Abstract
We investigated the occurrence and anatomy of the vomeronasal system (VNS) in tadpoles of 13 different anuran species. All of the species possessed a morphologically fully developed VNS with a highly conserved anatomical organisation. We found that a bean-shaped vomeronasal organ (VNO) developed early in the tadpoles, during the final embryonic stages, and was located in the anteromedial nasal region. Histology revealed the presence of bipolar chemosensory neurones in the VNO that were immunoreactive for the Gαo protein. Tract-tracing experiments demonstrated that chemosensory neurones from the VNO reach specific areas in the brain, where a discernible accessory olfactory bulb (AOB) could be observed. The AOB was located in the ventrolateral side of the anterior telencephalon, somewhat caudal to the main olfactory bulb. Synaptophysin-like immunodetection revealed that synaptic contacts between VNO and AOB are established during early larval stages. Moreover, using lectin staining, we identified glomerular structures in the AOB in most of the species that we examined. According to our findings, a significant maturation in the VNS is achieved in anuran larvae. Recent published evidence strongly suggests that the VNS appeared early in vertebrate evolution and was already present in the aquatic last common ancestor of lungfish and tetrapods. In this context, tadpoles may be a good model in which to investigate the anatomical, biochemical and functional aspects of the VNS in an aquatic environment.
Keywords: accessory olfactory system, chemical sensing, G protein, pheromones, vomeronasal organ
Introduction
In most terrestrial vertebrates, there are two anatomically distinct olfactory organs: the olfactory epithelium (OE) and the vomeronasal organ (VNO). The olfactory sensory neurones lining the OE and the vomeronasal sensory neurones lining the VNO are characterised as bipolar neurones that project to two different specific telencephalic areas: the main olfactory bulb (MOB) and the accessory olfactory bulb (AOB), respectively. The two sensory systems have anatomical, physiological, and molecular differences, which suggest different behavioural functions (Halpern & Martinez-Marcos, 2003). The VNO is commonly assumed to be specialised for detecting pheromones, whereas general odorants would be detected by the OE. However, this hypothesis is simplistic and not supported by the available behavioural and physiological data (Baxi et al., 2006). For example, the vomeronasal system (VNS) is crucial in mediating responses to foraging cues in snakes and salamanders (Alving & Kardong, 1996; Placyk & Graves, 2002; Halpern & Martinez-Marcos, 2003), whereas in mammals, some pheromones are detected by the OE (Dorries et al., 1997; Swann et al., 2001; Xu et al., 2005).
An anatomically discernible VNS, formed by a VNO in the nasal cavity and an AOB in the anterior brain, has not been recognised in fishes and it is generally believed that this accessory olfactory system exists only in tetrapods (Eisthen, 1992). This prompted the idea that the VNS arose over the course of tetrapod evolution as an adaptation to terrestrial life (Bertmar, 1981). Nevertheless, a growing body of evidence suggests that the VNS appeared in aquatic environments during vertebrate evolution (Eisthen, 2000; Grus & Zhang, 2009; Gonzalez et al., 2011). For example, VNS-specific genes have been identified in bony fishes, elasmobranches and lampreys, indicating that at least genetic components of the VNS arose early on in vertebrate evolution (Grus & Zhang, 2009). Moreover, the presence of a separate diverticulum in the nasal cavity that contains a sensory vomeronasal epithelium and an AOB in the telencephalon has been described in permanently aquatic amphibians (Eisthen, 2000). In addition, it was recently shown that the African lungfish Protopterus dolloi has epithelial crypts at the base of the lamellae of the OE; chemosensory neurones of these crypts express markers specific to the vomeronasal sensory neurones in tetrapods. Moreover, the projections of the chemosensory cells lining these crypts allowed the authors to identify an AOB on the lateral margin of the MOB (Gonzalez et al., 2011). These findings suggest that the VNS may play a role in chemosensory detection in aquatic environments, but nothing is known about the function of the VNS in water.
Among tetrapods, amphibians are unique in having an aquatic larval stage, followed by metamorphosis to a terrestrial adult. Thus, anuran larvae are excellent models in which to investigate the anatomical, biochemical and functional aspects of the VNS in an aquatic environment. However, there has been little research regarding the nasal chemosensory epithelia of tadpoles. Most classical and some recent works are restricted to descriptions of the general anatomy of the nasal cavity and do not analyse axonal projections to the brain or biochemical aspects of the olfactory and VNSs (Tsui, 1946; Yvroud, 1966; Taniguchi et al., 1996; Jermakowicz et al., 2004; Wang et al., 2008; Jungblut et al., 2011). By contrast, research that integrates anatomical, biochemical and molecular data has generally focused on the main olfactory system and overlooked the VNS (Reiss & Burd, 1997; Nezlin & Schild, 2005).
In the present work, we looked for the presence of VNO and AOB in tadpoles of 13 different anuran species. We found that a morphologically fully developed VNS was present in all of the species that we examined. The anatomical organisation of the VNS was highly conserved among anuran tadpoles and quite similar to the anatomy described in the VNS of adult anurans. To assess the degree of maturity of the VNS in tadpoles, we looked for expression of the G protein Gαo, a selective marker for vomeronasal sensory neurones in tetrapods (Halpern & Martinez-Marcos, 2003; Hagino-Yamagishi et al., 2004). Gαo protein was expressed in the VNO of tadpoles from the late embryonic stages onward, during organogenesis of the OE and the VNO. Moreover, tract-tracing techniques and synaptophysin immunoreactivity demonstrated that sensory neurones from the VNO are in synaptic contact with specific telencephalic areas, forming a histologically discernible AOB.
Materials and methods
Animals
Tadpoles of Rhinella arenarum, Hypsiboas pulchellus and Xenopus laevis were obtained by in vitro fertilisation according to methods previously described (Paz et al., 1995). Larvae were maintained in dechlorinated tap water with a constant photoperiod and temperature (12 : 12 h, dark : light; 22 °C) and fed ad libitum. Lithobates catesbeianus tadpoles were obtained from a local supplier and tadpoles of the other species that we examined were obtained from the Herpetological Collection of the Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’-CONICET (see Appendix 1). Animals were staged according to a generalised table for anuran embryos (Gosner, 1960) except for X. laevis tadpoles, which were staging according to a specific developmental table (Nieuwkoop & Faber, 1994) (Table 1). All of the experiments were performed in accordance with the principles of laboratory animal care of the Institutional Care and Use Committee of the Facultad de Ciencias Exactas y Naturales (UBA Res CD: 140/00) and the United States National Institutes of Health (Publication 8523, revised 1985).
Table 1.
Specimens analysed in the study
| Species | Family | Developmental stage used* |
|---|---|---|
| Rhinella arenarum | Bufonidae | 25–39 |
| Scinax acuminatus | Hylidae | 37 |
| Hypsiboas curupi | Hylidae | 29–30 |
| Hypsiboas pulchellus | Hylidae | 24–39 |
| Phyllomedusa azurea | Hylidae | 36–37 |
| Limnomedusa macroglossa | Cycloramphidae | 38 |
| Dermatonotus muelleri | Microhylidae | 28–30 |
| Crossodactylus schmidti | Hylodidae | 36–38 |
| Physalaemus sp. | Leiuperidae | 39 |
| Lepidobatrachus llanensis | Ceratophryidae | 36–37 |
| Leptodactylus latrans | Leptodactylidae | 37–38 |
| Lithobates catesbeianus | Ranidae | 33–36 |
| Xenopus laevis | Pipidae | 46–56 |
Light microscopy
Tadpoles were anaesthetised by immersion in a 0.1% solution of tricaine methanesulfonate (MS222; Sigma, St. Louis, MO, USA) and fixed in Bouin's solution for 24 h at 4 °C. They were then dehydrated, cleared in xylene, and embedded in Histoplast (Biopack, Buenos Aires, Argentina). Serial paraffin sections (7 μm thick) were cut, mounted on HiFix glass slides (HF-5001; InProt, Buenos Aires, Argentina), and subjected to immunohistochemistry and lectin histochemistry or stained using classical histological techniques (cresyl violet).
Immunohistochemistry and lectin histochemistry
General procedures for immunohistochemistry were followed as in our previous report (Jungblut et al., 2009). The primary antibodies used was rabbit anti-Gαo, 1 : 12 000 (sc-387; Santa Cruz). After primary antibody incubation (overnight at 4 °C), sections were treated with the appropriate biotinylated secondary antibody (Vector Laboratories, Burlingame, CA, USA), followed by avidin–biotin horseradish peroxidase complex (Vectastain ABC Kit; Vector Laboratories). The reaction was developed with the 3,3′-diaminobenzidine tetrahydrochloride (DAB) Staining Kit (Dako, Glostrup, Denmark). Omission of the primary antiserum (negative control) produced negligible background staining (data not shown).
To facilitate the identification of glomerular structures in the AOB, lectin histochemistry staining was performed using lectin soybean agglutinin (SBA), which has been shown to stain the AOB in several amphibian species (Meyer et al., 1996). After deparaffinisation and rehydration, tissue sections were blocked for nonspecific binding, as described above, incubated with biotin-labelled SBA (Vector Labs) overnight at 4 °C, and treated with streptavidin–FITC complex (Sigma).
FITC-treated sections were counterstained with propidium iodide (P-1304; Molecular Probes, Eugene, OR, USA), and images were captured with a confocal laser microscope (Olympus FV-300 attached to an Olympus Bx-61 microscope).
Tract-tracing techniques
Animals were transferred to a humidified chamber; under anaesthesia (see above), the remaining water in the olfactory cavity was carefully dried prior to the application of neuronal tracers. A dorsal approach through the external nares was used. Alexa Fluor 680 conjugated to dextran amine (680DA 3 kD; Molecular Probes) was applied by impaling the VNO with a glass micropipette (680DA had been recrystallised from a saturated solution in distilled water onto the tip of the micropipette). Tetramethylrhodamine-conjugated dextran amine (TMRDA 3 kDa; Molecular Probes) was applied to the contralateral OE following the methods described above. Dye-injected animals were maintained individually for 24 h at room temperature. After this survival time, tadpoles were fixed in Bouin's solution and processed for paraffin sections (15 μm thick), as described earlier. Images were captured with a confocal laser microscope (Olympus FV-300 attached to an Olympus Bx-61 microscope).
Combined tract-tracing/immunohistochemical techniques
Under anaesthesia, 680DA 3 kDa (Molecular Probes) was applied by impaling the VNO with a glass micropipette following methods described above. After 24 h at room temperature animals were fixed in Bouin's solution and processed for paraffin sections (15 μm thick) as described earlier. Sections were deparaffinised, rehydrated and incubated overnight at 4 °C with primary antibody (mouse anti-Synaptophysin 1/100; Sigma). Then, sections were treated with the appropriate biotinylated secondary antibody (Vector Laboratories) followed by incubation with FITC-labelled streptavidin (Sigma), and mounted for confocal analysis.
Results
Nasal region and vomeronasal organ
We analyzed the entire nasal region in tadpoles of 13 anuran species performing serial sections in the transverse and sagittal planes. In all 13 of the species that we examined, the VNO was a bean-shaped structure in the anteromedial nasal region (Fig. 1). Schematic illustration in Fig. 1A summarizes the general anatomical organization of the chemosensory nasal structures (OE and VNO) in a tadpole. The sensory epithelium of the VNO (composed of supporting, basal, and sensory cells) lay in a separate accessory chamber, or diverticulum, of the nasal cavity, located anteroventral to the OE of the principal chamber (Fig. 1A–D). Notably, the positioning of the VNO within the nasal region was remarkably conserved among the tadpoles. In a rostrocaudal direction, the VNO was the first nasal chemosensory structure and was located dorsolateral to the cornua trabeculae. As an example, histological parasagittal sections of the mesobatrachian X. laevis and the neobatrachians R. arenarum and L. catesbeianus are shown in Fig. 1B,C,D, respectively. The VNO lumen opened into the anterior wall of the principal nasal chamber (Fig. 1D).
Fig. 1.
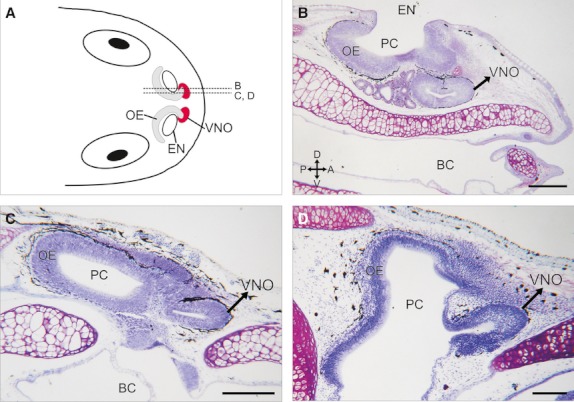
Nasal region in anuran tadpoles. (A) Schematic illustration of the nasal region of a generalised tadpole (dorsal view). Dotted lines (B, C, and D) indicate the estimated planes of the parasagittal sections shown in panels B–D. (B–D) Histological parasagittal sections (stained with cresyl violet) through the nasal region of X. laevis (B, stage N/F54), R. arenarum (C, stage G35), and L. catesbeianus (D, stage G34) showing the relative position of the bean-shaped VNO anteroventral to the olfactory epithelium in the principal chamber (PC). BC, buccal cavity; EN, external naris. Axes indicate: A, anterior; P, posterior; D, dorsal; V, ventral. Scale bar, 200 μm.
To evaluate the maturational state of the VNS in tadpoles, we assessed the expression of the second messenger protein Gαo, a marker specific to vomeronasal neurones in tetrapods (Fig. 2). The VNO originated as a ventral evagination of the rostral portion of the olfactory pit during the late embryonic stages, around the time of development of the operculum. From this early stage, developing sensory neurones from the VNO exhibited strong Gαo expression (Fig. 2A–C). Moreover, at the first larval stages (G26), these Gαo-expressing cells acquired the typical bipolar morphology of chemosensory neurones (Fig. 2D,E). Dendrites of the vomeronasal sensory neurones projected toward the luminal surface of the VNO, whereas axons ran toward the basal lamina of the VNO, forming obvious axon bundles at the lamina propria. As the larvae developed, the VNO continued growing and expanding medially, forming a medial diverticulum at the rostral end of the nasal region (Fig. 2G–L).
Fig. 2.
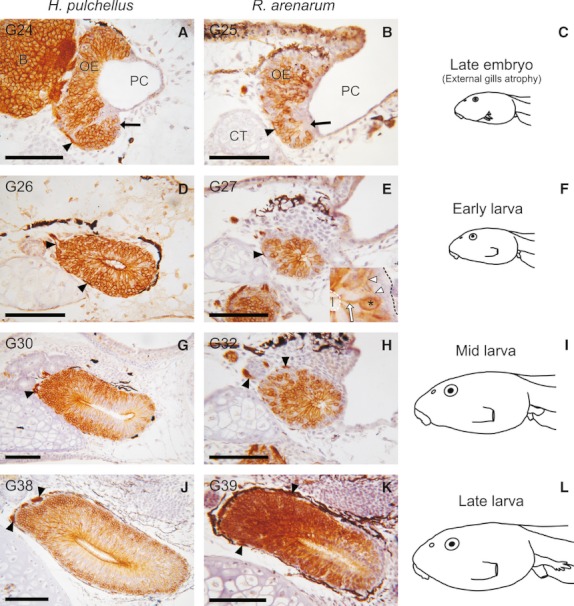
Gαo protein expression in the vomeronasal organ (cross sections, left side) of H. pulchellus (A, D, G, and J) and R. arenarum (B, E, H, and K) throughout larval development. Panels in the right column (C, F, I, and L) show a schematic lateral view of the estimated developmental stages used in both species. The specific developmental stage is shown in the top-left corner in each panel. (A and B) At the final embryonic stages (C), the vomeronasal organ (VNO) (arrows) evaginates from the rostral portion of the olfactory pit. Axons of the Gαo-expressing cells in the VNO project to the anterior telencephalon and form axon bundles in the lamina propria (arrowheads). (D, E) At early larval stages (F), the Gαo-expressing cells in the VNO acquire a bipolar morphology. Inset in (E) shows a higher magnification of a bipolar vomeronasal chemosensory neurone. Asterisk, cell body; white arrow, dendrite; white arrowheads, axon; black dotted line, basal lamina; white dotted line, luminal surface; l, lumen of the VNO. (G, H) VNO at mid-larval stages (I). (J, K) At late larval stages (L), Gαo protein is strongly expressed in the conspicuous VNO. B, brain; CT, cornua trabeculae; PC, principal chamber. Top is dorsal and left is medial. Scale bar, 100 μm.
Accessory olfactory bulb
The general morphology of the anterior portion of the telencephalon was very similar in all 13 species. The olfactory nerve entered the forebrain at the ventral zone in the rostral end of the telencephalon. The MOB was present as a laminar structure, as in all vertebrates (nerve, glomerular, external plexiform, mitral cell, internal plexiform, and granular layers). An AOB was apparent in the ventrolateral side of the anterior telencephalon, somewhat caudal to the MOB. The presence of an AOB was assessed by histological staining of the anterior brain serial sections (Fig. 3A,B). The AOB was represented as an anteroposteriorly elongated, ovoid nucleus. As in the MOB, the AOB was organised in layers. The glomerular layer was located anterolaterally in the AOB, whereas bodies of the mitral cells were localised in the mid-caudal portion of the AOB.
Fig. 3.
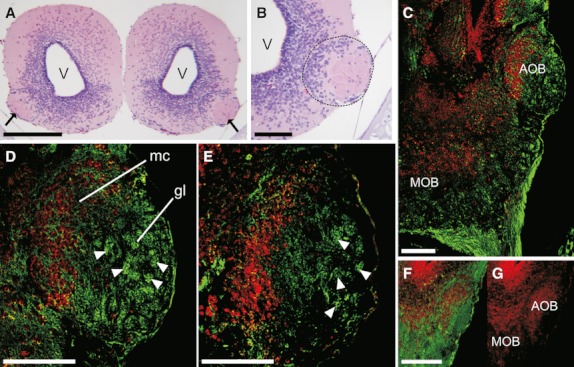
Accessory olfactory bulb in tadpoles. (A) Histological cross section of the anterior telencephalon of H. pulchellus (stage G34) showing a discernible accessory olfactory bulb (AOB) in the ventrolateral side (arrows); top is dorsal. (B) Higher magnification of the left AOB (demarcated by a black dotted line) shown in (A). (C–F) soybean agglutinin (SBA)-lectin staining (green) in tadpole horizontal sections. Nuclei were counterstained with propidium iodide (PI, red). (C) Anterior telencephalon (left side) of Crossodactylus schmidti. (D) Higher magnification of the AOB shown in (C). (E) Detail of the AOB of Leptodactylus latrans. (F and G) Anterior telencephalon (left side) of Dermatonotus muelleri; SBA-lectin and PI staining (F) and PI alone (G). Arrowheads show representative glomerular structures in the AOB. gl, glomerular layer; mc, mitral cell layer; MOB, main olfactory bulb. Scale bars, 200 μm (A and C) and 100 μm (B and D–F).
The use of lectin histochemistry has proven to be an excellent tool for the study of morphological aspects in the olfactory system of vertebrates (Endo et al., 2011). In particular, soybean agglutinin (SBA) has been used to stain specifically the AOB in several amphibian species (Meyer et al., 1996). SBA histochemistry revealed spherical neuropil structures, called glomeruli, in the AOB (Fig. 3C–E). Well-developed glomeruli were observed in the AOB of all of the tadpole species, except for Scinax acuminatus and Dermatonotus muelleri, in which the glomerular layer of the AOB appeared as a nonstructured fiber meshwork (Fig. 3F,G).
To characterise the VNS in tadpoles more thoroughly, we analysed the efferent projections of the VNO. Tract-tracing experiments demonstrated that axons of the vomeronasal sensory neurones reached the AOB in the anterior telecephalon, whereas sensory neurones from the OE projected their axons to the MOB (Fig. 4A–C). Axons of vomeronasal sensory neurones entered the AOB by the frontal side and formed spheroidal arborisations (glomeruli) in the glomerular layer. In some specimens, a few tract-positive glomeruli were observed in the MOB when the ipsilateral VNO was impaled (see Fig. 4E). Nevertheless, we do not consider that these are vomeronasal sensory neurones projecting to the MOB. Instead, this unexpected result may have been an artefact associated with the method of impaling we used. The anterior border of the OE is closely related to the VNO (see the schematic illustration of the nasal region in Fig. 1A); thus, the olfactory neurones of this zone are extremely difficult to avoid when the VNO is impaled in intact animals.
Fig. 4.
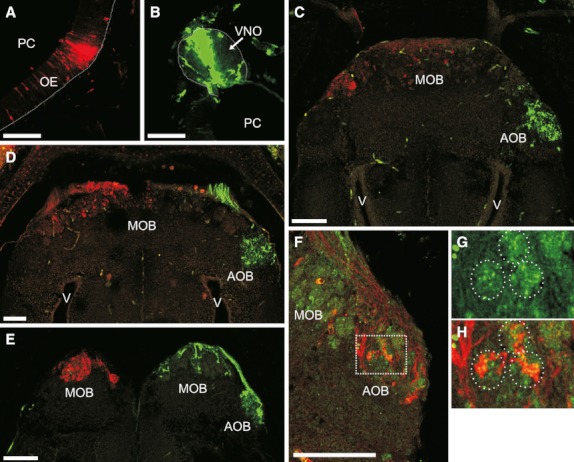
Vomeronasal sensory neurones establish synaptic contact with the accessory olfactory bulb (AOB). (A–E) Horizontal sections of tadpoles after application of the neuronal tracers TMRDA [left olfactory epithelium (OE), red] and 680DA [right vomeronasal organ (VNO), green]. (A–C) R. arenarum (stage G32) showing the injection sites in the OE (A) and VNO (B) and the corresponding projection sites in the anterior brain (the MOB and the AOB, respectively) (C). (D) Anterior brain of H. pulchellus (stage G30). (E) Anterior brain of X. laevis (stage N/F54). (F-H) Double labelling using 680DA (red) and synaptophysin-like immunodetection (green). (F) Horizontal sections of the anterior brain (right side) of R. arenarum at G35. G and H show high-magnification views of the boxed area in (F). The same three glomeruli are identified by circular dotted lines in G (synaptophysin-like immunoreactivity alone) and H (double labelling image). PC, principal chamber; MOB, main olfactory bulb; V, ventricle. Dotted line delimits the basal lamina in (A and B). Top is anterior in all panels, left is lateral in (A, F, and G) and medial in (B). Scale bar, 100 μm.
To evaluate synaptic connections between vomeronasal sensory neurones and the brain, we performed double labelling experiments using tract-tracing and synaptophysin-like immunodetection techniques. This experiment demonstrated that axons from the VNO had established synaptic contact with telencephalic neurones (mitral cells in the AOB) during the larval phase (Fig. 4F–H). As in the results obtained using lectin histochemistry, tract tracing and synaptophysin immunoreactivity revealed the presence of glomerular structures in the AOB (Fig. 4G,H).
Discussion
We found that tadpoles of all 13 of the species that we examined possess an anatomically fully developed VNS, with morphological and biochemical characteristics similar to those observed in adult anurans and other tetrapods. To the best of our knowledge, this is the first comparative study of the VNS in anuran larvae that integrates morphological and biochemical data. The general anatomical organisation of the VNS (summarised in Fig. 5) appears to be highly conserved among anuran tadpoles. It is represented by the VNO (bean-shaped structure), located in the medial nasal wall anteroventral to the OE, and the AOB, ventrolaterally positioned in the anterior telencephalon, somewhat caudal to the MOB. Interestingly, tadpoles of the basal anuran Ascaphus truei do not show this generalised organisation in the VNS (Benzekri & Reiss, 2011). In that species, the VNO has a completely different anatomical position; it is located in the ventrolateral side of the mid-nasal region. Ascaphus truei belong to the anuran family Leiopelmatidae, the most basal of extant anurans (Frost et al., 2006). They have various specific characteristics in the nasal organs that have not been observed in other anurans (Benzekri & Reiss, 2011).
Fig. 5.
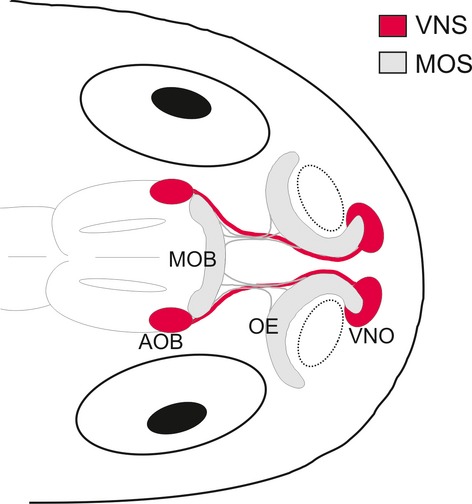
Schematic drawing representing the anatomical organisation of the vomeronasal system (VNS) in a generalised tadpole. The main olfactory system (MOS) is also shown. AOB, accessory olfactory bulb; OE, olfactory epithelium; MOB, main olfactory bulb; VNO, vomeronasal organ. Dotted-line circles indicate the position of the external nares.
Development of the VNO occurs very early in H. pulchellus and R. arenarum, during the final embryonic stages, meaning that the VNO, functional or not, is present during the entire larval phase. The early appearance of the VNO during development is a widespread characteristic of most tadpoles described to date (Cooper, 1943; Tsui, 1946; Nieuwkoop & Faber, 1994; Taniguchi et al., 1996; Jermakowicz et al., 2004; Wang et al., 2008; Jungblut et al., 2011). Moreover, the early appearance of the VNO during development seems to be a shared characteristic with other vertebrates, such as mammals (Garrosa et al., 1998) and reptiles (Holtzman & Halpern, 1990). Apparently, tadpoles of the amphibians Bufo americanus and Bufo regularis would be exceptional cases, in which the VNO appears during mid-larval stages, at G34 and G30–32, respectively (Khalil, 1978; Jermakowicz et al., 2004).
Robust evidence of the early appearance and maturation of the VNO in tadpoles was provided by Hansen et al. (1998), who performed an ontogenetic ultrastructural analysis of X. laevis olfactory organs. They found that differentiated neurones are present in the VNO as early as stage N/F42. Moreover, whereas neurones of the principal chamber dramatically change at metamorphosis (changing from water-sensitive to air-sensitive epithelium), the ultrastructural characteristics of the larval VNO remain in the adult (Hansen et al., 1998). This result strongly suggests that significant maturation of the VNO is achieved during the larval period in X. laevis.
A particularly interesting finding of the present work is the occurrence of the second messenger protein Gαo in the VNO of tadpoles, starting at early stages of development. Moreover, shortly after organogenesis of the VNO, Gαo-expressing cells acquire the typical bipolar morphology of chemical sensory neurones (olfactory and vomeronasal). These data indicate that a significant maturation of the transduction system occurs in larval vomeronasal sensory neurones.
Gαo-expressing neurones have been described in the VNO of mammals (Jia & Halpern, 1996; Halpern & Martinez-Marcos, 2003), reptiles (Luo et al., 1994; Murphy et al., 2001) and adult amphibians (Hagino-Yamagishi et al., 2004; Date-Ito et al., 2008; Hagino-Yamagishi & Nakazawa, 2011). Moreover, Gαo protein expression was described previously in the VNO of tadpoles of X. laevis and R. arenarum (Hagino-Yamagishi et al., 2004; Jungblut et al., 2009). Gαo-expressing neurones have even been found in the lungfish P. dolloi, in structures identified as ‘vomeronasal-like’ (Gonzalez et al., 2011).
G proteins play an important role in signal transduction in chemosensory neurones (Jia & Halpern, 1996) and different G protein transduction systems are coupled to different receptor families (Halpern & Martinez-Marcos, 2003). In the mammal VNO, two large and divergent families of receptors have been identified: vomeronasal receptor type I (VR1) and vomeronasal receptor type II (VR2), which encode G protein-coupled seven-transmembrane proteins (Matsunami & Buck, 1997; Ryba & Tirindelli, 1997). Neurones that express either V1R or V2R also coexpress Gαi2 or Gαo, respectively. V1R and Gαi2 coexpressing neurones have been described only in the VNO of mammals (Jia & Halpern, 1996; Halpern et al., 1998), whereas V2R and Gαo coexpressing neurones are a common feature in the VNO of mammal and non-mammal tetrapods (Hagino-Yamagishi et al., 2004; Date-Ito et al., 2008). Our findings suggest that V2R receptors (Gαo-expressing cells) are a common characteristic in the VNO of anuran larvae. This hypothesis has been corroborated in X. laevis tadpoles (Hagino-Yamagishi et al., 2004).
Tract-tracing experiments demonstrated that chemosensory neurones from the VNO reach specific areas in the telencephalon, in which there is a discernible AOB. Positioning of the AOB in the anterior brain was highly conserved among the anuran tadpoles included in our study. Moreover, this anatomical organisation is maintained in adult anurans (Meyer et al., 1996).
In the present study, we demonstrated the presence of glomerular structures in the AOB of tadpoles. Glomeruli are formed by synaptic contacts between axons of the chemosensory neurones and dendrites of the mitral/tufted cells. They represent the first relay station in the olfactory pathway and are suggested to represent functional units in olfactory information processing. In X. laevis tadpoles, specific glomeruli are activated in the MOB when the OE is exposed to amino acids, whereas only spontaneous activity has been reported in glomeruli of the AOB (Manzini & Schild, 2010). Interestingly, structural analysis of the olfactory bulb in premetamorphic tadpoles of X. laevis showed that minor differences exist between glomeruli in the AOB and MOB. Moreover, the total number of glomeruli in the AOB is higher than in the MOB (approximately 350 and 234, respectively) (Manzini & Schild, 2010). In our opinion, glomerular activity in the AOB has not been characterised in tadpoles because nothing is known about the natural odorants/pheromones that stimulate it, rather than because of physiological immaturity of the sensory system.
In conclusion, our work demonstrates that an accessory olfactory system that is anatomically fully developed is present in anuran larvae. Moreover, our results indicate that a significant degree of maturation is achieved in the larval VNS, which suggests that it could be a functional sensory system in tadpoles. Recent molecular data strongly suggest that the VNS appeared early in vertebrate evolution (Grus & Zhang, 2009). Moreover, the presence of a VNS in lungfish indicates that this sensory system was already present in the aquatic last common ancestor of lungfish and tetrapods (Gonzalez et al., 2011). Within this context, tadpoles could be helpful models with which to investigate the anatomical, biochemical and functional aspects of the VNS in an aquatic environment.
Acknowledgments
This study was supported by grants from Universidad de Buenos Aires (UBACyT 0793 and 0068) and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP-CONICET 00221). We gratefully acknowledge Dr Julian Faivovich (Museo Argentino de Ciencias Naturales Bernardino Rivadavia) for kindly providing us with material examined in this study.
Appendix 1
Specimens examined and collection data
JF1598: Scinax acuminatus from Laguna Yema, Formosa, Argentina. JF326: Hypsiboas curupi from Campo Experimental INTA ‘Cuartel Rio Victoria’, San Vicente, Departamento Guarani, Misiones, Argentina. JF1765: Phylomedusa azurea from Resistencia, Chaco, Argentina. JF220: Limnomedusa macroglossa from Misiones, Argentina. JF1153: Crossodactylus schmidti from Campo Experimental INTA ‘Cuartel Rio Victoria’, San Vicente, Departamento Guarani, Misiones, Argentina. JF1622: Physalaemus sp. from Laguna Yema, Formosa, Argentina. Not classified: Dermatonotus muelleri from Laguna Yema, Formosa, Argentina. JF1498: Lepidobatrachus llanensis from Laguna Yema, Formosa, Argentina. Not classified: Leptodactylus latrans, locality unknown.
References
- Alving WR, Kardong KV. The role of the vomeronasal organ in rattlesnake (Crotalus viridis oreganus) predatory behavior. Brain Behav Evol. 1996;48:165–172. doi: 10.1159/000113195. [DOI] [PubMed] [Google Scholar]
- Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 2006;29:1–7. doi: 10.1016/j.tins.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Benzekri NA, Reiss JO. Olfactory metamorphosis in the coastal tailed frog Ascaphus truei (Amphibia, Anura, Leiopelmatidae) J Morphol. 2011;273:68–87. doi: 10.1002/jmor.11008. [DOI] [PubMed] [Google Scholar]
- Bertmar G. Evolution of vomeronasal organs in vertebrates. Evolution. 1981;35:359–366. doi: 10.1111/j.1558-5646.1981.tb04893.x. [DOI] [PubMed] [Google Scholar]
- Cooper RS. An experimental study of the development of the larval olfactory organ of Rana pipiens Schreber. J Exp Zool. 1943;93:415–451. [Google Scholar]
- Date-Ito A, Ohara H, Ichikawa M, et al. Xenopus V1R vomeronasal receptor family is expressed in the main olfactory system. Chem Senses. 2008;33:339–346. doi: 10.1093/chemse/bjm090. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Adkins-Regan E, Halpern BP. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav Evol. 1997;49:53–62. doi: 10.1159/000112981. [DOI] [PubMed] [Google Scholar]
- Eisthen HL. Phylogeny of the vomeronasal system and of receptor cell types in the olfactory and vomeronasal epithelia of vertebrates. Microsc Res Tech. 1992;23:1–21. doi: 10.1002/jemt.1070230102. [DOI] [PubMed] [Google Scholar]
- Eisthen HL. Presence of the vomeronasal system in aquatic salamanders. Philos Trans R Soc Lond B Biol Sci. 2000;355:1209–1213. doi: 10.1098/rstb.2000.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo D, Yamamoto Y, Nakamuta N, et al. Developmental changes in lectin-binding patterns of three nasal sensory epithelia in Xenopus laevis. Anat Rec (Hoboken) 2011;294:839–846. doi: 10.1002/ar.21377. [DOI] [PubMed] [Google Scholar]
- Frost D, Grant T, Faivovich J, et al. The amphibian tree of life. Bull AMNH. 2006;297:1–370. [Google Scholar]
- Garrosa M, Gayoso MJ, Esteban FJ. Prenatal development of the mammalian vomeronasal organ. Microsc Res Tech. 1998;41:456–470. doi: 10.1002/(SICI)1097-0029(19980615)41:6<456::AID-JEMT2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Morona R, Lopez JM, et al. Lungfishes, like tetrapods, possess a vomeronasal system. Front Neuroanat. 2011;4:130. doi: 10.3389/fnana.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Grus WE, Zhang J. Origin of the genetic components of the vomeronasal system in the common ancestor of all extant vertebrates. Mol Biol Evol. 2009;26:407–419. doi: 10.1093/molbev/msn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagino-Yamagishi K, Nakazawa H. Involvement of Galpha(olf) -expressing neurons in the vomeronasal system of Bufo japonicus. J Comp Neurol. 2011;519:3189–3201. doi: 10.1002/cne.22671. [DOI] [PubMed] [Google Scholar]
- Hagino-Yamagishi K, Moriya K, Kubo H, et al. Expression of vomeronasal receptor genes in Xenopus laevis. J Comp Neurol. 2004;472:246–256. doi: 10.1002/cne.20073. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Halpern M, Jia C, Shapiro LS. Segregated pathways in the vomeronasal system. Microsc Res Tech. 1998;41:519–529. doi: 10.1002/(SICI)1097-0029(19980615)41:6<519::AID-JEMT7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hansen A, Reiss JO, Gentry CL, et al. Ultrastructure of the olfactory organ in the clawed frog, Xenopus laevis, during larval development and metamorphosis. J Comp Neurol. 1998;398:273–288. doi: 10.1002/(sici)1096-9861(19980824)398:2<273::aid-cne8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Holtzman DA, Halpern M. Embryonic and neonatal development of the vomeronasal and olfactory systems in garter snakes (Thamnophis spp.) J Morphol. 1990;203:123–140. doi: 10.1002/jmor.1052030202. [DOI] [PubMed] [Google Scholar]
- Jermakowicz WJ, III, Dorsey DA, Brown AL, et al. Development of the nasal chemosensory organs in two terrestrial anurans: the directly developing frog, Eleutherodactylus coqui (Anura: Leptodactylidae), and the metamorphosing toad, Bufo americanus (Anura: Bufonidae) J Morphol. 2004;261:225–248. doi: 10.1002/jmor.10246. [DOI] [PubMed] [Google Scholar]
- Jia C, Halpern M. Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Gi alpha 2 and G(o alpha)) and segregated projections to the accessory olfactory bulb. Brain Res. 1996;719:117–128. doi: 10.1016/0006-8993(96)00110-2. [DOI] [PubMed] [Google Scholar]
- Jungblut LD, Paz DA, Lopez-Costa JJ, et al. Heterogeneous distribution of G protein alpha subunits in the main olfactory and vomeronasal systems of Rhinella (Bufo) arenarum tadpoles. Zool Sci. 2009;26:722–728. doi: 10.2108/zsj.26.722. [DOI] [PubMed] [Google Scholar]
- Jungblut LD, Pozzi AG, Paz DA. Larval development and metamorphosis of the olfactory and vomeronasal organs in the toad Rhinella (Bufo) arenarum (Hensel, 1867) Acta Zool (Stockholm) 2011;92:305–315. [Google Scholar]
- Khalil SH. Development of the olfactory organ of the Egyptian Toad, Bufo regularis Reuss. I. Larval period. Folia Morphol (Prague) 1978;26:69–74. [PubMed] [Google Scholar]
- Luo Y, Lu S, Chen P, et al. Identification of chemoattractant receptors and G-proteins in the vomeronasal system of garter snakes. J Biol Chem. 1994;269:16867–16877. [PubMed] [Google Scholar]
- Manzini I, Schild D. Olfactory coding in larvae of the African Clawed Frog Xenopus laevis. In: Menini A, editor. The Neurobiology of Olfaction. Boca Raton, FL: CRC Press; 2010. pp. 113–119. Chapter 4. [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Meyer DL, Jadhao AG, Kicliter E. Soybean agglutinin binding by primary olfactory and primary accessory olfactory projections in different frogs. Brain Res. 1996;722:222–226. doi: 10.1016/0006-8993(96)00084-4. [DOI] [PubMed] [Google Scholar]
- Murphy FA, Tucker K, Fadool DA. Sexual dimorphism and developmental expression of signal-transduction machinery in the vomeronasal organ. J Comp Neurol. 2001;432:61–74. doi: 10.1002/cne.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlin LP, Schild D. Individual olfactory sensory neurons project into more than one glomerulus in Xenopus laevis tadpole olfactory bulb. J Comp Neurol. 2005;481:233–239. doi: 10.1002/cne.20390. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North-Holland; 1994. [Google Scholar]
- Paz DA, Alonso DG, Pisano A, et al. Expression of isoforms of the neural cell adhesion molecule (NCAM) and polysialic acid during the development of the Bufo arenarum olfactory system. Int J Dev Biol. 1995;39:1005–1013. [PubMed] [Google Scholar]
- Placyk JS, Jr, Graves BM. Prey detection by vomeronasal chemoreception in a plethodontid salamander. J Chem Ecol. 2002;28:1017–1036. doi: 10.1023/a:1015213918739. [DOI] [PubMed] [Google Scholar]
- Reiss JO, Burd GD. Metamorphic remodeling of the primary olfactory projection in Xenopus: developmental independence of projections from olfactory neuron subclasses. J Neurobiol. 1997;32:213–222. doi: 10.1002/(sici)1097-4695(199702)32:2<213::aid-neu6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron. 1997;19:371–379. doi: 10.1016/s0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Swann J, Rahaman F, Bijak T, et al. The main olfactory system mediates pheromone-induced fos expression in the extended amygdala and preoptic area of the male Syrian hamster. Neuroscience. 2001;105:695–706. doi: 10.1016/s0306-4522(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Toshima Y, Saito TR. Development of the olfactory epithelium and vomeronasal organ in the Japanese reddish frog, Rana japonica. J Vet Med Sci. 1996;58:7–15. doi: 10.1292/jvms.58.7. [DOI] [PubMed] [Google Scholar]
- Tsui CL. Development of olfactory organ in Rana nigromaculata. Q J Microsc Sci. 1946;87:61–90. [PubMed] [Google Scholar]
- Wang H, Zhao H, Tai F, et al. Postembryonic development of the olfactory and vomeronasal organs in the frog Rana chensinensis. Zool Sci. 2008;25:503–508. doi: 10.2108/zsj.25.503. [DOI] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, et al. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yvroud M. Développement de l'organe olfactif et des glandes annexes chez Alytes obstetricans Laurenti au cours de la vie larvaire et de la métamorphose. Arch Anat Microsc. 1966;55:387–410. [Google Scholar]


