Abstract
Adipose tissue is an important metabolic organ that is crucial for whole-body insulin sensitivity and energy homeostasis. Highly refined fructose intake increases visceral adiposity although the mechanism(s) remain unclear. Differentiation of preadipocytes to mature adipocytes is a highly regulated process that is associated with characteristic sequential changes in adipocyte gene expression. We demonstrate that fructose treatment of murine 3T3-L1 cells incubated in standard differentiation medium increases adipogenesis and adipocyte-related gene expression. We further show that the key fructose transporter, GluT5, is expressed in early-stage adipocyte differentiation but is not expressed in mature adipocytes. GluT5 overexpression or knockdown increased and decreased adipocyte differentiation, respectively, and treatment of 3T3-L1 cells with a specific GluT5 inhibitor decreased adipocyte differentiation. Epidymal white adipose tissue was reduced in GluT5−/− mice compared with wild-type mice, and mouse embryonic fibroblasts derived from GluT5−/− mice exhibited impaired adipocyte differentiation. Taken together, these results demonstrate that fructose and GluT5 play an important role in regulating adipose differentiation.
Obesity is an energy balance disorder in which nutrient intake chronically exceeds energy expenditure, resulting in excessive white adipose tissue (WAT) accumulation. It is associated with insulin resistance, development of type 2 diabetes, hypertension, hyperlipidemia, and atherosclerosis (1–3). Adipose tissue consists of WAT, which is crucial for whole-body insulin sensitivity and the primary site of energy storage and brown adipose tissue, which is involved in energy expenditure and homeostasis. WAT secretes an array of hormones (adipokines) including leptin, resistin, adiponectin, visfatin, and plasminogen activator inhibitor-1 and cytokines such as TNF-α, IL-6, and macrophage chemoattractant protein 4. These and other adipocyte-derived factors regulate physiological processes throughout the body including glucose metabolism, appetite, inflammatory responses, angiogenesis, blood pressure, and reproductive function (1, 2, 4).
During obesity development, adipocytes become hypertrophic and contribute to an altered adipocyte milieu that foster the cardiovascular and metabolic complications associated with obesity (5). For example, adipocytes in obesity produce reduced adiponectin levels and increased proatherogenic factors such as plasminogen activator inhibitor-1 (6, 7). Improved understanding of potentially correctable initiators and triggers of metabolic dysfunction in adipocytes is key to further insights into causes of obesity and associated morbidities (8–10).
Increased rates of obesity, insulin resistance, and metabolic syndrome are clearly multifactorial due to reduced exercise and poor dietary choices. It has been proposed that increased obesity rates may be due, in part, to increased consumption of fructose derived from dietary sugars, primarily sucrose and high-fructose corn syrup. Self-reported food intake data from the national Health and Nutrition Examination Survey (NHANES) suggests that approximately 15% of the US population consumes more than 25% of energy from added sugars. Importantly, increased fructose intake from sucrose or high-fructose corn syrup has been implicated in promoting weight gain, visceral adiposity, dyslipidemia, and insulin resistance, which are all components of the metabolic syndrome (11–16).
Metabolism of glucose and fructose share many metabolic pathways but some aspects of fructose metabolism are strikingly different from glucose. For example, in the liver, high fructose flux leads to enhanced hepatic triglyceride accumulation resulting in impaired glucose and lipid metabolism and increased proinflammatory cytokine expression (17–21). Dietary fructose is absorbed into the intestine via a specific saturable, facilitative glucose transporter (GLUT5) (22–24). Given the potential connections between fructose intake and obesity, we examined the effects of fructose on adipocyte differentiation. Differentiation of preadipocytes into adipocytes is a highly regulated process whereby a series of transcription factors including the peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα) trigger a cascade of transcriptional events that coordinate adipocyte differentiation. We demonstrate that addition of fructose to murine 3T3-L1 preadipocytes in standard differentiation medium enhanced adipocyte differentiation and increased the adipocyte marker genes PPARγ and C/EBPα. We further demonstrate that the specific fructose transporter, GluT5, plays a previously unrecognized role in adipogenesis.
Materials and Methods
3T3-L1 cell cultures and transfections
3T3-L1 preadipocytes, obtained from American Type Culture Collection (Manassas, VA) were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and grown to confluence. The cells were then treated with DMEM containing 10% FBS and 5 μg/ml insulin, 1 μm dexamethasone, and 0.5 mm 3-iso-butyl-1-methyxanthine. After 48 h incubation, culture medium was replaced with fresh 10% FBS DMEM containing 5 μg/ml insulin. After a further 48 h, the medium was changed to 10% FBS DMEM until completion of experiments.
Plasmid constructs and transfections
For Glut5 overexpression, the mouse GluT5 cDNA was amplified by PCR and cloned into the pEGFP-C1 vector. Control plasmid and enhanced green fluorescent protein-fusion Glut5 expression plasmid were transfected into 3T3L1 cells using the BioT plasmid DNA transfection protocol. For GluT5 knockdown studies, GluT5 small interfering RNA (siRNA) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and negative control siRNA were transfected into 3T3L1 murine preadipocytes using BioT (Bioland, Inc., Catonsville, MD) siRNA transfection protocol for mammalian cells (40 pmol of each siRNA oligo was added for every 1 μl of BioT). The GluT5 siRNA sequences were as follows: GAGUGAACGCGAUCUACUA, CAGACGUAUAUCCGAAGAA, and GUAAGCGAUACUACUUUGU. Cells were transferred 48 h after transfection to standard adipocyte differentiation medium as above containing 11.1 mm glucose and 550 μm fructose.
Animals
GluT5−/− mice on a C57BL/6J background were a gift from Dr. Jian Zuo (Department of Developmental Neurobiology, St. Jude Children's Research Hospital, Memphis, TN) (25). GluT5−/− mice were crossed with wild-type C57BL/6J mice (Jackson Lab) to generate GluT5+/− heterozygotes which were then interbred to generate littermate GluT5 −/− and wild-type littermates for mouse embryonic fibroblast (MEF) studies. Mice were housed under a 12-h light, 12-h dark cycle and fed a regular diet, and all studies were approved by the UCLA Animal Research Committee in compliance with all relevant federal guidelines and institutional policies.
MEF culture and adipocyte differentiation
MEF were generated from 14.5-d-old GluT5−/− and wild-type littermate embryos obtained from heterozygous GluT5+/− mice intercrosses. In brief, after dissection and visceral organ removal (heart, liver, etc) for genotyping, the embryos were minced and trypsinized for 10–15 min at 37 C. Genomic DNA was extracted and PCR analysis was performed to determine the MEF genotype. MEF were cultured in DMEM with 15% FBS at 37 C in an atmosphere of 5% CO2. All experiments were performed with wild-type (GluT5+/+) and knockout (GluT5−/−) MEF at second or third passages. For adipocyte differentiation studies, MEF cells were allowed to attain confluency (d 0) and were then treated with DMEM supplemented with 15% FBS, 5 μg/ml insulin, 1 μm dexamethasone, and 0.5 mm 3-iso-butyl-1-methyxanthine for 2 d. The culture medium was then replaced with DMEM containing 15% FBS, 5 μg/ml insulin, and 5 μm rosiglitazone for the next 48 h. Medium was renewed every 2 d with DMEM containing 15% FBS and 5 μm rosiglitazone until cells were analyzed.
Oil Red O staining
For Oil Red O staining, cells were fixed on plates with 3.7% (wt/vol) formaldehyde in PBS for 10 min, washed with PBS, and air dried. The fixed cells were then stained with Oil Red O staining solution (Sigma Chemical Co., St. Louis, MO) (0.5% Oil Red O in isopropanol, diluted 3:2 in water, and then filtered with a 0.22-μm filter) for 20 min at room temperature. The plates were then washed twice with PBS and photographed at ×20 magnification. To quantify intracellular lipid content, total lipids were extracted with isopropyl alcohol, and lipid content was measured using a colorimetric method at 540 nm.
Quantitative real-time PCR
Total RNA was extracted from 3T3-L1 cells or MEF using Rneasy RNA kit (QIAGEN, Chatsworth, CA), and cDNA was synthesized from 1.0 μg total RNA using Superscript III reverse transcriptase enzyme (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Real-time PCR was performed on MyiQ single-color real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) using SYBR green PCR mix (QIAGEN). The mRNA was normalized to actin expression, and the specific primer sequences are listed as supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org.
Western blotting
Protein aliquots from 3T3-L1 cells and MEF were harvested in RIPA buffer containing protease and phosphatase inhibitors (Sigma-Aldrich). Lysates were cleared by centrifugation and total protein concentration was determined using a Bio-Rad DC kit. Protein extracts were resolved by SDS-PAGE using an 8% gel and electrotransferred onto polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA). Membranes were blocked for 2 h at room temperature in TBS-Tween 20 [10 mm Tris, 0.15 mm NaCl, and 0.1% Tween 20 (pH 7.4)] containing 10% (wt/vol) nonfat dried milk, washed twice, and then incubated with primary antibodies against PPARγ, C/EBPα, GluT4, actin, or glyceraldehydes-3-phosphate dehydrogenase (all 1:200 from Cell Signaling Technology, Danvers, MA). After washing, membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies. Immunoreactive proteins were visualized using SuperSignal Chemiluminescence Assay kit (Pierce Chemical Co., Rockford, IL).
Statistical analysis
Data are presented as relative difference to control, and results are expressed as mean ± sem derived from at least three independent experiments. Statistical analysis was performed by ANOVA followed by Tukey post tests or nonparametric t test (two-tailed/unpaired) as indicated. P < 0.05 was considered significant.
Results
Fructose increases murine adipocyte differentiation
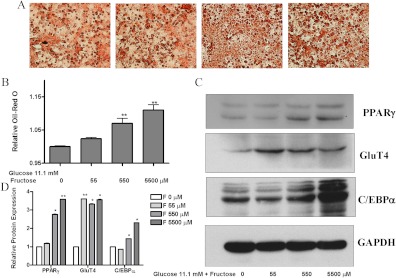
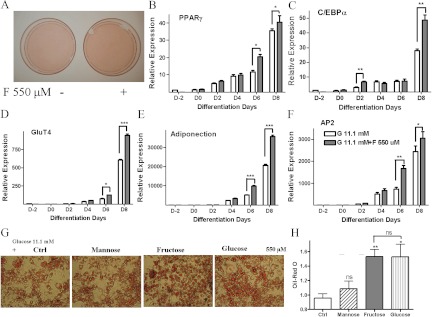
Addition of a range of fructose concentrations (0, 55, 550, 5500 μm) to murine 3T3-L1 preadipocytes incubated in standard differentiation medium containing 11.1 mm glucose resulted in a dose-dependant increase in lipid accumulation as measured by Oil Red O staining (depicted in Fig. 1A and quantitated in Fig. 1B). Western blot analysis of protein extracts demonstrated a 3.5-fold increase in the adipogenesis markers PPARγ and GluT4 and a 2-fold increase in C/EBPα levels upon fructose treatment (Fig. 1, C and D). To identify the onset of increased adipogenesis after fructose treatment, 3T3-L1 cells in standard differentiation medium were similarly treated with 550 μm fructose after which increased lipid accumulation was observed (Fig. 2A). Quantitative PCR analysis demonstrated increased PPARγ, C/EBPα, GluT4, adiponectin, and adipocyte fatty acid binding protein 2 (AP2) mRNA levels from d 2–d 4 of differentiation (Fig. 2, B–F) with approximately 1.5-fold increase in these transcription factors by d 8 in fructose-treated compared with standard conditions. These results suggested that fructose, a common dietary sugar, could contribute to adipogenesis.
Fig. 1.
Fructose induces murine 3T3-L1 cell adipogenesis. 3T3-L1 preadipocyte cells were differentiated under standard conditions as described in Materials and Methods. During differentiation, the medium contained 11.1 mm glucose and a range of fructose concentrations (left to right, 0, 55, 550, and 5500 μm). A and B, Depiction and quantification by OD measurement at 540 nm, respectively, for Oil Red O staining of fructose-treated 3T3-L1 cells. C, Western blot of adipogenesis marker proteins, PPARγ, C/EBPα, and GluT4 in the 3T3-L1 cells after treatment in differentiation medium and a range of fructose concentrations. D, Quantitification of Western blot PPARγ, C/EBPα, and GluT4 levels. Analysis was based on three independent experiments using one-way ANOVA followed by Tukey posttest. ns, Not significant; *, P < 0.05; **, P < 0.01. GAPDH, Glyceraldehyde-3 phosphate dehydrogenase. ns, P > 0.05.
Fig. 2.
Time course of fructose-induced adipocyte differentiation. A, Oil Red O staining of 3T3-L1 preadipocyte cells differentiated in medium contained 11.1 mm glucose with/without 550 μm fructose. B–F, PPARγ, C/EBPα, GluT4, adiponection, and AP2 mRNA measured by real-time PCR in the 3T3-L1 cells after treatment with differentiation medium and 550 μm fructose. (D, day of differentiation). Depiction (panel G) and quantitification (panel H) by OD measurement at 540 nm of Oil Red O staining of 3T3-L1 preadipocyte cells differentiated in medium contained 11.1 mm glucose or 11.1 mm glucose with addition of 550 μm mannose, fructose, or glucose. Analysis was based on three independent experiments using one-way ANOVA followed by Tukey posttest. ns, Not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Ctrl, Control. ns, P > 0.05.
We wanted to determine whether this effect of fructose was simply a sugar effect or whether there was some specificity to the adipogenic actions of fructose. Therefore, we also tested adipose differentiation in medium containing 11.1 mm glucose with addition of 550 μm fructose, glucose, or mannose. As shown in Fig. 2, G and H, whereas addition of fructose and glucose similarly increased adipocyte differentiation compared with 11.1 mm glucose alone, addition of mannose had no effect on adipose differentiation. This supported our prior studies and indicated that fructose, like glucose, can serve as a substrate to increase adipose differentiation.
GluT5 plays a role in adipocyte differentiation
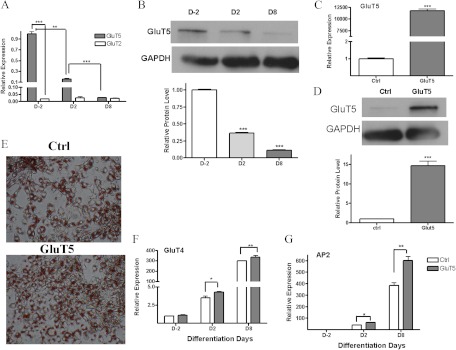
Although fructose can be transported via GluT2, GluT5, and GluT7, studies in GluT5 knockout mice indicate GluT5 is the main fructose transporter (24). We used quantitative PCR (Q-PCR) to characterize GluT2 and GluT5 expression during 3T3-L1 differentiation. As shown in Fig. 3A, GluT5 mRNA was abundantly expressed in preadipocytes (D-2) and then decreased rapidly during adipocyte differentiation so that by d 8, GluT5 levels were to 90% lower compared with preadipocytes. In contrast GluT2 expression was only approximately 1.7% of GluT5 levels in 3T3-L1 cells, and its expression did not alter during 3T3-L1 differentiation. This led us to hypothesize that GluT5 played a role in adipocyte differentiation, and we therefore characterized the effects of GluT5 overexpression on 3T3-L1 preadipocyte differentiation in standard differentiation medium containing 11.1 mm glucose and 550 μm fructose. Q-PCR confirmed GluT5 mRNA overexpression in our transfectants (Fig. 3B), and Oil Red O staining demonstrated that GluT5 transfectants exhibited higher lipid droplet number compared with vector controls (Fig. 3C). GluT5 overexpressing 3T3-L1 cells exhibited increased lipid accumulation (Fig. 3C), and Q-PCR demonstrated increased GluT4 and AP2 mRNA levels (Fig. 3, D and E) in keeping with increased adipocyte differentiation.
Fig. 3.
GluT5 overexpression increases 3T3-L1 adipocyte differentiation. A, Quantitative RT-PCR expression of GluT5 and GluT2mRNA and (B) GluT5 protein levels in 3T3-L1 adipocytes during differentiation in standard medium. C, Glut5 mRNA levels and (D) protein level in GluT5- and vector [control (Ctrl)]-transfected 3T3-L1 adipocytes. E, Oil Red O staining in Glut5-overexpressing or vector-transfected 3T3-L1 cells at d 8 in differentiation medium containing 11.1 mm glucose and 550 μm fructose. GluT4 (panel F) and AP2 mRNA (panel G) levels in 3T3-L1 GluT5 transfectants compared with vector-transfected (Ctrl) cells. Analysis based on three independent experiments using one-way ANOVA followed by Tukey posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001.GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
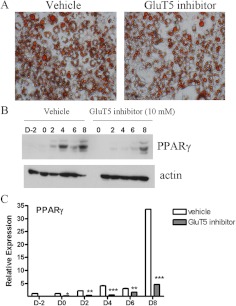
GluT5 knockdown or pharmacological blockade impairs murine adipocyte differentiation
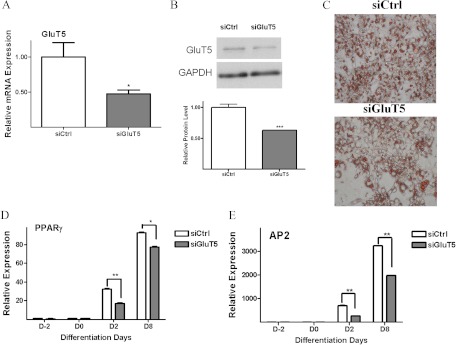
To further examine the role of GluT5 in adipogenesis, we used specific siRNA to knock down endogenous GluT5 levels in 3T3-L1 preadipocytes. Q-PCR confirmed approximately 50% siRNA-mediated GluT5 knockdown (Fig. 4A). At d 8 of differentiation in standard differentiation medium with 11.1 mm glucose and 550 μm fructose, lipid accumulation was lower in GluT5 knockdown cells compared with control siRNA transfected cells (Fig. 4B). We also observed decreased expression of the adipocyte differentiation markers, PPARγ and AP2, in the GluT5 knockdown cells (Fig. 4, C and D). These experiments indicated that decreased GluT5 expression impaired 3T3-L1 adipogenesis and so we next tested the effects of a specific GluT5 inhibitor, 1-O-Benzyl-2-N, 3-O-carbonyl-α-l-sorbofuranosylamine (L-Bn-OZO) [dissociation constant (Kd) 3 mm] on adipocyte differentiation (26, 27). On d 8 of differentiation, less lipid accumulation was observed in GluT5 inhibitor-treated (3 mm) compared with vehicle (dimethylsulfoxide)-treated 3T3-L1 cells cultured in standard differentiation medium with no added fructose (Fig. 5A). Western blot analysis demonstrated reduced PPARγ expression in protein lysates after GluT5 inhibitor, in comparison with vehicle treatment (Fig. 5, B and C). Taken together, these results indicated that inhibition of GluT5 function suppressed murine adipogenesis.
Fig. 4.
GluT5 knockdown impairs murine adipocyte differentiation. GluT5 mRNA levels (panel A) and protein levels (panel B) after siRNA-mediated GluT5 knockdown in 3T3-L1 adipocytes. C, Oil Red O staining in GluT5 knockdown and control SiRNA 3T3-L1 cells on d 8 in medium containing 11.1 mm glucose with 550 μm fructose. PPARγ (panel D) and AP2 mRNA (panel E) expression in GluT5 knockdown (siGluT5) and siRNA control (SiCtrl) 3T3-L1 cells. Analysis was based on three independent experiments using one-way ANOVA followed by Tukey posttest. ns, Not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, P > 0.05.
Fig. 5.
GluT5 inhibitor treatment suppressed murine adipocyte differentiation. Oil Red O staining (panel A) and Western blot analysis (panels B and C) of PPARγ expression in 3T3-L1 cells at d 8 in standard medium treated with or without the GluT5 inhibitor, L-Bn-OZO (10 mm), or vehicle (dimethylsulfoxide). Analysis was based on three independent experiments using one-way ANOVA followed by Tukey posttest. ns, Not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, P > 0.05.
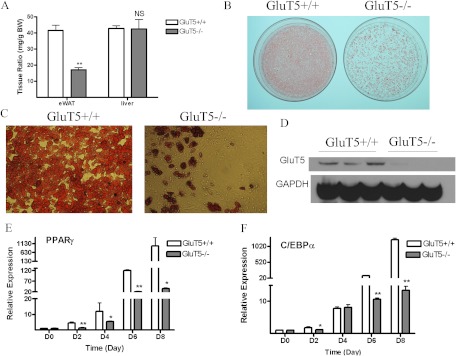
Decreased WAT and impaired adipogenesis in GluT5−/− mice
Given our in vitro findings demonstrating a role for GluT5 in adipogenesis, we examined white adipose fat tissue harvested from GluT5−/− mice. Although liver and total body (data not shown) weights did not differ from those of wild-type mice, GluT5−/− mice had 40% less epididymal WAT fat mass (Fig. 6A). To further investigate the role of GluT5 in adipocyte differentiation, MEF were derived from litter-matched wild-type (GluT5+/+) and knockout (GluT5−/−) embryos and induced to differentiate into adipocytes under standard conditions. Oil Red O staining demonstrated that GluT5+/+ MEF accumulated more lipid droplets than GluT5−/− MEF (Fig. 6, B and C). The absence of GluT5 in GluT5−/− MEF was determined by Western blot (Fig. 6D). Consistent with the Oil Red O staining pattern, expression of adipose marker genes PPARγ and C/EBPα was reduced in GluT5−/− compared with wild-type differentiated MEF (Fig. 6, E and F).
Fig. 6.
Mice lacking GluT5 showed reduced fat mass and impaired adipogenesis. A, Epidymal WAT (eWAT) to total body weight ratio in GluT5−/− and GluT5+/+ mice. B and C, Oil Red O staining in MEF cells derived from 14.5-d-old GluT5−/− and littermate GluT5+/+ embryos after 8 d treatment with adipocyte differentiation medium. D, Western blot GluT5 protein level in GluT5−/− and GluT5+/+ MEF cells. E and F, PPARγ and C/EBPα mRNA expression at d 8 after adipocyte induction (D, day of differentiation). Analysis was based on three independent experiments using one-way ANOVA followed by Tukey posttest. ns, Not significant; *, P < 0.05; **, P < 0.01. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. ns, P > 0.05.
Discussion
High refined sugar and particularly fructose intake have been associated with increased visceral fat that adversely affects metabolic risk factors including insulin resistance (11, 12, 15, 28–31). Prior studies have demonstrated that even moderate fructose consumption decreases muscle expression of early molecular markers of insulin resistance including GluT4 and stearyl coenzyme A desaturase-1 levels (32). GluT4 is a key mediator of insulin-stimulated muscle and adipocyte glucose uptake, and tissue-specific adipocyte or muscle GluT4 knockout results in impaired glucose tolerance (33, 34).
Preadipocytes are defined as cells that are restricted to becoming adipocytes but do not spontaneously undergo differentiation in the absence of exogenous adipogenic stimuli. Here we demonstrate that fructose, like glucose, can serve as a substrate for terminal differentiation of preadipocytes. Once preadipocytes have committed to the adipogenic program, a transcriptional cascade that induces the expression of metabolic genes and cytokines associated with the adipocyte is activated. We have observed that fructose treatment activates the adipocyte program and results in increased preadipocyte PPARγ, C/EBPα, and GluT4 expression. However, it is unclear from our studies whether fructose directly induces GluT4 or simply reflects earlier appearance of GluT4 due to fructose-induced adipocyte differentiation.
Fructose is extensively extracted and metabolized (50–75%) by insulin-independent pathways primarily in the liver, resulting in circulating fasting and postprandial fructose concentrations in the 10–60 μm and 1–2 mm range, respectively. However, concentrations in the portal circulation surrounding visceral adipocytes after a typical carbohydrate-rich meal can easily reach 5–10 mm (19, 35–37). Therefore, the concentrations of fructose that we have demonstrated alter adipocyte differentiation (0.005–0.550 mm) are easily attainable and physiologically relevant in both the portal and systemic circulation.
Unlike glucose, fructose ingestion does not stimulate insulin release or adipocyte leptin secretion, and administration of a fructose-rich diet to rats increased abdominal adipocyte mass and impaired insulin sensitivity. Consequently, fructose ingestion may suppress food intake to a lesser extent than glucose and result in a greater contribution to weight gain. Rhesus monkeys fed approximately 30% of their energy intake as fructose developed features of the metabolic syndrome including abdominal obesity, dyslipidemia, and inflammation within 6–12 months, and 15% of animals developed type 2 diabetes (38). Furthermore, in a 10-wk human study in which subjects consumed a 15% protein, 30% fat, and 55% carbohydrate diet in which 25% of calories were replaced with a glucose- or fructose-containing drink, subjects given fructose exhibited higher postprandial triglyceride levels and increased visceral adipose tissue (VAT) (39) compared with subjects fed glucose-sweetened drinks (40).
VAT deposition is of particular importance given it is closely related to development of metabolic syndrome and cardiovascular disease. Although fructose can be absorbed via three glucose transporters (GluT), namely 2, 5, and possibly GluT7, absence of GluT5 results in significant fructose malabsorption confirming GluT5 as the main fructose transporter and underpinning the focus of our adipocyte studies (24, 41–43). GluT5-knockout mice had less VAT mass compared with wild-type mice, which supports a role for GluT5 in visceral fat adipogenesis.
A potential role for fructose and GluT5 in adipocyte function was previously reported in a study that demonstrated higher GluT5 levels and increased fructose uptake in adipocytes derived from young obese (fa/fa) insulin-responsive Zucker rats compared with their lean counterparts. As these rats aged and became insulin resistant, adipocyte GluT5 levels fell, suggesting a temporal role for GluT5-mediated adipocyte effects (22). Our study provides further insight into temporal GluT5 expression in adipocyte differentiation. First, we noted that whereas GluT5 is expressed in the preadipocyte before emergence of GluT4, GluT5 is not expressed in mature adipocytes. This may suggest that GluT5 is primarily involved in adipocyte development and/or expansion. This is further supported by the finding of reduced epidymal adipose tissue deposits in GluT5−/− mice compared with wild-type mice. We also demonstrated that modulating preadipocyte GluT5 expression resulted in altered adipogenesis whereby high GluT5 increased, and knockdown impaired, murine adipogenesis. Additionally, MEF cells derived from GluT5−/− mice have diminished adipogenic potential. Whether GluT5−/− mice are resistant to diet-induced obesity is unclear and is a subject of our ongoing studies.
We also noted that treatment of differentiating preadipocytes with a GluT5 inhibitor suppressed adipocyte differentiation even though fructose was not present in the medium. Because L-Bn-OZO is a specific GluT5 inhibitor, we speculate that this may have been due to its effects to block glucose uptake, which can also occur via GluT5. However, we cannot exclude other possible mechanisms given its comparatively low binding affinity (Kd of 3 mm). Taken together, these findings demonstrate a previously unknown role for GluT5 in the adipocyte.
Insulin plays a pivotal role in adipocyte differentiation, and the phosphoinositide-3-kinase phosphatidyl inositol-3-kinase/Akt pathway is required for adipose differentiation. We examined Akt phosphorylation in wild-type and GluT5−/− MEF preincubated in Opti-MEM for 2 h before treatment with insulin (100 nm). Total Akt (t-Akt) and p-Akt(Ser473) expression were similar in wild-type and GluT5−/− MEF suggesting that altered insulin signaling is not involved in the effects of fructose and/or GluT5 on adipogenesis.
In summary, our study demonstrates a novel function for GluT5 and fructose in adipocyte differentiation whereby GluT5 overexpression increased and GluT5 knockdown inhibited adipocyte differentiation. Furthermore, pharmacological GluT5 inhibition impaired adipocyte differentiation, suggesting that GluT5 could represent a potential therapeutic target in obesity.
Supplementary Material
Acknowledgments
We thank Dr. Jian Zuo, St. Jude Children's Research Hospital (Memphis, TN) for providing GluT5−/− mice.
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants P30 DK063491-07 P and F (to A.P.H.).
Authors' contributions: L.D. conducted the in vitro and in vivo studies and drafted the manuscript. A.P.H. conceived and designed the study and helped write the manuscript. All authors read and approved the final manuscript.
Disclosure Summary: The authors have nothing to disclose and have no competing interests.
Footnotes
- AP2
- Adipocyte fatty acid binding protein 2
- C/EBPα
- CCAAT/enhancer-binding protein α
- FBS
- fetal bovine serum
- GLUT
- glucose transporter
- l-Bn-OZO
- l-O-Benzyl-2-N, 3-O-carbonyl-α-l-sorbofuranosylamine
- MEF
- mouse embryonic fibroblast
- PPARγ
- peroxisome proliferator-activated receptor γ
- Q-PCR
- quantitative PCR
- siRNA
- small interfering RNA
- VAT
- visceral adipose tissue
- WAT
- white adipose tissue.
References
- 1. Hotamisligil GS, Shargill NS, Spiegelman BM. 1993. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91 [DOI] [PubMed] [Google Scholar]
- 2. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 4. Wellen KE, Hotamisligil GS. 2003. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112:1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Britton KA, Fox CS. 2011. Ectopic fat depots and cardiovascular disease. Circulation 124:e837–e841 [DOI] [PubMed] [Google Scholar]
- 6. Koistinen HA, Dusserre E, Ebeling P, Vallier P, Koivisto VA, Vidal H. 2000. Subcutaneous adipose tissue expression of plasminogen activator inhibitor-1 (PAI-1) in nondiabetic and Type 2 diabetic subjects. Diabetes Metab Res Rev 16:364–369 [DOI] [PubMed] [Google Scholar]
- 7. Mangge H, Almer G, Truschnig-Wilders M, Schmidt A, Gasser R, Fuchs D. 2012. Inflammation, adiponectin, obesity and cardiovascular risk. Curr Med Chem 17:4511–4520 [DOI] [PubMed] [Google Scholar]
- 8. Tontonoz P, Hu E, Spiegelman BM. 1994. Stimulation of adipogenesis in fibroblasts by PPAR γ 2, a lipid-activated transcription factor. Cell 79:1147–1156 [DOI] [PubMed] [Google Scholar]
- 9. Spiegelman BM, Flier JS. 1996. Adipogenesis and obesity: rounding out the big picture. Cell 87:377–389 [DOI] [PubMed] [Google Scholar]
- 10. Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. 1999. Cross-regulation of C/EBP α and PPAR γ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell 3:151–158 [DOI] [PubMed] [Google Scholar]
- 11. Teff KL, Elliott SS, Tschöp M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D'Alessio D, Havel PJ. 2004. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 89:2963–2972 [DOI] [PubMed] [Google Scholar]
- 12. Abdel-Sayed A, Binnert C, Lê KA, Bortolotti M, Schneiter P, Tappy L. 2008. A high-fructose diet impairs basal and stress-mediated lipid metabolism in healthy male subjects. Br J Nutr 100:393–399 [DOI] [PubMed] [Google Scholar]
- 13. Couchepin C, Lê KA, Bortolotti M, da Encarnaçao JA, Oboni JB, Tran C, Schneiter P, Tappy L. 2008. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care 31:1254–1256 [DOI] [PubMed] [Google Scholar]
- 14. Lê KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. 2009. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 89:1760–1765 [DOI] [PubMed] [Google Scholar]
- 15. Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. 2009. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dolan LC, Potter SM, Burdock GA. 2010. Evidence-based review on the effect of normal dietary consumption of fructose on development of hyperlipidemia and obesity in healthy, normal weight individuals. Crit Rev Food Sci Nutr 50:53–84 [DOI] [PubMed] [Google Scholar]
- 17. Basciano H, Federico L, Adeli K. 2005. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hallfrisch J. 1990. Metabolic effects of dietary fructose. FASEB J 4:2652–2660 [DOI] [PubMed] [Google Scholar]
- 19. Mayes PA. 1993. Intermediary metabolism of fructose. Am J Clin Nutr 58:754S–765S [DOI] [PubMed] [Google Scholar]
- 20. Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. 2008. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol 295:G987–G995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rutledge AC, Adeli K. 2007. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65:S13–S23 [DOI] [PubMed] [Google Scholar]
- 22. Litherland GJ, Hajduch E, Gould GW, Hundal HS. 2004. Fructose transport and metabolism in adipose tissue of Zucker rats: diminished GLUT5 activity during obesity and insulin resistance. Mol Cell Biochem 261:23–33 [DOI] [PubMed] [Google Scholar]
- 23. Douard V, Choi HI, Elshenawy S, Lagunoff D, Ferraris RP. 2008. Developmental reprogramming of rat GLUT5 requires glucocorticoid receptor translocation to the nucleus. J Physiol 586:3657–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Douard V, Ferraris RP. 2008. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295:E227–E237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu X, Wang X, Gao J, Yu Y, Jia S, Zheng J, Dallos P, He DZ, Cheatham M, Zuo J. 2008. Glucose transporter 5 is undetectable in outer hair cells and does not contribute to cochlear amplification. Brain Res 1210:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang J, Dowden J, Tatibouët A, Hatanaka Y, Holman GD. 2002. Development of high-affinity ligands and photoaffinity labels for the D-fructose transporter GLUT5. Biochem J 367:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Girniene J, Tatibouët A, Sackus A, Yang J, Holman GD, Rollin P. 2003. Inhibition of the D-fructose transporter protein GLUT5 by fused-ring glyco-1,3-oxazolidin-2-thiones and -oxazolidin-2-ones. Carbohydr Res 338:711–719 [DOI] [PubMed] [Google Scholar]
- 28. Faeh D, Minehira K, Schwarz JM, Periasamy R, Perisami R, Park S, Seongsu P, Tappy L. 2005. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 54:1907–1913 [DOI] [PubMed] [Google Scholar]
- 29. Lê KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. 2006. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr 84:1374–1379 [DOI] [PubMed] [Google Scholar]
- 30. Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, Christiansen MP, Griffen SC, Keim NL, Havel PJ. 2008. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr 100:947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. 2009. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab 94:1562–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rizkalla SW. 2010. Health implications of fructose consumption: a review of recent data. Nutr Metab (Lond) 7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. 2004. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest 114:1666–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stenbit AE, Tsao TS, Li J, Burcelin R, Geenen DL, Factor SM, Houseknecht K, Katz EB, Charron MJ. 1997. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat Med 3:1096–1101 [DOI] [PubMed] [Google Scholar]
- 35. Bizeau ME, Pagliassotti MJ. 2005. Hepatic adaptations to sucrose and fructose. Metabolism 54:1189–1201 [DOI] [PubMed] [Google Scholar]
- 36. Macdonald I, Keyser A, Pacy D. 1978. Some effects, in man, of varying the load of glucose, sucrose, fructose, or sorbitol on various metabolites in blood. Am J Clin Nutr 31:1305–1311 [DOI] [PubMed] [Google Scholar]
- 37. Burant CF, Saxena M. 1994. Rapid reversible substrate regulation of fructose transporter expression in rat small intestine and kidney. Am J Physiol 267:G71–G79 [DOI] [PubMed] [Google Scholar]
- 38. Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel PJ. 2011. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci 4:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bayturan O, Tuzcu EM, Uno K, Lavoie AJ, Hu T, Shreevatsa A, Wolski K, Schoenhagen P, Kapadia S, Nissen SE, Nicholls SJ. 2010. Comparison of rates of progression of coronary atherosclerosis in patients with diabetes mellitus versus those with the metabolic syndrome. Am J Cardiol 105:1735–1739 [DOI] [PubMed] [Google Scholar]
- 40. Van Schaftingen E. 1993. Glycolysis revisited. Diabetologia 36:581–588 [DOI] [PubMed] [Google Scholar]
- 41. Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C, Wu X, Yu Y, Amlal H, Seidler U, Zuo J, Soleimani M. 2009. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem 284:5056–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheeseman C. 2008. GLUT7: a new intestinal facilitated hexose transporter. Am J Physiol Endocrinol Metab 295:E238–E241 [DOI] [PubMed] [Google Scholar]
- 43. Helliwell PA, Richardson M, Affleck J, Kellett GL. 2000. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J 350:163–169 [PMC free article] [PubMed] [Google Scholar]
- 44. London E, Castonguay TW. 2011. High fructose diets increase 11β-hydroxysteroid dehydrogenase type 1 in liver and visceral adipose in rats within 24-h exposure. Obesity (Silver Spring) 19:925–932 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.