Abstract
Tendon lesions induce muscular atrophy, the nature of which has not yet been clearly related to lesion etiology and entity. In the present study, tendon and muscle alterations were assessed after experimental tendon lesion of the Infraspinatus muscle in young rats. The consequences of lesions differed on the basis of both extension and injured tissue vascularization, that is apoptosis and/or degeneration, differing mainly by energy demands: apoptosis requires high energy levels (proportional to vascular supply), but degeneration does not. It is well known that tendons are poorly supplied with blood compared with muscular masses, which are abundantly vascularized. Five weeks after tendon surgical section, tendon/muscle samples were taken for TUNEL and transmission electron microscopy. The structural results reported here identified different tendon/muscle alterations: degeneration of tendon without signs of apoptosis, and atrophy of muscle fibers due only to apoptosis. This led to the formulation of the following hypothetical sequence of events: a tendon lesion, not recovering quickly due to the poor tendon blood supply, results in degeneration of the injured tendon, which, in turn, induces a partial disuse of the muscle mass, which consequently atrophies (proportionally to the severity of tendon lesion) by striated muscular fiber apoptosis. The authors suggest that the different behavior of the two tissues depends on the marked difference in their vascularization.
Keywords: apoptosis, degeneration, rat model, striated muscle, transmission electron microscopy, tendon lesion, TUNEL
Introduction
It is well known that long-term tendon pathologies (like degeneration, inflammatory reaction, lesion, etc.) induce various degrees of alteration in the muscular mass, identifiable by instrumental evaluation [magnetic nuclear risonance (MNR), computerized tomography (CT)], eventually leading to muscular atrophy. The literature includes a number of papers on the topic regarding both human clinical studies (Fukuda et al. 1994; Nakagaki et al. 1996; Hashimoto et al. 2003) and experimental animal models (Björkenheim, 1989; Fabis et al. 1998; Barton et al. 2005). However, the type and evolution of muscular alterations are not yet well defined in relation to the etiology and entity of the tendon pathology. Another consideration is the fact that even if surgical repair of a tendon is performed a significant time after the lesion, full recovery of the muscular mass trophism has never been observed (Goutallier et al. 1994), particularly in chronic lesions (Buchmann et al. 2011).
The present study investigated the fate of tendon and muscular mass after experimental tendon lesion in a rat model. It is important to underline that the two tissues making up skeletal muscle have very different blood supplies: notwithstanding the controversial theory about tendon neovascularization during wound healing (Rees et al. 2006), tendon is a very poorly vascularized fibrous tissue and this negatively influences its healing processes. Striated muscle fibers are instead surrounded by a rich network of blood capillaries inside the perimysium and endomysium (Jozsa et al. 1980). Different possibilities exist according to both the extension of the lesion and the vascular supply to the injured tissues, that is degeneration and/or apoptosis, which differ mainly by energy demands: the process of apoptosis requires a high level of energy (Trump & Berezesky, 1996; Sandri & Carraro, 1999; Lyu et al. 2007), while degeneration does not. Moreover, the possibility of benefiting from high levels of energy is directly correlated to the vascular supply of the tissue: the more abundant the blood vessels in a tissue, the higher the level of energy it can obtain. On the basis of these considerations, the types of muscular alterations are investigated, as well as the different resulting muscle/tendon structures of the myotendinous unit, subsequent to experimental lesions of the tendon of the Infraspinatus muscle in six young rats. Five weeks after tendon surgical section, the rats were killed, and samples of tendons and muscles were taken for structural, histochemical (in-situ end labeling-TUNEL) and ultrastructural analyses under both light and transmission electron microscopy (LM and TEM).
The aim of the study, conceived as a prerequisite to subsequent functional investigations, was to identify the type of outcomes occurring in fibrous and muscular tissues in order to propose a working hypothesis to explain the structural alterations of striated muscular mass after experimental tendon lesions, as well as to define an appropriate animal model for comparison with outcomes recorded in human rotary cuff alterations.
Materials and methods
Animals and treatments
Six young (2 months) male Sprague–Dawley rats, weighing 230–250 g, were purchased from Harlan Laboratories (S. Pietro al Natisone UD, Italy). They were housed in a single cage and maintained for the whole experimental period in standard conditions, with a 12 : 12 h light : dark cycle, at a temperature of 22 ± 1 °C and 55–60% relative humidity. Commercial rat pellets and drinking water were available ad libitum. After a 7-day adaptation period to the controlled laboratory conditions, the animals were submitted to surgical section of infraspinatus tendon, as previously suggested (Soslowsky et al. 1996), under ketamine hydrochloride (Ketavet 100®; Farmaceutici Gellini S.p.a, Aprilia, Italy) plus xylazine hydrochloride (Rompun®; Bayer, Leverkusen, Germany) anesthesia. Three animals were operated on the left side, three on the right side, as quadrupeds do not have a dominant antimer and shoulder trophism, and biomechanics do not present significant differences between the two sides (Soslowsky et al. 1996). The contralateral side of each animal was used as control. In particular, after dorsal surgical access to the shoulder in correspondence to the scapular spine and the detachment of subcutaneous plane and partially of the Trapezius muscle, the insertion of the Infraspinatus muscle was dissected preserving innervation; the section plane was marked with a point of 5/0 nylon suture as a landmark. Five weeks after tendon surgical section, the rats were killed, and samples of both muscular fibers and tendon of the Infraspinatus muscle were removed from all the anterior limbs of the animals and performed for both in-situ end labeling (TUNEL) under LM and ultrastructural analysis under TEM.
The Infraspinatus muscle was chosen for the following reasons: first, in man, after lesion of the tendon, Infraspinatus muscle undergoes phenomena of atrophy, clinically and instrumentally more evident with respect to other cuff muscles; second, Infraspinatus muscle is easier to identify and dissect, being immediately below the spine of the scapula; and third, the section of Infraspinatus muscle does not especially aggravate the deficit in gait of the animals used for the experiment, as it is predominantly an extra rotator muscle.
Animal care, maintenance and treatments were conducted in accordance with Italian law (D.L. n. 116/1992) and European legislation (EEC n. 86/609). The experimental design and procedures received the approval of the Bioethical Committee of the Italian National Institute of Health.
In-situ end-labeling analysis (TUNEL)
Samples of both tendon and muscular fibers were fixed in 4% buffered paraformaldehyde (0.1 m phosphate buffer, pH 7.2) overnight at 4 °C, embedded in paraffin via a standard method, sectioned on a microtome (5 μm thick) and mounted on silanized slides. After being deparaffinated and rehydrated, the sections were treated in a humidified chamber with proteinase K (Boehringer, Mannheim, Germany) at 20 μg mL−1 for 15 min at room temperature, washed in bidistilled water, treated with 2% H2O2 in methanol for 10 min at room temperature, and then washed in bidistilled water. The slides were pre-incubated with terminal deoxynucleotidyl transferase (TdT) buffer and 1 mm CoCl2 for 5 min at room temperature, and then incubated for 60 min in a humidified chamber at 37 °C with 50 μL TdT and biotinylated deoxyuridine triphosphate (Bio dUTP; Boehringer; TdT 0.3 U μL−1, Bio dUTP 8 μm in TdT buffer and CoCl2 1 mm). The sections were then washed four times in bidistilled apyrogen water (for 2 min each), twice in phosphate-buffered saline (PBS; 5 min each), in human serum albumin 2% (5 min) and in PBS (5 min), then covered with streptavidin–biotinylated peroxidase complex (Boehringer) diluted 1 : 100 in a humidified chamber for 45 min at room temperature, washed in PBS and stained with diaminobenzidine 50 mm (0.05%). The slides were then washed in water and counterstained with 0.5% methyl green for 10 min. Positive and negative controls were included in each experiment. For the positive controls, sections were treated with DNase I (1 μg mL−1; Boehringer) in DNase buffer for 10 min at room temperature before exposure to Bio dUTP and TdT. Sections were incubated without the TdT enzyme as negative control.
Ultrastructural analysis (TEM)
Samples of both tendon and muscular fibers reduced in small pieces were fixed for 2 h with 4% paraformaldehyde in 0.13 m phosphate buffer, pH 7.4, postfixed for 1 h with 1% osmium tetroxide in 0.13 m phosphate buffer, pH 7.4, dehydrated in graded ethanol and embedded in epoxy resin (Durcupan ACM), and sectioned with a diamond knife mounted in an Ultracut-Reichert Microtome. Thin sections (1 μm) were stained with toluidine blue, and examined under an Axiophot-Zeiss LM in order to select the microscopic fields of interest. Ultrathin sections (70–80 μm) were mounted on Formvar- and carbon-coated copper grids, stained with 1% uranyl acetate and lead citrate, and examined under a Zeiss EM109 TEM.
Results
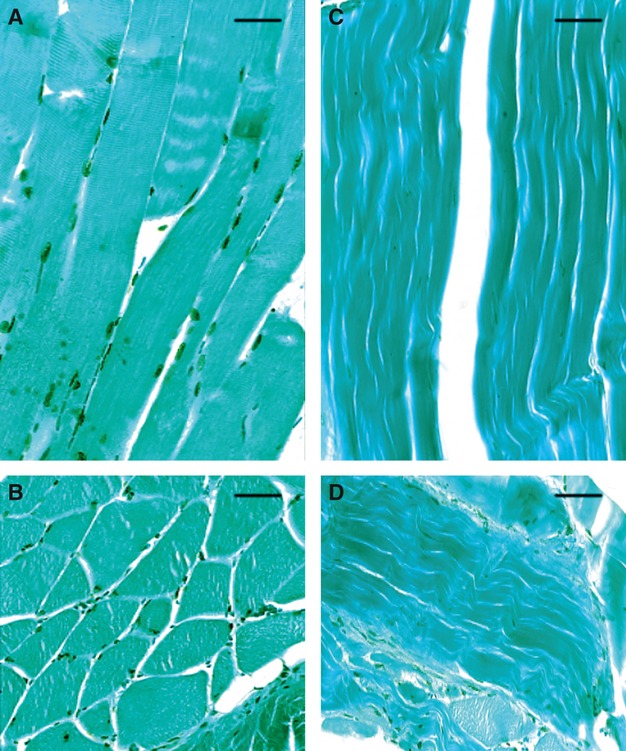
Observations of histological sections of the Infraspinatus muscle revealed the classic aspect of a pennate muscle with muscular fibers running obliquely to the tendon insertion, and numerous capillary vessels present inside the endomysium and perimysium (Fig. 1A); in contrast, the tendon, as classically described, appeared as fibrous tissue, mostly devoid of capillaries (Fig. 1B).
Fig. 1.

Histological sections of Infraspinatus muscle showing (A) the classical aspect of pennate muscle with muscular fibers running obliquely to the tendon insertion (marked by the black line) and abundant vessels inside the endomysium (head arrows) and perimysium (arrows). (B) The fibrous tissue forming the tendon appears poorly vascularized. Stain: toluidine blue. Scale bars: 25 μm (A); 15 μm (B).
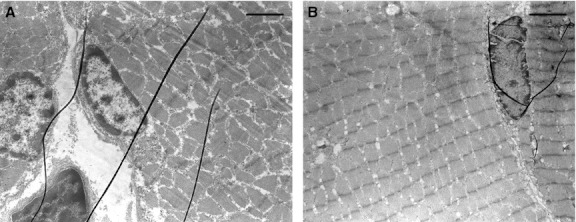
The TUNEL method, which labels cells with DNA fragmentations brown, identified the presence of apoptotic phenomena affecting the nuclei of muscular fibers, clearly evident both in longitudinal (Fig. 2A) and transverse (Fig. 2B) sections; conversely, tendon produced a TUNEL negative reaction (Fig. 2C,D).
Fig. 2.
In-situ end labeling on longitudinal (top) and transversal (bottom) sections of muscular fibers (A, B) and tendon (C, D). Note the TUNEL positive reaction (labeled cells exhibit a brownish color) only in the muscular fiber nuclei. LM micrographs counterstained with methyl green. Scale bars: 10 μm (A, B); 15 μm (C, D).
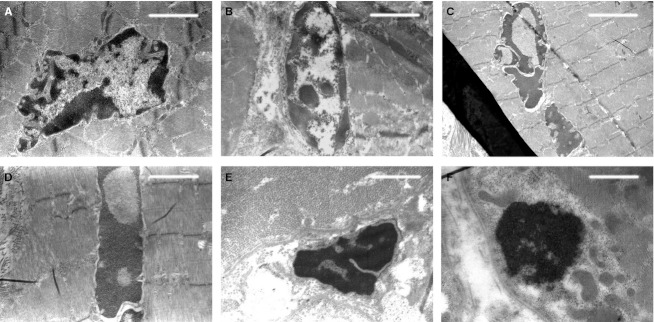
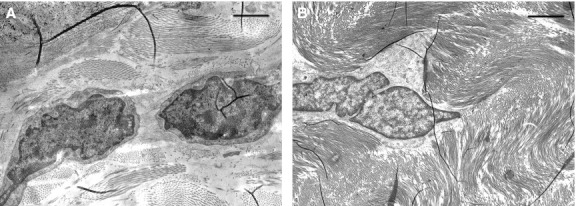
Transmission electron microscopic observations of the same microscopic fields showed, in addition to the presence of muscular fibers with normally structured nuclei and cytoplasm (Fig. 3), also the presence of striated muscle fiber nuclei in apoptosis with an ultrastructure exhibiting a variable morphology of chromatin masses due to the different degrees of condensation (Fig. 4). At the beginning of the apoptotic processes, the muscular fibers had well-preserved sarcomeric units arranged serially (Fig. 4C,D), whereas with increasing progressive nuclear apoptotic phenomena, it was possible to observe progressive alterations of sarcomeres, whose arrangement appeared disrupted (Fig. 4E,F). As regards the tendon, TEM observations confirmed the absence of apoptotic phenomena evidenced by the negative TUNEL reaction, both remote and proximal to the experimental lesion. Moreover, from an ultrastructural viewpoint, tenocytes remote from the lesion appeared well preserved (Fig. 5), with evident nucleoli and well-developed cytomembranes, in particular the rough endoplasmic reticulum and the Golgi complex. Instead, tenocytes proximal to the lesion exhibited a degenerative aspect, particularly evident in the many membrane-bound vacuoles (similar to interconnected caveolae) contained in the cytoplasm close to the plasma membrane (Fig. 6).
Fig. 3.
TEM micrographs showing well-preserved muscular fibers with a normal ultrastructure of the nuclei, located peripherally in the zone immediately beneath the cell membrane. Note the different aspect of the serially arranged sarcomeric units in transversal (left) and longitudinal (right) section. Scale bars: 1.7 μm (A); 1.8 μm (B).
Fig. 4.
TEM micrographs showing, from (A) to (F), muscular fiber nuclei in apoptosis at different progressive stages of chromatin condensation. Note in (E) and (F), with respect to the previous stages, the disruption of the sarcomeric units. Scale bars: 1.5 μm (A, B, D); 2 μm (C); 1 μm (E); 0.75 μm (F).
Fig. 5.
TEM micrographs of tenocytes taken from a microscopic field far from the lesion. Note the well-preserved ultrastructure. Scale bars: 1.4 μm (A, B).
Fig. 6.
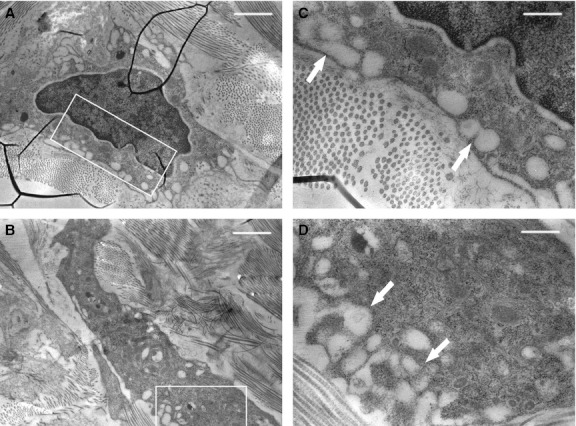
TEM micrographs of tenocytes taken from a microscopic field near the lesion. Note the cytoplasm vacuolization (arrows), indicative of degeneration. (C and D) Enlargements of the squared areas, respectively, in (A) and (B). Scale bars: 1.4 μm (A, B); 0.4 μm (C, D).
Discussion
This study investigated histological changes in rat Infraspinatus tendon and muscle after experimental tendon lesion. The observations reported clearly underline important morphofunctional correlations between tendon and muscle fibers, not only in the physiology of skeletal muscles, but also in the different prospects for these two components after injury.
The first point of discussion is the importance of the differing vascular supply to the two tissues comprising skeletal muscles. This consideration formed the basis for interpreting the results, which revealed two different possible occurrences of degeneration and/or apoptosis in injured tissues, and the energy required for each to occur. As regards apoptosis, it has been suggested that it requires a high level of energy (Jozsa et al. 1980; Rees et al. 2006), while degeneration does not. For this reason, as expected, only degeneration was observed in the poorly vascularized fibrous tissue of the tendon of the Infraspinatus muscle experimentally lesioned. In contrast, and more difficult to explain, are the structural alterations recorded in muscular fibers after tendon injury. A possible sequence of events could be the following: a tendon lesion, not recovering quickly due to the poor blood supply to the tendon, induces a degeneration of the injured tendon, which, in turn, causes partial disuse of the muscle mass, which atrophises (proportionally to the severity of the tendon lesion) by striated muscular fiber apoptosis, the high energy demand of which is satisfied by the abundant blood supply to the muscle mass. The fact that both TUNEL and TEM analyses confirmed the presence of apoptotic phenomena only inside the muscle mass concurs with this hypothesis. The occurrence of apoptosis in the muscular fibers within 5 weeks of the tenotomy is in line with data reported in the literature, according to which capillary density slowly decreases in muscular tissue following disuse produced by tenotomy or immobilization (Jozsa et al. 1980).
The present demonstration that muscle fibers underwent rapid alterations due to apoptosis within 5 weeks in contrast with tendon degeneration does not permit prediction of what happens after this period of time. According to data from the literature (Buchmann et al. 2011), in the short term (3–6 weeks) alterations in muscle and tendon occur, while over the longer term (especially between 6 and 9 weeks) it is possible to observe partial regeneration due to the increased healing potential of rat tissues; the same authors also refer that in their rat model the level of atrophy showed a peak in the early period (3 weeks), decreasing with time. Equivalent findings were documented by Barton et al. (2005): they found the highest level of atrophy after 4 weeks with a subsequent recovery of the muscle. Other authors reported that, in the rabbit model, contractile response tests produced results comparable with a continuous recovery of the muscle until contraction values reached the level of the control group after 12 weeks (Björkenheim, 1989). In human rotator cuff repair, the atrophy of the muscle can recover with time, but this process takes at least 6 months (Hata et al. 2005).
The healing of the rat Infraspinatus tendon differs from human tendons in some respects, one being that tendon lesions, though severe, almost always self-heal in the rat model, while human tendon lesions do not follow the same trend (Walch & Levigne, 1997). Not insignificant in the case of human tendon damage involving joints is the role of synovial fluid, which hinders cicatrisation causing a weak or absent healing response (Buchmann et al. 2011).
As regards tendon repair, there are various possible consequences, according to the etiology of the lesion. In the present model, the experimental surgical lesion leads to degeneration of tendon tissue not associated with the inflammatory process, as also observed by other authors (Zamora & Marini, 1988; Soslowsky et al. 2000). Otherwise, in recurrent microdamage from tendon overuse, the evolution is analogous to the process that results in bone stress fractures. However, a bone fracture has the potential for very good recovery, thanks to the abundant vascularization of bone tissue, so that the increased osteoblastic activity can give excellent results. A damaged tendon, instead, is subject to fibroplasia, which combined with its poor vascularization results in scar tissue formation and weakening (Rees et al. 2006).
It should be noted that alterations in muscular mass often have multifactor origins that induce different and often correlated consequences. For example, decreased motoneuron activity associated with loss of tension of the tendon, combined with proprioceptive feedback, leads to an inhibition of reflexes, and finally to inactivity and disuse; the same effect is obtained with a surgical lesion of the tendon, which eventually leads to decreased tendon tension and consequently muscular hypofunction.
The authors also refer that data are reported in the literature suggesting that osteocyte apoptosis could be involved in triggering bone turnover (Noble et al. 1997; Jilka et al. 1998; Noble & Reeve, 2000); it cannot be excluded that such an improvement of turnover might also occur in other tissues of the locomotor apparatus, like the myotendinous unit, although data are not yet published on this topic. Work is in progress in the authors' laboratory to clarify this interesting aspect.
Notwithstanding the intriguing question of the causal factors inducing apoptosis (i.e. programmed cell death) instead of simple degeneration, the present study, based on an animal experimental approach, could be very useful in defining a proper model for comparing the outcomes recorded in human rotary cuff alterations, in order to better clarify in further studies the issue of human rotator cuff repair and tendon healing combined with biological augmentation.
Acknowledgments
The authors thank Dr Marta Benincasa for her valuable help in setting iconography. This study was supported by funds of Fondazione of Vignola and Banca Popolare of Emilia Romagna.
References
- Barton ER, Gimbel JA, Williams GR, et al. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23:259–265. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Björkenheim JM. Structure and function of the rabbit's supraspinatus muscle after resection of its tendon. Acta Orthop Scand. 1989;60:461–463. doi: 10.3109/17453678909149320. [DOI] [PubMed] [Google Scholar]
- Buchmann S, Walz L, Sandmann GH, et al. Rotator cuff changes in a full thickness tear rat model: verification of the optimal time interval until reconstruction for comparison to the healing process of chronic lesions in humans. Arch Orthop Trauma Surg. 2011;131:429–435. doi: 10.1007/s00402-010-1246-5. [DOI] [PubMed] [Google Scholar]
- Fabis J, Kordek P, Bogucki A, et al. Function of the rabbit supraspinatus muscle after detachment of its tendon from the greater tubercle. Observations up to 6 months. Acta Orthop Scand. 1998;69:570–574. doi: 10.3109/17453679808999257. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Hamada K, Nakajima T, et al. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;304:60–67. [PubMed] [Google Scholar]
- Goutallier D, Postel JM, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop. 1994;304:78–83. [PubMed] [Google Scholar]
- Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;415:111–120. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- Hata Y, Saitoh S, Murakami N, et al. Volume changes of supraspinatus and infraspinatus muscles after supraspinatus tendon repair: a magnetic resonance imaging study. J Shoulder Elbow Surg. 2005;14:631–635. doi: 10.1016/j.jse.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, et al. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Jarvinen M, Kvist M, et al. Capillary density of tenotomized skeletal muscles. I. Experimental study in the rat. Eur J Appl Physiol Occup Physiol. 1980;44:175–181. doi: 10.1007/BF00421096. [DOI] [PubMed] [Google Scholar]
- Lyu BN, Lyu MB, Ismailov BI, et al. Four hypotheses on mitochondria's role in the development and regulation of oxidative stress in the normal state, cell pathology and reversion of tumor cells. Med Hypotheses. 2007;69:186–194. doi: 10.1016/j.mehy.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Nakagaki K, Ozaki J, Tomita Y, et al. Fatty degeneration in the supraspinatus muscle after rotator cuff tear. J Shoulder Elbow Surg. 1996;5:194–200. doi: 10.1016/s1058-2746(05)80005-9. [DOI] [PubMed] [Google Scholar]
- Noble BS, Reeve J. Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol. 2000;159:7–13. doi: 10.1016/s0303-7207(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Noble BS, Stevens H, Loveridge N, et al. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone. 1997;20:273–282. doi: 10.1016/s8756-3282(96)00365-1. [DOI] [PubMed] [Google Scholar]
- Rees JD, Wilson1 AM, Wolman RV. Current concepts in the management of tendon disorders. Rheumatology. 2006;45:508–521. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- Sandri M, Carraro U. Apoptosis of skeletal muscles during development and disease. Int J Biochem Mol Biol. 1999;31:1373–1390. doi: 10.1016/s1357-2725(99)00063-1. [DOI] [PubMed] [Google Scholar]
- Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- Soslowsky LJ, Thomopoulous S, Tun S, et al. Neer Award 1999. Overuse activity injuries the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- Trump BF, Berezesky IK. The mechanisms of calcium-mediated cell injury and cell death. New Horiz. 1996;4:139–150. [PubMed] [Google Scholar]
- Walch G, Levigne C. Introduction to partial thickness tears of the supraspinatus tendon. In: Gazielly DF, Gleyze P, Thomas TI, editors. The Cuff. Amsterdam: Elsevier Masson; 1997. pp. 231–233. [Google Scholar]
- Zamora AJ, Marini JF. Tendon and myo-tendinous junction in an overloaded skeletal muscle of the rat. Anat Embryol. 1988;179:89–96. doi: 10.1007/BF00305103. [DOI] [PubMed] [Google Scholar]