Abstract
The study of cranial design and development in Gymnophthalmidae is important to understand the ontogenetic processes behind the morphological diversity of the group and to examine the possible effects of microhabitat use and other ecological parameters, as well as phylogenetic constraints, on skull anatomy. Complete morphological descriptions of embryonic skull development within Gymnophthalmidae are non-existent. Likewise, very little is known about the complete chondrocranium of the family. Herein, the development of the skull of the semi-fossorial lizard Ptychoglossus bicolor is described along with an examination of the chondrocranium of other gymnophthalmid taxa and the teiid Cnemidophorus lemniscatus. Cranial chondrification begins with early condensations in the ethmoid, orbitotemporal and occipital regions of the chondrocranium as well as the viscerocranium. Ossification of the skull starts with elements of the dermatocranium (pterygoid, prefrontal, maxilla and jugal). The orbitosphenoid is the last chondral bone to appear. At birth, the skull is almost completely ossified and exhibits a large frontoparietal fontanelle. In general terms, the chondrocranium of the gymnophthalmids studied is characteristic of lacertiform terrestrial lizards, in spite of their life habits, and resembles the chondrocranium of C. lemniscatus in many aspects. However, the gymnophthalmids show great variation in the orbitosphenoid and a complex nasal capsule. The latter exhibits greater development of some nasal cartilages, which make it more complex than in C. lemniscatus. These characteristics might be related to microhabitat use and the well-developed olfactory and vomeronasal systems observed within this clade.
Keywords: chondrocranium, cranial chondrification, gymnophthalmidae, skull development
Introduction
Gymnophthalmidae is a morphologically and ecologically diverse group of Neotropical lizards (Pianka & Vitt, 2003; Donnelly et al. 2006) with nearly 223 species and 45 genera (Uetz et al. 2011). Within this lineage, changes in body shape have repeatedly evolved in a scenario where the species' lifestyle (e.g. fossoriality, burrowing) acts as a strong selective pressure (Pellegrino et al. 2001; Barros et al. 2011). In this sense, among gymnophthalmid lizards we find variations from lacertiform to serpentiform body plans, which are related to terrestrial and fossorial lifestyles. Recently, Barros et al. (2011) found that in this family cranial design evolved in response to selective pressures imposed primarily by microhabitat use. Thus, specialized snake-like burrowing forms exhibit shorter and acute heads in contrast with lacertiform terrestrial forms. Despite that these analyses show important variations in head morphology in Gymnophthalmidae, there are few studies about skull anatomy in this clade. Roscito & Rodrigues (2010) analyze the skull of the fossorial and serpentiform lizards Nothobachia ablephara, Scriptosaura catimbau and Calyptommatus nicterus. Other authors describe the skull morphology of lacertiform species such as Euspondylus acutirostris, Potamites ecpleopus and Vanzosaura rubricauda (Montero et al. 2002; Bell et al. 2003; Guerra & Montero, 2009); the latter being the first and only description of the entire chondrocranium of a gymnophthalmid lizard.
Studies of skull development in this family include only a description of the postembryonic development of the dermatocranium and the orbitotemporal region of the chondrocranium in Bachia bicolor (Tarazona & Ramírez-Pinilla, 2008; Tarazona et al. 2008). Tarazona et al. (2008) found that the skull in this burrowing snake-like lizard presents characteristics similar to those of specialized burrowers like amphisbaenians. Therefore, the study of skull development in Gymnophthalmidae is interesting in an evolutionary context; nevertheless, we do not know anything regarding embryonic skull development in this clade.
The main purpose of this work is to describe the development of the embryonic skull of Ptychoglossus bicolor. In addition, the chondrocranium of P. bicolor is compared with the chondrocranium of other gymnophthalmid lizards. This species is particularly important to study considering the fact that it belongs to the clade Alopoglossinae, which is basal in the phylogeny of Gymnophthalmidae (Castoe et al. 2004), and that this lacertiform species exhibits terrestrial and semi-fossorial habits (Anaya-Rojas et al. 2010). In this sense, information about the developmental pattern and processes behind skull morphology in P. bicolor will provide insight into the ancestral characteristics of the family and the morphological diversification of the skull in this group of lizards. Furthermore, within gymnophthalmids where variations in head shape are related to microhabitat use (Barros et al. 2011), the study of the development in lizard-like and basal species like P. bicolor comprises a baseline for future comparative works (i.e. heterochrony analysis) with fossorial snake-like species, to assess the factors associated with the evolution of serpentiform body plan in gymnophthalmids, including cranial reductions, body elongation and limb reduction.
Materials and methods
The embryonic material was collected from a coffee shade plantation at the Hacienda El Roble from the municipality of Los Santos (Department of Santander; 06°52′N; 73°03′W), at an altitude of 1700 m, on the western slopes of the Cordillera Oriental of the Colombian Andes. In this locality, the only lizard species inhabiting the leaf litter of the coffee plantation is Ptychoglossus bicolor. The embryos were either fixed in 10% neutralized formalin or Bouin's fixative. Afterwards, they were staged according to Dufaure & Hubert (D&H) (1961), and processed by clearing and double-staining of cartilage and bone following Wassersug (1976). Descriptions included 23 embryos from stages 31, 32–33, 33–34, 35, 39, 39–40, 40, and four hatchlings. For each stage we only mention major morphological changes relative to the previous one. Anatomical descriptions followed the nomenclature from Bellairs & Kamal (1981) for the chondrocranium and Bell et al. (2003) for the dermal bones. Collected specimens were deposited in the Colección Herpetológica of the Museo de Historia Natural, Universidad Industrial de Santander (UIS-R).
The chondrocranium of Ptychoglossus bicolor neonates was compared with that of 18 other gymnophthalmid species to obtain a general view of the chondrocranium of Gymnophthalmidae. These specimens were loaned from the Museo de Herpetología of the Universidad de Antioquia (MHUA), the Colección de Reptiles of the Instituto de Ciencias Naturales, Universidad Nacional de Colombia (ICN), and the Colección Herpetológica of the Universidad Industrial de Santander (UIS-R). The gymnophthalmid species analyzed included Ptychoglossus vallensis (MHUA10188), P. festae (MHUA10461), Alopoglossus copii (ICN8089), Anadia ocellata (MHUA10493), A. bogotensis (ICN4516, 4517), Cercosaura vertebralis (MHUA10183, 11628), C. ampuedae (UIS-R-2603), Echinosaura horrida (MHUA12251, 12330), Riama striata (MHUA10577), Bachia bicolor (UIS-R-2599), Potamites cochranae (ICN9453), Pholidobolus montium (ICN5655), Leposoma rugiceps (UIS-R-2600), L. southi (MHUA11401), Gymnophthalmus speciosus (MHUA11327), Iphisa elegans (ICN7084) and Tretioscincus bifasciatus (UIS-R-2577). The specimens were selected based on availability in museums, choosing those that based on body size probably correspond to neonates or juveniles. The data of Vanzosaura rubricauda were obtained from the work of Guerra & Montero (2009). The gymnophthalmid species were classified into four subfamilies according to the phylogenetic classification provided by Castoe et al. (2004) (Alopoglossinae, Cercosaurinae, Ecpleopinae and Gymnophthalminae). In addition, the chondrocranium of these gymnophthalmid lizards was compared with the teiid Cnemidophorus lemniscatus (UIS-R-251), a close external group. All of these specimens were cleared and stained with the same protocol used for P. bicolor. Due to the number of species analyzed, the comparative data were organized in a table to show, in a concise way, the characteristics of the chondrocranium of the gymnophthalmids considered in this study.
Results
Descriptions follow an anterior to posterior direction dividing the chondrocranium into three regions: an anterior ethmoid region; a medial orbitotemporal region; and a posterior occipital region.
Skull development
Chondrification of the skull starts with the early condensations of elements from the orbitotemporal region, viscerocranium, and the prechondrogenic condensations of the nasal septum (ns) and parietotectal cartilage (ptc). Later, in the neonate, the chondrocranium resembles the adult form. Cranial ossification begins at stage 35 with the formation of the prefrontal (prf), maxilla (mx), jugal (jg) and pterygoid bones (pt). Subsequently, at stage 40, all of the remaining dermal elements are ossifying.
Stage 31
In this stage, the three regions of the chondrocranium begin to condense: the anterior ethmoid region; the intermediate orbitotemporal region; and the posterior occipital region (Fig. 1a,b). The floor of the chondrocranium in the orbitotemporal and occipital regions is more developed. Also, the elements of the viscerocranium, i.e. quadrate, ascending process of the pterygoquadrate (asp), Meckel's cartilage, are present.
Fig. 1.
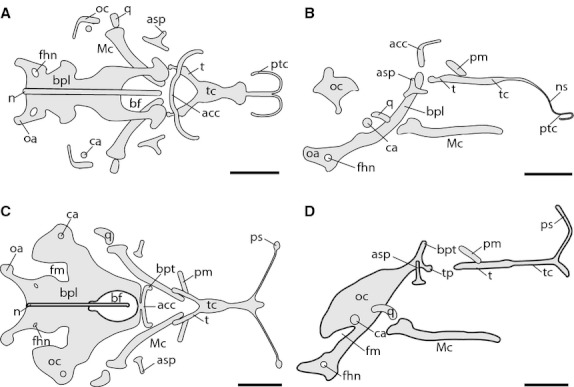
Chondrocranium of Ptychoglossus bicolor at stages 31 and 32–33. Stage 31: (A) dorsal view, (B) lateral view. Stage 32–33: (C) dorsal view, (D) lateral view. Abbreviations: acc, acrochordal cartilage; asp, ascending process of pterygoquadrate; bf, basicranial fenestra; bpl, basal plate; bpt, basipterygoid process; ca, columella auris; fhn, hypoglossal foramen; fm, fissura metotica; Mc, Meckel's cartilage; n, notochord; ns, nasal septum; oa, occipital arch; oc, otic capsule; pm, pila metoptica; ps, planum supraseptale; ptc, parietotectal cartilage; q, quadrate; t, trabecula; tc, trabecula communis; tp, trabecular process of acrochordal cartilage. Scale bar: 0.5 mm.
The anterior region of the chondrocranium consists of a very thin prechondrogenic condensation of the nasal septum and the rudiment of the developing parietotectal cartilage enclosing most of what will become the fenestra superior (fs); we were unable to identify these prechondrogenic structures in the following two stages.
In the orbitotemporal region, the trabeculae cranii are merged together between the orbits forming the trabecula communis (tc), referred to as the tropitrabic (tropibasic) state, consistent with the condition of most reptiles. In dorsal view, the anterior region of the trabecula cranii appears as a broad cartilaginous plate (trabecular plate). Posteriorly, the trabecula cranii diverge freely into two trabeculae (t). The pila metoptica (pm) is the only cartilage from the orbitotemporal scaffolding present at this early stage of development; this is observed on each side of the trabecula cranii, yet both are separated from each other.
In the occipital region, the skull floor consists of a slim and concave basal plate (bp) and a separate long and narrow transversal bar in front of it, just behind and above the trabecula cranii corresponding to the acrochordal cartilage (acc; posterior orbital cartilage). In the basal plate, the basicranial fenestra is partially formed; only the posterior and lateral margins are present. In the acrochordal cartilage, the lateral edges are rostrally oriented, parallel to the trabecula cranii. Also, the notochord (n) appears embedded in the medial aspect of the basal plate, projecting rostrally into the basicranial fenestra without contacting the acrochordal cartilage. Distally, the basal plate is continuous with the developing occipital arch (oa); also in this region the first of the three openings for the roots of the hypoglossal nerves, i.e. the first hypoglossal foramen (fhn), is open at the back of the basal plate, on each side of the notochord. Laterally, the sidewall of the skull is partly formed by the early condensation of the cochlear portion of the auditory (otic) capsule (oc), which chondrifies independently from the basal plate and in this stage remains separate from one another. Also laterally, the small spherical rudiment of the columella auris (ca) rests just below the developing otic capsule, on each side of the skull.
Regarding the elements of the viscerocranium, Meckel's cartilage (Mc) and the palatoquadrate (pterygoquadrate) complex are present; however, the pterygoquadrate (or intermediate part) that connects the quadrate with the rest of the complex is regressed and so the quadrate cartilage (q) is separated. Therefore, at this stage the palatoquadrate complex comprises only the ascending process dorsally, a reduced pterygoid process rostrally, and posteriorly what appears to be the remains of the intermediate part.
Stage 32–33
In this stage, the main changes are observed in the orbitotemporal region with the development of the planum supraseptale (ps) and the occipital region where the basal plate and otic capsule are more developed (Fig. 1c,d). In the orbitotemporal region, the main change consists of the chondrification of the planum supraseptale, which is now underway, projecting dorsally from the lateral flanks of the anterior aspect of the trabecula communis as two separate narrow cartilaginous bars, one on each side of the skull. Also, the trabecula communis has further elongated and now appears narrower.
In the occipital region, the basal plate has partly fused rostrally with the medial portion of the acrochordal cartilage, thus closing the anterior border of the basicranial fenestra. Furthermore, the lateral edges of the acrochordal cartilage remain rostrally oriented in an almost vertical position, forming the basipterygoid processes (bpt). Also in this stage, an extra pair of short horizontal processes that we named as trabecular processes (tp) have appeared below the basipterygoid processes also projecting forward so that the anterior tips lie just behind the hind end of each trabecula; these trabecular processes must not be confused with the basipterygoid (basitrabecular) processes. The cochlear portion of the auditory capsule has expanded, and in this stage appears fused with the anterior lateral margins of the basal plate at the basicapsular commissure but leaving a gap behind known as the fissura metotica (fm).
In regard to the viscerocranium, the ascending process of the pterygoquadrate complex is now more prominent, while the pterygoid process and the remains of the intermediate part have further shortened.
Stage 33–34
In this stage, the three regions of the chondrocranium are more advanced (Fig. 2). In the ethmoid region, the nasal septum and parietotectal cartilage are now apparent. In the orbitotemporal region, the interorbital septum begins to develop as well as other cartilages from the orbitotemporal scaffolding. In addition, the planum supraseptale is more advanced in contrast with the previous stage. In the occipital region, the otic capsule is more developed.
Fig. 2.
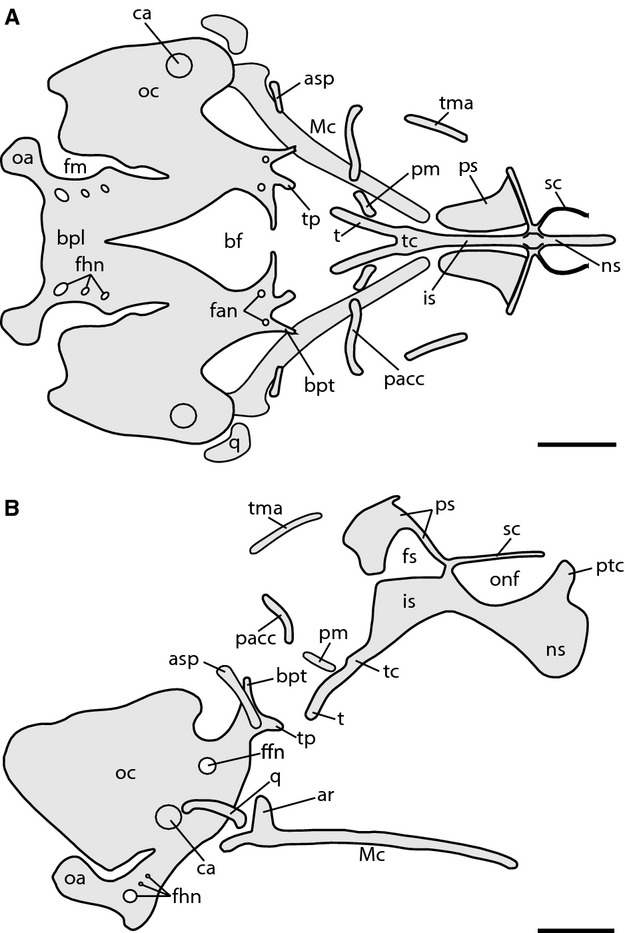
Chondrocranium of Ptychoglossus bicolor at stage 33–34. (A) Dorsal view. (B) Lateral view. Abbreviations: ar, articular; asp, ascending process of pterygoquadrate; bf, basicranial fenestra; bpl, basal plate; bpt, basipterygoid process; ca, columella auris; fan, foramina for abducens nerve; ffn, foramen for facial nerve; fhn, hypoglossal foramina; fm, fissura metotica; fs, fenestra in nasal or interorbital septum; is, interorbital septum; Mc, Meckel's cartilage; ns, nasal septum; oa, occipital arch; oc, otic capsule; onf, orbitonasal fissure; pacc, pila accesoria; pm, pila metoptica; ps, planum supraseptale; ptc, parietotectal cartilage; q, quadrate; sc, sphenethmoid commisure; t, trabecula; tc, trabecula communis; tma, taenia marginalis; tp, trabecular process of acrochordal cartilage. Scale bar: 0.5 mm.
As development proceeds, the interorbital septum (is) appears continuous with the trabecula communis and extends into the ethmoid region of the chondrocranium, thus forming the rudiment of the nasal septum, which becomes tall by means of a prechondrogenic dorsal expansion. The two halves of the developing planum supraseptale resemble a slim plate and persist as separate structures. Also in this stage, a pair of arched slender cartilaginous rods corresponding to the rudiment of the sphenethmoid commissures (sc) is continuous with the rostral aspect of each half of the planum supraseptale while anteriorly they end freely. The dorsal aspect of the interorbital septum has increased in height, now forming a thin prechondrogenic wall in the midline of the skull, which is evident in dorsal view between the two halves of the planum supraseptale. Further behind, the rudiments of the taenia marginalis (tm) and pila accesoria (pacc) appear separated from every element of the chondrocranium. In the occipital region, the acrochordal cartilage appears completely fused with the basal plate. Furthermore, the medial portion of the acrochordal cartilage has regressed a little bit, so that the basicranial fenestra is open again anteriorly in the midline. Also, in the rostral aspect of the basal plate (previously acrochordal cartilage), the foramina for the abducens nerve (fan) are evident. The trabecular processes have extended rostrally, and the lateral projections of the acrochordal cartilage form the rudiments of the basipterygoid processes. Toward the occipital arch, the three hypoglossal foramina are now open in the basal plate. In the developing auditory capsule the foramen for the facial nerve (ffn) is open on both sides, and is evident in lateral view. The ascending process of the pterygoquadrate complex has further increased in size, and now the anterior and posterior processes of the complex are not evident anymore. Meckel's cartilage has extended anteriorly, and at this time the articular process (ar) projects dorsally from the posterior region.
Stage 35
In this stage, the elements of the chondrocranium belonging to the orbitotemporal and occipital regions are more developed, while the structures of the ethmoid region are still very rudimentary (Fig. 3).
Fig. 3.
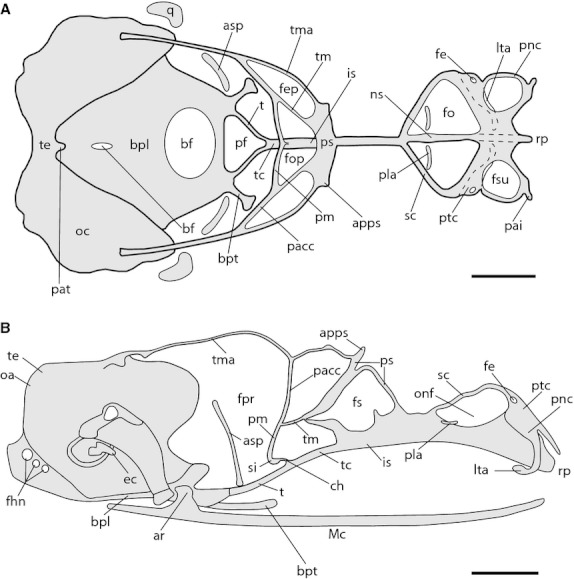
Chondrocranium of Ptychoglossus bicolor at stage 35. Dashed lines denote subjacent cartilage. (A) Dorsal view. (B) Lateral view. Abbreviations: apps, anterior process of planum supraseptale; ar, articular; asp, ascending process of pterygoquadrate; bf, basicranial fenestra; bpl, basal plate; bpt, basipterygoid process; ch, cartilago hypochiasmatica; ec, extracolumella; fe, foramen epiphaniale; fep, fenestra epioptica; fhn, hypoglossal foramina; fo, fenestra olfactoria; fop, fenestra optica; fs, fenestra in nasal or interorbital septum; fsu, fenestra superior; is, interorbital septum; lta, lamina transversalis anterior; Mc, Meckel's cartilage; ns, nasal septum; oa, occipital arch; oc, otic capsule; onf, orbitonasal fissure; pacc, pila accesoria; pai, processus alaris inferior; pat, ascending process of tectum; pf, pituitary fossa; pla, planum antorbitale; pm, pila metoptica; pnc, paranasal cartilage; ps, planum supraseptale; ptc, parietotectal cartilage; q, quadrate; rp, rostral process; sc, sphenethmoid commisure; si, subiculum infundibuli; t, trabecula; tc, trabecula communis; te, tectum; tm, taenia medialis; tma, taenia marginalis. Scale bar: 0.5 mm.
In the ethmoid region, the nasal capsule is underway showing a large fenestra superior surrounded by the early parietotectal and paranasal (pnc) cartilages, which are continuous with the nasal septum forming part of the roof and lateral wall, respectively. The foramen epiphaniale (fe) is open behind the fenestra superior and lateral to this foramen, the paranasal cartilage extends ventrally to form the anterior contour of the fenestra lateralis (fl). Anteriorly, the dorsal extension of the nasal septum has now chondrified, projecting towards the roof of the capsule forming a consistent wall, which contacts the parietotectal cartilage in the region between the paired fenestra superior. Lateral to the nasal septum, the rudiments of the paired lamina transversalis anterior (lta) and planum antorbitale (pla) appear as two slender cartilaginous condensations. The lamina transversalis anterior lies at the floor of the nasal capsule; it is continuous with the lateral wall of the capsule and is separated from the nasal septum. Ventrally and medially the lamina transversalis anterior shows the cartilage of Jacobson's organ densely chondrified. The planum antorbitale appears as a short independent condensation in the back of the nasal capsule. Posteriorly, the nasal capsule contacts the anterior portion of the sphenethmoid commissures, forming a large fenestra olfactoria (fo) for the olfactory and vomeronasal nerves.
In the orbitotemporal region, the taenia marginalis contacts the dorsal surface of the otic capsule posteriorly, the dorsal aspect of the planum supraseptale anteriorly and the pila accesoria ventrally. Also, the taenia medialis is continuous with the pila accesoria, the pila metoptica and the posterior border of the planum supraseptale. Medially, the pila metoptica has joined its counterpart forming the subiculum infundibuli (si), which contacts the dorsal margin of the trabecula communis by means of the cartilago hypochiasmatica (ch). The planum supraseptale is completely formed and barely contacts the interorbital septum rostrally and caudally, leaving a large space between the dorsal margin of the interorbital septum and the ventral surface of the planum supraseptale, namely the fenestra in the interorbital septum. Posteriorly, the trabeculae are complete, contacting the rostral aspect of the basal plate, specifically the trabecular processes of the acrochordal cartilage. Furthermore, the pituitary fossa (pf; hypophyseal fenestra) is now complete, formed anteriorly and laterally by the trabeculae and posteriorly by the rostral aspect of the basal plate.
The roof of the occipital region of the cartilaginous skull shows a closed occipital arch forming the tectum posterius and the otic capsules fused together dorsally forming the tectum synoticum. The tectum posterius projects forward and contacts the tectum synoticum anteriorly forming a single tectum (te; tectum synoticum plus posterius), from which the cartilaginous ascending process of tectum (pat; processus anterior tecti) projects rostrally. The basicranial fenestra has become reduced and split into two by chondrification from the medial region of the basal plate.
In this stage, changes associated with elements of the viscerocranium are related to the quadrate, which is complete and the extracolumella (ec) that is now formed.
Stage 39
The most relevant changes are observed toward the ethmoid region, which exhibits great transformations in the nasal cartilages (Fig. 4). In the occipital region, there is further ossification of the elements that comprise the braincase. In general, the chondrocranium resembles the configuration observed in the neonate and adult.
Fig. 4.
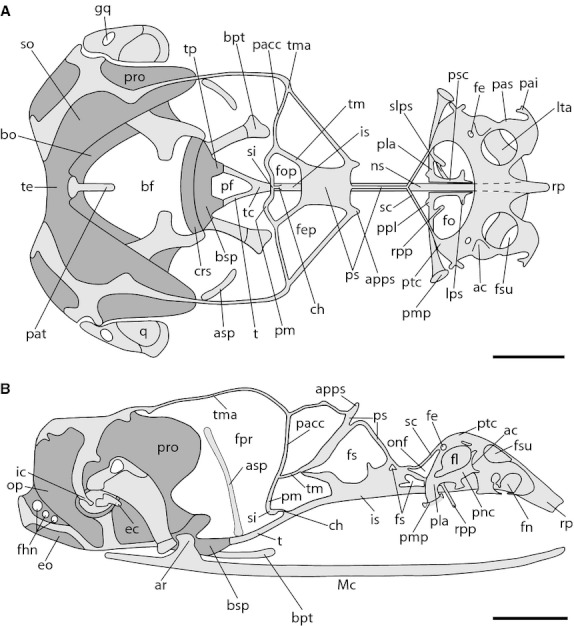
Chondrocranium of Ptychoglossus bicolor at stage 39. Dashed lines denote subjacent cartilage and dark-gray structures denote ossified cartilage. (A) Dorsal view. (B) Lateral view. Abbreviations: ac, aditus conchae; apps, anterior process of planum supraseptale; ar, articular; asp, ascending process of pterygoquadrate; bf, basicranial fenestra; bo, basioccipital; bpt, basipterygoid process; bsp, basisphenoid; ch, cartilago hypochiasmatica; crs, crista sellaris; ec, extracolumella; eo; exoccipital; fe, foramen epiphaniale; fep, fenestra epioptica; fhn, hypoglossal foramina; fn, fenestra narina; fo, fenestra olfactoria; fop, fenestra optica; fpr, fenestra prootica; fs, fenestra in nasal or interorbital septum; gq, gap in quadrate; ic, intercalary; is, interorbital septum; lps, lateral process of paraseptal cartilage; lta, lamina transversalis anterior; Mc, Meckel's cartilage; ns, nasal septum; onf, orbitonasal fissure; op, opisthotic; pacc, pila accesoria; pai, processus alaris inferior; pas, processus alaris superior; pat, ascending process of tectum; pf, pituitary fossa; pla, planum antorbitale; pm, pila metoptica; pmp, posterior maxillary process; ppl, posterior process of planum antorbitale; pro, prootic; ps, planum supraseptale; psc, paraseptal cartilage; ptc, parietotectal cartilage; q, quadrate; rp, rostral process; rpp, rostral process of planum antorbitale; sc, sphenethmoid commisure; si, subiculum infundibuli; slps, secondary lateral process of paraseptal cartilage; so, supraoccipital; t, trabecula; tc, trabecula communis; te, tectum; tm, taenia medialis; tma, taenia marginalis; tp, trabecular process of acrochordal cartilage. Scale bar: 0.5 mm.
In the nasal capsule of the ethmoid region, the parietotectal cartilage has broadened, extending further over the nasal septum. In the sidewall, the nasal concha is completely formed by the paranasal cartilage and opens to the outside through the aditus conchae (ac), which abuts the lateral aspect of the lamina transversalis anterior. Also, on the lateral aspects of the paranasal cartilage the processus alaris superior (pas) is well formed. Posterolaterally, the fenestra lateralis is completely formed. The planum antorbitale is continuous medially with the paraseptal cartilage (psc) and laterally it forms the posterior maxillary process (pmp). Also, the planum antorbital now forms a small wall in the back of the nasal capsule and contacts the posterior and lateral margins of the nasal concha. Mesially, the planum antorbitale has short posterior and rostral processes. In respect to the viscerocranium, the ascending process of the pterygoquadrate is quite long now, extending dorsally near the taenia marginalis. The intercalary cartilage (ic) appears as a small structure whose apical tip lies dorsal to the extracolumella and is joined at its base to the crista parotica.
In relation to the occipital region, the basipterygoid processes have increased in length. The rostral aspect of the basal plate is now ossified into the crista sellaris (crs) including the trabecular processes of the acrochordal cartilage. The otic capsule, occipital arch and basal plate have begun to ossify, forming the prootic (pro), supraoccipital (so), exoccipital (eo), opisthotic, basioccipital (bo) and basisphenoid (bsp), respectively, which are joined together by synchondrosis. The intercalary cartilage appears as a small structure whose apical tip lies dorsal to the extracolumella and is joined at its base to the crista parotica.
Stage 39–40
In regard to the chondrocranium, the most important events evident at this stage are restricted to ossification events; everything else resembles the previous stage (Fig. 5). In the occipital region, the basipterygoid processes appear ossified, except for their articular edges. The ascending process – one element of the viscerocranium – has ossified into the epipterygoid bone (ep) with its epiphyses still cartilaginous. Moreover, the quadrate appears partly ossified medially and Meckel's cartilage has become invested by the membrane bone of the lower jaw (mandible) (md).
Fig. 5.
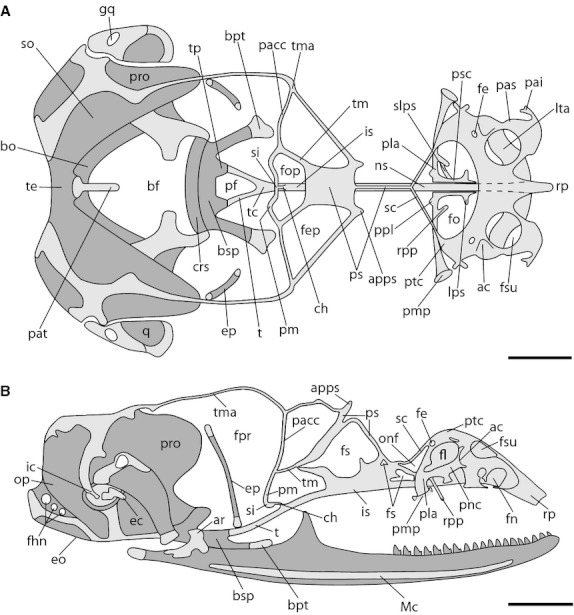
Chondrocranium of Ptychoglossus bicolor at stage 39–40. Dashed lines denote subjacent cartilage and dark-gray structures denote ossified cartilage. (A) Dorsal view. (B) Lateral view. Abbreviations: ac, aditus conchae; apps, anterior process of planum supraseptale; ar, articular; bf, basicranial fenestra; bo, basioccipital; bpt, basipterygoid process; bsp, basisphenoid; ch, cartilago hypochiasmatica; crs, crista sellaris; ec, extracolumella; eo; exoccipital; ep, epipterygoid; fe, foramen epiphaniale; fep, fenestra epioptica; fhn, hypoglossal foramina; fl, fenestra lateralis; fn, fenestra narina; fo, fenestra olfactoria; fop, fenestra optica; fpr, fenestra prootica; fs, fenestra in nasal or interorbital septum; fsu, fenestra superior; gq, gap in quadrate; ic, intercalary; is, interorbital septum; lps, lateral process of paraseptal cartilage; lta, lamina transversalis anterior; Mc, Meckel's cartilage; ns, nasal septum; onf, orbitonasal fissure; op, opisthotic; pacc, pila accesoria; pai, processus alaris inferior; pas, processus alaris superior; pat, ascending process of tectum; pf, pituitary fossa; pla, planum antorbitale; pm, pila metoptica; pmp, posterior maxillary process; pnc, paranasal cartilage; ppl, posterior process of planum antorbitale; pro, prootic; ps, planum supraseptale; psc, paraseptal cartilage; ptc, parietotectal cartilage; q, quadrate; rp, rostral process; rpp, rostral process of planum antorbitale; sc, sphenethmoid commisure; si, subiculum infundibuli; slps, secondary lateral process of paraseptal cartilage; so, supraoccipital; t, trabecula; tc, trabecula communis; te, tectum; tm, taenia medialis; tma, taenia marginalis; tp, trabecular process of acrochordal cartilage. Scale bar: 0.5 mm.
Stage 40
In this stage, the major changes correspond to the development of the orbitosphenoid (os) in the orbitotemporal region (Fig. 6). This element begins to form by ossification of the anterior part of the pila metoptica near the region of junction between this cartilage, the taenia medialis and the pila accesoria. Likewise, in the occipital region the posterior bases of the bifurcated trabecula ossify forming the trabecular crest.
Fig. 6.
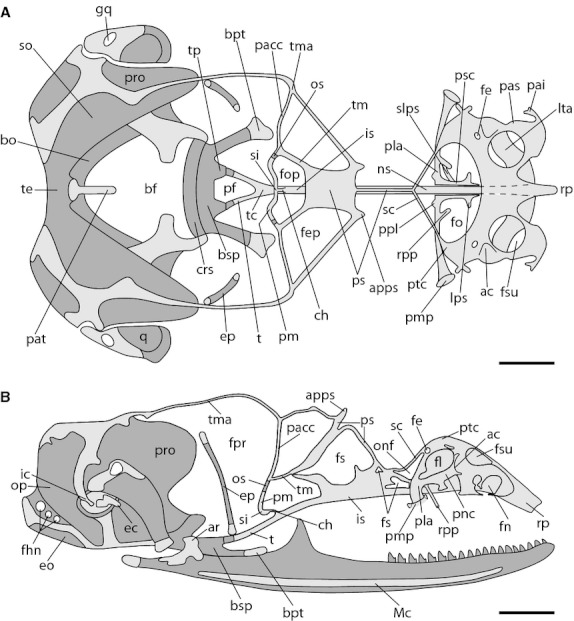
Chondrocranium of Ptychoglossus bicolor at stage 40. Dashed lines denote subjacent cartilage and dark-gray structures denote ossified cartilage. (A) Dorsal view. (B) Lateral view. Abbreviations: ac, aditus conchae; apps, anterior process of planum supraseptale; ar, articular; bf, basicranial fenestra; bo, basioccipital; bpt, basipterygoid process; bsp, basisphenoid; ch, cartilago hypochiasmatica; crs, crista sellaris; ec, extracolumella; eo; exoccipital; ep, epipterygoid; fe, foramen epiphaniale; fep, fenestra epioptica; fhn, hypoglossal foramina; fl, fenestra lateralis; fn, fenestra narina; fo, fenestra olfactoria; fop, fenestra optica; fpr, fenestra prootica; fs, fenestra in nasal or interorbital septum; fsu, fenestra superior; gq, gap in quadrate; ic, intercalary; is, interorbital septum; lps, lateral process of paraseptal cartilage; lta, lamina transversalis anterior; Mc, Meckel's cartilage; ns, nasal septum; onf, orbitonasal fissure; op, opisthotic; os, orbitosphenoid; pacc, pila accesoria; pai, processus alaris inferior; pas, processus alaris superior; pat, ascending process of tectum; pf, pituitary fossa; pla, planum antorbitale; pm, pila metoptica; pmp, posterior maxillary process; pnc, paranasal cartilage; ppl, posterior process of planum antorbitale; pro, prootic; ps, planum supraseptale; psc, paraseptal cartilage; ptc, parietotectal cartilage; q, quadrate; rp, rostral process; rpp, rostral process of planum antorbitale; sc, sphenethmoid commisure; si, subiculum infundibuli; slps, secondary lateral process of paraseptal cartilage; so, supraoccipital; t, trabecula; tc, trabecula communis; te, tectum; tm, taenia medialis; tma, taenia marginalis; tp, trabecular process of acrochordal cartilage. Scale bar: 0.5 mm.
Neonate
In this developmental stage, the chondrocranium of Ptychoglossus bicolor resembles the condition of the adult stage (Fig. 7).
Fig. 7.
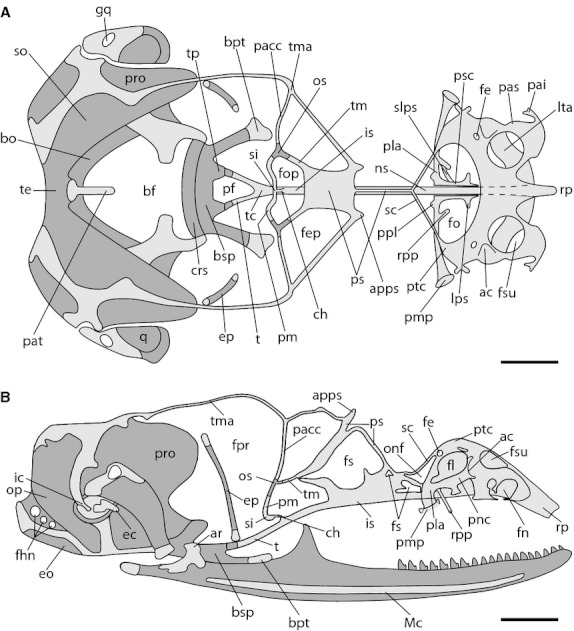
Neonatal chondrocranium of Ptychoglossus bicolor. Dashed lines denote subjacent cartilage and dark-gray structures denote ossified cartilage. (A) Dorsal view. (B) Lateral view. Abbreviations: ac, aditus conchae; apps, anterior process of planum supraseptale; ar, articular; bf, basicranial fenestra; bo, basioccipital; bpt, basipterygoid process; bsp, basisphenoid; ch, cartilago hypochiasmatica; crs, crista sellaris; ec, extracolumella; eo; exoccipital; ep, epipterygoid; fe, foramen epiphaniale; fep, fenestra epioptica; fhn, hypoglossal foramina; fl, fenestra lateralis; fn, fenestra narina; fo, fenestra olfactoria; fop, fenestra optica; fpr, fenestra prootica; fs, fenestra in nasal or interorbital septum; fsu, fenestra superior; gq, gap in quadrate; ic, intercalary; is, interorbital septum; lps, lateral process of paraseptal cartilage; lta, lamina transversalis anterior; Mc, Meckel's cartilage; ns, nasal septum; onf, orbitonasal fissure; op, opisthotic; os, orbitosphenoid; pa, planum antorbitale; pacc, pila accesoria; pai, processus alaris inferior; pas, processus alaris superior; pat, ascending process of tectum; pf, pituitary fossa; pla, planum antorbitale; pm, pila metoptica; pmp, posterior maxillary process; pnc, paranasal cartilage; ppl, posterior process of planum antorbitale; pro, prootic; ps, planum supraseptale; psc, paraseptal cartilage; ptc, parietotectal cartilage; q, quadrate; rp, rostral process; rpp, rostral process of planum antorbitale; sc, sphenethmoid commisure; si, subiculum infundibuli; slps, secondary lateral process of paraseptal cartilage; so, supraoccipital; t, trabecula; tc, trabecula communis; te, tectum; tm, taenia medialis; tma, taenia marginalis; tp, trabecular process of acrochordal cartilage. Scale bar: 0.5 mm.
In the ethmoid region, the cartilages of the nasal capsule exhibit an advanced development. The parietotectal cartilage roofs the elements of the nasal capsule; it is continuous medially with the nasal septum and laterally with the paranasal cartilage. Dorsally, a large rostral fenestra superior and a small posterior foramen epiphaniale are present over the parietotectal cartilage on each side. The sidewall of the capsule is pierced on both flanks of the skull by two large openings, which lie on opposite extremes, an anterior fenestra narina and a posterior fenestra lateralis. The anterior region of the fenestra narina (fn) shows the processus alaris inferior and processus alaris superior. The nasal capsule is divided medially by a high slender nasal septum, which is continuous posteriorly with the interorbital septum. The backside of the nasal capsule consists of the planum antorbitale, which forms a very thin and low cartilaginous wall. Additionally, the middle portion of the planum antorbitale bears two paired processes on its base, one short posterior process, and one long and slender anterior process; the latter projects rostrally in an oblique direction until it contacts the dorsal aspect of the vomer (vo). Medially, the planum antorbitale is continuous with the paired paraseptal cartilages, which run anteriorly and parallel to the nasal septum, bearing one short rostral process at the anterior tip. An additional process, namely the secondary lateral process of paraseptal cartilage (slps), was also found connecting the paraseptal cartilage with the rostral process of the planum antorbitale, thus forming a fenestra. Nevertheless, this condition was only documented in the right side of the skull of some embryos and is depicted as such in Fig. 7. Behind the planum antorbitale, the paired, narrow sphenethmoid commissures outline a large fenestra olfactoria. On each side, the floor of the nasal capsule consists of the lamina transversalis anterior, which is continuous with the anterior sidewall of the paranasal cartilage, forming a complete cartilaginous ring (zona annularis). Also in the lamina transversalis anterior, the cartilage of Jacobson's organ appears medially as well as the ectochoanal cartilage, which looks like a large cartilaginous process projecting posteriorly.
The orbitotemporal region of Ptychoglossus bicolor consists of the planum supraseptale and a scaffolding of narrow cartilaginous bars. The planum supraseptale bends in the midline dividing the structure into two distinct regions: an anterior cylindrical and slender portion, which contacts the interorbital septum at the point where the paired sphenethmoid commissures converge; and a posterior broad shield-shaped region, which contacts the interorbital septum and is posteriorly continuous with the taenia medialis. A short rostrally oriented lateral process arises from both sides of the planum supraseptale, namely the anterior process of the planum supraseptale (apps). The long taenia marginalis contacts the roof of the auditory capsule posteriorly and the lateral margin of the planum supraseptale anteriorly. The interorbital septum is continuous anteriorly with the nasal septum and posteriorly with the trabecula communis. In lateral view, the interorbital septum looks like a thin, high longitudinal wall, pierced by three membrane-filled fenestrae of different size. Moreover, the orbitosphenoid is now a tripartite element, formed by endochondral ossification where the pila accesoria, taenia medialis and pila metoptica converge. The paired pila metoptica contact each other medially forming the subiculum infundibuli, which joins the interorbital septum by means of the cartilago hypochiasmatica. Posteriorly, the two trabeculae contact the ossified trabecular processes at the rostral aspect of the basal plate delineating the pituitary fossa. The epipterygoid appears almost fully ossified, with the epiphyses still cartilaginous.
The occipital region of the chondrocranium of Ptychoglossus bicolor is almost entirely ossified in the neonate, and the prootic, opisthotic, supraoccipital, exoccipital, basioccipital and basisphenoid are present; however, some areas remain cartilaginous and a portion of the basicranial fenestra persists. Posteriorly, the roof of the occipital region derives from the fusion between the tectum posterius and the tectum synoticum of the otic capsule. Therefore, a single tectum (tectum synoticum plus posterius) is formed outlining the dorsal aspect of the foramen magnum; this tectum is also ossified at this stage forming the supraoccipital. The ascending process of tectum rests in the anteriormost margin of the supraoccipital and extends rostrally. The otic capsule is ossified forming the prootic, which is joined ventrally by synchondrosis to the exoccipital and basioccipital, and thus the fissura metotica is completely closed. The cartilaginous footplate of the columella auris rests over the fenestra ovalis, which lies on the sidewall of the otic capsule. Ventrally, the basipterygoid process, which remains cartilaginous at its articular region, rises at each side of the basisphenoid.
The gymnophthalmid chondrocranium
The more generalized gymnophthalmid chondrocranium shares many characteristics with the chondrocranium of the teiid Cnemidophorus lemniscatus (Table 1). Below we summarize: first, the characteristics of the chondrocranium shared by Ptychoglossus bicolor with most (more than 70%) of the other gymnophthalmid species included in this study; and second, the most relevant features of the gymnophthalmid chondrocranium that showed variation among the taxa.
Table 1.
Chondrocranial trait distribution in four subfamilies of gymnophthalmid lizards and their sister taxon
 |
Most of the overall features of the gymnophthalmid chondrocranium correspond to elements of the nasal capsule. 1. In the lateral wall of the nasal capsule, a cartilaginous invagination corresponding to the nasal concha is opened to the outside through the aditus conchae. 2. A long and broad ectochoanal cartilage projects from the lamina transversalis anterior and extends distally near the planum antorbitale. 3. A small foramen epiphaniale is present on each side of the nasal septum near the aditus conchae. 4. As in Cnemidophorus lemniscatus, the nasal septum projects forwards as a rostral process, which varies in size and is largest in Bachia bicolor (Table 1). 5. The paraseptal cartilage is present running parallel to the nasal septum and extending ventrally forming a thin wall; posteriorly it is continuous with the planum antorbitale. 6. The lamina transversalis anterior is wide and large; it contacts the lateral wall of the nasal capsule, it appears to be in contact with the nasal septum thus forming a complete zona annularis. 7. The fenestra superior consists of a large space present in the roof of the nasal capsule. However, the fenestra superior is absent in Leposoma rugiceps, Gymnophthalmus speciosus, Tretioscincus bifasciatus, Vanzosaura rubricauda and C. lemniscatus. 8. The cartilage of Jacobson's organ is well developed. 9. As in C. lemniscatus, a large fenestra lateralis is present. 10. A paired, long, rod-like rostral process projects from the planum antorbitale and abuts the dorsal aspect of the vomer ventrally. This process was found in each of the gymnophthalmid species studied and in C. lemniscatus, but in the latter it is short and fails to reach the vomer, thus it was considered incomplete. In some species (Ptychoglossus bicolor, Leposoma southi and Tretioscincus bifasciatus), this process abuts the vomer, and additionally contacts the paraseptal cartilage laterally forming a fenestra. However, in P. bicolor this condition was not always evident and, when it was, it was always observed only on the left side of the nasal septum. 11. Within Gymnophthalmidae, the size of the fenestra olfactoria (advehens and evehens) was generally large, consistent with the large size of the main and accessory olfactory bulbs of the species studied (pers. obs.). The fenestra olfactoria was smallest in Echinosaura horrida due to the comparatively smaller olfactory bulbs in comparison with the other gymnophthalmid taxa.
Additionally, in the orbitotemporal region of the gymnophthalmid chondrocranium, we find some general features as well. 12. The planum supraseptale bears two laterally oriented wings, which project dorsally and contact the rostral ends of the taenia marginalis, as described by Guerra & Montero (2009) for Vanzosaura rubricauda; these lateral wings are largest in B. bicolor due to a low planum supraseptale given the absence of an interorbital septum. 13. The anteriormost portion of the planum supraseptale consists of a long and slender trough-like process, almost as thin as the sphenethmoid commissures; however, in B. bicolor the planum supraseptale as a whole is very narrow in contrast to the other gymnophthalmids. 14. The sphenethmoid commissures contact the planum supraseptale posteriorly and the nasal capsule rostrally. However, in some species the sphenethmoid commissures fail to reach either the planum supraseptale or the nasal capsule. 15. Dorsally, an anteriorly oriented process projects from the rostral border of the lateral wings of the planum supraseptale.
Ossification sequence
The sequence of ossification events is presented in Table 2. Ossification starts in stage 35 embryos with four dermal elements: prefrontal; maxilla; jugal; and pterygoid. In more advanced stages, the elements of the braincase form by endochondral ossification of the prootic, opisthotic, supraoccipital, exoccipital, basioccipital and basisphenoid, all of which are joined by synchondrosis. At stage 39–40 the mandible starts to ossify. The orbitosphenoid and lacrimal are, respectively, the last chondral and dermal bones to begin the ossification process. Later, in the neonate dermatocranium, all of the elements show distinct degrees of ossification, but most are almost completely formed (Fig. 8). The frontal (fr) and parietal (pa) bones are not yet fully differentiated (i.e. only the margins show some ossification); therefore, a large frontoparietal fenestra (fpf; frontoparietal fontanelle) remains open.
Table 2.
Ossification sequence of the skull of Ptychoglossus bicolor
 |
Fig. 8.
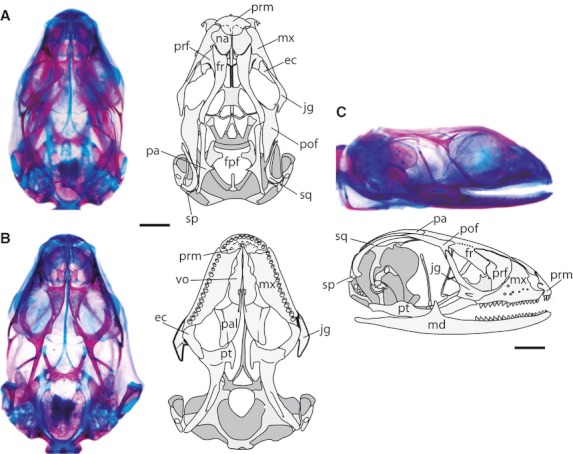
Neonatal dermatocranium of Ptychoglossus bicolor. (A) Dorsal view. (B) Ventral view. (C) Lateral view. Abbreviations: ec, ectopterygoid; fpf, frontoparietal fenestra; fr, frontal; jg, jugal; md, mandible; mx, maxilla; na, nasal; pa, parietal; pal, palatine; pof, postorbitofrontal; prf, prefrontal; prm, premaxilla; pt, pterygoid; sp, supratemporal; sq, squamosal; vo, vomer. Scale bar: 0.5 mm.
Discussion
Development of the chondrocranium of Ptychoglossus bicolor
In general terms, the development of the skull in Ptychoglossus bicolor follows the pattern observed in other studied lizards (de Beer, 1930; El-Toubi & Kamal, 1959; Kamal & Abdeen, 1972; Skinner, 1973; Jerez, 2007). Initially, at stage 31, some cartilages from the three regions of the chondrocranium are present as well as from the viscerocranium. The elements of the dermatocranium appear later at stage 35. Finally, in the neonate the cranium resembles the adult stage, showing a complete skull without any reduction or loss of cranial elements. The nasal capsule in the ethmoid region of the chondrocranium is complete and complex, the elements of the cartilaginous scaffolding in the orbitotemporal region are connected to each other, and in the occipital region the braincase exhibits the typical conformation in lizards. The dermatocranium is complete, and only shows the fusion of the postorbital and postfrontal bones. Thus, P. bicolor conserves the characteristics of lacertiform terrestrial lizards, which is very interesting as this species exhibits terrestrial to semi-fossorial habits, and can build galleries when put in captivity in a terrarium with soft soil and litter (pers. obs.). However, the development of some chondrocranial structures is different from other lizard species that have been well characterized. These characteristics could be common within Gymnophthalmidae, but similar studies are necessary in other species within this clade.
Below we compare the characteristics of the embryonic development of the chondrocranium of Ptychoglossus bicolor to other lizards whose development of this structure has been studied in depth.
In lizards the basipterygoid processes usually originate either from the pterygoid process of the pterygoquadrate complex, as observed in Trachylepis capensis, or from the posterior ends of the trabeculae, as observed in Lacerta agilis and Acanthodactylus boskianus (de Beer, 1930; Kamal & Abdeen, 1972; Skinner, 1973). Nonetheless, in Ptychoglossus bicolor the basipterygoid processes are formed from the acrochordal cartilage (posterior orbital cartilage). Therefore, at least three different patterns in the development of the basipterygoid processes have been described in Squamata. This is very interesting considering that this structure is always present in the squamate skull and is functionally important for cranial kinesis in protraction and retraction movements (Metzger, 2002).
Furthermore, we find variation with respect to other species in the development of the viscerocranium. In Ptychoglossus bicolor all of the elements of the viscerocranium develop concomitantly with the neurocranium, resembling the condition of the turtle Caretta caretta (Kuratani, 1999). In contrast, in Chalcides ocellatus, Acanthodactylus boskianus and Trachylepis capensis, only Meckel's cartilage and the pterygoquadrate complex develop at the same time as the neurocranium (El-Toubi & Kamal, 1959; Kamal & Abdeen, 1972; Skinner, 1973). More differences are observed in Lacerta vivipara and Calotes versicolor, in which Meckel's cartilage chondrifies much earlier than the neurocranium (de Beer, 1937; Ramaswami, 1946). There is also diversity in the timing of development of the elements of the viscerocranium. In P. bicolor, Chalcides ocellatus and Mabuya sp., the quadrate is separated from the other elements of the pterygoquadrate complex at the earliest stage analyzed (El-Toubi & Kamal, 1959; Jerez, 2007). In contrast, in other lizard taxa like Ptyodactylus hasselquistii, A. boskianus and T. capensis, the quadrate is continuous with the pterygoquadrate complex via the intermediate element from the very first stages analyzed (El-Toubi & Kamal, 1961; Kamal & Abdeen, 1972; Skinner, 1973). This indicates that there are probably some heterochronic factors affecting the ontogenetic formation of the viscerocranium with respect to the chondrocranium.
In regard to the palatoquadrate complex, in Ptychoglossus bicolor the quadrate is separated from the complex at the earliest stage analyzed, and the intermediate element is greatly reduced. This situation is similar to the one observed in Mabuya sp. and Chalcides ocellatus (El-Toubi & Kamal, 1959; Jerez, 2007). In contrast, in Ptyodactylus hasselquistii, Acanthodactylus boskianus and Trachylepis capensis (El-Toubi & Kamal, 1961; Kamal & Abdeen, 1972; Skinner, 1973), the quadrate is continuous with the pterygoquadrate complex via the intermediate element from the first stages analyzed.
Bellairs & Kamal (1981) suggested that generally in lizards the taenia marginalis arises from the back of the planum supraseptale and then elongates to contact the otic capsule. Conversely, de Beer (1930) observed the taenia marginalis projecting rostrally from the otic capsule to the planum supraseptale in Lacerta vivipara. However, in P. bicolor the taenia marginalis originates independently near the planum supraseptale, and then elongates posteriorly and rostrally to contact the otic capsule and the planum supraseptale. This pattern is somewhat similar to Trachylepis capensis (Skinner, 1973). This diversity indicates that there is more than one pattern regarding the development of the taenia marginalis. Further evidence may throw light on the variations of this pattern in other lizards, given that we see such great variation in the morphogenesis of the orbital cartilages within the chondrocranium. This condition becomes relevant when observing burrowing species, i.e. Anguis fragilis, Aniella pulchra and Acontias meleagris (Bellairs & Kamal, 1981), where reductions in the chondrocranium compromise primarily cartilages from the orbitotemporal region.
Bellairs & Kamal (1981) suggested that frequently the cochlear part of the otic capsule is attached to the basal plate in lizards. This is observed in the development of the chondrocranium in Acanthodactylus boskianus and Trachylepis capensis (Kamal & Abdeen, 1972; Skinner, 1973). However, in Ptychoglossus bicolor the otic capsule originates independently, starting with the cochlear portion, which is formed from a chondrification center separated from the basal plate (parachordal). It would be interesting to investigate whether this pattern is observed in other gymnophthalmid species, which would indicate a diagnostic character.
The gymnophthalmid chondrocranium
In general terms, the gymnophthalmids included in this study exhibit a complete chondrocranium without any reduction or loss of elements associated with microhabitat use, unlike burrowing lizards such as Anguis fragilis, Aniella pulchra and Acontias meleagris (Bellairs & Kamal, 1981). Therefore, the gymnophthalmid chondrocranium more resembles the chondrocranium of terrestrial lacertiform lizards. Despite this similarity, the gymnophthalmid lizards also exhibit unique characteristics. First, the nasal capsule is characterized by more developed nasal cartilages like the parietotectal and paranasal cartilages, which widen anteriorly and laterally; second, the presence of a large fenestra superior in the anterior dorsal region; third, the presence of a complete zona annularis formed ventrally by a large lamina transversalis anterior; and fourth, a well-developed cartilage of Jacobson's organ. Additionally, the gymnophthalmid nasal capsule is wider than long, in comparison with Cnemidophorus lemniscatus, which shows a simplified nasal capsule without a fenestra superior similar to Tupinambis (Bellairs & Kamal, 1981). The greater development of the nasal cartilages in gymnophthalmids (see above) is probably related to the well-developed olfactory bulbs and vomeronasal organ. This characteristic might play an important role in exploiting different microhabitats like the leaf litter and the underground, which in turn may have favored the great diversification observed within gymnophthalmids inhabiting the Andean and Amazonian forests. Other chondrocranial features, like the nasal septum, are more developed in gymnophthalmids than in teiids. For example, the rostral process of the nasal septum observed in Cnemidophorus lemniscatus, Ameiva undulata, Teius teyou and Aspidoscelis sexlineata (Malan, 1946; Bellairs & Kamal, 1981) is much more simplified than in gymnophthalmids where it is more developed and is the largest in the fossorial lizard Bachia bicolor (Table 1). The presence of a large rostral process in the snake-like B. bicolor probably has no functional role as it is absent in other snake-like lizards such as Aniella pulchra, Anguis fragilis and Acontias meleagris (Bellairs & Kamal, 1981), although biomechanical studies are necessary to directly test this hypothesis.
Within Gymnophthalmidae, the size of the fenestra olfactoria (advehens and evehens) is generally larger than in Cnemidophorus lemniscatus. This is consistent with the large size of the main and accessory olfactory bulbs of the gymnophthalmid species studied here. The fenestra olfactoria is smallest in Echinosaura horrida, due to the comparatively smaller olfactory bulbs in this species than in the other gymnophthalmid taxa. Scleroglossans are generally active foragers, which use a combination of vision and vomerolfaction (Cooper, 1994; Vitt & Pianka, 2005). In addition to the gymnophthalmids, this group includes lacertids, scincids, anguids, helodermatids and varanids, which all show a medium to large fenestra olfactoria and probably exhibit well-developed olfactory bulbs (Bellairs & Kamal, 1981; Schwenk, 1993; Bernstein, 1999). In contrast, ambush-foraging iguanians like chamaeleonids, polychrotids, iguanids and agamids generally use visual detection to locate prey (Cooper, 1994; Vitt & Pianka, 2005). As a consequence, the sensory olfactory apparatus in this latter group is poorly developed and the chondrocranium exhibits a fenestra olfactoria that is comparatively very small or absent (Bellairs & Kamal, 1981).
In the orbitotemporal region of the gymnophthalmid chondrocranium, we find some generalized features as well. The planum supraseptale is very thin anteriorly and posteriorly it is wide, forming a shield. This condition is also observed in C. lemniscatus and in scincids (Bellairs & Kamal, 1981). There are reductions of some orbitotemporal cartilages in Bachia bicolor including a very thin planum supraseptale, and the absence of the sphenethmoid commissures and interorbital septum. Similar reductions are observed in other serpentiform lizards like Anguis fragilis, Aniella pulchra and Acontias meleagris (Bellairs & Kamal, 1981).
The orbitosphenoid of Ptychoglossus bicolor is formed by endochondral ossification and begins to ossify during late embryonic development (Stage 40). This process starts with the ossification of the anterior surface of the pila metoptica and then extends into a portion of the taenia medialis and pila accesoria, forming a small triradiate bone. The orbitosphenoid of Bachia bicolor, Elgaria coerulea, Liolaemus scapularis and L. quilmes also appears at late embryonic stages (Good, 1995; Lobo et al. 1995; Abdala et al. 1997; Jerez, 2007; Tarazona & Ramírez-Pinilla, 2008).
There is significant variation concerning the orbitotemporal cartilages involved in the development of the orbitosphenoid. For instance, the orbitosphenoid is formed by ossification of the pila metoptica in some terrestrial lizards such as Trachylepis capensis, Liolaemus scapularis, Stenocercus guentheri and Mabuya sp. (de Beer, 1937; Skinner, 1973; Lobo et al. 1995; Torres-Carvajal, 2003; Jerez, 2007). Within the gymnophthalmid species studied here (Table 1; traits 24, 28 and 37), there is great variation in the orbitosphenoid and we observed four different patterns of development.
In the first type, the orbitosphenoid is formed by ossification of the anterior surface of the pila metoptica, and then extends into a portion of the taenia medialis and pila accesoria, forming a small triradiate bone. This is observed in 50% of the species studied here, including Ptychoglossus bicolor, P. vallensis, P. festae, Alopoglossus copii, Anadia ocellata, A. bogotensis, Cercosaura argulus and Cnemidophorus lemniscatus. This has also been observed in the fossorial snake-like gymnophthalmids Nothobachia ablephara and Scriptosaura catimbau (Roscito & Rodrigues, 2010). The fact that this condition is observed in the three Ptychoglossus species, Alopoglossus copii and C. lemniscatus might indicate that this feature is observed within Gymnophthalmidae due to common ancestry and that it evolved independently in different clades like Anadia. This pattern of development is probably not associated with ecology due to the fact that these species are generally found inhabiting the ground, leaf-litter, buried and under or in rotting logs, while at the same time arboreal taxa like Anadia ocellata exhibit the same pattern (Oftedal, 1974; Castro-Herrera et al. 2007; Anaya-Rojas et al. 2010; Layche et al. 2010).
On the other hand, 40% of the taxa showed a second pattern in which the orbitosphenoid forms by ossification of a portion of the pila metoptica and taenia medialis. These species include Cercosaura ampuedae, Echinosaura horrida, Riama striata, Pholidobolus montium, Leposoma rugiceps, Leposoma southi, Iphisa elegans and Tretioscincus bifasciatus. These terrestrial species generally inhabit the leaf-litter and some exploit semi-aquatic microhabitats (Ruthven & Gaige, 1924; Avila-Pires, 1995; Ortega-Andrade, 2006; pers. obs.). It would be interesting to evaluate whether the condition of the orbitosphenoid in these species is related to their life habits, as they belong to non-basal groups within gymnophthalmids.
A third type of orbitosphenoid formation corresponds to the condition of the orbitosphenoid in Vanzosaura rubricauda, which is formed only by the ossification of the taenia medialis (Guerra & Montero, 2009). This condition is interesting as this species is widely distributed in open areas within various habitats and substrates. Nevertheless, because only a few gymnophthalmid species have been studied, it remains unclear whether this condition in Vanzosaura rubricauda is common or rare in this family.
The last type of orbitosphenoid development we observed was found in the fossorial snake-like Bachia bicolor. In this species the orbitosphenoid is markedly broader than that of all other gymnophthalmids studied, as it involves the ossification of the taenia medialis and pila metoptica, as well as two–three additional orbitotemporal cartilages. These latter cartilages are hardly comparable to orbitotemporal cartilages such as the pila accesoria and pila antotica (Tarazona & Ramírez-Pinilla, 2008). The broad orbitosphenoid of B. bicolor resembles both structurally and ontogenetically the orbitosphenoid of amphisbaenians, and thus it has been hypothesized that the morphology and origin of this bone is related to fossorial habits (Tarazona & Ramírez-Pinilla, 2008). Nevertheless, the orbitosphenoid of other snake-like fossorial gymnophthalmids such as Scriptosaura catimbau and Nothobachia ablephara resembles that in Ptychoglossus bicolor. This is probably because these two species are fossorial but show adaptations to life in sand (Roscito & Rodrigues, 2010).
The condition of the pila antotica within Gymnophthalmidae is also variable. This cartilage can be long or short, and it is always separated from the basal plate. However, it may also be absent, as in Bachia bicolor and Ptychoglossus bicolor. In the latter species, the pila antotica is absent even in early stages of development. This cartilaginous bar is also absent in Plestiodon fasciatus, Cordylus sp., Acontias meleagris, Bradypodion pumilum, Anguis fragilis, Aniella pulchra, Agama mutabilis and Ptyodactylus hasselquistii (Rice, 1920; Brock, 1941; Van-Pletzen, 1946; Bellairs & Kamal, 1981). Furthermore, the pila antotica may appear early during embryonic development and disappear in later stages, as is the case in Plestiodon latiscutatus (Rice, 1920). This situation indicates that the condition of the pila antotica is variable among groups and is subject to change during ontogeny.
Ossification sequence of Ptychoglossus bicolor
The ossification of the skull of Ptychoglossus bicolor consists of two different processes: the development of the dermatocranium and the ossification of the chondrocranium. In Ptychoglossus bicolor the pterygoid is the first or among the first bones to ossify in the skull. This occurs also in Trachylepis capensis, Liolaemus scapularis, L. quilmes, Tupinambis rufescens, T. merianae, Lacerta agilis, Elgaria coerulea and Liopholis whitii (Skinner, 1973; Rieppel, 1994; Good, 1995; Lobo et al. 1995; Abdala et al. 1997; Arias & Lobo, 2006; Hugi et al. 2010). On the other hand, the lacrimal, postorbital and postfrontal bones are among the last elements of the dermatocranium to start to ossify during embryonic development, similar to the condition in Tupinambis merianae, T. rufescens, Liolaemus scapularis and L. quilmes (Lobo et al. 1995; Abdala et al. 1997; Arias & Lobo, 2006). The last dermal bones to fully differentiate are generally those from the frontoparietal region, which remain incompletely differentiated in the neonate (Maisano, 2001; Jerez, 2007; Tarazona et al. 2008). This suggests that the sequence of onset and termination of the ossification of the dermatocranium is somewhat conserved among the lizards studied so far. In P. bicolor the postorbitofrontal ossifies as a single bone. This is also the case in the gymnophthalmid Calyptommatus nicterus (Roscito & Rodrigues, 2010), whereas in other species like Euspondylus acutirostris, Potamites ecpleopus, Vanzosaura rubricauda, Nothobachia ablephara and Scriptosaura catimbau, the postfrontal and postorbital bones are distinct (Montero et al. 2002; Bell et al. 2003; Guerra & Montero, 2009; Roscito & Rodrigues, 2010).
The size of the frontoparietal fontanelle in the neonate lizard is variable, and depends upon the differentiation of the frontals and parietals. Thus, this fenestra may be large, small or closed at the time of birth (Maisano, 2001). Herein, we found that a large frontoparietal fontanelle was characteristic of Ptychoglossus bicolor neonates. This condition is similar to the neonate skull of Bachia bicolor (Tarazona et al. 2008). However, in Potamites ecpleopus the ossification of the frontals and parietals is more advanced (Maisano, 2001). Within Scleroglossa, the size of the neonate frontoparietal fontanelle varies from small to large, and closes early during postnatal ontogeny, unlike in Iguania where the frontoparietal fenestra closes in adult stages (Maisano, 2001; Torres-Carvajal, 2003). Particularly, the neonates of serpentiform burrowing species (Acontias meleagris, Aniella pulchra and Bipes biporus) exhibit a fully differentiated skull roof, apparently without any traces of the frontoparietal fontanelle (Bellairs & Kamal, 1981; Maisano, 2001; Torres-Carvajal, 2003). In this sense, the accelerated ossification of the dermatocranium in specialized burrowers is characteristic of scleroglossans, and represents an association between variation in body form at the cranial level and microhabitat use (Bellairs & Kamal, 1981). Nonetheless, this is not the pattern observed in Ptychoglossus bicolor and the fossorial snake-like gymnophthalmids Calyptommatus nicterus, Scriptosaura catimbau and Nothobachia ablephara (Tarazona et al. 2008; Roscito & Rodrigues, 2010). This may be due to the fact that these snake-like gymnophthalmid species inhabit soft sandy soils, and exhibit a small and complete skull, without drastic transformations.
Conclusions
The fully formed chondrocranium of Ptychoglossus bicolor and the overall ossification sequence pattern of the skull resemble that of other lizard species, especially that of teiids. However, certain features regarding the development of the chondrocranium during early embryogenesis, such as the development of the basipterygoid processes from the acrochordal cartilage, the early ossification of the crista sellaris and the formation and ossification of the trabecular processes also from the acrochordal cartilage, differ from other reptilian embryos described in the literature.
When looking at the gymnophthalmid orbitosphenoid we find different states in respect to its origin and the orbitotemporal cartilages that comprise this bone, with Vanzosaura rubricauda at one extreme and Bachia bicolor at the other. In most of the other gymnophthalmid species, including Ptychoglossus bicolor (Table 1), we see an intermediate state, suggesting that there might be a tendency within Gymnophthalmidae towards the expansion of the orbitosphenoid by means of the ossification of neighboring orbitotemporal cartilages. These variations in the orbitosphenoid are probably related to the gymnophthalmid life habits, which vary from terrestrial to fossorial species; comparative studies that incorporate this feature could explain whether it evolved in response to microhabitat use.
The nasal capsule of Ptychoglossus bicolor and the other gymnophthalmids included in this work is considered here as complete and structurally complex. This characteristic of the nasal capsule is related to a well-developed cartilage of Jacobson's organ and big olfactory bulbs. These morphological features as a whole are probably related to the fact that scleroglossans use the olfactory and vomeronasal organs for prey detection and discrimination (Cooper, 1994; Pianka & Vitt, 2003; Vitt & Pianka, 2005). This contrasts with iguanian lizards with arboreal habits, in which the nasal capsule is relatively smaller and simplified showing reduction and loss of elements (Bellairs & Kamal, 1981), which is probably related to poorly developed olfactory and vomeronasal senses and visual prey detection (Cooper, 1994; Vitt & Pianka, 2005).
Acknowledgments
Special thanks to V. Páez, J. M. Daza from the Universidad de Antioquia, and M. Calderón from the Universidad Nacional de Colombia, for the loan of gymnophthalmid specimens. We thank R. Montero, F. Leal, O. Tarazona and two anonymous reviewers who helped with valuable suggestions and comments on the manuscript. D. Moen for his comments on the language. We also thank L. Meza, E. Ramos, F. Cediel and J. Lozano for their assistance collecting some Ptychoglossus bicolor embryos. The people from the Hacienda El Roble kindly allowed us to collect specimens in the coffee plantation. The Corporación para la Defensa de la Meseta de Bucaramanga (CDMB) provided the collecting permits.
Author contributions
All of the authors contributed equally to the production of this work.
References
- Abdala F, Lobo F, Scrocchi G. Patterns of ossification in the skeleton of Liolaemus quilmes (Iguania: Tropiduridae) Amphib-Reptil. 1997;18:75–83. [Google Scholar]
- Anaya-Rojas JM, Serrano-Cardozo VH, Ramírez-Pinilla MP. Diet, microhabitat use, and thermal preferences of Ptychoglossus bicolor (Squamata, Gymnophthalmidae) in an organic coffee shade plantation in Colombia. Pap Avulsos Zool. 2010;50:159–166. [Google Scholar]
- Arias F, Lobo F. Patrones de osificación en Tupinambis merianae y Tupinambis rufescens (Squamata, Teiidae) y patrones generales en Squamata. Cuad Herpetol. 2006;20:3–23. [Google Scholar]
- Avila-Pires TCS. Lizards of Brazilian Amazonia (Reptilia: Squamata) Zool Verh. 1995;299:1–706. [Google Scholar]
- Barros FC, Herrel A, Kohlsdorf T. Head shape evolution in Gymnophthalmidae: does habitat use constrain the evolution of cranial design in fossorial lizards? J Evol Biol. 2011;24:2423–2433. doi: 10.1111/j.1420-9101.2011.02372.x. [DOI] [PubMed] [Google Scholar]
- de Beer GR. The early development of the chondrocranium of the lizard. Q J Microsc Sci. 1930;73:707–739. [Google Scholar]
- de Beer GR. The Development of the Vertebrate Skull. Oxford: Clarendon Press; 1937. p. 552. [Google Scholar]
- Bell CJ, Evans SE, Maisano JA. The skull of the gymnophthalmid lizard Neusticurus ecpleopus (Reptilia: Squamata) Zool J Linn Soc. 2003;139:283–304. [Google Scholar]
- Bellairs Ad'A, Kamal AM. The chondrocranium and the development of the skull in recent reptiles. In: Gans C, Parsons T, editors. Biology of the Reptilia, vol. 11. Morphology F. New York: Academic Press; 1981. pp. 1–263. [Google Scholar]
- Bernstein P. Morphology of the nasal capsule of Heloderma suspectum with comments on the systematic position of helodermatids (Squamata: Helodermatidae) Acta Zool. 1999;80:219–230. [Google Scholar]
- Brock GT. The skull of Acontias meleagris, with a study of the affinities between lizards and snakes. Zool J Linn Soc. 1941;41:71–88. [Google Scholar]
- Castoe TA, Doan TM, Parkinson CL. Data partitions and complex models in bayesian analysis: the phylogeny of gymnophthalmid lizards. Syst Biol. 2004;53:448–469. doi: 10.1080/10635150490445797. [DOI] [PubMed] [Google Scholar]
- Castro-Herrera F, Bolívar-García W, Herrera-Montes MI. Guía de anfibios y reptiles del bosque de Yotoco, Valle del Cauca, Colombia. Cali: Grupo de Investigación Laboratorio de Herpetología, Universidad del Valle; 2007. p. 70. [Google Scholar]
- Cooper WE., Jr Chemical discrimination by tongue-flicking in lizards: a review with hypotheses on its origin and its ecological and phylogenetic relationships. J Chem Ecol. 1994;20:439–487. doi: 10.1007/BF02064449. [DOI] [PubMed] [Google Scholar]
- Donnelly MA, MacCulloch RD, Ugarte CA, et al. A new riparian gymnophthalmid (Squamata) from Guyana. Copeia. 2006;2006:396–403. [Google Scholar]
- Dufaure JP, Hubert J. Table de développement du lézard vivipare: Lacerta (Zootoca) vivipara Jaquin. Arch Anat Micr Morph Exp. 1961;50:309–328. [Google Scholar]
- El-Toubi MR, Kamal AM. The development of the skull of Chalcides ocellatus. I. The development of the chondrocranium. J Morphol. 1959;104:269–306. doi: 10.1002/jmor.1051040205. [DOI] [PubMed] [Google Scholar]
- El-Toubi MR, Kamal AM. The development of the skull of Ptyodactylus hasselquistii. I. The development of the chondrocranium. J Morphol. 1961;108:63–94. doi: 10.1002/jmor.1051080204. [DOI] [PubMed] [Google Scholar]
- Good DA. Cranial ossification in the northern alligator lizard, Elgaria coerulea (Squamata, Anguidae) Amphib-Reptil. 1995;16:157–166. [Google Scholar]
- Guerra C, Montero R. The skull of Vanzosaura rubricauda (Squamata: Gymnophthalmidae) Acta Zool. 2009;90:359–371. [Google Scholar]
- Hugi J, Mitgutsch C, Sánchez-Villagra MR. Chondrogenic and ossification patterns and sequences in White's skink Liopholis whitii (Scincidae, Reptilia) Zoosyst Evol. 2010;86:21–32. [Google Scholar]
- Jerez A. Desarrollo del Plan Corporal en Mabuya mabouya. Argentina: Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán; 2007. Doctoral dissertation. [Google Scholar]
- Kamal AM, Abdeen AM. The development of the chondrocranium of the lacertid lizard, Acanthodactylus boskiana. J Morphol. 1972;137:289–334. doi: 10.1002/jmor.1051370304. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Development of the chondrocranium of the loggerhead turtle, Caretta caretta. Zool Sci. 1999;16:803–818. [Google Scholar]
- Layche JF, Ribeyro BO, Acosta A. Herpetofauna en bosque de varillal del centro de investigación y enseñanza forestal (CIEFOR) – puerto Almendras, Iquitos – Perú. Acta Biol Herrer. 2010;1:35–42. [Google Scholar]
- Lobo F, Abdala F, Scrocchi GJ. Desarrollo del esqueleto de Liolaemus scapularis (Iguania: Tropiduridae) Boll Mus reg Sci nat Torino. 1995;13:77–104. [Google Scholar]
- Maisano JA. A survey of state of ossification in neonatal squamates. Herpetol Monogr. 2001;15:135–157. [Google Scholar]
- Malan ME. Contributions to the comparative anatomy of the nasal capsule and the organ of Jacobson of the Lacertilia. Annale Univ Stellenbosch. 1946;24:69–137. [Google Scholar]
- Metzger KA. Cranial kinesis in lepidosaurs: skulls in motion. In: Aerts P, Herrel A, D'Aout K, Van Damme R, editors. Topics in Vertebrate Functional and Ecological Morphology. Maastricht: Shaker; 2002. pp. 15–46. [Google Scholar]
- Montero R, Moro SA, Abdala V. Cranial anatomy of Euspondylus acutirostris (Squamata: Gymnophthalmidae) and its placement in a modern phylogenetic hypothesis. Russ J Herpetol. 2002;9:215–228. [Google Scholar]
- Oftedal OT. A revision of the genus Anadia (Sauria, Teiidae) Arq Zool S Paulo. 1974;25:203–265. [Google Scholar]
- Ortega-Andrade HM. Reptilia, Squamata, Gymnophthalmidae, Echinosaura horrida: distribution extension and new geographic distribution map for Ecuador. Check List. 2006;2:2–4. [Google Scholar]
- Pellegrino KCM, Rodrigues MT, Yonenaga-Yassuda Y, et al. A molecular perspective on the evolution of microteiid lizards (Squamata, Gymnophthalmidae), and a new classification for the family. Biol J Linn Soc. 2001;74:315–338. [Google Scholar]
- Pianka ER, Vitt LJ. Lizards: Windows to the Evolution of Diversity. 1st edn. Berkeley: University of California Press; 2003. p. 348. [Google Scholar]
- Ramaswami LS. The chondrocranium of Calotes versicolor (Daud.) with a description of the osteocranium of a just-hatched young. Q J Microsc Sci. 1946;87:237–297. [PubMed] [Google Scholar]
- Rice EL. The development of the skull in the skink Eumeces quinquelineatus. J Morphol. 1920;34:120–243. [Google Scholar]
- Rieppel O. Studies on skeleton formation in Reptiles. Patterns of ossification in the skeleton of Lacerta agilis exigua Eichwald (Reptilia, Squamata) J Herpetol. 1994;28:145–153. [Google Scholar]
- Roscito JG, Rodrigues MT. Comparative cranial osteology of fossorial lizards from the tribe Gymnophthalmini (Squamata, Gymnophthalmidae) J Morphol. 2010;271:1352–1365. doi: 10.1002/jmor.10878. [DOI] [PubMed] [Google Scholar]
- Ruthven AG, Gaige HT. A new Leposoma from Panama. Occas Pap Mus Zool Univ Mich. 1924;147:1–3. [Google Scholar]
- Schwenk K. Are geckos olfactory specialists? J Zool Lond. 1993;229:289–302. [Google Scholar]
- Skinner MM. Ontogeny and adult morphology of the skull of the South African skink, Mabuya capensis (Gray) Ann Univ Stellenbosch. 1973;48:1–116. [Google Scholar]
- Tarazona OA, Ramírez-Pinilla MP. The unusual orbitosphenoid of the snakelike lizard Bachia bicolor. J Anat. 2008;213:120–130. doi: 10.1111/j.1469-7580.2008.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazona OA, Fabrezi M, Ramírez-Pinilla MP. Cranial morphology of Bachia bicolor (Squamata, Gymnophthalmidae) and its postnatal development. Zool J Linn Soc. 2008;152:775–792. [Google Scholar]
- Torres-Carvajal O. Cranial osteology of the Andean lizard Stenocercus guentheri (Squamata: Tropiduridae) and its postembryonic development. J Morphol. 2003;255:94–113. doi: 10.1002/jmor.10051. [DOI] [PubMed] [Google Scholar]
- Uetz P, Goll J, Hallermann J. The reptile database. 2011. http://www.reptile-database.org, accessed February 5, 2011.
- Van-Pletzen R. The cranial morphology of Cordylus with special reference to cranial kinesis. Ann Uni Stellenbosch. 1946;24:41–68. [Google Scholar]
- Vitt LJ, Pianka ER. Deep history impacts present-day ecology and biodiversity. Proc Natl Acad Sci USA. 2005;102:7877–7881. doi: 10.1073/pnas.0501104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassersug RJ. A procedure for differential staining of cartilage and bone in whole formalin-fixed vertebrates. Stain Technol. 1976;51:131–134. doi: 10.3109/10520297609116684. [DOI] [PubMed] [Google Scholar]


