Abstract
LH triggers the biosynthesis of androgens in the theca-interstitial (T-I) cells of ovary through the activation of a cAMP-dependent pathway. We have previously shown that LH/human chorionic gonadotropin (hCG) activates mammalian target of rapamycin complex 1 (mTORC1) signaling network, leading to cell proliferation. In the present study, we provide evidence that the LH/hCG-mediated activation of the mTORC1 signaling cascade is involved in the regulation of steroidogenic enzymes in androgen biosynthesis. Treatment with LH/hCG increased the expression of downstream targets of mTORC1, ribosomal protein S6 kinase 1, and eukaryotic initiation factor 4E as well as steroidogenic enzymes. LH/hCG-mediated stimulation of the steroidogenic enzyme mRNA was blocked by the mTORC1 inhibitor, rapamycin. This inhibitory effect was selective because rapamycin failed to block hCG-mediated increase in the expression of Star mRNA levels. Furthermore, pharmacological targeting of mTORC1 with rapamycin also blocked LH/hCG- or forskolin-induced expression of cAMP response element-binding protein (CREB) and steroidogenic enzymes (P450 side-chain cleavage enzyme, 3β-hydroxysteroid dehydrogenase type 1, and 17α-hydroxylase/17,20 lyase) but produced no effect on steroidogenic acute regulatory protein levels. These results were further confirmed by demonstrating that the knockdown of mTOR using small interfering RNA selectively abrogated the LH/hCG-induced increase in steroidogenic enzyme expression, without affecting steroidogenic acute regulatory protein expression. LH/hCG-stimulated androgen production was also blocked by rapamycin. Furthermore, the pharmacological inhibition of mTORC1 or ribosomal protein S6 kinase 1 signaling prevented the LH/hCG-induced phosphorylation of CREB. Chromatin immunoprecipitation assays revealed the association of CREB with the proximal promoter of the Cyp17a1 gene in response to hCG, and this association was reduced by rapamycin treatment. Taken together, our findings show for the first time that LH/hCG-mediated activation of androgen biosynthesis is regulated by the mTORC1 signaling pathway in T-I cells.
It is well established that LH regulates androgen production by the theca-interstitial (T-I) cells of the ovary, which then serves as substrate for estrogen synthesis in granulosa cells (1–4). LH transduces the intracellular signal through its receptor, a member of the G-protein coupled receptor family, and subsequently increases intracellular cAMP levels, which, in turn, activate protein kinases (5–12). These protein kinases can trigger the expression of steroidogenic enzymes directly or by activating the downstream targets (13–19). Recently we reported that LH/human chorionic gonadotropin (hCG) activates mammalian target of rapamycin complex 1 (mTORC1) signaling by the cAMP/phosphatidylinositol-3-kinase (PI3-kinase)/AKT-dependent pathway in T-I cells (20). Furthermore, LH-induced phosphorylation of downstream targets of mTORC1, ribosomal protein S6 kinase 1 (S6K1), and eukaryotic initiation factor 4E (eIF4E) binding protein 1 was blocked by inhibiting either the AKT or mTORC1 pathways (20).
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that plays a central role in regulating many cellular processes including protein and lipid synthesis as well as growth and proliferation in response to growth factors and hormones (20–23). mTOR is the catalytic subunit of two multiprotein complexes, mTORC1 and mTORC2. mTORC1 is sensitive to rapamycin, whereas mTORC2 is insensitive (24). In response to growth factors and hormones, mTORC1 activates p70 S6K1 and eIF4E binding protein 1, which, in turn, increase protein synthesis (22, 23). Our previous work shows that LH/hCG-mediated activation of the cAMP/PI3-kinase/AKT/mTORC1 signaling pathway regulates T-I cell proliferation (20). However, the role of LH/hCG-mediated activation of mTORC1 on steroid hormone biosynthesis has not yet been examined in steroidogenic cells. In this study, we provide evidence for the first time that LH/hCG-mediated activation of mTORC1 signaling is required for T-I cell androgen production. We have shown that rapamycin, a specific inhibitor of mTORC1, or small interfering RNA (siRNA)-mediated knockdown of mTOR selectively inhibits LH/hCG-induced induction of steroidogenic enzymes [P450 side-chain cleavage enzyme (P450scc), 3β-hydroxysteroid dehydrogenase type 1 (HSD3B1), and 17α-hydroxylase/17,20lyase (P450c17)] but leaving steroidogenic acute regulatory protein (StAR) protein expression unaffected. Furthermore, our results also show that rapamycin inhibits cAMP response element-binding protein (CREB) phosphorylation and the interaction of CREB with the Cyp17a1 promoter, which occur in response to LH/hCG. Thus, LH/hCG-mediated activation of the mTORC1 signal is essential for steroidogenic enzyme expression, which, in turn, regulates androgen biosynthesis in T-I cells.
Materials and Methods
Medium 199, McCoy's 5A medium, l-glutamine, and HEPES buffer were purchased from Invitrogen/GIBCO (Carlsbad, CA). Penicillin-streptomycin was purchased from Roche Diagnostics (Indianapolis, IN). Collagenase (CLS I) and deoxyribonuclease I were obtained from Worthington Biochemical Corp. (Freehold, NJ). BSA, PF-4708671, and β-tubulin antibody (1:10,000 dilution) were purchased from Sigma Chemical Co. (St. Louis, MO). Purified hCG was purchased from Dr. A. F. Parlow (National Hormone and Peptide Program, Torrance, CA). Forskolin was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). The mTORC1 inhibitor, rapamycin, and antibodies against phosphorylated CREB (Ser133) (1:4000 dilution), total CREB (1:5000 dilution), eIF4E (1:2000 dilution), S6K1 (1:3000 dilution), and the mTOR siRNA kit were purchased from Cell Signaling Technology (Beverly, MA). The StAR antibody (1:50000 dilution) was obtained as a gift from Dr. D. M. Stocco (Texas Tech University Health Center, Lubbock, TX), and HSD3B1antibody (1:2000 dilution) was purchased from Novus Biologicals (Littleton, CO). Anti-P450scc (1:10,000 dilution) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:10,000 dilution) were obtained from Chemicon (Temecula, CA). The antibody against P450c17 (1:750 dilution) and anti-goat IgG horseradish peroxidase conjugates (1:7500 dilution) were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). EZ-Magna chromatin immunoprecipitation (ChIP) assay kits were purchased from Millipore/ Upstate (Lake Placid, NY). Anti-mouse (1:10,000 dilution), antirabbit (1:10,000 dilution) IgG horseradish peroxidase conjugates, enhanced chemiluminescence using the Femto SuperSignal substrate system and Restore Western blot stripping buffer were purchased from Pierce (Rockford, IL). Reagents as well as the primers and probes for Star, Cyp11a1, Hsd3b1, and Cyp17a1 were procured from Applied Biosystems (Foster City, CA). All other reagents used were conventional commercial products.
Animals
Sprague Dawley female rats (25 d old) were purchased from Charles River Laboratories (Wilmington, MA). All the experimental protocols used in this study were approved by the University Committee on the Use and Care of Animals (University of Michigan). Animals were housed in a temperature-controlled room with proper dark-light cycles as per the guidelines provided by the University Committee on the Use and Care of Animals. The animals were euthanized by CO2 asphyxiation. The ovaries were removed under sterile conditions and were processed immediately for the isolation of T-I cells.
Isolation and culture of T-I cells
The T-I cells were isolated, dispersed, and cultured following a protocol previously published from our laboratory (20, 25). Briefly, freshly collected ovaries were placed in Medium 199 containing 25 mm HEPES (pH 7.4), 2 mm l-glutamine, 1 mg/ml BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin. The ovaries were then freed from adhering fat and actively punctured with a 27-gauge needle under a dissecting microscope to release the granulosa and blood cells. The remaining ovarian tissue was then washed three times with medium to release any remaining granulosa cells. The tissue was then minced and incubated for 30 min at 37 C in the same medium, supplemented with 0.65 mg/ml collagenase type 1 plus 10 μg/ml deoxyribonuclease. The dispersion was further facilitated by mechanically pipetting the ovarian tissue suspension with a 10-ml pipette. The T-I cells released by this digestion were centrifuged at 250 × g for 5 min and washed in medium twice to eliminate the remaining collagenase. The dispersed cells were then resuspended in McCoy's 5A medium containing 2 mm l-glutamine, 1 mg/ml BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin and then subjected to unit gravity sedimentation for 5 min to eliminate the small fragments of undispersed ovarian tissue. Cell viability was assessed by trypan blue exclusion and was always greater than 90%. The dispersed cells were seeded in 60-mm plates (3 × 106 viable cells). The plated cells were maintained overnight in McCoy's 5A medium containing 2 mm l-glutamine, 1 mg/ml BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 95% air-5% CO2 at 37 C. After allowing cells to attach, they were treated with hCG at different time intervals, and inhibitors were used as indicated in the figure legends.
Cell viability assay
T-I cells were seeded into 96-well plates and cultured overnight with McCoy's medium containing 0.1% BSA. After attachment, cells were pretreated with rapamycin for 1 h followed by hCG (50 ng/ml) for 24 h. After the treatment periods, cell viability was determined by a dimethylthiazol diphenyltetetrazolium bromide assay as previously described (26).
Real-time PCR
The role of mTORC1 in hCG-mediated Star, Cyp11a1, Hsd3b1, and Cyp17a1 mRNA expression was examined by pretreating the cells with or without rapamycin (20 nm) for 1 h, followed by hCG for 6 h. At the end of the incubation, the cells were harvested, and total RNA was extracted using TRIzol reagent following the manufacturer's instructions (Life Technologies, Grand Island, NY). Aliquots of total RNA (100 ng) extracted from the control and treated groups were reverse transcribed in a reaction volume of 20 μl using 2.5 μm random hexamer, 500 μm deoxynucleotide triphosphates, 5.5 mm MgCl2, 8 U ribonuclease inhibitor, and 25 U Multiscribe reverse transcriptase (Applied Biosystems). The reactions were carried out in a PTC-100 thermal controller (MJ Research, Watertown, MA) (25 C for 10 min, 48 C for 30 min, and 95 C for 5 min). The resulting cDNA were diluted with water. The real-time PCR quantification was then performed using 5 μl of the diluted cDNA in triplicate with predesigned primers and probes for rat Star, Cyp11a1, Hsd3b1, and Cyp17a1 (TaqMan Assay on Demand gene expression products; Applied Biosystems). Reactions were carried out in a final volume of 25 μl using an Applied Biosystems 7300 real-time PCR system (95 C for 15 sec and 60 C for 1 min) after an initial incubation for 10 min at 95 C. The changes in Star, Cyp11a1, Hsd3b1, and Cyp17a1 expression were calculated using the ΔΔCt method (27) with 18S rRNA as the internal control.
Western blot analysis
After various treatments described in the respective figure legends, cell monolayers were washed with PBS and then were solubilized using radioimmunoprecipitation assay (RIPA) buffer (PBS containing 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate). Cell lysates were then sonicated and centrifuged for 10 min at 13,000 × g. The protein content of the supernatants was determined using BCA reagent (Pierce). Proteins (30–50 μg/lane) were separated by electrophoresis using 10 or 4–20% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad) before immunoblot analysis. Membranes were blocked in 5% fat-free milk in 20 mm Tris base (pH 7.45), 137 mm NaCl, and 0.1% Tween 20 (TBST) for 1 h at room temperature and then incubated overnight at 4 C with primary antibody in 5% fat-free milk/TBST. After three 5-min washes with TBST, membranes were incubated in appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. After three 5-min washes with TBST, membrane-bound antibodies were detected with the Femto SuperSignal substrate system Western blotting detection kit (Pierce). Protein loading was monitored by reprobing the same blots with appropriate antibodies (loading control) as indicated in the figure legends.
RIA of androstenedione
To determine the effect of mTORC1 inhibition on hCG-stimulated androstenedione production, T-I cells were grown in 60-mm plates with McCoy's medium containing 0.1% BSA. After attachment, cells were pretreated with mTORC1 inhibitor, rapamycin (20 nm) for 1 h followed by hCG treatment for 24 h. At the end of treatments, the media were collected, trace amounts of 3H androstenedione were added to monitor recovery, and then they were extracted for the steroid using diethyl ether. The extraction recovery was greater than 85%. Androstenedione production was determined using RIA kits (Diagnostic System Laboratories, Webster, TX), following the manufacturer's instructions. The intraassay and interassay coefficients of variation for androstenedione were 2.7–5.9 and 4.8–7.0%, respectively.
siRNA-mediated silencing of mTOR
The protocol for siRNA-mediated knockdown of mTOR in T-I cells was previously published in our laboratory (20). Briefly, T-I cells were transfected with control siRNA (nontargeted) or mTOR siRNA (targeted) using a Nucleofector transfection reagent (Amaxa, Walkersville, MD), as per the manufacturer's instructions. After transfection, cells were resuspended in 5% FBS in McCoy's medium and plated. Forty-eight hours later, the media were replaced with serum-free medium for overnight culture and then treated without or with hCG (50 ng/ml) for an additional 24 h. mTOR, StAR, P450scc, HSD3B1, and P450c17 were analyzed by Western blot analysis using specific antibodies.
Chromatin immunoprecipitation assay
ChIP assays were performed using the EZ-magna ChIP kit from Millipore/Upstate. Briefly, T-I cells (1 × 107) were cultured in 100-mm dishes. After overnight attachment, cells were pretreated with or without rapamycin (20 nm) for 1 h followed by stimulation with hCG (50 ng/ml) for 2 h. After treatments, cells were incubated with formaldehyde (1%) for 10 min at room temperature to cross-link DNA and its associated proteins. The cross-linking was stopped by one 20th V of 2.5 m glycine, and then cells were lysed and sonicated for six cycles of 15-sec pulses (Fisher Scientific, Pittsburgh, PA). One tenth of the sample was removed for input and one tenth removed to evaluate sonication efficiency. Remaining samples were immunoprecipitated with CREB antibody (rabbit monoclonal; Cell Signaling; catalog no. 4820) overnight at 4 C. Rabbit IgG was used as a negative control. Reversal of cross-linking was carried out at 65 C for 3 h followed by DNA isolation. The purified DNA samples were amplified by PCR using rat primer sequences for the Cyp17a1 and Gapdh gene promoters. The primers designed for the proximal promoter regions of Cyp17a1 and Gapdh are as follows: [rat Cyp17a1 (forward) 5′-TTGAAATGCTACTCTGTGGGTTT-3′; (reverse) 5′-ACGGCAGCTCATGATTTTCT-3′ or rat Gapdh (forward) 5′-CGTAGCTCAGGCCTCTGCGCCCTT-3′; (reverse) 5′-CTGGCACTGCACAAGAAGATGCGGCTG-3′]. The PCR products were separated on 2% agarose/ethidium bromide gels and visualized.
Statistical analysis
Statistical analysis was carried out using one-way ANOVA followed by the Tukey multiple comparison test using Prism software (GraphPad Prism, version 3.0; GraphPad Inc., San Diego, CA). Values were considered statistically significant at P < 0.05. Each experiment was repeated at least three times, with similar results. Blots are representative of one experiment, and graphs represent the mean ± se of three independent experiments.
Results
LH/hCG stimulates downstream targets of mTORC1 and steroidogenic enzyme expression
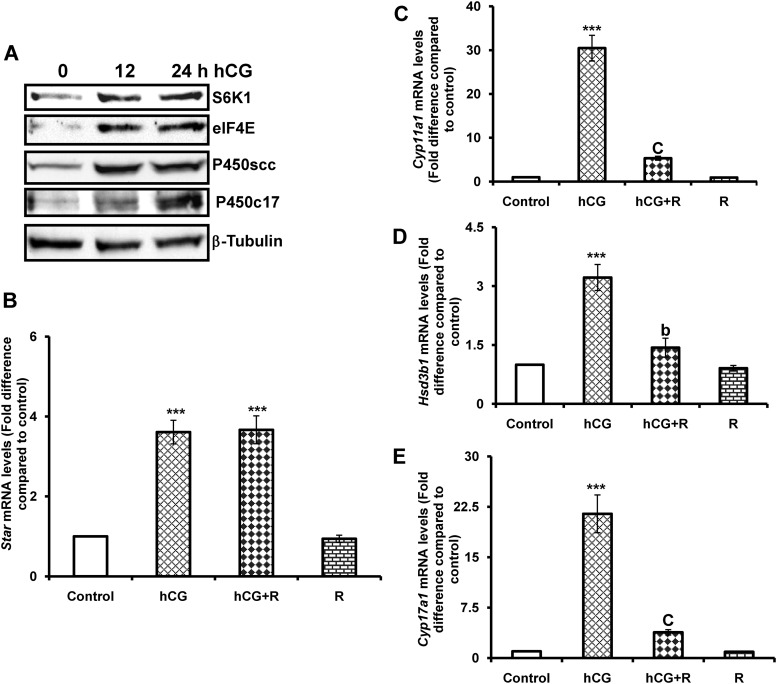
The initial experiments examined the possible relationship between mTORC1 activation and steroidogenic enzyme expression in response to hCG. To test this, T-I cells isolated from immature rats were cultured with hCG for different time intervals, and the expression of downstream targets, S6K1 and eIF4E, as well as steroidogenic enzymes such as P450scc and P450c17 were examined by Western blot analysis. The results presented in Fig. 1A show that hCG treatment for 12 h produced an increase in the expression S6K1, eIF4E, and P450scc. At 24 h, these protein levels were sustained. However, maximal P450c17 expression was observed at 24 h after hCG treatment. These results indicate that hCG treatment induces the downstream targets of mTORC1, S6K1, and eIF4E as well as the expression of steroidogenic enzymes.
Fig. 1.
Time-course study of hCG effect on downstream targets of mTORC1 and steroidogenic enzyme expression and rapamycin sensitivity. Ovaries were collected from 25-d-old Sprague Dawley rats. T-I cells were isolated by collagenase digestion and cells were plated with 0.1% BSA in McCoy's medium. After 24 h attachment, cells were treated with hCG (50 ng/ml) for 0, 12, and 24 h. A, Cells were lysed using RIPA buffer and subjected to Western blot analysis for S6K1, eIF4E, P450scc, and P450c17 expression using specific antibodies. Protein loading was monitored by stripping and reprobing the same blot with β-tubulin antibody. B–E, T-I cells were pretreated without or with rapamycin (20 nm) for 1 h before 6 h treatment with hCG (50 ng/ml). Control groups were treated with vehicle (dimethylsulfoxide). Total RNA was reverse transcribed, and the resulting cDNA was subjected to real-time PCR using predesigned primers and probes for rat Star, Cyp11a1, Hsd3b1, and Cyp17a1as described in Materials and Methods. The graph in B shows changes in Star mRNA expression normalized for 18S rRNA. The graphs in C, D, and E show changes in Cyp11a1, Hsd3b1, and Cyp17a1 mRNA expression (respectively), normalized for 18S rRNA. Blots are representative of one of three independent experiments, and the graphs represent the mean of three separate experiments. Error bars represent mean ± se. ***, P < 0.001 vs. control. b and c represent significant differences (P < 0.01 and P < 0.001, respectively), compared with hCG treatment. R, Rapamycin.
LH/hCG-stimulated steroidogenic enzyme requires mTORC1 signaling
The studies were then extended to determine whether hCG-mediated activation of mTORC1 signaling affects steroidogenic enzyme mRNA expression. To examine this, cells were pretreated with the mTORC1inhibitor, rapamycin (20 nm), for 1 h followed by stimulation with hCG for an additional 6 h, total RNA extracted, and Star and steroidogenic enzyme (Cyp11a1, Hsd3b1, and Cyp17a1) mRNA levels were measured by real-time PCR. As expected, hCG treatment caused a significant increase in mRNA levels of Star and steroidogenic enzyme expression. By contrast, pretreatment with rapamycin blocked hCG-induced Cyp11a1, Hsd3b1, and Cyp17a1expression. However, pretreatment with the mTORC1 inhibitor failed to block Star mRNA levels in response to hCG (Fig. 1, B–E). These results suggest that mTORC1 signaling is involved in LH/hCG-induced steroidogenic enzyme mRNA expression.
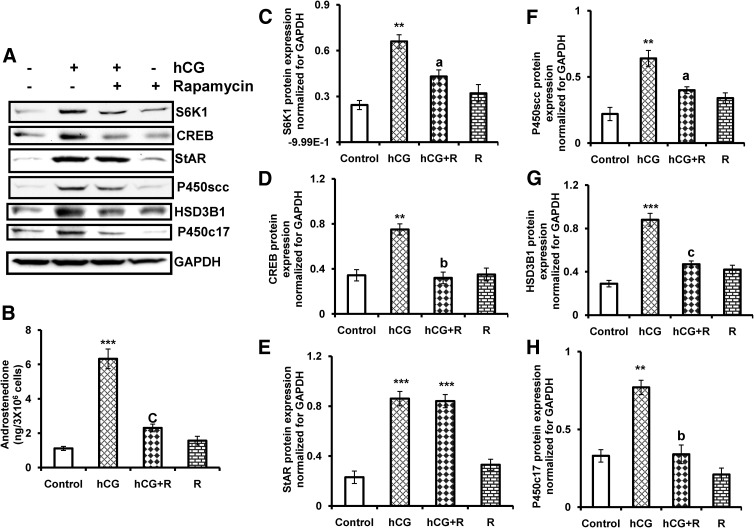
To test a role for mTORC1 in the context of regulation of steroidogenesis, we also examined the protein levels of steroidogenic enzymes, which are involved in androgen biosynthesis. The initial experiments examined the toxicity of rapamycin treatment in cultured T-I cells using cell viability assay. To test this, cultured T-I cells were preincubated with or without rapamycin (20 nm) for 1 h followed by treatment with hCG for 24 h. Cell viability was analyzed by a dimethylthiazol diphenyltetetrazolium bromide assay. The results show that rapamycin treatment at 20 nm concentration did not reduce cell viability compared with control or hCG treatment group (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). The cell lysates were examined for downstream target of mTORC1, S6K1, and CREB, StAR, P450scc, HSD3B1, and P450c17 by immunoblot analysis using specific antibodies. The results showed that hCG treatment dramatically increases S6K1, CREB, StAR, and steroidogenic enzyme expression and that pretreatment with rapamycin reduces all of these hCG-induced increases except for StAR protein (Fig. 2A). StAR protein has been identified as a transport protein that regulates cholesterol transport within the mitochondria (28). It has been shown that LH/hCG stimulates StAR protein expression in gonadal cells (29, 30). Our results strongly suggest that hCG-mediated activation of mTORC1 signal is necessary for steroidogenic enzyme expression but not for StAR protein expression.
Fig. 2.
Effect of rapamycin on hCG-stimulated CREB, StAR, steroidogenic enzyme expression, and androstenedione production in T-I cells. Cells were pretreated without or with rapamycin (20 nm) for 1 h followed by hCG (50 ng/ml) treatment for 24 h. Control groups were treated with vehicle (dimethylsulfoxide). A, The cell lysates were examined for S6K1, CREB, StAR, and steroidogenic enzyme (P450scc, HSD3B1, and P450c17) expression by Western blot analysis. The level of GAPDH was used as loading control. B, The media were collected and androstenedione was extracted and measured by a RIA as described in Materials and Methods. The graphs (C–H) represent densitrometric scans of S6K1, CREB, StAR, P450scc, HSD3B1, and P450c17 protein expression normalized for GAPDH as seen in A. The blots in A represent one of three separate experiments, and the graphs represent the mean of three experiments. Error bars represent mean + se. **, P < 0.01, ***, P < 0.001 vs. control. a, b, and c represent significant differences (P < 0.05, P < 0.01, and P < 0.001, respectively), compared with hCG. R, Rapamycin.
Rapamycin blocks LH/hCG-stimulated androstenedione synthesis
The role of mTORC1 in hCG-stimulated androstenedione secretion was then examined. Primary cultures of T-I cells were pretreated with vehicle or rapamycin (20 nm) for 1 h followed by stimulation with hCG for 24 h. The incubation media were collected and androstenedione levels were analyzed by RIA. As shown in Fig. 2B, androstenedione secretion increased greater than 6-fold in hCG treated T-I cells. However, this dramatic increase was significantly diminished by pretreatment with rapamycin followed by hCG treatment. These results suggest that LH-stimulated androgen synthesis requires mTORC1 signaling in the T-I cells.
Forskolin-induced steroidogenic enzymes are rapamycin sensitive
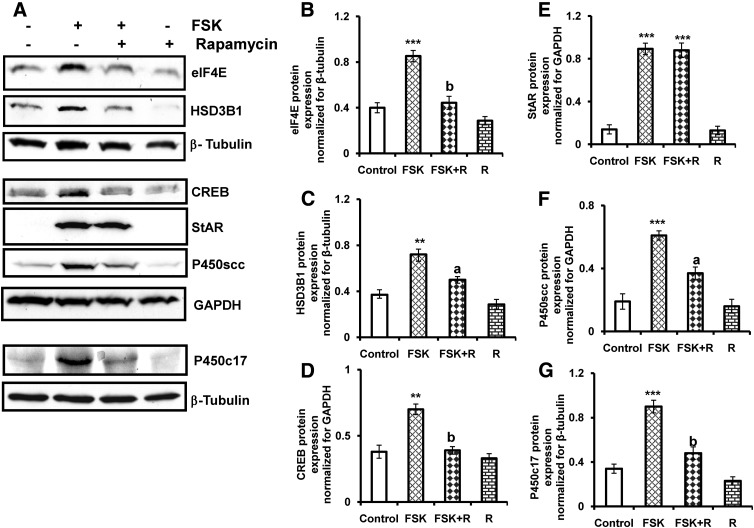
Because we have recently shown that a pharmacological activator of adenylate cyclase, forskolin, activates mTORC1 signaling in T-I cells (20), the possible role of the mTORC1 signaling pathway in forskolin-stimulated increase in steroidogenic enzyme expression was then examined. Cells were pretreated with rapamycin for 1 h followed by stimulation with forskolin for 24 h. The cell lysates were analyzed for the expression of downstream target of mTORC1, eIF4E as well as CREB, StAR, and steroidogenic enzymes (P450scc, HSD3B1, and P450c17) by immunoblot analysis using specific antibodies. As expected, treatment with forskolin promotes eIF4E, CREB, StAR, P450scc, HSD3B1, and P450c17 expression. Pretreatment with rapamycin dramatically reduced forskolin-induced eIF4E, CREB, and steroidogenic enzyme expression but not StAR protein expression (Fig. 3). These data again provide evidence that mTORC1 activation selectively regulates steroidogenic enzyme expression in T-I cells.
Fig. 3.
Effect of rapamycin on forskolin-stimulated CREB, StAR, and steroidogenic enzyme expression. Cells were pretreated without or with rapamycin (20 nm) for 1 h followed by forskolin (10 μm) treatment for 24 h. Control groups were treated with vehicle (dimethylsulfoxide). A, The cell lysates were examined for downstream target of mTORC1, eIF4E, CREB, StAR, and steroidogenic enzyme (P450scc, HSD3B1, and P450c17) expression by Western blot analysis. β-Tubulin or GAPDH was used as loading control. The graphs (B–G) represent densitrometric scans of eIF4E, HSD3B1, CREB, StAR, P450scc, and P450c17 protein expression normalized for β-tubulin or GAPDH as seen in A. The blots in A represent one of three separate experiments, and the graphs represent the mean of three experiments. Error bars represent mean + se. **, P < 0.01, ***, P < 0.001 vs. control. a, and b represent significant differences (P < 0.05 and P < 0.01, respectively), compared with FSK. FSK, Forskolin; R, rapamycin.
siRNA-mediated knockdown of mTOR and its effect on hCG-induced steroidogenic enzymes
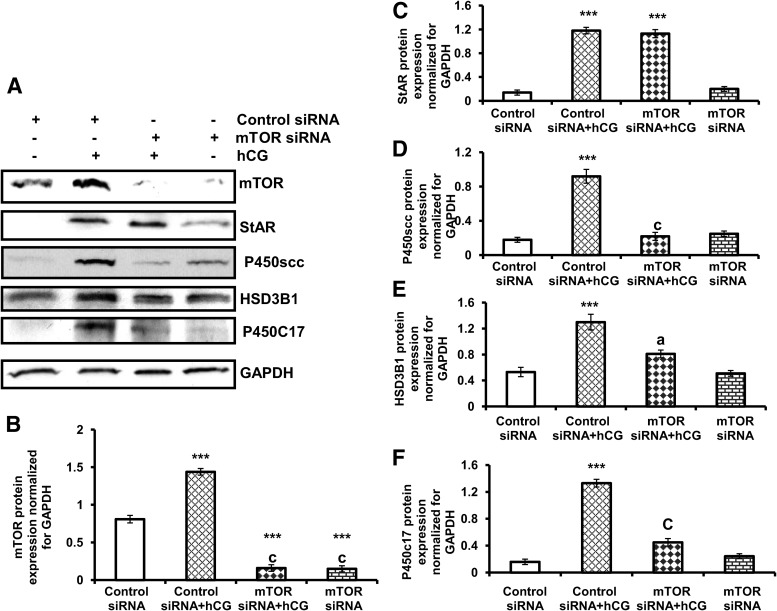
To provide further evidence that mTOR signaling is involved in steroidogenic enzyme expression, primary cultures of T-I cells were transfected with control siRNA (nontargeted) or mTOR siRNA (targeted) for 48 h followed by hCG treatment for 24 h. Cell lysates were examined for mTOR, StAR, and steroidogenic enzymes (P450scc, HSD3B1, and P450c17) by Western blot analysis. As shown in Fig. 4, the level of mTOR expression was blocked in cells transfected with the siRNA targeting mTOR, compared with cells transfected with nontargeting control siRNA in either the presence or absence of hCG treatment. Furthermore, hCG-induced P450scc, HSD3B1, and P450c17 expression was reduced by silencing mTOR by mTOR siRNA. As expected, knockdown of mTOR did not block hCG-induced StAR protein expression. These data provide conclusive evidence for a role of mTOR in steroidogenic enzyme expression in response to LH/hCG.
Fig. 4.
mTOR siRNA inhibits hCG-induced steroidogenic enzyme expression. T-I cells were transfected with 100 nm of control siRNA or mTOR siRNA for 48 h and then treated without or with hCG for another 24 h. A, Proteins (30 μg) were separated by SDS-PAGE (4–20%) and then immunoblotted with mTOR, StAR, P450scc, HSD3B1, and P450c17 antibodies. Protein loading was monitored by stripping and reprobing the same blot with antibody for GAPDH. The graphs (B–F) represent densitrometric scans of mTOR, StAR, P450scc, HSD3B1, and P450c17 protein expression normalized for GAPDH as seen in A. The blots in A represent one of three separate experiments, and the graphs represent the mean of three experiments. Error bars represent mean + se. ***, P < 0.001 vs. control siRNA. a, and c represent significant differences (P < 0.05 and P < 0.001, respectively), compared with control siRNA plus hCG treatment.
LH/hCG-stimulated CREB phosphorylation is sensitive to rapamycin
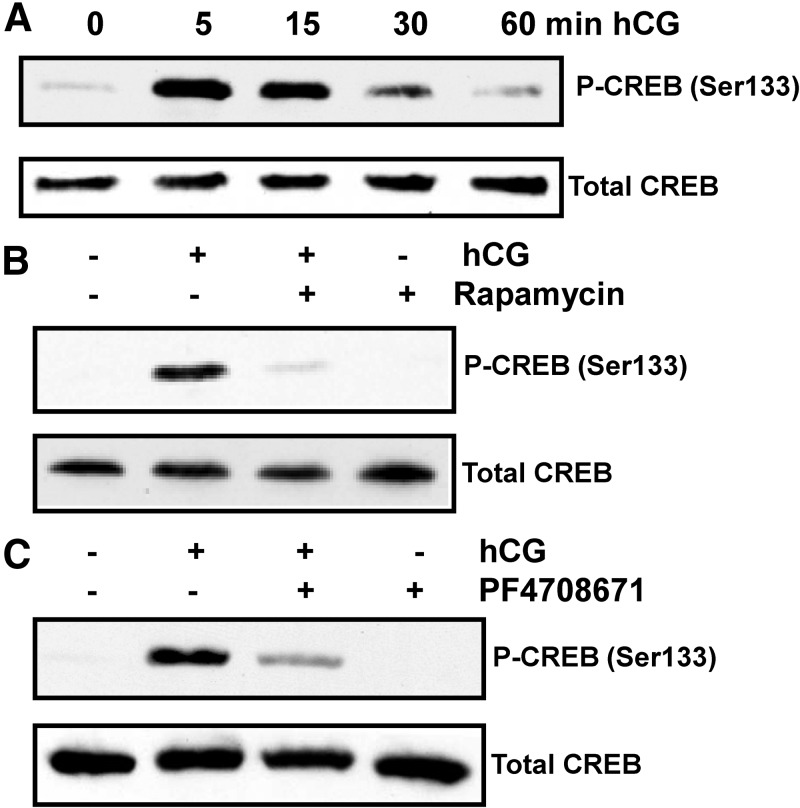
Because LH/hCG-mediated activation of cAMP is known to increase CREB activation leading to steroidogenic enzyme expression, for the next set of experiments, we used the mTORC1 inhibitor, rapamycin, to determine whether phosphorylation of CREB (Ser133) occurs downstream of mTORC1 in response to hCG. To test this, the initial experiment was conducted as a time-course study of hCG's effect on CREB phosphorylation. T-I cells were cultured with hCG for different time intervals and phosphorylation of CREB was examined by immunoblot analysis using a phospho-specific antibody that recognizes CREB phosphorylation at Ser133. The results demonstrated that within 5 min of hCG addition, there was a robust phosphorylation of CREB at Ser133. At 15 min, the extent of CREB phosphorylation was slightly lower than that seen at 5 min. The amount of CREB phosphorylation decreased at 60 min after hCG treatment (Fig. 5A).
Fig. 5.
Effect of rapamycin on hCG-stimulated phosphorylation of CREB. A, T-I cells were treated with hCG (50 ng/ml) for different time periods. Cells were lysed using RIPA buffer and subjected to Western blot analysis using phospho-specific CREB (Ser133) antibody. B, T-I cells were pretreated without or with rapamycin (20 nm) for 1 h before treatment for 15 min with hCG (50 ng/ml). Control groups were treated with vehicle (dimethylsulfoxide). Cells were lysed and subjected to Western blot analysis using phosphorylation site-specific antibody for CREB (Ser133). C, T-I cells were pretreated without or with the S6K1 inhibitor, PF4708671 (10 μm), for 1 h followed by hCG (50 ng/ml) treatment for 15 min. Cell lysates were examined for phospho-specific CREB (Ser133) by Western blotting. The levels of CREB protein were used as internal controls. Results in each panel are representative of three separate experiments.
To determine whether mTORC1 signaling plays a role in hCG-induced CREB phosphorylation, T-I cells were pretreated with the mTORC1 inhibitor, rapamycin, for 1 h followed by hCG treatment for 15 min. Cell lysates were examined for CREB phosphorylation by Western blot analysis. As expected, hCG treatment increased CREB phosphorylation, whereas pretreatment with rapamycin completely blocked this response, further suggesting that CREB is a downstream target of mTORC1 (Fig. 5B).
For the next set of experiments, we used the S6K1 inhibitor, PF4708671, to further validate that phosphorylation of CREB occurs downstream of S6K1 activation in hCG-stimulated cells. To test this, T-I cells were pretreated with PF4708671 for 1 h followed by hCG stimulation for 15 min. Immunoblot analysis was performed using phospho-specific CREB (Ser133) antibody. The result presented in Fig. 5C shows that hCG produced robust phosphorylation of CREB, whereas inhibition of S6K1 by PF4708671 abrogated the hCG-induced phosphorylation of CREB. These results suggest that hCG-mediated activation of mTORC1/S6K1 signaling is required for CREB activation. The inhibitory potential of the S6K1 inhibitor, PF4708671, was also verified by examining the phosphorylation of S6K1 under the experimental conditions described above. The results showed that cells treated with hCG increased phosphorylation of S6K1(Thr389), whereas pretreatment with PF4708671 blocked hCG-induced phosphorylation of S6K1 (Supplemental Fig. 2).
mTORC1 signaling involves CREB-mediated transcription of the Cyp17a1 gene
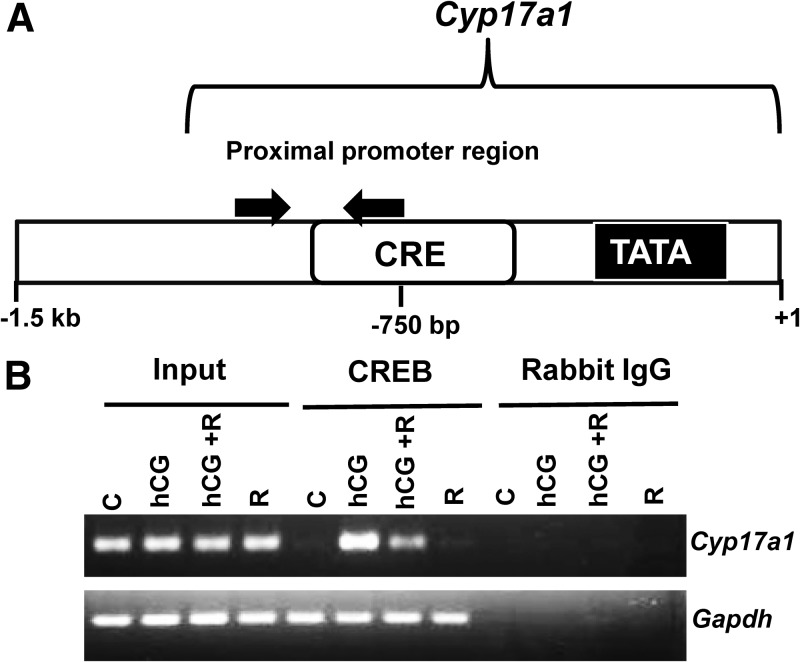
LH/hCG has been known to activate CREB, which leads to transcriptional activation of genes which are involved in androgen biosynthesis (31, 32). To determine whether mTORC1-mediated activation of CREB recruits the Cyp17a1 promoter, ChIP assays were performed in T-I cells. To examine this, T-I cells were pretreated with rapamycin for 1 h followed by hCG treatment for 2 h. As shown in Fig. 6B, the results demonstrated that treatment with hCG resulted in a dramatic increase in the association of CREB with the proximal Cyp17a1 promoter. This increased association was reduced by pretreatment with rapamycin followed by hCG treatment. Immunoprecipitation with normal rabbit IgG failed to show an association with the proximal Cyp17a1 gene under any treatment conditions. These results suggest that mTORC1-mediated activation of CREB, at least in part, regulates the expression of Cyp17a1 gene in T-I cells.
Fig. 6.
Effect of rapamycin on hCG regulation of CREB binding to Cyp17a1. Cells were pretreated without or with rapamycin (20 nm) for 1 h followed by hCG (50 ng/ml) treatment for 2 h. Control groups were treated with vehicle (dimethylsulfoxide). Cells were cross-linked, and ChIP assays were performed with CREB antibody or normal rabbit IgG (negative control) as described in Materials and Methods. A, Schematic representation of the DNA amplified by PCR using primers in the proximal region of the Cyp17a1 gene. B, The PCR products were analyzed by 2% agarose gel. Results in each panel are representative of three separate experiments. C, Control; R, rapamycin.
Discussion
The present study provides novel insights into the role of the LH/hCG-mediated activation of the mTORC1 signaling network in androgen synthesis by T-I cells. The results clearly show that hCG activates downstream targets of mTORC1, S6K1, and eIF4E as well as steroidogenic enzyme expression. These hCG-induced responses were blocked by the mTORC1 inhibitor, rapamycin, and also by siRNA-mediated knockdown of mTOR. However, mTORC1 inhibition failed to block hCG- or forskolin-induced StAR protein expression, suggesting that not all events involved in steroidogenesis are blocked by rapamycin.
mTORC1 is a serine/threonine kinase and plays a vital role in regulating cellular processes such as growth, proliferation, transcription, protein, and lipid synthesis (23, 33, 34). We and others have shown that gonadotropin activates the PI3-kinase/AKT/mTORC1 pathway via cAMP, and this pathway plays a key role in ovarian cell proliferation and differentiation (12, 20, 35). The present study shows that mTORC1 activation is not limited to inducing cell growth and proliferation but that it also plays a role in androgen biosynthesis by regulating the expression of genes encoding steroidogenic enzymes. Inhibition of mTORC1 by rapamycin reduced the expression of mRNAs of Cyp11a1, Hsd3b1, and Cyp17a1 in response to hCG, suggesting that mTORC1 activation is required for hCG-stimulated steroidogenic enzyme induction. The effect of mTORC1 in regulating the expression of enzymes involved in androgen synthesis appears to be selective because the expression of Star mRNA was not affected by inhibiting this pathway.
The involvement of mTORC1 signaling in hCG-mediated activation of genes involved in steroidogenesis is consistent with the recent findings of Duvel et al. (36), who showed that the activation of mTORC1 leads to the induction of genes involved in lipid/sterol biosynthesis. Furthermore, Li et al. (37) have reported that rapamycin blocks insulin-stimulated sterol regulatory element-binding protein 1c mRNA expression in primary cultures of rat hepatocytes. It has also been reported that activation of mTORC1 not only increases the translational machinery but also up-regulates transcriptional activity (34, 36, 38–41). Although the exact mechanism of mTORC1-mediated induction of gene expression remains to be determined, the present evidence suggests that this process may be mediated through the activation of transcription factors. This is further supported by our findings that hCG-stimulated CREB phosphorylation was abrogated by rapamycin or the S6K1 inhibitor, suggesting that hCG-induced CREB activation occurs through mTORC1/S6K1-dependent pathway. S6K1-mediated inhibition of CREB phosphorylation further suggests that CREB is a downstream target of S6K1. Several lines of evidence have suggested that CREB is a transcriptional activator for the genes involved in steroidogenesis in response to gonadotropin (32, 42–45). Furthermore, the results of ChIP assay demonstrated that hCG treatment caused increased association of CREB with the proximal promoter of Cyp17a1 and this association was reduced by rapamycin. These results suggest that mTORC1-mediated activation of CREB regulates Cyp17a1 gene expression, which is involved in androgen biosynthesis in T-I cells. The inhibitory effect of rapamycin or siRNA-mediated silencing of mTOR on androgen synthesis was also associated with a reduction in P450scc, HSD3B1, and P450c17 protein expression. It is important to mention that these reductions of steroidogenic enzyme levels were not due to a general inhibition of protein synthesis because hCG-induced StAR protein expression was not affected by inhibiting mTORC1.
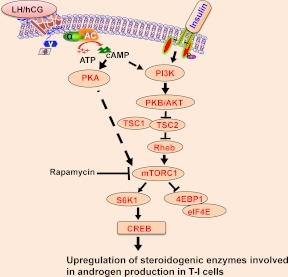
In summary, these findings highlight that LH/hCG-mediated activation of mTORC1 is involved in androgen biosynthesis by up-regulating steroidogenic enzyme expression as shown in Fig. 7. The present findings clearly show that LH/hCG-mediated mTORC1 signaling not only regulates cell growth and proliferation but also controls androgen synthesis by regulating the expression of steroidogenic enzyme expression in T-I cells. Thus, LH/hCG-mediated activation of multiple signaling pathways might converge on mTORC1, which in turn regulates steroidogenic enzyme expression.
Fig. 7.
Proposed model of LH/hCG-mediated activation of mTORC1 signal regulating androgen synthesis in T-I cells. The results of the present study support the schematic model by which LH/hCG activates mTORC1. As a result, mTORC1 enhances downstream targets, S6K1 and eIF4E as well as CREB and steroidogenic enzyme expression. Inhibition of mTORC1 blocks LH/hCG-stimulated androgen production by reducing the expression of steroidogenic enzymes.
Supplementary Material
Acknowledgments
We express our appreciation to Helle Peegel and Dr. Bindu Menon for their critical reading of the manuscript and valuable comments.
This work was supported by National Institutes of Health Grant HD 38424.
Current address for M.P.: Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Ligands: Dihydrotestosterone.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- ChIP
- Chromatin immunoprecipitation
- CREB
- cAMP response element binding
- eIF4E
- eukaryotic translation initiation factor 4E
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- hCG
- human chorionic gonadotropin
- HSD3B1
- 3β-hydroxysteroid dehydrogenase
- mTOR
- mammalian target of rapamycin
- mTORC1
- mTOR complex 1
- P450c17
- 17α-hydroxylase/17,20 lyase
- PI3-kinase
- phosphatidylinositol-3-kinase
- P450scc
- cholesterol side-chain cleavage enzyme
- RIPA
- radioimmunoprecipitation assay
- siRNA
- small interfering RNA
- S6K1
- ribosomal protein S6 kinase 1
- StAR
- steroid acute regulatory protein
- TBST
- Tris base, NaCl, and Tween 20
- T-I
- theca-interstitial.
References
- 1. Magoffin DA. 2002. The ovarian androgen-producing cells: a 2001 perspective. Rev Endocr Metab Disord 3:47–53 [DOI] [PubMed] [Google Scholar]
- 2. Magoffin DA. 2005. Ovarian theca cell. Int J Biochem Cell Biol 37:1344–1349 [DOI] [PubMed] [Google Scholar]
- 3. Magoffin DA. 2006. Ovarian enzyme activities in women with polycystic ovary syndrome. Fertil Steril 86(Suppl 1):S9–S11 [DOI] [PubMed] [Google Scholar]
- 4. Young JM, McNeilly AS. 2010. Theca: the forgotten cell of the ovarian follicle. Reproduction 140:489–504 [DOI] [PubMed] [Google Scholar]
- 5. Leung PC, Steele GL. 1992. Intracellular signaling in the gonads. Endocr Rev 13:476–498 [DOI] [PubMed] [Google Scholar]
- 6. Wood JR, Strauss JF., 3rd 2002. Multiple signal transduction pathways regulate ovarian steroidogenesis. Rev Endocr Metab Disord 3:33–46 [DOI] [PubMed] [Google Scholar]
- 7. Ascoli M, Fanelli F, Segaloff DL. 2002. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141–174 [DOI] [PubMed] [Google Scholar]
- 8. Richards JS. 2001. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- 9. Shiraishi K, Ascoli M. 2006. Activation of the lutropin/choriogonadotropin receptor in MA-10 cells stimulates tyrosine kinase cascades that activate ras and the extracellular signal regulated kinases (ERK1/2). Endocrinology 147:3419–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panigone S, Hsieh M, Fu M, Persani L, Conti M. 2008. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tai P, Shiraishi K, Ascoli M. 2009. Activation of the lutropin/choriogonadotropin receptor inhibits apoptosis of immature Leydig cells in primary culture. Endocrinology 150:3766–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeleznik AJ, Saxena D, Little-Ihrig L. 2003. Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology 144:3985–3994 [DOI] [PubMed] [Google Scholar]
- 13. Tajima K, Yoshii K, Fukuda S, Orisaka M, Miyamoto K, Amsterdam A, Kotsuji F. 2005. Luteinizing hormone-induced extracellular-signal regulated kinase activation differently modulates progesterone and androstenedione production in bovine theca cells. Endocrinology 146:2903–2910 [DOI] [PubMed] [Google Scholar]
- 14. Manna PR, Stocco DM. 2005. Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metab Disord 5:93–108 [DOI] [PubMed] [Google Scholar]
- 15. Stocco DM, Wang X, Jo Y, Manna PR. 2005. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- 16. Evaul K, Hammes SR. 2008. Cross-talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem 283:27525–27533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy L, McDonald CA, Jiang C, Maroni D, Zeleznik AJ, Wyatt TA, Hou X, Davis JS. 2009. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3β/β-catenin signaling in corpus luteum progesterone synthesis. Endocrinology 150:5036–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manna PR, Soh JW, Stocco DM. 2011. The involvement of specific PKC isoenzymes in phorbol ester-mediated regulation of steroidogenic acute regulatory protein expression and steroid synthesis in mouse Leydig cells. Endocrinology 152:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller WL, Auchus RJ. 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palaniappan M, Menon KM. 2010. Human chorionic gonadotropin stimulates theca-interstitial cell proliferation and cell cycle regulatory proteins by a cAMP-dependent activation of AKT/mTORC1 signaling pathway. Mol Endocrinol 24:1782–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fingar DC, Blenis J. 2004. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23:3151–3171 [DOI] [PubMed] [Google Scholar]
- 22. Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. 2004. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 24:200–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma XM, Blenis J. 2009. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10:307–318 [DOI] [PubMed] [Google Scholar]
- 24. Guertin DA, Sabatini DM. 2007. Defining the role of mTOR in cancer. Cancer Cell 12:9–22 [DOI] [PubMed] [Google Scholar]
- 25. Palaniappan M, Menon KM. 2009. Regulation of sterol regulatory element-binding transcription factor 1a by human chorionic gonadotropin and insulin in cultured rat theca-interstitial cells. Biol Reprod 81:284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63 [DOI] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 28. Clark BJ, Wells J, King SR, Stocco DM. 1994. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- 29. Clark BJ, Stocco DM. 1995. Expression of the steroidogenic acute regulatory (StAR) protein: a novel LH-induced mitochondrial protein required for the acute regulation of steroidogenesis in mouse Leydig tumor cells. Endocr Res 21:243–257 [DOI] [PubMed] [Google Scholar]
- 30. Rekawiecki R, Nowik M, Kotwica J. 2005. Stimulatory effect of LH, PGE2 and progesterone on StAR protein, cytochrome P450 cholesterol side chain cleavage and 3β hydroxysteroid dehydrogenase gene expression in bovine luteal cells. Prostaglandins Other Lipid Mediat 78:169–184 [DOI] [PubMed] [Google Scholar]
- 31. Towns R, Azhar S, Peegel H, Menon KM. 2005. LH/hCG-stimulated androgen production and selective HDL-cholesterol transport are inhibited by a dominant-negative CREB construct in primary cultures of rat theca-interstitial cells. Endocrine 27:269–277 [DOI] [PubMed] [Google Scholar]
- 32. Sands WA, Palmer TM. 2008. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal 20:460–466 [DOI] [PubMed] [Google Scholar]
- 33. Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. 2008. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. 2010. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta 1804:433–439 [DOI] [PubMed] [Google Scholar]
- 35. Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M. 2004. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem 279:19431–19440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. 2010. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39:171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li S, Brown MS, Goldstein JL. 2010. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA 107:3441–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lewis CA, Griffiths B, Santos CR, Pende M, Schulze A. 2011. Regulation of the SREBP transcription factors by mTORC1. Biochem Soc Trans 39:495–499 [DOI] [PubMed] [Google Scholar]
- 39. Laplante M, Sabatini DM. 2009. An emerging role of mTOR in lipid biosynthesis. Curr Biol 19:R1046–R1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gingras AC, Raught B, Sonenberg N. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev 15:807–826 [DOI] [PubMed] [Google Scholar]
- 41. Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. 2010. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328:1172–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manna PR, Huhtaniemi IT, Stocco DM. 2009. Mechanisms of protein kinase C signaling in the modulation of 3′,5′-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells. Endocrinology 150:3308–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. 2002. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol 16:184–199 [DOI] [PubMed] [Google Scholar]
- 44. Jo Y, King SR, Khan SA, Stocco DM. 2005. Involvement of protein kinase C and cyclic adenosine 3′,5′-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol Reprod 73:244–255 [DOI] [PubMed] [Google Scholar]
- 45. Guo IC, Huang CY, Wang CK, Chung BC. 2007. Activating protein-1 cooperates with steroidogenic factor-1 to regulate 3′,5′-cyclic adenosine 5′-monophosphate-dependent human P450SCC transcription in vitro and in vivo. Endocrinology 148:1804–1812 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.