Abstract
Adaptation to constant vibration (acoustic oscillation) is likely to confer a specific morphology at the bone–tendon and bone–ligament interfaces at the ear ossicles, which therefore represent an exciting target of enthesis research. We histologically examined (i) the bone attachments of the tensor tympani and stapedius muscles and (ii) the annular ligament of the incudostapedial joint obtained from seven elderly donated cadavers. Notably, both aldehyde-fuchsin and elastic-Masson staining demonstrated that the major fibrous component of the entheses was not collagen fibers but mature elastic fibers. The positive controls for elastic fiber staining were the arterial wall elastic laminae included in the temporal bone materials. The elastic fibers were inserted deeply into the type II collagen-poor fibrocartilage covering the ear ossicles. The muscle tendons were composed of an outer thin layer of collagen fibers and an inner thick core of elastic fibers near the malleus or stapes. In the unique elastic fiber-mediated entheses, hyaluronan, versican and fibronectin were expressed strongly along the elastic fibers. The hyaluronan seemed to act as a friction-reducing lubricant for the elastic fibers. Aggrecan was labeled strongly in a disk- or plica-like fibrous mass on the inner side of the elastic fiber-rich ligament, possibly due to compression stress from the ligament. Tenascin-c was not evident in the entheses. The elastic fiber-mediated entheses appeared resistant to tissue destruction in an environment exposed to constant vibration. The morphology was unlikely to be the result of age-related degeneration.
Keywords: ear ossicles, elastic fibers, enthesis, human anatomy, hyaluronan, stapedius muscle, tensor tympani muscle
Introduction
At bone–tendon and bone–ligament interfaces, or entheses, collagens show a type-dependent and site-specific distribution, and various glycosaminoglycans and proteoglycans are also expressed in a region-specific manner (Waggett et al. 1998; Benjamin & McGonagle, 2001; Benjamin et al. 2006). A typical example is the type II collagen that is restricted to fibrocartilage in order to receive type I collagen fibers from a ligament or tendon, a morphology considered to allow accommodation of both tensile and compressive stress. In their excellent review, Milz et al. (2005) summarized variations of proteoglycans for adaptation to mechanical stress; under conditions of gradually intensifying compression stress, versican decreases and aggrecan becomes prominent. Similarly, all parts of the human scapholunate interosseous ligament are labeled for type I, III and VI collagens, versican and tenascin, but the attached fibrocartilage expresses only type I collagen and aggrecan (Milz et al. 2006). Such localization is more evident in the triangular fibrocartilage complex of the human wrist: aggrecan and type II collagen are restricted to the radial side of the fibrocartilage complex (Milz et al. 2007). In the middle ear, there are two muscle insertions to the ear ossicles and several ligaments reinforcing the articulations among the malleus, incus and stapes bones. However, studies of the entheses of the ear ossicles have been very limited (see the paragraph below), despite their unique features that have allowed them to adapt to the constant vibrations or acoustic oscillation conducted from the tympanic membrane. Moreover, these two muscle insertions have evolved to dampen excess vibration resulting from violent noise levels.
Ohashi et al. (2008) demonstrated that, in the rat ear, the most medial part of the middle ear entheses (the annular ligament of the stapediovestibular joint that fixes the stapes to the vestibular window of the temporal bone) contains abundant hyaluronan (hyaluronic acid) and fibrillin. Fibrillin is essential for formation of elastic fibers, but Ohashi et al. did not confirm the presence of elastin or elastic fibers at the joint, especially at the enthesis. In fact, some joint capsules in the human body are known to contain elastic fibers, for example at the facet joint of the vertebral column (Shiraishi et al. 2003) and at the temporomandibular joint (Caltabiano et al. 1990). However, knowledge of the hyaluronan content of the joint capsule is less extensive than might be expected. Another well known structure that shows adaptation to vibration is the vocal fold. Notably, the lamina propria of the human vocal fold is characterized by an abundance of both elastin and hyaluronan (Hahn et al. 2006; Yamashita et al. 2010). The amounts vary according to age and gender (Hammond et al. 1997; Korn et al. 2011). Likewise, both elastin and hyaluronan are predominant in the extracellular matrix of the syrinx in songbirds (Riede & Goller, 2010). Therefore, for adaptation to vibration, many or some parts of the middle ear entheses are likely to contain both elastin and hyaluronan.
In the present study, using materials obtained from elderly donated cadavers, our aim was to examine histologically the entheses at and along the ear ossicles, including (i) attachments of the tensor tympani and stapedius muscles and (ii) the annular ligament of the incudostapedial joint. Among the ossicle articulations, the incudostapedial joint seems to demonstrate the greatest capacity for motion in accordance with the shapes of the bones, i.e. it is a ball attached to a shallow socket, similar to the glenohumeral joint of the shoulder. Thus, the mechanical stress imposed on the capsule (i.e. the annular ligament) may also be potentially considerable. Because of the suggested need to withstand vibration (see above), we considered that identification of elastic fibers, as well as hyaluronan, should be included in this study. To identify elastic fibers, we chose both aldehyde-fuchsin and elastica-Masson staining.
Materials and methods
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined paraffin-embedded sections of the ear ossicles obtained from seven donated cadavers (age 78–93 years; mean 85 years; four males and three females). The cause of death had been heart failure in all cases. All the cadavers had been donated to Sapporo Medical University for anatomical research and education, and their use for research had been approved by the university ethics committee. The cadavers had been fixed by perfusion with 10% v/v formalin solution and stocked in 50% ethanol solution for more than 3 months.
After removal of the brain, we removed the left or right side of the petrosal portion of the temporal bone from the cadavers. With careful drilling, we removed (i) the malleus along with the attachment of the tensor tympani muscle and (ii) the incudostapedial joint along with the tendon of the stapedius muscle. These small specimens were decalcified by incubating them at 4 °C in 0.5 m EDTA solution (pH 7.5; Decalcifying solution B; Wako, Tokyo, Japan) for 4–5 days. Routine procedures for paraffin-embedded histology (using sections 5 μm thick) were conducted. To provide a good orientation of the topographical anatomy in a two-dimensional section, we tried to create a sectional plane along the tendon or along the long crus of the incus bone, but the planes usually became oblique because of difficulty in maintaining precise positioning of the small specimens (maximum size 3 mm) during histological procedures. In addition, the branches of the external carotid artery were included during removal of the temporal bone for use as a positive control for the two types of elastic fiber staining we employed (see below).
Ten serial sections (5 μm thick) were prepared at 200-μm intervals and stained with (i) hematoxylin and eosin, and also (ii) two types of elastic fiber staining, (iii) immunohistochemistry for matrix substances and (iv) another staining for hyaluronan. In the last case, a biotinylated hyaluronan-binding protein (2 μg mL−1; Seikagaku Corp., Tokyo, Japan) was used after immersion of the sections in chondroitinase ABC (10 μU mL−1; Seikagaku Corp.) in 0.1 m Tris-acetate buffer (pH 8.0, 37 °C) for 30 min (Shibata et al. 2003). To identify elastic fibers, we used two different types of staining: (i) aldehyde-fuchsin with post-fixation after attachment to the glass slide using Bouin's fixative overnight (Fujita, 1959; Sunami-Kataoka et al. 2001) and (ii) elastica-Masson staining (a variation of Masson-Goldner staining; Motohashi et al. 1995; Okada et al. 2002; Hayashi et al. 2010).
The primary antibodies used were (i) mouse monoclonal anti-versican core protein (12C5) from Developmental Studies Hybridoma Bank (Iowa City, IA, USA; dilution 1 : 25); (ii) mouse monoclonal anti-aggrecan core protein (12/21/1C6) from Developmental Studies Hybridoma Bank (dilution 1 : 25); (iii) rabbit polyclonal anti-rat type I collagen (dilution 1 : 400; LSL, Tokyo, Japan); (iv) rabbit polyclonal anti-rat type II collagen (dilution 1 : 400; LSL); (v) rabbit polyclonal anti-rat tenascin-c (dilution 1 : 100; Chemicon, Temecula, CA, USA); and (vi) rabbit polyclonal anti-rat fibronectin (dilution 1 : 400; LSL). These antibodies were used in our previous immunohistochemical studies (Shibata et al. 2003; Nakasone et al. 2007; Yokohama-Tamaki et al. 2011). All sections were treated with testicular hyaluronidase (25 mg mL−1, Sigma type I-S; Sigma Chemicals, St. Louis, MO, USA) in phosphate-buffered saline for 30 min at 37 °C before addition of the primary antibodies. After incubation with the secondary antibody employing a Histofine SAB kit (Nichirei, Tokyo, Japan), the sections were treated with 3-amino-9-ethylcarbazole (AEC) to form the red immunocomplex. Negative controls that were not treated with the first antibody were also prepared.
Results
As a positive control for the two types of staining for elastic fibers, we employed the elastic laminae of arterial walls: these were selectively stained and colored black by elastica-Masson (Fig. 1A) or bright violet by aldehyde-fuchsin (Fig. 1B). In contrast, collagen fibers were stained pale pink by aldehyde-fuchsin or green by elastica-Masson. The latter stained the arterial smooth muscle dark red. Unfortunately, the sectional planes of the small specimens were usually not aligned along the tendon or the long crus of the incus, but were oblique (Fig. 2). Thus, the topographical anatomy was often difficult to interpret. However, the planes of section were good in two of the specimens (the incudostapedial joint and the tensor tympani muscle insertion; Figs 3 and 4). All specimens of the stapedius muscle insertion were cut obliquely (Fig. 5). In addition, oblique sections of the distal part (muscle side) of the tensor tympani tendon are shown in Fig. 6.
Fig. 1.
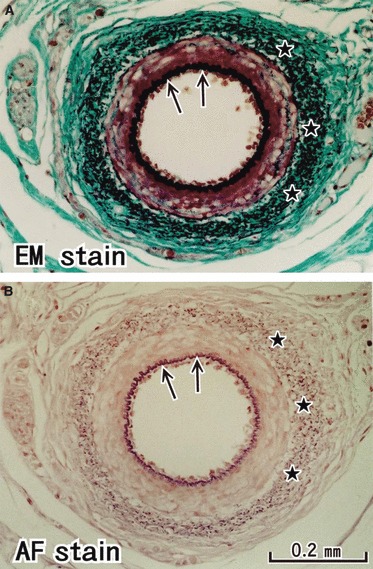
Elastic fibers in the arterial wall: a positive control of the present staining methods. The inner elastic lamina (arrows) of a branch of the external carotid artery is clearly identified as a black line in elastica-Masson staining (panel A) and as a bright violet line in aldehyde-fuchsin staining (panel B). The outer lamina was identified as black or violet dots (stars). These panels are prepared at the same magnification: scale bar in panel B.
Fig. 2.
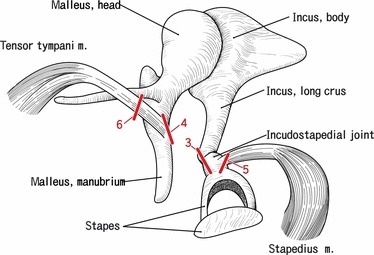
A diagram showing the present sectional planes. Right ear ossicles with attachments of the tensor tympani and stapedius muscles are shown, although in the present study, we used both the left and the right sides of the specimens. Bar with 3, 4, 5 or 6 corresponds to the sectional plane shown in the Figs 3, 4, 5 or 6.
Fig. 4.
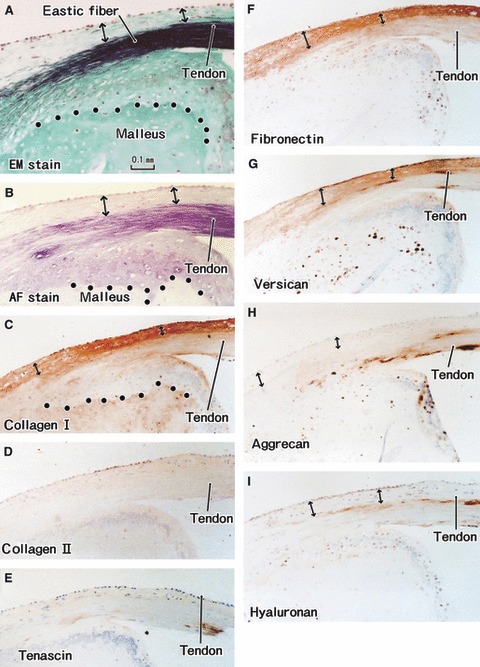
Tendon insertion of the tensor tympani muscle to the malleus. A specimen from an 85-year-old man. All panels are adjacent or near sections. (A) Elastica-Masson staining (EM stain). (B) Aldehyde-fuchsin staining (AF stain). (C) Immunohistochemistry (IHC) for type I collagen. (D) IHC for type II collagen. (E) IHC for tenascin-c. (F) IHC for fibronectin. (G) IHC for versican. (H) IHC for aggrecan. (I) IHC for hyaluronan. (C–I) are prepared at the same magnification (scale bars in A–C). Dotted line in (A–C) indicate the tidemark: elastic fibers insert deeply into the uncalcified fibrocartilage. Type I collagen, fibronectin and versican are expressed in the superficial layer of the tendon (double-headed arrow).
Fig. 5.
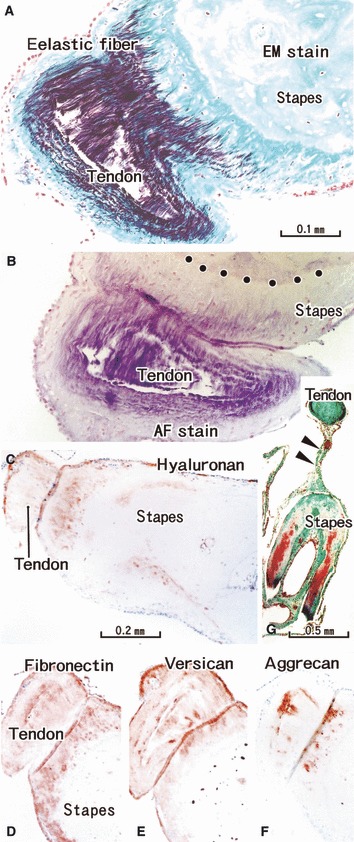
Tendon insertion of the stapedius muscle to the stapes. A specimen from an 81-year-old man (the same specimen as shown in Fig. 3). (A,G) Elastica-Masson staining (EM stain). (B) Aldehyde-fuchsin staining (AF stain). (C) Immunohistochemistry (IHC) for hyaluronan. (D) IHC for fibronectin. (E) IHC for versican. (F) IHC for aggrecan. (A–F) Near sections. (A,B) (or C–F) are prepared at the same magnification (scale bars in panels A, C and G). (G) A mesentery-like structure to fix the tendon (arrowheads): it contains no elastic fibers. Dotted line in (B,C) indicate the tidemark: elastic fibers insert deeply into the uncalcified fibrocartilage. Hyaluronan, fibronectin, versican and aggrecan appear to be expressed in the same area of the tendon and cartilage.
Fig. 6.
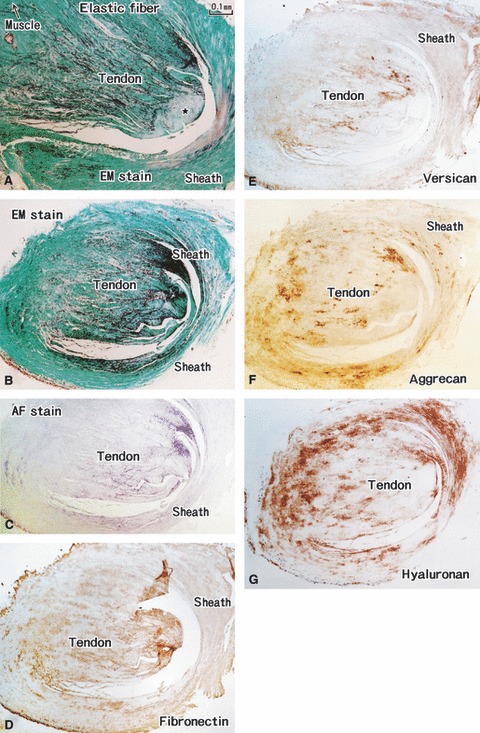
Distal parts of the tensor tympani muscle tendon. A specimen from an 85-years-old man (the same specimen as shown in Fig. 4). (A,B) Elastica-Masson staining (EM stain). (C) Aldehyde-fuchsin staining (AF stain). (D) Immunohistochemistry (IHC) for fibronectin. (E) IHC for versican. (F) IHC for aggrecan. (G) IHC for hyaluronan. All panels are prepared at the same magnification (scale bar in A). (A) Muscle–tendon interface of the tensor tympani muscle. (B–G) Adjacent or near sections. At the muscle–tendon interface, elastic fibers converge to a collagenous core (star in A); near the insertion, the fibers gradually occupy almost all cut surface of the tendon (B,C). The tendon sheath also contains abundant elastic fibers. Hyaluronan, versican and fiibronectin display a laminar distribution between the elastic fibers (D–F). Many aggrecan-positive spots are seen in the sheath of the tendon (G).
Both aldehyde-fuchsin and elastica-Masson staining demonstrated that the annular ligament of the incudostapedial joint contained abundant elastic fibers (Fig. 3A,B). The former staining clearly demonstrated the tidemark between the calcified and uncalcified cartilages (Fig. 3B). The annular ligament was lined superficially by a thin layer containing type 1 collagen, but almost all of the ligament was composed of elastic fibers (Fig. 3A,C). With a spray-like configuration, the elastic fibers were inserted deeply into the uncalcified fibrocartilage covering the incus and stapes (Fig. 3A,B). The elastic fibers did not pass through the tidemark to penetrate into the calcified tissue. Notably, type II collagen expression was weak in the fibrocartilage (Fig. 3D). Versican, fibronectin and hyaluronan were present among the elastic fibers (Fig. 3F,G,I). Aggrecan was labeled strongly in a disk- or plica-like fibrous mass on the inner side of the elastic fiber-rich ligament (Fig. 3H). In addition, spotty expression of aggrecan was seen at the base of the ligament near the tidemark. Tenascin expression was not evident in the ligament or its enthesis (Fig. 3E).
Fig. 3.
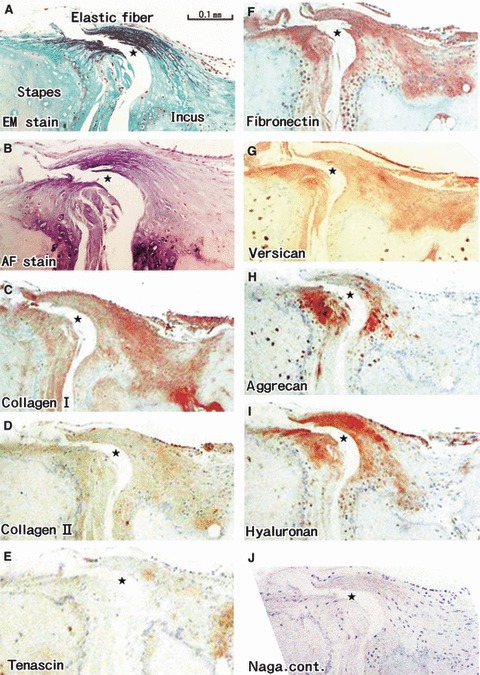
Annular ligament of the incudostapedial joint. A specimen from an 81-year-old man. All panels are adjacent or near sections. The ligament was accidentally cut during the histological procedure. Thus, the joint cavity (star) looks larger than the original. (A) Elastica-Masson staining (EM stain). (B) Aldehyde-fuchsin staining (AF stain). (C) Immunohistochemistry (IHC) for type I collagen. (D) IHC for type II collagen. (E) IHC for tenascin-c. (F) IHC for fibronectin. (G) IHC for versican. (H) IHC for aggrecan. (I) IHC for hyaluronan. (J) Negative control without the challenge of the first antibody. (A,B) (or C–J) are prepared at the same magnification (scale bars in A and C). The tidemark is clearly seen in (B): elastic fibers insert deeply into the uncalcified fibrocartilage. Note that fibronectin, versican and hyaluronan are expressed along the elastic fibers.
Both aldehyde-fuchsin and elastic-Masson staining demonstrated that the tendons of the tensor tympani and stapedius muscles contained abundant elastic fibers (Figs 4–6). Moreover, a laminar distribution of matrix substances was seen in the tensor tympani muscle tendon (Fig. 4). At the bony insertion of the tensor tympani muscle, the superficial layer, continuous with the periosteum-like tissue of the ear ossicles, was composed of type I collagen (Fig. 4C). Similarly to the annular ligament, the elastic fibers were inserted deeply into the uncalcified fibrocartilage covering the malleus or stapes (Figs 4 and 5). In contrast, however, versican and fibronectin were labeled in the superficial layer of the tensor tympani tendon (Fig. 4F,G). Hyaluronan exhibited linear expression between the superficial layer and the elastic fiber-rich core (Fig. 4I). An aggrecan-positive layer was seen in the flexion or bony side of the tendon (Fig. 4H). In the stapedius muscle tendon, the distribution patterns of the matrices were slightly different. Although, similarly to the annular ligament, hyaluronan, versican and fibronectin appeared to coexist among the elastic fibers (Fig. 5C–F), no longitudinal section along the tendon was obtained for the stapedius muscle.
The elastic fibers appeared in the center of the tendons immediately before the entrance into the tympanic cavity, and the amount was increased at sites nearer the bone insertion, finally occupying almost the entire tendon except for the thin superficial layer (Figs 5 and 6). The muscles themselves contained no elastic fibers: the endomysium appeared continuous with an elastic fiber. In the tendon distant from the bone, elastic fibers initially converged to a collagenous core (Fig. 6A) and then gradually occupied almost the entire cut surface of the tendon (Fig. 6B,C). Hyaluronan, versican and fibronectin displayed a laminar distribution between the elastic fibers (Fig. 5D,E,G). Aggrecan-positive spots were intermingled with the elastic fibers but most of the spots were located in the sheath of the tendon (Fig. 6F). The tendon sheath of the tensor tympani muscle contained elastic fibers (Fig. 6A–C), whereas near the bone, that of the stapedius did not (Fig. 5A,B). However, in the distal part of the latter, which was connected with the stapes by a mesentery-like structure containing vessels (Fig. 5G), elastic fibers tended to distribute at the surface.
In the ossicles distant from the entheses, versican and fibronectin were widely expressed along the periosteum and perichondrium, as well as in peninsula-like soft areas protruding into the bone along the nutrient vessels. Type II collagen-positive areas were not evident in the periosteum and perichondrium, but only in the peninsula-like areas. Tenascin-positive spots were also found along the vessels in the peninsula-like areas. Aggrecan was labeled in most of the osteocytes and some chondrocytes. Finally, hyaluronan expression was restricted to the entheses (see above). Although we prepared a negative control by omitting the first antibody each time immunohistochemistry was performed, only one representative photo is shown in Fig. 3J.
Discussion
The present study seems to be the first to have specifically investigated entheses that are made up of mature elastic fibers inserted into the uncalcified fibrocartilage and ending before the tidemark. To our knowledge, only one previously published photo has shown a similar morphology in the ligamentum flavum attachment to the vertebral arch in the human adult (Viejo-Fuertes et al. 1998; Yayama et al. 2007). However, in contrast to the type II collagen-poor fibrocartilage in the middle ear entheses, the fibrocartilage at the ligamentum flavum attachment expresses the collagen (Yayama et al. 2007) usually seen in entheses (see Introduction). Although Hough (1958) often or sometimes found absence, ossification and variation in length or attachment site of the stapedius muscle in his surgical mobilization cases of the bone, we did not found such variations in the present materials: one reason may be related to the fact that potential donors of the present study included only a few auditory handicapped individuals.
Mature elastic fibers can be identified using either aldehyde-fuchsin or elastica-Masson staining. However, these methods are not able to identify elastin (composite protein of elastic fibers) or immature elastic fibers such as oxytalan and elaunin (Cotta-Pereira et al. 1976). Therefore, there is a possibility that elastin or immature fibers might have been distributed in the calcified cartilage as well as in the middle ear muscles. Moreover, because of the difficulty in handling such small specimens, only a few of the sections we prepared clearly showed the topographical anatomy of the middle ear entheses. Nevertheless, we believe that we are able to discuss the present observations, especially the colocalization of elastic fibers with other matrix substances. The morphology we observed was unlikely to have resulted from age-related degeneration because, in human fetuses at 25–30 weeks of gestation, we found abundant elastic fibers in the tendons of the tensor tympani and stapedius muscles, as well as around the incudostapedial joint (Murakami G, unpublished data). In contrast to the human pelvic floor muscles (DeLancey, 2002; Hirata et al. 2011a, b), the middle ear muscles did not carry elastic fibers either in the epimysium or the perimysium. Conversely, elastic fibers were restricted to the entheses. Thus, our discussion is able to focus on ‘elastic fiber-mediated enthesis’.
The most striking feature of the matrix distribution seemed to be the presence of hyaluronan among elastic fibers. The co-presence of elastic fibers and hyaluronan in the human vocal fold is well known (Hammond et al. 1997; Hahn et al. 2006; Yamashita et al. 2010; Korn et al. 2011), although the vocal fold elastic elements are composed predominantly of elaunin and oxytalan fibers (Hammond et al. 1997). The combination of elastic component and hyaluronan has been considered to be an adaptation to vibration of the vocal fold. In the human body, the same combination of substances has also been reported in the annular and cruciform pulleys of the finger (Katzman et al. 1999), the extensor retinaculum of the wrist and ankle (Klein et al. 1999), and the skin dermis (Rodrigo & Cotta-Pereira, 1979; Frei et al. 1998; Veiga et al. 2011). Hyaluronan acts as a lubricant in the joint cavity, and a similar role in reducing friction stress between inner ear cells has been reported (Anniko & Arnold, 1995). Hyaluronan has recently been used as a scaffold for regeneration of joint cartilage (Fan et al. 2010; Jakobsen et al. 2010). In this context, for wound healing of the vocal fold, favorable restoration of both elastin and hyaluronan is necessary for prevention of scarring after injury (Ohno et al. 2012). In the field of bioengineering, a positive role of elastic fibers and hyaluronan in combination for repair of the aortic wall has also been reported (Gacchina & Ramamurthi, 2011), as well as in the nucleus pulposus of the intervertebral disc (Moss et al. 2011). In the middle ear entheses, the constant vibration or oscillation from the tympanic membrane seems to facilitate differentiation of the elastic fiber-hyaluronan combination, which in turn appears to prevent tissue destruction resulting from such vibration.
Hyaluronan usually forms a complex with aggrecan or versican (Matsumoto et al. 2006): the present results suggest that in the middle ear entheses, hyaluronan most likely combines with versican. In contrast, aggrecan expression was restricted to a plica-like structure in the icudostapedial joint, possibly for adaptation to compression stress. It has been reported that versican colocalizes with both elastin and hyaluronan in the heart mitral valve (Stephens et al. 2010) and vascular smooth muscle under conditions of inflammation (Kuznetsova et al. 2006). Finally, like the situation in the middle ear entheses, quadruple colocalization of versican, fibronectin, elastin and hyaluronan has been reported in human airway cartilages (Johnson, 2001) and the trabecular meshwork of the corneoscleral junction in the human eye (Ueda & Yue, 2003). A common feature of all these structures is that they are composed of strong but soft tissues receiving multidirectional mechanical stress. Unfortunately, we were unable to obtain detailed information about the composite matrices of the ligamentum flavum attachment (see also the first paragraph), which are often associated with age-related calcification (Nakamura et al. 1990; Viejo-Fuertes et al. 1998; Yayama et al. 2007).
Osteopontin, an acidic matrix protein expressed mainly in mineralized tissues, is a constitutive component of normal elastic fibers (Baccarani-Contri et al. 1995), and thus elastic fibers facilitate calcification (reviewed by Perrotta et al. 2011). Moreover, osteopontin is involved in the generation of T-lymphocyte subpopulations that are responsible for various autoimmune diseases (reviewed by Scatena et al. 2007; Uede, 2011). The enthesis is known to be a target of inflammation, especially when immunity is disturbed (reviewed by Benjamin & McGonagle, 2001; Milz et al. 2005) and, as a result, the elastic fiber-mediated enthesis becomes calcified, in contrast to its usual collagen-rich composition. However, some reports of such pathology in ear ossicles may exist. One possibly relevant feature is the low expression of tenascin-c in normal middle ear entheses. Tenascin is known to be expressed under conditions of degeneration or repair (Pfander et al. 2004; Nakoshi et al. 2010). Goepel (2008) has reported an interesting correlation between elastin and tenascin in the intrapelvic connective tissue: greater amounts of tenascin and smaller amounts of elastin were found in patients with pelvic organ prolapse. Further investigations will be necessary to clarify this relationship in the middle ear entheses under conditions of inflammation.
Finally, we should discuss the ‘insertion angle change’ of the tendon or ligament to the bone during the joint movement because it exerts great stress on the enthesis (Benjamin et al. 2006). Notably, according to a finite-element model of the ear ossicle articulations (Koike et al. 2002), positional changes at the incudostapedial joint seems to be < 0.1° in the joint angle (angle between the two bones) and < 1 μm in the sliding distance. Such small changes are never seen in any other joints in the human body. Therefore, the insertion angle changes of the middle ear muscle tendons are also likely to be very small. This fact may be one of major reasons why the elastic fiber-mediated enthesis can exist in the middle ear, in addition to the constant vibration stress. Although the present results included a region-specific manner of elastic fiber distribution in the tendons, we can't deny the possibility of individual variations: actually, a study limitation was the small number of specimens. However, the small insertion angle change may allow variations.
Acknowledgments
We are grateful to Mr. Hiroyuki Oosugi (Department of Anatomy, Okayama University School of Medicine) for his assistance with elastic fiber staining.
References
- Anniko M, Arnold W. Hyaluronic acid as a molecular filter and friction-reducing lubricant in the human inner ear. ORL J Otorhinolaryngol Relat Spec. 1995;57:82–86. doi: 10.1159/000276716. [DOI] [PubMed] [Google Scholar]
- Baccarani-Contri M, Taparelli F, Pasquail-Ronchetti I. Osteopontin is a constitutive component of normal elastic fibers in human skin and aorta. Matrix Biol. 1995;14:553–560. doi: 10.1016/s0945-053x(05)80004-6. [DOI] [PubMed] [Google Scholar]
- Benjamin M, McGonagle D. The anatomical basis for disease localization in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Toumi H, Ralphs JR, et al. Where tendons and ligaments meet bone: attachment sites (enthesis) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltabiano M, Caltabiano C, Martinez G, et al. Histology of the cartilage of the human fetal condyle. Mondo Ortod. 1990;15:439–442. (Italian with English abstract) [PubMed] [Google Scholar]
- Cotta-Pereira G, Guerra Rodrigo F, Bittencourt-Sampaio S. Oxytalan, elaunin, and elastic fibers in the human skin. J Invest Dermatol. 1976;66:143–148. doi: 10.1111/1523-1747.ep12481882. [DOI] [PubMed] [Google Scholar]
- DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93–98. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- Fan H, Tao H, Wu Y, et al. TGF-β3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid scaffold for cartilage regeneration. J Biomed Mater Res A. 2010;95:982–992. doi: 10.1002/jbm.a.32899. [DOI] [PubMed] [Google Scholar]
- Frei V, Perrier E, Orly I, et al. Activation of fibroblast metabolism in a dermal and skin equivalent model: a screening test for activity of peptides. Int J Cosmet Sci. 1998;20:159–173. doi: 10.1046/j.1467-2494.1998.171748.x. [DOI] [PubMed] [Google Scholar]
- Fujita T. Histological studies on the neuro-insular complex in the pancreas of some mammals. Z Zellforsch Mikrosk Anat. 1959;50:94–109. [Google Scholar]
- Gacchina CE, Ramamurthi A. Impact of pre-existing elastic matrix on TGFbeta1 and HA oligomer-induced regenerative elastin repair by rat aortic smooth muscle cells. J Tissue Eng Regen Med. 2011;5:85–96. doi: 10.1002/term.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepel C. Differential elastin and tenascin immunolabeling in the uterosacral ligaments in postmenopausal women with and without pelvic organ prolapse. Acta Histochem. 2008;110:204–209. doi: 10.1016/j.acthis.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Hahn MS, Kobler JB, Starcher BC, et al. Quantitative and comparative studies of the vocal fold extracellular matrix. 1. Elastin and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006;115:156–164. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- Hammond TH, Zhou R, Hammond EH, et al. The intermediate layer: a morphologic study of the elastin and hyaluronic acid constituents of normal human vocal folds. J Voice. 1997;11:59–66. doi: 10.1016/s0892-1997(97)80024-0. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kumasaka T, Mitani K, et al. Loss of heterozygosity tuberous sclerosis complex genes in multifocal micronodular pneumatocyte hyperplasia. Mod Pathol. 2010;23:1251–1260. doi: 10.1038/modpathol.2010.114. [DOI] [PubMed] [Google Scholar]
- Hirata E, Fujiwara H, Hayashi S, et al. Intergender difference in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011a;24:469–477. doi: 10.1002/ca.21042. [DOI] [PubMed] [Google Scholar]
- Hirata E, Koyama M, Murakami G, et al. A comparative histological study of the level 1–3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011b;37:13–23. doi: 10.1111/j.1447-0756.2010.01298.x. [DOI] [PubMed] [Google Scholar]
- Hough JVD. Malformations and anatomical variations seen in the middle ear during the operation for mobilization of the stapes. Laryngoscope. 1958;68:1337–1379. doi: 10.1288/00005537-195808000-00001. [DOI] [PubMed] [Google Scholar]
- Jakobsen RB, Shahdadfar A, Reinholt FP, et al. Chondrogenesis in a hyaluronic acid scaffold: comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg Sports Traumatol Arthrosc. 2010;18:1407–1416. doi: 10.1007/s00167-009-1017-4. [DOI] [PubMed] [Google Scholar]
- Johnson PR. Role of human airway smooth muscle in altered extracellular matrix production in asthma. Clin Exp Pharmacol Physiol. 2001;28:233–236. doi: 10.1046/j.1440-1681.2001.03426.x. [DOI] [PubMed] [Google Scholar]
- Katzman BM, Klein DM, Garven TC, et al. Comparative histology of the annular and cruciform pulleys. J Hand Surg Br. 1999;24:272–274. doi: 10.1054/jhsb.1999.0069. [DOI] [PubMed] [Google Scholar]
- Klein DM, Katzman BM, Mesa JA, et al. Histology of the extensor retinaculum of the wrist and the ankle. J Hand Surg Am. 1999;24:799–802. doi: 10.1053/jhsu.1999.0799. [DOI] [PubMed] [Google Scholar]
- Koike T, Wada H, Kobayashi T. Modeling of the human middle ear using the finite-element method. J Acoust Soc Am. 2002;111:1306–1317. doi: 10.1121/1.1451073. [DOI] [PubMed] [Google Scholar]
- Korn GP, Martins JR, Park SW, et al. Concentration of hyaluronic acid in human vocal folds in young and old subjects. Otolaryngol Head Neck Surg. 2011;145:981–986. doi: 10.1177/0194599811419457. [DOI] [PubMed] [Google Scholar]
- Kuznetsova SA, Issa O, Perruccio EM, et al. Versican-thrombospondin-1 binding in vitro and colocalization in microfibrils induced by inflammation on vascular smooth muscle cells. J Cell Sci. 2006;119:4499–4509. doi: 10.1242/jcs.03171. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Kamiya N, Suwan K, et al. Identification and characterization of versican/PG-M aggregates in cartilage. J Biol Chem. 2006;281:18257–18263. doi: 10.1074/jbc.M510330200. [DOI] [PubMed] [Google Scholar]
- Milz S, Benjamin M, Putz R. Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol. 2005;178:1–71. [PubMed] [Google Scholar]
- Milz S, Aktas T, Putz R, et al. Expression of extracellular matrix molecules typical of articular cartilage in the human scapholunate interosseous ligament. J Anat. 2006;208:671–679. doi: 10.1111/j.1469-7580.2006.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milz S, Sicking B, Sprecher CM, et al. An immunohistcohemical study of the triangular fibrocartilage complex of the wrist: regional variations in cartilage phenotype. J Anat. 2007;211:1–7. doi: 10.1111/j.1469-7580.2007.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss IL, Gordon L, Woodhouse KA, et al. A novel thiol-modified hyaluronan and elastin-like polypeptide composite material for tissue engineering of the nucleus pulposus of the intervertebral disc. Spine. 2011;36:1022–1029. doi: 10.1097/BRS.0b013e3181e7b705. [DOI] [PubMed] [Google Scholar]
- Motohashi O, Suzuki M, Shida N, et al. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Acta Neurochir. 1995;136:88–91. doi: 10.1007/BF01411441. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hashimoto N, Maeda Y, et al. Degeneration and ossification of the yellow ligament in unstable spine. J Spinal Disord. 1990;3:288–292. [PubMed] [Google Scholar]
- Nakasone A, Shibata S, Suzuki S, et al. Laser burn wound healing in naso-labial region of fetal and neonatal mice. Oral Dis. 2007;13:45–50. doi: 10.1111/j.1601-0825.2006.01245.x. [DOI] [PubMed] [Google Scholar]
- Nakoshi Y, Hasegawa M, Akeda K, et al. Distribution and role of tenascin-C in human osteoarthritic cartilage. J Orthop Sci. 2010;15:666–673. doi: 10.1007/s00776-010-1513-x. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Ide S, Sawaguchi A, et al. Histochemical localization of the extracellular matrix components in the annular ligament of rat stapediovestibular joint with special reference to fibrillin, 36-kDa microfibril-associated glycoprotein (MAGP-36), and hyaluronic acid. Med Mol Morphol. 2008;41:28–33. doi: 10.1007/s00795-007-0394-3. [DOI] [PubMed] [Google Scholar]
- Ohno S, Hirano S, Kanemaru S, et al. Transforming growth factor β3 for the prevention of vocal fold scaring. Laryngoscope. 2012;122:583–589. doi: 10.1002/lary.22389. [DOI] [PubMed] [Google Scholar]
- Okada R, Arima K, Kawai M. Arterial changes in cerebral autosomal dominant arteriopathy with subbcortical infarcts and leukoencephalopathy (CADASIL) in relation to pathogenesis of diffuse myelin loss of cerebral white matter. Stroke. 2002;33:2565–2569. doi: 10.1161/01.str.0000032620.91848.1c. [DOI] [PubMed] [Google Scholar]
- Perrotta I, Russo E, Camastra C, et al. New evidence for a critical role of elastin in calcification of native heart valves: immunohistochemical and ultrastructural study with literature review. Histopathology. 2011;59:504–513. doi: 10.1111/j.1365-2559.2011.03977.x. [DOI] [PubMed] [Google Scholar]
- Pfander D, Heinz N, Rothe P, et al. Tenascin and aggrecan expression by articular chondrocytes is influenced by interleukin 1β: a possible explanation for the changes in matrix synthesis during osteoarthritis. Ann Rheum Dis. 2004;63:240–244. doi: 10.1136/ard.2002.003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Goller F. Functional morphology of the sound-generating labia in the syrinx of two songbird species. J Anat. 2010;216:23–36. doi: 10.1111/j.1469-7580.2009.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo FG, Cotta-Pereira G. Connective fibers involved in the dermoepidermal anchorage. An electron microscopical study. Dermatologica. 1979;158:13–23. doi: 10.1159/000250738. [DOI] [PubMed] [Google Scholar]
- Scatena M, Liaw L, Giachelli CM, et al. A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Imai H, et al. In situ hybridization and immunohistochemistry of versican, aggrecan, and link protein and histochemistry of hyaluronan in the developing mouse limb bud cartilage. J Anat. 2003;203:425–432. doi: 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y, Kobayashi M, Yasui M, et al. Innervation and functional characteristics of connective tissues, especially elastic fibers, in human fetal thoracic intervertebral articular capsule and its surroundings. Anat Embryol. 2003;206:437–445. doi: 10.1007/s00429-003-0320-y. [DOI] [PubMed] [Google Scholar]
- Stephens EH, Post AD, Laucirica DR, et al. Perinatal changes in mitral and aortic valve structure and composition. Pediatr Dev Pathol. 2010;13:447–458. doi: 10.2350/09-11-0749-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami-Kataoka Y, Akagi H, Nishizaki K, et al. Chondritin sulfate proteoglycan at the basal lamina beneath high endothelial cells in human palate tonsils: a light and electron microscopic study using the cationic colloidal iron method. Arch Histol Cytol. 2001;64:535–543. doi: 10.1679/aohc.64.535. [DOI] [PubMed] [Google Scholar]
- Ueda J, Yue BY. Distribution of myocilin and extracellular matrix components in the corneoscleral meshwork of human eyes. Invest Ophthalmol Vis Sci. 2003;44:4772–4779. doi: 10.1167/iovs.02-1002. [DOI] [PubMed] [Google Scholar]
- Uede T. Osteopontin intrinsic tissue regulator or intractable inflammatory diseases. Pathol Int. 2011;61:265–280. doi: 10.1111/j.1440-1827.2011.02649.x. [DOI] [PubMed] [Google Scholar]
- Veiga DF, Bussolaro RA, Kobayashi EY, et al. Glycosaminoglycans of abdominal skin after massive weight loss in post-bariatric female patients. Obes Surg. 2011;21:774–782. doi: 10.1007/s11695-011-0405-2. [DOI] [PubMed] [Google Scholar]
- Viejo-Fuertes D, Liguoro D, Rivel J, et al. Morphologic and histologic study of the ligamentum flavum in the thoraco-lumbar region. Surg Radiol Anat. 1998;20:171–176. doi: 10.1007/BF01628891. [DOI] [PubMed] [Google Scholar]
- Waggett A, Kwan A, Woodnutt D, et al. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 1998;16:457–470. doi: 10.1016/s0945-053x(98)90017-8. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Bless DM, Welham NV. Morphological and extracellular matrix changes following vocal fold injury in mice. Cells Tissues Organs. 2010;192:262–271. doi: 10.1159/000315476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayama T, Uchida K, Kobayashi S. Thoracic ossification of the human ligamentum flavum: histopathological and immunohistochemical findings around the ossification lesion. J Neurosurg Spine. 2007;7:184–193. doi: 10.3171/SPI-07/08/184. [DOI] [PubMed] [Google Scholar]
- Yokohama-Tamaki T, Maeda T, Tanaka TS, et al. Functional analysis of CTRP3/cartducin in Meckel's cartilage and developing condylar cartilage in the fetal mouse mandible. J Anat. 2011;218:517–533. doi: 10.1111/j.1469-7580.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


