Abstract
The aim of this study was to visualize and describe the vascular architecture of the vaginal and supravaginal parts of the human uterine cervix. Uteri collected at autopsy (n = 42) were perfused via the afferent vessels with fixative followed by Mercox resin. After polymerization of the resin, corrosion was performed. The obtained vascular casts of the cervix, visualizing all vessels including capillaries, were examined using scanning electron microscopy. Both in the vaginal and supravaginal parts of the cervix, four distinct vascular zones were distinguished – the outer zone containing large arteries and veins, the arteriole and venule zone, the endocervical mucosal capillaries zone and the pericanalar zone containing small veins and capillaries. In the pericanalar zone ran small veins, responsible for draining the mucosal capillaries. Both in the muscular layer, as well as in the pericanalar zone, arterioles and venules passed close to each other, often adjoining. This study introduces the idea of two systems responsible for draining blood from the mucosal capillaries. It is also the first to suggest the possible existence of a countercurrent transport between adjoining veins and arteries.
Keywords: blood vessels, cervix, corrosion-casting, scanning electron microscopy, uterus
Introduction
The uterine cervix receives most of its blood supply from the uterine artery (Pilarczyk et al. 2002). The classic approach divides the course of the uterine artery into three parts – the descending, transverse and ascending. Lateral branches originate mostly from the transverse and ascending parts. Among those branches are also vessels supplying the uterine cervix (Pilarczyk et al. 2002).
Palacios Jaraquemada et al. (2007) reported that blood is supplied to the cervix via the uterine artery, the cervical arteries (in 67% originating from the uterine artery) and the vaginal arteries (originating from the uterine artery or the internal illiac artery).
Due to its unique remodelling capabilities, associated with the menstrual cycle, implantation and pregnancy (Osol & Mandala, 2009), the vascular structure of the uterus has been the target of many studies. The earliest of them date back to the 19th century (Manconi et al. 2010). The microvascular structure of the uterus was also the subject of extensive studies (Walocha et al. 2003; Hamid et al. 2006) but most of the studies dealt with the microvascular structure of the uterine corpus. In contrast to the abundance of studies dealing with the microvasculature of the uterine corpus, there are few studies on the subject of uterine cervix microvasculature (Sekiba et al. 1979).
Thus, the aim of this study was to visualize the microvasculature of the human uterine cervix, using corrosion casting together with scanning electron microscopy (SEM), and to describe the vascular architecture of the vaginal and supravaginal parts of the cervix.
Materials and methods
The material for this study was obtained from women (ages 20–45 years) during autopsies performed at the Department of Forensic Medicine, Jagiellonian University Medical College (JUMC). Women who died due to disorders of the reproductive system were not included in the study. The material was collected not later than 24 h post-mortem. This study was performed using uteri from cadavers of menstruating nulliparas (17 uteri) and menstruating multiparas (25 uteri).
Of the 42 uteri, only 35 were found suitable for SEM assessment. Five were excluded because of decomposition changes and two because of active menstruation at the time of the donors' death. The research protocol was approved by the Jagiellonian University Ethics Committee (registry KBET/121/8/2007).
Each uterus together with the ovaries and the cervical portion of the vagina was removed in such a way that relatively long fragments of uterine and ovarian vessels (both arteries and veins) were retained.
Preparing material for scanning electron microscopy assessment
Immediately after removal, the uteri were perfused via the afferent arteries with prewarmed (37 °C), heparinized saline (Heparin, Polfa, Poland, 12 IU mL−1) containing 3% dextrane (70 kDa) and 0.025% lidocaine (Lignocaine, Polfa) until the fluid outflowing via the veins was completely transparent (5–10 min). Next, perfusion was continued using a solution of 0.66% paraformaldehyde/0.08% glutaraldehyde (Sigma) in 0.1 m cacodylate buffer (pH 7.4) supplemented with 0.2% lidocaine. Finally, the vascular system was injected with 60–80 mL of Mercox CL-2R resin (Vilene Comp. Ltd., Japan) containing 0.0625 mg mL−1 methyl methacrylate with the polymerization initiator (Vilene Comp. Ltd.). The uteri were left in a warm water bath (56 °C) for 12 h to allow polymerization and tempering of the resin (Składzień et al. 1995). After completion of polymerization, the uterine tissues were macerated for 5–6 days by repeated soaking in 10% potassium hydroxide at 37 °C, followed by washing with warm (50–55 °C) running tap water. The obtained vascular casts were washed in several changes of distilled water, cleaned in 5% trichloroacetic acid for 1–2 days, washed again in distilled water for 2–3 days and freeze-dried in a lyophilizer (Liovag G2, Aqua Fina, Germany). Parts of the freeze-dried casts corresponding to the uterine cervix and a small fragment of the uterine body were excised and examined macroscopically. To facilitate sectioning of the casts, they were then embedded at 55 °C in a mixture of polyethylene glycols (PEG 2000/PEG 600, 20 : 1), cooled to room temperature to solidify PEG (Walocha et al. 2002) and gently dissected longitudinally in the plane of the endocervical canal to expose the vasculature of the cervix wall. The sectioned fragments were washed with stirred distilled water to remove PEG and stored in an exsiccator containing phosphorus pentoxide until microscopic examination. The fragments were then mounted onto copper plates using colloidal silver and ‘conductive bridges’ (Lametschwandtner et al. 1980) and coated with gold. The casts were examined in a JEOL SEM 35-CF scanning electron microscope at 20–25 kV. Casts of arteries and arterioles were distinguished from those of veins and venules on the basis of different imprints of endothelial cell nuclei (Miodoński et al. 1976).
Results
Cervical supravaginal vascular architecture
When studying the sagittal sections of the cervix, one could identify densely packed arteries and veins (diameter ∼ 500 μm) located right below the serous membrane. Medially, towards the canal of the cervix, a zone of large vessels (diameter 0.8–1 mm) was found. The vessels were surrounded by empty spaces, most probably places where perivascular connective tissue was located.
At the junction between the supravaginal part of the cervix and the corpus of the uterus, four distinct vascular zones could be distinguished (Fig. 1). Going from lateral to medial (towards the cervix canal) the zones were identified as the:
Fig. 1.
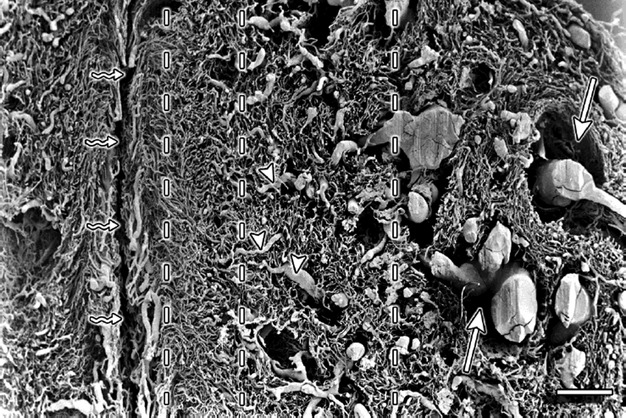
Cervix sagittal section – supravaginal part. Vertical interrupted lines divide the vasculature into four zones. The outermost zone is on the far right of the figure. The straight arrows point to the places where perivascular connective tissue was located. The triangles mark the vessels that run transversely and supply the pericanalar vessels. ‘Serpent’ arrows point to the cervical canal. Scale bar: 1000 μm.
outer zone containing large arteries and veins;
zone of arterioles and venules of the muscular layer, characterized by loose and irregular texture;
zone of endocervical mucosal capillaries, characterized by dense texture (Fig. 2);
pericanalar zone containing small veins and capillaries (Fig. 3).
Fig. 2.
Cervix sagittal section – supravaginal part. The straight arrows mark the zone of endocervical mucosal capillaries. Scale bar: 1000 μm.
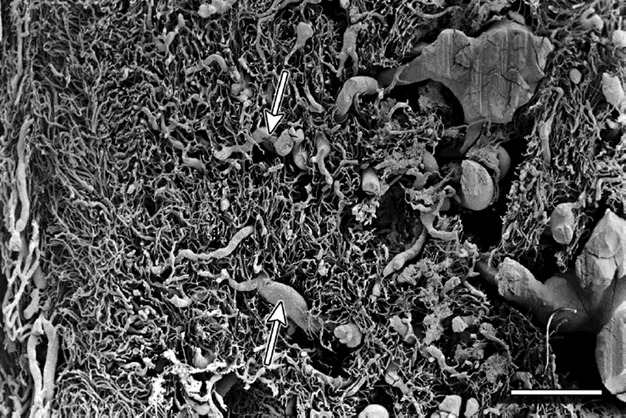
Fig. 3.
Cervix sagittal section – supravaginal part. The straight arrows point to the pericanalar veins. The asterisks mark the vessels draining blood from the pericanalar zone. Scale bar: 100 μm.
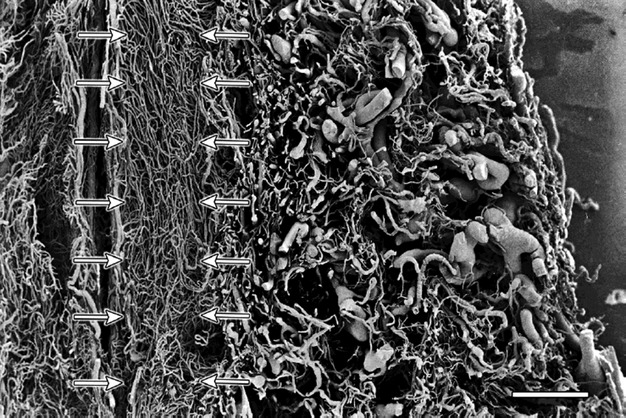
Large arteries and veins from the outermost zone gave off branches that ran towards the canal of the cervix and supplied or drained the mucosal capillaries. The branches ran almost perpendicular to the long axis of the canal (Fig. 4). Along their course the branches divided and formed capillaries (diameter 12–18 μm) arranged in a ‘ladder’ or ‘H’-shaped fashion (Fig. 5). Towards the vaginal portion of the cervix the vessels assumed a pattern of parallel capillaries running increasingly obliquely to the axis of the endocervical canal (Fig. 6) and showing relatively few interconnections.
Fig. 4.
Cervix sagittal section – supravaginal part. The figure shows the outer zone containing large arteries and veins. The arrows mark the perpendicular vessel branches that run towards the pericanalar zone. Scale bar: 1000 μm.
Fig. 5.
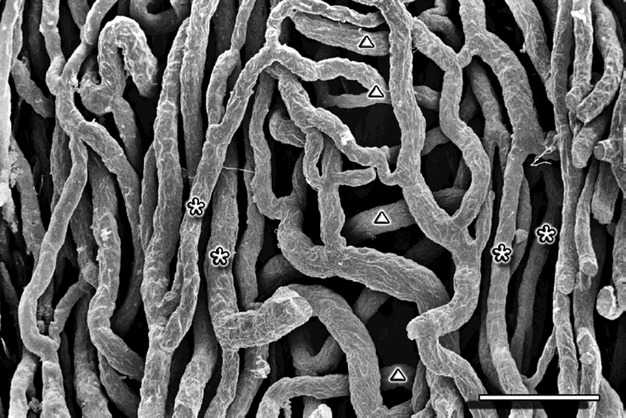
Cervix sagittal section – supravaginal part. Perivascular zone – capillaries arranged in a ‘ladder’ or ‘H’-shaped fashion. The asterisks mark the vertical and the triangles mark the transverse capillaries. Scale bar: 100 μm.
Fig. 6.
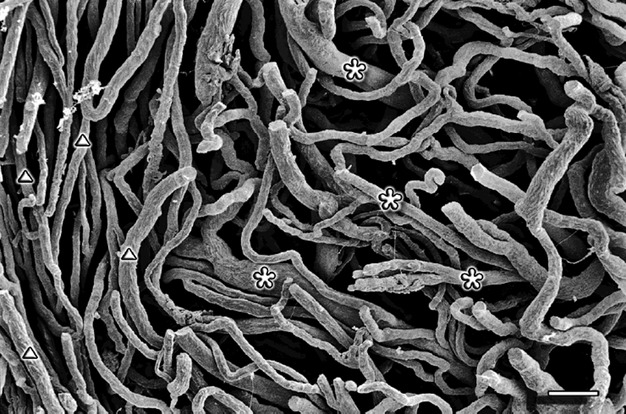
Cervix sagittal section – vaginal part. Small capillaries (marked by traingles) and venules (marked by asterisks). Scale bar: 100 μm.
In the subepithelial region of the cervical canal ran veins (diameter 80–150 μm), which drained the mucosal capillaries. The veins ran parallel to the long axis of the cervical canal and were joined by capillaries (diameter 12–20 μm) that ran perpendicular to their course (Fig. 7). The capillaries covered the veins, forming a sort of plexus around the larger vessels (Fig. 8). The subepithelial veins were observed along the whole length of the cervical canal with the exception of its last segment close to the external os. Their number, depending on canal segment, ranged from one to four.
Fig. 7.
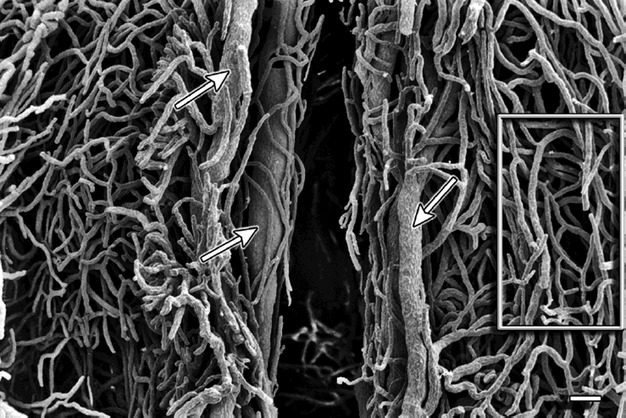
Cervix sagittal section – supravaginal part. The arrows mark the pericanalar veins. Surrounded by the box are capillaries arranged in a ‘ladder’ or ‘H’-shaped fashion. Scale bar: 100 μm.
Fig. 8.
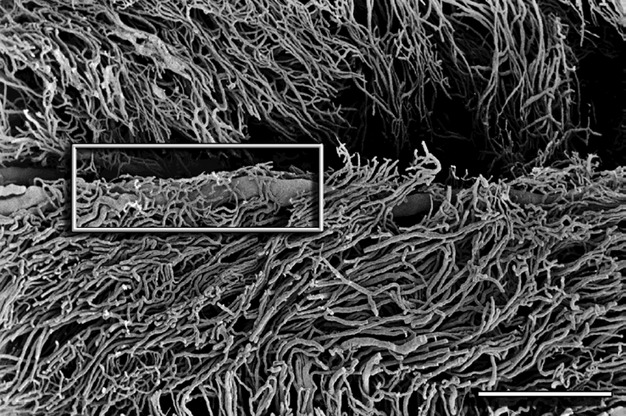
Cervix sagittal section – supravaginal part. Pericanalar vein covered by capillary plexus (marked by box). Scale bar: 1000 μm.
Both in the muscular layer as well as in the pericanalar zone, there were numerous places where the arterioles and venules passed close to each other, often adjoining.
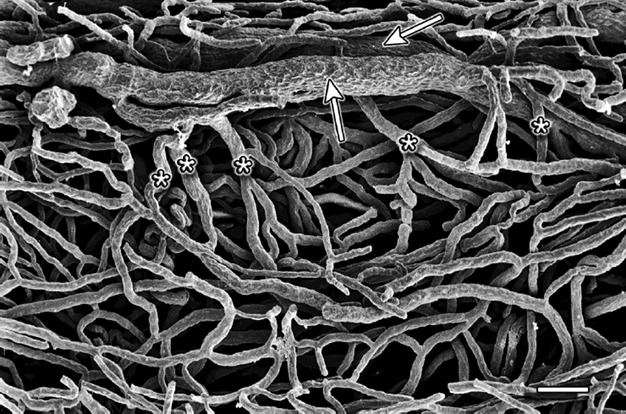
The cervical glands were surrounded by a dense capillary network (Fig. 9). The plexuses were composed either of parallel capillaries (right side of Fig. 9) or irregularly formed vessels (left side of Fig. 9).
Fig. 9.
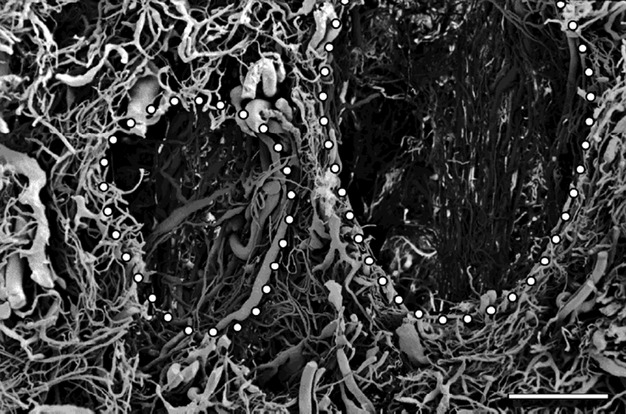
Cervix coronal section – supravaginal part. The dots mark the spaces were cervical glands were located. The glands were separated from each other by characteristically flattened veins. Scale bar: 1000 μm.
Cervical vaginal vascular architecture
Vessel arrangement in the vaginal part of the cervix was similar to the one described in the supravaginal part. However, the arteries and veins of the outer zone were of significantly smaller caliber and the veins of the pericanalar zone were much more exposed (Fig. 10). The capillaries joining the pericanalar veins ran a parallel course to the large subepithelial vessels (Fig. 10), in opposition to the perpendicular course that characterized the capillaries in the supravaginal part.
Fig. 10.
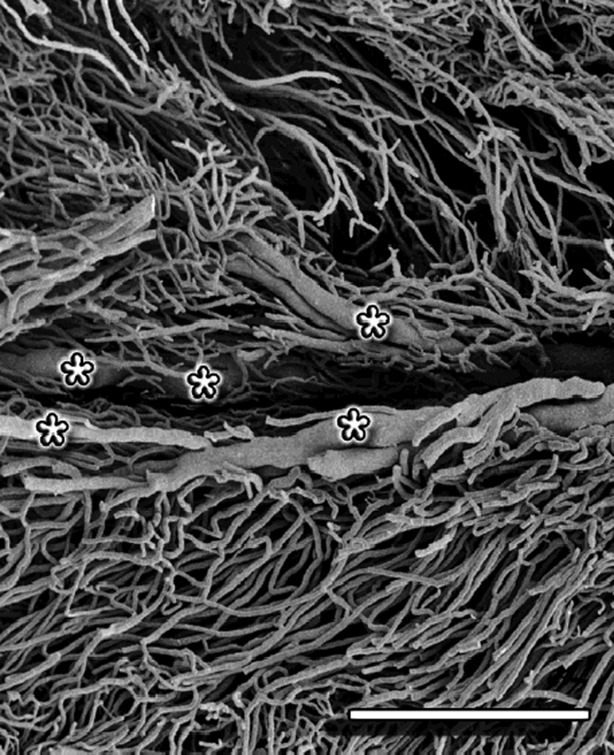
Cervix sagittal section – vaginal part. The asterisk mark the large, exposed, pericanalar veins. The capillaries joining the pericanalar veins run parallel to the subepithelial vessels. Scale bar: 1000 μm.
It was noted that the vessels of the supravaginal part of the cervix, near the external os of the uterus, formed three separate layers:
supplying vessels – characterized by relatively large diameters;
oblique or perpendicular small arteries and veins;
subepithelial capillaries joining the, above mentioned, oblique and perpendicular vessels.
In the layer of supplying vessels, veins were found (diameter ∼ 500–800 μm) that did not join the pericanalar veins. No differences between multiparas and nulliparas were noted in the vascular architecture of the cervix.
Discussion
This study describes the vascular architecture of the vaginal and supravaginal parts of the human uterine cervix, as obtained by corrosion casting and assessed in scanning electron microscopy. This is the first study to describe in detail the vaginal and supravaginal vasculature of the human uterine cervix. Only one similar study has been made before, which fragmentarily described the vascular architecture of the endocervical mucosa (Gillet et al. 1973).
Corrosion casting combined with SEM assessment is currently the best available technique to visualize vascular architecture (Lametschwandtner et al. 1990). It offers high resolution and quasi-three-dimensional images of the vessels, without interference from the non-vascular tissue.
Cicinelli & de Ziegler (1999) introduced the term ‘portal system’ to describe a new functional system flowing from the vagina to the uterus. However, our study is the first to show that there is no anatomical basis for such a theory. SEM assessment clearly visualized capillaries that are drained by venules, proving that the vasculature of the cervix is a part of the classic circulatory system. This is also the first study to report areas (in the muscular layer and the pericanalar zone) where venules and arterioles adjoin, which might suggest that countercurrent transport may exist between veins and arteries.
Small subepithelial veins running along the endocervical canal have not been described in previous studies that dealt with cervical vasculature. In our observations these veins seem to be a frequent finding in the human cervix. This might suggest the existence of two drainage systems of the mucosal capillary plexus, one using venules and veins of the middle and peripheral zones, and the other based on small pericanalar veins. The veins of the second drainage system might contribute to the formation of pregnancy-induced cervical varices, which may lead to thrombosis (Sammour et al. 2011).
Radiological studies point to the existence of a centrally orientated (medial plane), poorly vascularized zone in the cervix (Pilarczyk et al. 2002). This zone is supposed to exist regardless of a woman's gynecological history. Our study is the first to deny the existence of such a layer. It is very likely that this zone is an artifact, created when relatively large contrast medium molecules fail to pass further through the small blood vessels. The other reason why such an artifact might develop is that, in vivo, microclots might exist in the vessels, blocking the passage of the contrast medium. This problem does not exist when applying resin because of the thorough study material preparation.
In this study we have described the arrangement of cervical blood vessels, which are divided into four zones. The existence of these zones has been demonstrated in magnetic resonance imaging by DeSouza et al. (1994) and this study details their microvascular anatomy. The zones of the cervix are a continuation of the uterine body wall layers. The zone of larger blood vessels corresponds to the vascular layer of the myometrium, whereas the other three zones belong to the mucosal layer.
The current study has three limitations. Although several attempts have been made, the authors were unable to obtain satisfactory transverse sections of the cervix. The gold coating did not fully cover the surface of the cast, especially in the lumen of the cervical canal. The second limitation was that only in two cases of uteri from multiparous women, were the authors able to recreate fully the vascular architecture of the cervix. The rest of the casts were characterized by a varying percentage of completely visualized vascular architecture. Although not fully recreated, these samples were found to be still suitable for SEM assessment, and supported our findings as stated in the conclusions. The third problem was that the fragile microvasculature of the ectocervical mucosa was lost during the corrosion step. However, this does not affect the outcome of this study as the vascular architecture of the ectocervical mucosa has already been described in detail by other authors (Gillet et al. 1973; Sekiba et al. 1979).
Apart from its purely anatomical value, this study presents facts that can also be useful to a clinician. First, as mentioned earlier, the pericanalar veins might contribute to the formation of pregnancy-induced cervical varices, which may lead to thrombosis (Sammour et al. 2011). Secondly, the rich blood supply to the endocervical region might promote the development of certain cervical pathologies such as placenta praevia.
In conclusion, apart from describing the vascular architecture of the vaginal and supravaginal parts of the human uterine cervix, this study introduces the idea of two systems responsible for draining blood from the mucosal capillaries. It is also the first to refute the theory of a functional ‘portal system’ between the vagina and the uterus, and at the same time the study is the first to suggest the existence of countercurrent transport between adjoining veins and arteries.
Author contributions
Tomasz Bereza was involved in study design, material acquisition, analysis and interpretation of data and revision of the manuscript. Krzysztof Andrzej Tomaszewski was involved in data analysis and interpretation, drafting of the manuscript and manuscript revision. Marta Bałajewicz-Nowak was involved in material acquisition and data analysis. Ewa Mizia was involved in material acquisition and data analysis. Artur Pasternak was involved in material acquisition. Jerzy Walocha was involved in study design, data analysis and interpretation and critical manuscript revision. All authors approved the final version of the manuscript.
Funding source
This study was supported by the statutory funds granted by the Jagiellonian University Medical College.
Conflict of interest
The authors have no conflict of interest or financial relationship to disclose.
References
- Cicinelli E, de Ziegler D. Transvaginal progesterone: evidence for a new functional ‘portal system’ flowing from the vagina to the uterus. Hum Reprod Update. 1999;5:365–372. doi: 10.1093/humupd/5.4.365. [DOI] [PubMed] [Google Scholar]
- DeSouza NM, Hawley IC, Schwieso JE, et al. The uterine cervix on in vitro and in vivo MR images: a study of zonal anatomy and vascularity using an enveloping cervical coil. Am J Roentgenol. 1994;163:607–612. doi: 10.2214/ajr.163.3.8079853. [DOI] [PubMed] [Google Scholar]
- Gillet JY, Rihm G, Koritke JG, et al. La vascularisation du col de l'uterus chez la femme. Rev Fr Gynecol Obstet. 1973;68:13–24. [PubMed] [Google Scholar]
- Hamid SA, Ferguson LE, McGavigan CJ, et al. Observing three-dimensional human microvascular and myogenic architecture using conventional fluorescence microscopy. Micron. 2006;37:134–138. doi: 10.1016/j.micron.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Lametschwandtner A, Miodoński A, Simonsberger P. On the prevention of specimen charging in scanning electron microscopy of vascular corrosion casts by attaching conductive bridges. Mikroskopie. 1980;36:270–273. [PubMed] [Google Scholar]
- Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts – technique and applications: updated review. Scanning Microsc. 1990;4:889–940. discussion 941. [PubMed] [Google Scholar]
- Manconi F, Thomas GA, Fraser IS. A historical overview of the study and representation of uterine microvascular structures. Microvasc Res. 2010;79:80–89. doi: 10.1016/j.mvr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Miodoński A, Hodde KC, Bakker C. Rasterelektronenmikroskopie von Plastik-Korrosions-Praeparaten: morphologische Unterschiede zwischen Arterien and Venen. BEDO. 1976;9:435–442. [Google Scholar]
- Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios Jaraquemada JM, Garcia Monaco R, Barbosa NE, et al. Lower uterine blood supply: extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet Gynecol Scand. 2007;86:228–234. doi: 10.1080/00016340601089875. [DOI] [PubMed] [Google Scholar]
- Pilarczyk K, Kozik W, Czerwiński F. Arteries of the uterine cervix in reproductive age in microangiographic studies. Ginekol Pol. 2002;73b:1179–1183. [PubMed] [Google Scholar]
- Sammour RN, Gonen R, Ohel G, et al. Cervical varices complicated by thrombosis in pregnancy. Ultrasound Obstet Gynecol. 2011;37:614–616. doi: 10.1002/uog.8946. [DOI] [PubMed] [Google Scholar]
- Sekiba K, Okuda H, Fukui H, et al. A scanning electron microscope study of the fine angioarchitecture of the uterine cervix using a newly established cast formation technique. Obstet Gynecol Surv. 1979;34:823–826. doi: 10.1097/00006254-197911000-00012. [DOI] [PubMed] [Google Scholar]
- Składzień J, Litwin JA, Nowogrodzka-Zagórska M, et al. Corrosion casting study on the vasculature of nasal mucosa in the human fetus. Anat Rec. 1995;242:411–416. doi: 10.1002/ar.1092420313. [DOI] [PubMed] [Google Scholar]
- Walocha JA, Miodoński AJ, Nowogrodzka-Zagórska M, et al. Application of a mixture of glycol polyethylenes for the preparation of microcorrosion casts – an observation. Folia Morphol (Warsz) 2002;61:313–316. [PubMed] [Google Scholar]
- Walocha JA, Litwin JA, Miodoński AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088–1093. doi: 10.1093/humrep/deg213. [DOI] [PubMed] [Google Scholar]


