-significance, whereas *, ** and ***, respectively, indicate significance at the 0.05, 0.01 and 0.001 levels of confidence. Nd = not determined.
Fig. s4.
Transmission electron micrographs showing the subcellular distribution of cysteine in Nicotiana tabacum cv. Samsun NN leaf cells of mock- (a) and TMV-inoculated (b-d) plants grown with sulfate at the day of inoculation (a), 1 (b), 2 (c) and 4 (d) days after inoculation. C = chloroplasts with and without starch (St), CW = cell walls, M = mitochondria, N = nuclei, Px = peroxisomes, V = vacuoles. Bars = 1 μm.
Fig. s5.
Transmission electron micrographs showing the subcellular distribution of cysteine in Nicotiana tabacum cv. Samsun NN leaf cells of mock- (a) and TMV-inoculated (b-d) plants grown without sulfate at the day of inoculation (a), 1 (b), 2 (c) and 4 (d) days after inoculation. C = chloroplasts, CW = cell walls, M = mitochondria, N = nuclei, Px = peroxisomes, V = vacuoles. Bars = 1 μm.
Total amounts of sulfur in mg in leaves of Nicotiana tabacum cv. Samsun NN mock-inoculated plants grown with (gray columns) and without (diagonally striped columns) sulfate. Plants grown without sulfate received one single dose (5 ml) of Hoagland solution with sulfate when transferred onto the sand-vermiculite mixture. Measurements were performed over a time period of 4 days post inoculation (dpi). N ≥ 6 replicate samples for each measurement. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; *** indicates significance at the 0.001 level of confidence.
Changes in total sulfur contents in leaves of TMV-inoculated Nicotiana tabacum cv. Samsun NN plants grown with (gray columns) or without sulfate (diagonally striped columns) in comparison to mock-inoculated plants. Measurements were performed over a time period of 4 days post inoculation (dpi). N ≥ 6 replicate samples for each measurement. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; ns indicates non-significance.
Total amount of sulfur in mg/kg dry weight (DW) in the soil of Nicotiana tabacum cv. Samsun NN plants grown with (gray columns) and without (diagonally striped columns) sulfate. Measurements were performed at the time of TMV-inoculation and 4 days post inoculation (dpi) in plants inoculated with TMV and in mock inoculated control plants (C). Plants grown without sulfate received one single dose (5 ml) of Hoagland solution with sulfate when transferred onto the sand-vermiculite mixture which did not contain measureable amounts of sulfur. Data are means with standard errors. Different lowercase letters indicate significant differences (P < 0.05) analyzed with the Kruskal–Wallis test followed by post-hoc comparison according to Conover.
References
- 1.Leustek T., Martin M.N., Bick J.A., Davies J.P. Pathways and regulation of sulfur metabolism revealed through molecular studies. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:141–166. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- 2.Leustek T. Sulfate metabolism. In: Somerville C.R., Meyerowitz E.M., editors. The Arabidopsis Book. American Society of Plant Biologists; Rockville, MD: 2002. [Google Scholar]
- 3.Kopriva S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann. Bot. 2006;97:479–495. doi: 10.1093/aob/mcl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl C., Hell R., Leustek E., Knaff D. Introduction to sulfur metabolism in phototrophic organisms. In: Hell R., Dahl C., Knaff D., Leustek T., editors. vol. 27. Springer; Dordrecht: 2008. pp. 1–14. (Advances in Photosynthesis and Respiration, Sulfur Metabolism in Phototrophic Organisms). [Google Scholar]
- 5.Kopriva S., Mugford S.G., Matthewman C., Koprivova A. Plant sulfate assimilation genes: redundancy versus specialization. Plant Cell Rep. 2009;28:1769–1780. doi: 10.1007/s00299-009-0793-0. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H., Kopriva S., Giordano M., Saito K., Hell R. Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Ann. Rev. Plant Biol. 2011;62:157–184. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- 7.Rausch T., Gromes R., Liedschulte V., Müller I., Bogs J., Galovic V., Wachter A. Novel insight into the regulation of GSH biosynthesis in higher plants. Plant Biol. 2007;9:565–572. doi: 10.1055/s-2007-965580. [DOI] [PubMed] [Google Scholar]
- 8.Hell R., Wirtz M. Metabolism of cysteine in plants and phototrophic bacteria. In: Hell R., Dahl C., Knaff D., Leustek T., editors. vol. 27. Springer; Dordrecht: 2008. pp. 59–91. (Advances in Photosynthesis and Respiration, Sulfur Metabolism in Phototrophic Organisms). [Google Scholar]
- 9.Kopriva S., Wiedemann G., Reski R. Sulfate assimilation in basal land plants. What does genomic sequencing tell us? Plant Biol. 2007;9:556–564. doi: 10.1055/s-2007-965430. [DOI] [PubMed] [Google Scholar]
- 10.Schnug E., Haneklaus S., Borchers A., Polle A. Relations between sulphur supply and glutathione, ascorbate and glucosinolate concentrations in Brassica napus varieties. J. Plant Nut. Soil Sci. 1995;158:67–70. [Google Scholar]
- 11.Bloem E., Haneklaus S., Schnug E. Significance of sulfur compounds in the protection of plants against pests and diseases. J. Plant Nut. 2005;28:763–784. [Google Scholar]
- 12.Dubuis P.H., Marazzi C., Städler E., Mauch F. Sulphur deficiency causes a reduction in antimicrobial potential and leads to increased disease susceptibility of oilseed rape. J. Phytopathol. 2005;153:27–36. [Google Scholar]
- 13.Klikocka H., Haneklaus S., Bloem E., Schnug E. Influence of sulfur fertilization on infection of potato tubers with Rhizoctonia solani and Streptomyces scabies. J. Plant Nut. 2005;28:819–833. [Google Scholar]
- 14.Schnug E., Haneklaus S. Sulphur deficiency symptoms in oilseed rape (Brassica napus L.) – the aesthetics of starvation. Phyton (Horn, Austria) 2005;45:79–95. [Google Scholar]
- 15.Zhao F.J., Tausz M., De Kok L.J. Role of sulfur for plant production in agricultural and natural ecosystems. In: Hell R., Dahl C., Knaff D., Leustek T., editors. vol. 27. Springer; Dordrecht: 2008. pp. 417–435. (Advances in Photosynthesis and Respiration, Sulfur Metabolism in Phototrophic Organisms). [Google Scholar]
- 16.Bloem E., Haneklaus S., Salac I., Wickenhäuser P., Schnug E. Facts and fiction about sulfur metabolism in relation to plant-pathogen interactions. Plant Biol. 2007;9:596–607. doi: 10.1055/s-2007-965420. [DOI] [PubMed] [Google Scholar]
- 17.Haneklaus S., Bloem E., Schnug E. Plant disease control by nutrient management: sulphur. In: Walters D., editor. Disease Control in Crops: Biological and Environmentally Friendly Approaches. Wiley-Blackwell; Oxford: 2009. pp. 221–234. [Google Scholar]
- 18.Kruse C., Jost R., Lipschis M., Kopp B., Hartmann M., Hell R. Sulfur-enhanced defence: effects of sulfur metabolism, nitrogen supply, and pathogen lifestyle. Plant Biol. 2007;9:608–619. doi: 10.1055/s-2007-965432. [DOI] [PubMed] [Google Scholar]
- 19.Schlaeppi K., Abou-Mansour E., Buchala A. Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J. 2010;62:840–851. doi: 10.1111/j.1365-313X.2010.04197.x. [DOI] [PubMed] [Google Scholar]
- 20.Höller K., Király L., Künstler A., Müller M., Gullner G., Fattinger M., Zechmann B. Enhanced glutathione metabolism is correlated with sulfur induced resistance in Tobacco mosaic virus-infected genetically susceptible Nicotiana tabacum plants. Mol. Plant-Microb. Inter. 2010;23:1448–1459. doi: 10.1094/MPMI-05-10-0117. [DOI] [PubMed] [Google Scholar]
- 21.Foyer C.H., Noctor G. Redox regulation and photosynthetic organisms: signaling, acclimation, and practical implications. Antiox. Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- 22.Noctor G., Queval G., Mhamdi A., Chaouch S., Foyer C.H. The Arabidopsis Book 9. American Society of Plant Biologists Rockville; MD: 2011. Glutathione; p. e0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noctor G., Foyer C.H. Ascorbate and glutathione: keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:229–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 24.Noctor G., Gomez L., Vanacker H., Foyer C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J. Exp. Bot. 2002;53:1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama A., Nishimura J., Mochizuki Y., Inagaki K., Sekiya J. Homoglutathione synthesis in transgenic tobacco plants expressing soybean homoglutathione synthetase. Plant Biotech. 2004;21:79–83. [Google Scholar]
- 26.Wachter A., Wolf S., Steininger H., Bogs J., Rausch T. Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J. 2005;41:15–30. doi: 10.1111/j.1365-313X.2004.02269.x. [DOI] [PubMed] [Google Scholar]
- 27.Zechmann B., Müller M., Zellnig G. Modified levels of cysteine affect glutathione metabolism in plant cells. In: Khan N.A., Singh S., Umar S., editors. Sulfur Assimilation and Abiotic Stress in Plants. Springer; Berlin, Heidelberg: 2008. pp. 193–206. [Google Scholar]
- 28.Foyer C.H., Rennenberg H. Regulation of glutathione synthesis and its role in abiotic and biotic stress defence. In: Brunold C., Rennenberg H., De Kok L.J., Stulen I., Davidian J.C., editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. Paul Haupt Publishers; Bern: 2000. pp. 127–153. [Google Scholar]
- 29.Kopriva S., Rennenberg H. Control of sulphate assimilation and glutathione synthesis: interaction with N and C metabolism. J. Exp. Bot. 2004;55:1831–1842. doi: 10.1093/jxb/erh203. [DOI] [PubMed] [Google Scholar]
- 30.De Kok L.J., De Kan J.L., Tánczos O.G., Kuiper P.J.C. Sulphate induced accumulation of glutathione and frost tolerance of spinach leaf tissue. Physiol. Plant. 1981;53:435–438. [Google Scholar]
- 31.Blake-Kalff M.M.A., Hawkesford M.J., Zhao F.J., McGrath S.P. Diagnosing sulfur deficiency in field-grown oilseed rape (Brassica napus L.) and wheat (Triticum aestivum L.) Plant Soil. 2000;225:95–107. [Google Scholar]
- 32.Bloem E., Riemenschneider A., Volker J., Papenbrock J., Schmidt A., Salac I., Haneklaus S., Schnug E. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J. Exp. Bot. 2004;55:2305–2312. doi: 10.1093/jxb/erh236. [DOI] [PubMed] [Google Scholar]
- 33.Bloem E., Haneklaus S., Schnug E. Sulphur-induced resistance (SIR) - Sulphur fertilization as a sustainable strategy for keeping plants healthy. J. Consumer Prot. Food Saf. 2007;2:7–12. [Schwefel-induzierte Resistenz (SIR) - Schwefeldungung als nachhaltige Strategie zur Gesunderhaltung von Pflanzen] [Google Scholar]
- 34.Blake-Kalff M.M.A., Harrison K.R., Hawkesford M.J., Zhao F.J., McGrath S.P. Distribution of sulfur within oilseed rape leaves in response to sulfur deficiency during vegetative growth. Plant Physiol. 1998;118:1337–1344. doi: 10.1104/pp.118.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikiforova V.J., Bielecka M., Gakiere B., Krueger S., Rinder J., Kempa S., Morcuende R., Scheible W.R., Hesse H., Hoefgen R. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids. 2006;30:173–183. doi: 10.1007/s00726-005-0251-4. [DOI] [PubMed] [Google Scholar]
- 36.Parmar S., Buchner P., Hawkesford M.J. Leaf developmental stage affects sulfate depletion and specific sulfate transporter expression during sulfur deprivation in Brassica napus L. Plant Biol. 2007;9:647–653. doi: 10.1055/s-2007-965428. [DOI] [PubMed] [Google Scholar]
- 37.Gullner G., Tóbiás I., Fodor J., Kömives T. Elevation of glutathione level and activation of glutathione-related enzymes affect virus infection in tobacco. Free Rad. Res. 1999;31:155–161. doi: 10.1080/10715769900301451. [DOI] [PubMed] [Google Scholar]
- 38.Harms K., von Ballmoos P., Brunold C., Höfgen R., Hesse H. Expression of a bacterial serine acetyltransferase in transgenic potato plants leads to increased levels of cysteine and glutathione. Plant J. 2000;22:335–343. doi: 10.1046/j.1365-313x.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- 39.Zechmann B., Zellnig G., Urbanek-Krajnc A., Müller M. Artificial elevation of glutathione affects symptom development in ZYMV-infected Cucurbita pepo L. plants. Arch. Virol. 2007;152:747–762. doi: 10.1007/s00705-006-0880-2. [DOI] [PubMed] [Google Scholar]
- 40.Loebenstein G. Local lesions and induced resistance. Adv. Virus Res. 2009;75:74–117. doi: 10.1016/S0065-3527(09)07503-4. [DOI] [PubMed] [Google Scholar]
- 41.Hammerschmidt R. Systemic acquired resistance. Adv. Bot. Res. 2009;51:173–222. [Google Scholar]
- 42.Culver J.N., Lehto K., Close S.M., Hilf M.E., Dawson W.O. Genomic position affects the expression of tobacco mosaic virus movement and coat protein genes. Proc. Natl. Acad. Sci. USA. 1993;90:2055–2059. doi: 10.1073/pnas.90.5.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nejidat A., Beachy R.N. Decreased levels of TMV coat protein in transgenic tobacco plants at elevated temperatures reduce resistance to TMV infection. Virology. 1989;173:531–538. doi: 10.1016/0042-6822(89)90565-5. [DOI] [PubMed] [Google Scholar]
- 44.Ghanta S., Bhattacharyya D., Sinha R., Banerjee A. Chattopadhyay, Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid-mediated pathway. Planta. 2011;233:895–910. doi: 10.1007/s00425-011-1349-4. [DOI] [PubMed] [Google Scholar]
- 45.Willekens H., Chamnongpol S., Davey M., Schraudner M., Langebartels C., Van Montagu M., Inzé D., Van Camp W. Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. EMBO J. 1997;16:4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauch F., Dudler R. Differential induction of distinct glutathione-S-tramsferases of wheat by xenobiotics and by pathogen attack. Plant Physiol. 1993;102:1193–1201. doi: 10.1104/pp.102.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards R., Dixon D., Walbot B. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000;5:193–198. doi: 10.1016/s1360-1385(00)01601-0. [DOI] [PubMed] [Google Scholar]
- 48.Fodor J., Gullner G., Ádám A.L., Barna B., Kőmíves T., Király Z. Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid. Plant Physiol. 1997;114:1443–1451. doi: 10.1104/pp.114.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi S.Y., Yu S.H., Choi D. Molecular cloning of a catalase cDNA from Nicotiana glutinosa L. and its repression by tobacco mosaic virus infection. Mol. Cells. 1999;9:320–325. [PubMed] [Google Scholar]
- 50.Király Z., Barna B., Kecskés A., Fodor J. Down-regulation of antioxidative capacity in a transgenic tobacco which fails to develop acquired resistance to necrotization caused by tobacco mosaic virus. Free Rad. Res. 2002;36:981–991. doi: 10.1080/1071576021000006581. [DOI] [PubMed] [Google Scholar]
- 51.Zechmann B., Müller M., Zellnig G. Intracellular adaptations of glutathione content in Cucurbita pepo (L.) induced by reduced glutathione and buthionine sulfoximine treatment. Protoplasma. 2006;227:197–209. doi: 10.1007/s00709-005-0129-z. [DOI] [PubMed] [Google Scholar]
- 52.Vaca R.A., de Pinto M.C., Valenti D., Passarella S., Ersilia M., de Gara L. Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco bright-yellow 2 cells. Plant Physiol. 2004;134:1100–1112. doi: 10.1104/pp.103.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amirsadeghi S., Robson C.A., Vanlerberghe G.C. The role of the mitochondrion in plant responses to biotic stress. Physiol. Plant. 2007;129:253–266. [Google Scholar]
- 54.Vianello A., Zancani M., Peresson C., Petrussa E., Casolo V., Krajnakova J., Patui S., Braidot E., Macri F. Plant mitochondrial pathway leading to programmed cell death. Physiol. Plant. 2007;129:242–252. [Google Scholar]
- 55.Go Y.M., Jones D.P. Redox control system in the nucleus: mechanisms and functions. Antiox. Redox Signal. 2010;12:489–509. doi: 10.1089/ars.2009.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz-Vivancos P., Wolff T., Markovic J., Pallardo F.V., Foyer C.H. A nuclear glutathione cycle within the cell cycle. Biochem. J. 2011;431:169–178. doi: 10.1042/BJ20100409. [DOI] [PubMed] [Google Scholar]
- 57.Green R.M., Graham M., O’Donovan M.R., Chipman J.K., Hodges N.J. Subcellular compartmentalization of glutathione: correlations with parameters of oxidative stress related to genotoxicity. Mutagenesis. 2006;21:383–390. doi: 10.1093/mutage/gel043. [DOI] [PubMed] [Google Scholar]
- 58.Queval G., Jaillard D., Zechmann B., Noctor G. Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ. 2011;34:21–32. doi: 10.1111/j.1365-3040.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 59.Kataoka T., Watanabe-Takahashi A., Hayashi N., Ohnishi M., Mimura T., Buchner P., Hawkesford M.J., Yamaya T., Takahashi H. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell. 2004;16:2693–2704. doi: 10.1105/tpc.104.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bortz J., Lienert G.A., Bohenke K. Springer; Heidelberg: 2008. Verteilungsfreie Methoden in der Biostatistik. [Google Scholar]
- 61.Zechmann B., Müller M. Effects of zucchini yellow mosaic virus infection on the subcellular distribution of glutathione and its precursors in a highly tolerant Cucurbita pepo cultivar. Botany. 2008;86:1092–1100. [Google Scholar]
- 62.Zechmann B., Mauch F., Sticher L., Müller M. Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. J. Exp. Bot. 2008;59:4017–4027. doi: 10.1093/jxb/ern243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zechmann B., Müller M. Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma. 2010;246:15–24. doi: 10.1007/s00709-010-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirsten W.J., Nordenmark B.S. Rapid, automatic, high-precision method for micro, ultramicro, and trace determinations of sulfur. Analyt. Chim. Acta. 1987;196:59–68. [Google Scholar]
- 65.Pilch B., Grill D. Determination of organic and inorganic sulphur by ion chromatography in small quantities of plant material. J. Plant Physiol. 1995;146:10–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in glutathione and cysteine labeling density in mesophyll cells of TMV-inoculated Nicotiana tabacum cv. Samsun NN grown with sulfate when compared to TMV-inoculated plants grown without sulfate. Measurements were performed 1, 2 and 4 days post inoculation (dpi). n > 20 for peroxisomes and n > 60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; ns indicates non-significance, whereas *, ** and ***, respectively, indicate significance at the 0.05, 0.01 and 0.001 levels of confidence. Nd = not determined.
Total amounts of sulfur in mg in leaves of Nicotiana tabacum cv. Samsun NN mock-inoculated plants grown with (gray columns) and without (diagonally striped columns) sulfate. Plants grown without sulfate received one single dose (5 ml) of Hoagland solution with sulfate when transferred onto the sand-vermiculite mixture. Measurements were performed over a time period of 4 days post inoculation (dpi). N ≥ 6 replicate samples for each measurement. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; *** indicates significance at the 0.001 level of confidence.
Changes in total sulfur contents in leaves of TMV-inoculated Nicotiana tabacum cv. Samsun NN plants grown with (gray columns) or without sulfate (diagonally striped columns) in comparison to mock-inoculated plants. Measurements were performed over a time period of 4 days post inoculation (dpi). N ≥ 6 replicate samples for each measurement. Data are means with standard errors. Significant differences were calculated using the Mann Whitney U-test; ns indicates non-significance.
Total amount of sulfur in mg/kg dry weight (DW) in the soil of Nicotiana tabacum cv. Samsun NN plants grown with (gray columns) and without (diagonally striped columns) sulfate. Measurements were performed at the time of TMV-inoculation and 4 days post inoculation (dpi) in plants inoculated with TMV and in mock inoculated control plants (C). Plants grown without sulfate received one single dose (5 ml) of Hoagland solution with sulfate when transferred onto the sand-vermiculite mixture which did not contain measureable amounts of sulfur. Data are means with standard errors. Different lowercase letters indicate significant differences (P < 0.05) analyzed with the Kruskal–Wallis test followed by post-hoc comparison according to Conover.